Abstract
Major depressive disorder (MDD) is one of the leading causes of morbidity and all-cause mortality (including suicide) worldwide, and, unfortunately, first-line monoaminergic antidepressants and evidence-based psychotherapies are not effective for all patients. Subanesthetic doses of the N-methyl-D-aspartate receptor antagonists and glutamate modulators ketamine and S-ketamine have rapid and robust antidepressant efficacy in such treatment-resistant depressed patients (TRD). Yet, as with all antidepressant treatments including electroconvulsive therapy (ECT), not all TRD patients adequately respond, and we are presently unable to a priori predict who will respond or not respond to ketamine. Therefore, antidepressant treatment response biomarkers to ketamine have been a major focus of research for over a decade. In this article, we review the evidence in support of treatment response biomarkers, with a particular focus on genetics, functional magnetic resonance imaging, and neurophysiological studies, i.e. electroencephalography and magnetoencephalography. The studies outlined here lay the groundwork for replication and dissemination.
Keywords: ketamine, glutamate, major depressive disorder, treatment resistant depression, genetics, functional magnetic resonance imaging, electroencephalography, magnetoencephalography
Introduction
Major depressive disorder (MDD) is a disabling and potentially lethal psychiatric illness with, in 2017, a global prevalence of 2-6%. 1 In the United States, this prevalence was 7%, with 64% of those having severe impairment as a result. 2 Given the high burden of suffering and increased risk of suicidal thoughts and behaviors, fast-acting and highly efficacious treatments are needed. Most available medications for MDD are monoamine reuptake inhibitors; although effective for many patients, e.g. 40-60% response, 3 they are not effective for all patients. In 2013, up to 20% of depressed patients were treatment resistant (TRD), defined as failing two or more adequate antidepressant trials. 4 Moreover, antidepressant response is often delayed with monoamine-based medications, which is problematic for patients with severe depression and/or acute suicidality, especially if managed on an outpatient basis.
The N-methyl-D-aspartate receptor antagonist and glutamate modulator racemic ketamine and its (S)-enantiomer at subanesthetic doses have antidepressant efficacy in TRD. 56 The response rate has been shown to be as high as 70% in mostly Caucasian samples, 7 making it more effective than monoaminergic drugs, though there is variability in ketamine’s efficacy depending on the study. Antidepressant efficacy is also seen within hours-to-days, rather than weeks-to-months compared to standard antidepressant medications. 8 For these reasons, subanesthetic dose ketamine has been described as the “most important discovery in half a century” for the treatment of major depression. 9 Still, not all patients have an antidepressant response to subanesthetic dose ketamine; therefore, it is critical to have reliable and reproducible biomarkers to guide treatment selection and monitor biological response.
This manuscript reviews the ketamine antidepressant biomarker literature in clinical studies, with emphases on genetics, neuroimaging, and neurophysiology. PubMed was searched from inception until December 2020, with a specific focus on publications that report associations between depression score changes and genetics, functional magnetic resonance imaging, electroencephalography, and magnetoencephalography. Publications were excluded if they did not fit these methodological categories, if they did not explicitly mention correlation with antidepressant efficacy, or if they were done in non-depressed samples, e.g., healthy volunteers. Some example PubMed searches included “ketamine AND depression,” “ketamine AND genetics AND depression,” “ketamine AND functional connectivity AND depression,” “ketamine AND functional magnetic resonance imaging AND depression,” “ketamine AND electroencephalography AND depression,” and “ketamine AND magnetoencephalography AND depression.”
Genetics
Overall, the ketamine depression literature is unfortunately sparse on this topic. The single nucleotide polymorphism (SNP) that has been studied most extensively is the rs6265 (val66met) allele in the brain-derived neurotrophic factor (BDNF) gene. The met allele of this polymorphism is associated with impairments in the activity-dependent secretion of BDNF, 10 resulting in reduced synaptogenesis and dendritic complexity in rodents. 11 Because ketamine’s mechanism of action is thought to require BDNF, this SNP is a candidate for response prediction. Laje et al. found that depressed met heterozygous participants of European ancestry had less improvement in depression scores 4 hours after ketamine infusion than val/val patients. 12 In this sample, BDNF rs6265 genotype accounted for 28% of the response variance.
As alluded to above, there are differences in the prevalence of val and met alleles depending on ethnicity. For example, the met allele frequency in Taiwanese and Han Chinese populations is around 50% 13 while in Caucasian populations it is around 20%. 14 Su et al. explored this relationship, where they looked specifically at treatment resistant Taiwanese patients who received 0.2 or 0.5 mg/kg ketamine infusions. 13 Albeit likely underpowered, there was no statistically significant antidepressant difference between val homozygous and met allele carriers. Using the same dataset, Chen et al. assessed improvement in suicidal ideation items on the Hamilton Depression Rating Scale (HAM-D) and Montgomery Åsberg Depression Rating Scale (MADRS) based on rs6265 genotype. 15 Although the strength of their results varied by measure, val allele carriers displayed an anti-suicidal effect to both ketamine doses, while met homozygotes either improved less overall or required the higher dose for an anti-suicidal effect.
Other studies have attempted to demonstrate a genetic underpinning of ketamine’s antidepressant response. Guo used 157 participants of European descent in a genome wide association study (GWAS) to find SNPs or other genetic variants. Although no marker reached genome-wide significance, 31 SNPs and 8 linkage disequilibrium (LD)-independent loci had a trending relationship. 16 These cumulative results reaffirm the hypothesis that, like major depression itself, the genetic factors mediating treatment response are heterogeneous, and such complex relationships are likely multifactorial and combinatorial. As an example, Niciu et al. correlated subcortical brain volume and genotype to antidepressant response to ketamine. 17 Su et al. 13 and Chen et al. 15 also considered ketamine blood concentration and serum BDNF levels, respectively, and found no associations with genotype and treatment response. In the future, preclinical and clinical studies may want to consider how glutamate pathway genes and other prosynaptic plasticity pathways influence the antidepressant response to ketamine.
Functional Connectivity
Functional Magnetic Resonance Imaging (fMRI)
Resting-State
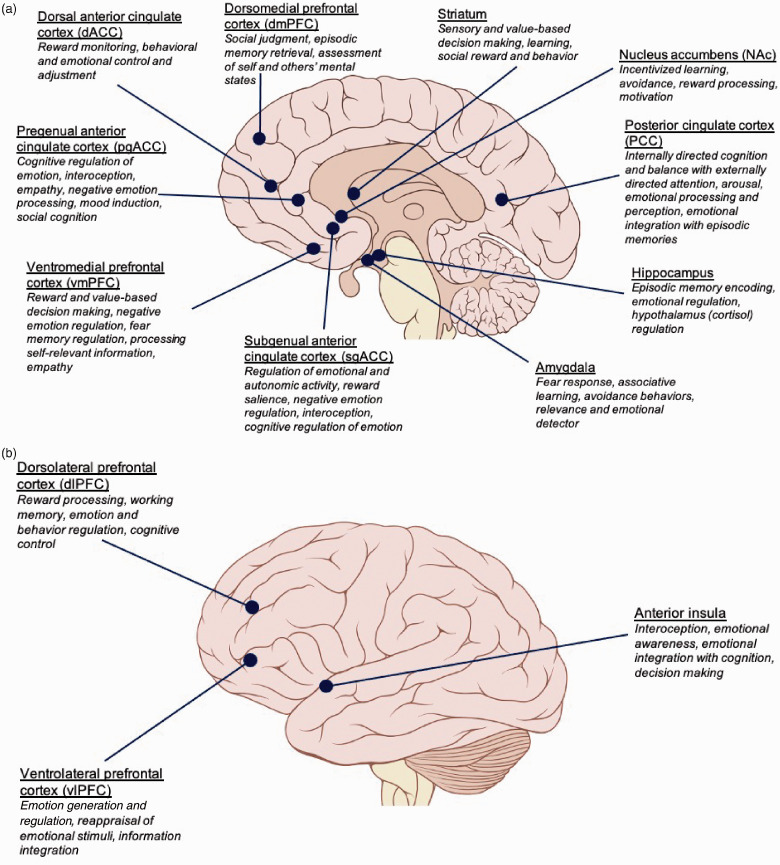
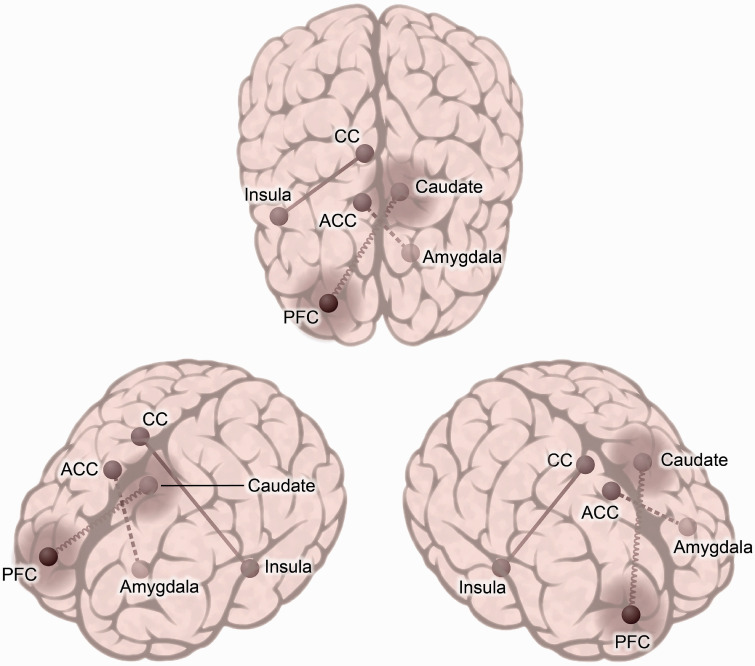
Ketamine has been shown to modulate neural networks involved in depression pathology, and its efficacy correlates with normalization of region and pathway deficits. 18 The studies to be discussed used both seed-based/region of interest (ROI) analyses and seed-independent approaches, e.g. global brain connectivity (GBC). ROIs in MDD pathology and ketamine depression studies and their proposed functions are diagrammed in Figure 1 and the connectivity relationships found most consistently are diagrammed in Figure 2.
Figure 1.
Brain regions implicated in the antidepressant response to ketamine and their emotional processing correlates. Sagittal (a) and lateral (b) views of the brain outlining the main emotional functions of regions implicated in ketamine’s antidepressant efficacy.
Figure 2.
Common functional connectivity pathways implicated in ketamine’s antidepressant response. Functional connections outlined here are referenced throughout this review and have been found to be correlated with ketamine’s antidepressant effect. The relationship of the connection to antidepressant response is indicated by the line type, where a solid straight line corresponds to increased connectivity, a dotted line represents decreased connectivity, and a coiled line signifies mixed reports, i.e. some studies with increased and others with decreased connectivity in this circuit. A “halo” effect represents global brain connectivity (GBC), where the strength of the connectivity of the prefrontal cortex and caudate with the rest of the brain is related to antidepressant response. The laterality of these regions were mixed across the studies, except for the left amygdala, which was consistently reported. As alluded to above, there is a broad range of heterogeneity, i.e. task used, time of measurement (in relation to ketamine administration), and functional analysis methodology. CC: cingulate cortex, ACC: anterior cingulate cortex, PFC: prefrontal cortex.
Functional connections outlined here are referenced throughout this review and have been found to be correlated with ketamine's antidepressant effect. The relationship of the connection to antidepressant response is indicated by the line type, where a solid straight line corresponds to increased connectivity, a dotted line represents decreased connectivity, and a coiled line signifies mixed reports, i.e. some studies with increased and others with decreased connectivity in this circuit. A “halo” effect represents global brain connectivity (GBC), where the strength of the connectivity of the prefrontal cortex and caudate with the rest of the brain is related to antidepressant response. The laterality of these regions were mixed across the studies, except for the left amygdala, which was consistently reported. As alluded to above, there is a broad range of heterogeneity, i.e. task used, time of measurement (in relation to ketamine administration), and functional analysis methodology. CC: cingulate cortex, ACC: anterior cingulate cortex, PFC: prefrontal cortex.
Gärtner et al. found that lower baseline functional connectivity between the subgenual anterior cingulate cortex (sgACC) and right lateral prefrontal cortex (PFC) correlated with greater improvement in depression scores 24 hours post-ketamine. 19 At baseline, all responders had more negative connectivity between these regions compared to non-responders. Post-infusion functional connectivity increased in the right lateral PFC and sgACC, which positively correlated with treatment response. Similarly, Downey et al. reported that increased blood-oxygen level dependent (BOLD) signal in the sgACC and rostral ACC correlated with percent change improvement in the Beck Depression Inventory (BDI), but not MADRS, 1 and 7 days after ketamine infusion. 20 However, they did not observe any significant changes in mood from ketamine separate from placebo, questioning the specificity of this region in ketamine response. Nugent et al. found that lower functional connectivity between the left amygdala and sgACC correlated with MADRS improvement, again implicating the sgACC in the antidepressant response to ketamine. 21 Interestingly, McMillan et al. recorded EEG and fMRI simultaneously during ketamine infusion and only found significant correlations with treatment response when associating the two modalities. 22 In their study, BOLD signal variance explained by high gamma power in the insula, ACC, and posterior cingulate cortex (PCC) correlated with antidepressant response, where responders had a smaller change in BOLD signal than non-responders. They were also unable to replicate Downey et al.’s increased sgACC BOLD signal. 20 Instead, they observed a decrease in sgACC BOLD activity that did not correlate with antidepressant response. Yet, there were no post-infusion changes in sgACC BOLD signal after motion correction, suggesting that effects here could be due to cardiovascular processes. Continuing the emphasis on the insula and cingulate, Evans and colleagues correlated MADRS improvement from baseline to 2 days post-ketamine with BOLD signal normalization between the right posterior insula and posterior cingulate. 23 Finally, based on preclinical evidence of decreased burst firing in the lateral habenula in the antidepressant-like effects of ketamine, 24 Rivas-Grajales et al. found that increased functional connectivity between the right habenula with several cortical regions and parahippocampal gyrus correlated with an enhanced antidepressant response to ketamine. 25
Similar observations have been made with the anti-suicidal and hedonic effects of ketamine. When looking specifically at the suicidal ideation item on the MADRS (item 10), Chen found that decreased suicidal thinking after 0.5 mg/kg, but not 0.2 mg/kg, correlated with reduced connectivity between left dorsal ACC (dACC) and right ACC. 26 Instead, in the 0.2 mg/kg group, increased connectivity between right dorsolateral PFC (dlPFC) and left superior parietal region correlated with a reduction in suicidal ideation. In the striatal system, Mkrtchian et al. found a relationship with anhedonia, as evident by Snaith-Hamilton Pleasure Scale (SHAPS) improvement, but not MADRS, and increased connectivity between the dorsal caudate and ventrolateral PFC (vlPFC). 27 At day 10, SHAPS improvement trended with increased connectivity between the dorsal caudate and pregenual ACC (pgACC) and other regions. Vasavada et al. focused on the cortico-limbic system and major brain networks after repeated infusions. 28 Increased functional connectivity between the right hippocampus and the left central executive network (CEN) after the first infusion predicted percent change in SHAPS at the end of treatment. Additionally, connectivity changes between the left amygdala and the salience network (SN) pre- and post-treatment correlated with percent change in inhibition and avoidance behaviors as measured by the Behavioral Inhibition System (BIS), but not the HDRS nor the Rumination Scale. However, in this study, there were no overall group differences in BIS scores with ketamine treatment. Finally, Roy et al. used entropy to measure neural flexibility and signal variability in adolescents with TRD after 6 ketamine infusions, finding that responders had greater right nucleus accumbens entropy than non-responders, while non-responders had decreased entropy. 29
Although hypothesis driven, ROI-centeric studies can fall victim to selection bias and do not typically take into account whole brain activity. In contrast, data-driven analyses without a priori defined ROIs, like global brain connectivity with global signal regression (GBCr), have been proven to be fecund approaches in the ketamine depression literature. Chen et al. 30 used data previously reported in their 2019 paper 26 for a GBCr analysis and found that baseline hypoconnectivity between the bilateral superior frontal cortex and the striatum correlated with percent reduction of HAMD in participants receiving 0.2 mg/kg, but not 0.5 mg/kg, ketamine. Next, Abdallah et al. found an overall normalization in GBCr after ketamine in MDD patients, i.e. ketamine increased GBCr in the PFC but reduced GBCr in the cerebellum. 31 Ketamine responders had higher post-treatment GBCr in clusters involving lateral PFC, left middle temporal cortex, and caudate, though there was only a trending correlation with percent change in MADRS. They then used these implicated regions as ROIs in seed-based analyses. In responders, there was increased lateral PFC and caudate connectivity with subcortical areas, and lower connectivity within the PFC and subcortical regions. Continuing the data-driven approach with treatment-resistant bipolar patients, Zhuo did not reveal a relationship between global functional connectivity and HAMD scores in response to ketamine. 32 Oddly in this study, many patients relapsed in the second week of treatment, and the average depression score was higher post-treatment. The authors cited that the atypical clinical findings may be a result of glutamate exhaustion due to multiple glutamate modulating medications. An additional study from Abdallah and colleagues in 2017 found that baseline bilateral dorsomedial PFC and left frontolateral PFC GBCr predicted change in MADRS, but not the self-reported Inventory of Depressive Symptomatology (IDS), after ketamine and placebo treatment. 33 Subsequent seeded analysis showed that past treatment failure, greater MADRS improvement, and a lower response to placebo in this study correlated with higher baseline vPFC GBCr. In 2018, Abdallah et al. reported that baseline PFC GBCr trended with BDI improvement to ketamine, though this correlation was stronger with lanicemine, another non-competitve NMDA receptor antagonist. 34 They also observed a correlation between dorsal PFC GBCr during infusion and BDI improvement at 24 hours as well as a negative correlation with GBCr in the ventral PFC during infusion, though these p-values were not corrected for multiple comparisons.
Taken together, both the seed-based and data-driven resting state fMRI literature indicate the importance of the cingulate and prefrontal cortices in the antidepressant response to ketamine. Even so, there has been an unfortunate inability to replicate. As an example, Kraus and colleagues were unable to replicate Abdallah et al.’s findings,31,33,34 and instead found that there were no differences in GBCr post-ketamine or placebo. 35 This aligns more with the findings from Zhuo et al. 32 Baseline differences between patients and controls were also not replicated from Abdallah et al. 31 Although these are small effects in modest sized samples, the inconsistency may also result from scan timing post-ketamine, i.e. Kraus et al. scanned participants at least 2 days after infusion, where the other studies scanned 24 hours post-infusion. 35 They further discuss the importance of preprocessing strategies, especially since they only found significant results between MDD and HC groups, irrespective of treatment response, after GBC regression, rather than other methods of preprocessing. However, this lends to question how ketamine can have longer-lasting antidepressant effects but observable GBCr connectivity changes only at 24 hours and under very specific processing protocols. These findings, therefore, highlight the importance of physiologically-relevant timing of study procedures and consistent image processing/analyses across studies/sites.
Task-Based
Depression salient-tasks, e.g. emotional and cognitive testing, during fMRI have also been employed to investigate the antidepressant response to ketamine. Sahib et al. used an inhibitory control task to reveal changes in brain regions responsible for inhibitory and executive functioning in response to four ketamine infusions, and these findings correlated with treatment response. 36 More specifically, in the precentral gyrus, remitters had lower baseline BOLD signal that normalized to healthy control levels, whereas non-remitters had baseline BOLD signals that resembled healthy controls but decreased after the ketamine series. Supplemental motor area changes in BOLD signal corresponded to reductions in depressive symptoms. In post-hoc ROI analyses, they found that precentral gyrus activation negatively correlated with percent change in HDRS at the end of treatment. This suggests that ketamine promotes neuroplasticity in executive control networks, and that the normalization of inhibitory network activity is related to antidepressant response. Murrough et al. used positive and negative emotional perception tasks and found that greater connectivity of the right caudate during positive, but not negative, emotional perception was associated with depression improvement. 37 Next, using an attention bias task with angry and happy faces, Reed and colleagues did not find any differences in caudate activation after ketamine. 38 Overall, they found that MADRS percent improvement was associated with less activation of the left parahippocampal gyrus, amygdala, cingulate gyrus, precuneus, left medial, and middle frontal gyri during the angry cues and greater activation of these regions with happy cues, though they did not see attentional bias differences between patients and controls. The effect on the left frontal gyrus and its relationship to MADRS improvement was not specific to ketamine, as they saw the same effect in the placebo condition. Loureiro et al. looked specifically at amygdala reactivity during an emotional face matching task. 39 Here, baseline amygdala activity did not predict clinical outcome, but post-treatment decreases in the right amygdala during both positive and negative face presentation correlated with depression and anhedonia improvement. In the posterior superior temporal cortex, BOLD changes during the happy condition correlated with percent HDRS improvement. In the insula, dlPFC, and a central posterior cluster, they found that greater BOLD signal after ketamine in the fearful condition correlated with improved depression scores. Finally, BOLD decreases in the dlPFC in the happy condition correlated with percent improvement in SHAPS. Unlike many other studies, they did not notice a normalization in brain activation. In adolescents with TRD, Thai et al. used an emotional Word Face Stroop task with congruent or incongruent emotional words and faces to look specifically at changes in corticolimbic, corticostriatal, and brain areas implicated in the default mode networks (DMN) after six ketamine infusions. 40 Decreased activation in corticolimbic and corticostriatal regions with each task correlated with improvements in depression and anhedonia, which was most strongly led by negative emotion trials. The right nucleus accumbens, amygdala, and subcallosal cortex were the most consistent regions implicated across all conditions. The right hippocampus had increased activation during congruent positive trials, which corresponded to improved depression and pleasure, and the DMN had decreased activation to incongruent conditions, corresponding to improved depression scores. There were slight decreases in DMN activation across all conditions relating to improved pleasure scores. Like in Reed et al., 38 the participants in Thai and colleagues’s study did not show a negative attention bias in their performance on the task. In sum, they suggest that the overall decreased DMN activity after ketamine administration validates inefficient recruitment of brain regions in TRD, and that this network is a target of the antidepressant and anhedonic responses to ketamine.
Continuing task-based studies with reward processing, Morris et al. used an incentive processing task to reveal that higher baseline sgACC activity to positive, but not negative, feedback was correlated with anhedonia improvement. 41 sgACC hyperactivity normalized after ketamine infusion, though it was not reported if this correlated with antidepressant response. This study was also unable to replicate the increased post-ketamine resting state sgACC activity seen in Downey et al.’s results. 20 Additionally, Downey and colleagues measured resting state fMRI while Morris et al. used an incentive processing task, so this may confound the comparison.
Taken together from task-based fMRI ketamine literature, the most widely replicated finding is decreased amygdala activation during emotional tasks, thereby normalizing “bottom-up” amygdala overactivity in active major depression. Additionally, several brain regions were implicated across different tasks as well as in the task-free studies, i.e. sgACC and caudate, further emphasizing emotional control/processing as being critically involved in the antidepressant response to ketamine. And, as always, independent replication strengthens confidence in the potential association between emotional processing brain areas and the antidepressant response to ketamine.
Neurophysiology
Electroencephalography (EEG) and magnetoencephalography (MEG) measure electrical events with a higher degree of temporal precision than BOLD signal in fMRI. This can aid in understanding brain activity during acute phases of ketamine infusion and how to best predict treatment response. Table 1 outlines brain frequencies and their commonly described clinical correlates. Much of the EEG/MEG literature focuses specifically on assessing glutamate-based synaptic plasticity, which is a widely agreed-upon mechanism of ketamine’s antidepressant effects.
Table 1.
Neural frequencies and their clinical correlates. This table summarizes the frequency bands seen on electroencephalography and magnetoencephalography as well as the behavioral and cognitive activities that correlate with these frequencies. The gamma band is highlighted in this context for its relationship to AMPA and NMDA activity and functional connectivity, which are strongly implicated in ketamine’s antidepressant mechanisms of action.
| Frequency | Evoked activity | Neuronal and cognitive correlates |
|---|---|---|
| Delta (0.2–3 Hz) | Deep sleep, relaxation | Awareness, cortical plasticity |
| Slow wave activity (0.75–4.5 Hz) | Non-REM sleep | Synaptic number, strength, and homeostasis |
| Theta (4–7 Hz) | Sleep, meditation | Learning, memory |
| Alpha (8–13 Hz) | Awake resting state | Cognitive abilities, calmness, mental coordination |
| Beta (14–31 Hz) | Alert behavior, tasks that require attention | Decision making, anxiety, excitement |
| Gamma (32–100 Hz) | Simultaneous information processing | NMDA and AMPA activity, functional connectivity, BOLD signal, inhibition/excitation balance, synaptic potentiation |
Electroencephalography (EEG)
Resting-State
Baseline differences in EEG, especially in frontal electrodes, have been shown in MDD and subsequent treatment response to traditional antidepressants and non-invasive neuromodulatory approaches, e.g. transcranial magnetic stimulation.42,43 Zehong et al. used four frontal dry electrodes (potentially making more clinically useful as reducing set-up time and effort) to specifically look at frontal frequencies 240 minutes after a ketamine infusion in TRD patients receiving either 0.2 or 0.5 mg/kg of ketamine or placebo. 44 At baseline, ketamine responders had weaker relative, rather than absolute, theta power. After treatment, responders had higher alpha power in all channels, reduced alpha asymmetry, i.e. the difference in alpha power between the left and right frontal hemispheres, between Fp1 and Fp2, and lower theta concordance, i.e. normalized combined absolute and relative theta power, in all channels. The authors created a model to predict response vs. non-response, and the best model (81% accuracy, 82% sensitivity, and 91% specificity) used baseline relative theta and low alpha powers. Yet, there were differing predictors of treatment response depending on channel and dose. Responders to 0.2 mg/kg ketamine had baseline weaker theta power at Fp2. 0.5 mg/kg responders had lower relative theta and alpha power at baseline summed across all electrodes, and, after treatment, they had higher theta power in Fp1 and Fp2, findings not observed in 0.2 mg/kg responders. In McMillan et al.’s simultaneous EEG and fMRI study 22 discussed above, from the EEG perspective, a trending relationship was observed with increased frontal theta power and antidepressant response. Yet, like their fMRI results, these were sensitive to noise correction, calling for the standardization of processing techniques, but these preliminary findings corroborates Zehong and colleagues’s increased theta power in ketamine responders. 44
Sleep EEG studies have also been used to evaluate ketamine’s antidepressant mechanisms and identify efficacy biomarkers. First, Duncan et al. reported a positive correlation between increased serum BDNF and slow wave EEG activity, a biomarker of enhanced synaptic plasticity, in ketamine responders. 45 Specifically, those in the lowest BDNF quartile were more likely to respond and saw larger increases in BDNF and slow wave activity (SWA). Unfortunately, they did not find differences in baseline sleep measures that predicted response. The same group previously assessed delta sleep ratio (non-REM SWA in the first sleep epoch divided by the second sleep epoch) and found that lower baseline delta sleep ratio corresponded to a reduction in MADRS at 24 hours, but not 230 minutes, after ketamine infusion. 46 Taken together, synaptic strengthening during sleep may be a critical mechanism underlying ketamine’s antidepressant response, and, if replicated in independent samples, has potential for use as a treatment response biomarker, i.e. predicting early response in an index infusion series.
Task-Based
Task-based EEG and dynamic causal modeling have been used to assess neuroplasticity mediating the antidepressant actions of ketamine. Sumner et al. used an auditory mismatch negativity task to evaluate EEG signal change associated with sensory prediction error after hearing an unexpected tone. 47 They found that neither the P3a event related potential (ERP) amplitude change nor mismatch negativity seen after ketamine during the task correlated with MADRS improvement at 24 hours post-infusion. Instead, they found that antidepressant response correlated with greater modulation in forward connectivity between the right primary auditory cortex and right inferior temporal cortex after the unexpected tone. Finally, those with greater antidepressant efficacy had stronger modulation of these connections during expected tones. The same group also presented high-frequency visual stimulation to depressed patients and measured changes in visual evoked potential, a potential marker of long-term potentiation. 48 Surprisingly, they did not observe a correlation between MADRS reduction and the change in visual evoked potentials after ketamine.
To conclude the EEG section, there was an overall theme of ketamine-induced synaptic plasticity, though a correlation with antidepressant treatment response was not consistently apparent.
Magnetoencephalography (MEG)
Resting-State
Magnetoencephalography (MEG) measures magnetic fields created by electrical currents with greater spatial localization than EEG and greater temporal precision than fMRI. Nugent and colleagues used the same sample as Reed et al. 38 to measure functional connectivity and gamma power as a proxy for excitatory/inhibitory balance after a single ketamine infusion. 49 They found that higher gamma power after ketamine was associated with raw depression score improvement 40 minutes post-infusion in subjects with lower baseline gamma power in several regions including the thalamus and right insula. There was also a trending association in the dorsomedial PFC and right parietal cortex with depression improvement 230 minutes post-infusion. Farmer et al. assessed serum ketamine metabolite levels and their relationships with MEG gamma power and antidepressant response. 50 In this study, there was no association between gamma power and MADRS improvement, though there was a positive relationship between hydroxynorketamine and gamma power and a negative relationship between hydroxynorketamine and antidepressant efficacy. Next, Nugent et al. assessed beta band functional connectivity across ROIs implicated in MDD pathophysiology in response to a single ketamine infusion but observed a lack of correlation with MADRS improvement. 51 In 2020, with the same dataset, Nugent and colleagues investigated functional connectivity at other frequencies. 52 Alpha and beta band connectivity did not correlate with antidepressant response, which is in line with their previous study. 51 At baseline, non-responders had greater connectivity in beta and theta bands than controls but only slight reductions after ketamine. Similar to healthy controls, antidepressant responders had increased delta-alpha, delta-gamma, and delta-beta connectivity 40 minutes and 24 hours after both ketamine and placebo treatment. Responders also had lower baseline alpha and beta connectivity. However, in this report, the response criteria was lower (30%) than is typical for most ketamine depression studies. In another resting-state MEG report from the same group, Gilbert et al. looked specifically at insula and anterior cingulate using dynamic causal modeling to correlate gamma power with changes in depression scores. 53 They modeled AMPA and NMDA connectivity and found that lower AMPA connectivity from anterior insula to anterior cingulate was associated with depression, but not suicidal ideation, reduction, though this was not corrected for multiple comparisons. These results also support the fMRI literature indicating that the anterior cingulate and insula are critical regions in the antidepressant response to ketamine as well as further implicating AMPA receptor involvement in ketamine’s mechanism of action, though admittedly in the opposite direction as hypothesized from the preclinical literature.
In sum, even though the ketamine resting-state MEG literature is suggestive of enhanced functional connectivity and depression improvement, the associated frequency bands did not converge across all studies.
Task-Based
Lundin et al. used an emotional evaluation task to assess event-related electrical changes in the calcarine sulcus, occipital, and fusiform cortices to assess early visual responses to emotional stimuli and their potential relationship with ketamine’s antidepressant response. 54 In this report, there were no associations between MADRS change and event-related field amplitudes in the calcarine sulcus nor with latency in the left middle occipital cortex. In the fusiform cortex, however, higher baseline M170 amplitudes that normalized to levels similar to healthy controls post-ketamine corresponded with greater antidepressant response. Furthermore, lower M170 amplitudes in response to sad/angry faces and higher M170 amplitudes to happy/neutral faces were affiliated with a greater improvement. Next, Salvadore et al. rapidly presented fearful faces to activate the rostral ACC and amygdala before ketamine treatment to determine if baseline activity in these regions could predict antidepressant response. 55 Greater ACC and decreased amygdala activities during this task correlated with an antidepressant effect at 230 minutes. In 2010, Salvadore and colleagues used a working memory task with increasing memory load to determine how baseline pgACC hyperactivity and its functional connectivity to the amygdala could predict treatment response 4 hours after a single ketamine infusion; they found that depressed subjects with larger beta desynchronization in the pgACC and sgACC during the task were less likely to respond to ketamine, and those with less change in beta activity were more likely to respond to ketamine. 56 Additionally, decreased beta coherence (functional connectivity) between pgACC and the left amygdala correlated with greater antidepressant response, and those subjects displaying the least engagement of the pgACC as working memory load increased had the greatest symptom improvement. These results suggest an interesting task-dependency, i.e. with emotional provocation, increased and, in non-emotional tasks, decreased pgACC activity predicting rapid antidepressant response to subanesthetic dose ketamine.55,56
Tactile stimulation tasks have also been used to measure cortical excitability and synaptic plasticity in response to ketamine. A replication study by Nugent et al. saw increased gamma power after ketamine when the right hand was stimulated in responders; non-responders displayed increased gamma power during left hand stimulation. 57 Compared to healthy controls, next-day responders had significant differences in gamma power for right hand stimulation, but only trending differences for left hand stimulation. There was also an association between the difference in peak gamma power after right hand stimulation between ketamine and placebo and antidepressant response at both 230 minutes and 24 hours post-infusion. Cornwell et al. found increased stimulus evoked somatosensory cortical responses independent of spontaneous cortical gamma activity in responders 230 minutes after infusion. 58 Based on these results, they hypothesized that increased AMPA-mediated glutamatergic transmission may be a main mechanism in ketamine’s antidepressant response. Contrastingly, Gilbert et al. used dynamic causal modeling to measure AMPA and NMDA mediated connectivity and found that reduced NMDA connectivity between left and right primary somatosensory cortex and reduced AMPA connectivity between the left primary somatosensory cortex and left frontal cortex post-ketamine were associated with MADRS improvement. 59 The relationship with AMPA connectivity was long lasting, with trending significance 11 days after ketamine exposure. Although this opposes Cornwell et al.’s supposition of increased AMPA-mediated connectivity, 58 it affirms Gilbert and colleagues’s 2020 resting state results on lower ketamine-evoked AMPA connectivity as an antidepressant biomarker. 53 Taken together, these tactile stimulation task results suggest that ketamine alters synaptic plasticity and cortical excitability, which plays an important role in its rapid-acting antidepressant effects.
Conclusion
A summary of the findings from this review is presented in Table 2. Although ketamine is an effective antidepressant for many refractory patients, not all respond and some even worsen. 60 The identification of reproducible and mechanistically relevant biomarkers would satisfy a critical need for a priori treatment response prediction. The preponderance of the genetic, neuroimaging, and neurophysiological studies reviewed here support ketamine-induced normalization of MDD pathophysiology via the homeostatic reestablishment of synaptic plasticity and functional connectivity.
Table 2.
Main findings of the current review.
| • There is a strong need to predict treatment outcome to ketamine’s rapid acting antidepressant properties |
| • The genetics literature has only found a weak relationship with the val66met polymorphism and antidepressant response, though there is likely an ethnicity bias |
| • Neuroimaging potentially implicates emotional processing regions such as the anterior cingulate and prefrontal cortex in ketamine’s antidepressant response |
| • Electrophysiological data suggests that ketamine’s antidepressant efficacy relies on modulating whole-brain synaptic plasticity |
| • Overall, due to the heterogenous nature of major depressive disorder and small sample sizes in existing studies, additional studdies are needed to identify and replicate biomarkers of ketamine’s antidepressant response |
Yet, as mentioned throughout, the results have been variable with a dearth of replication. Among many potential factors, these discrepancies could be due to differences in inclusion criteria, depression scales, treatment response cut-off, measurement timing, and response rates. As an example, the use of different depression rating scales, e.g. MADRS, HAMD and BDI, limits the generalizability of these studies, since each scale explores slightly differing aspects of depression. Additionally, there are questions about the utility of current depression scales for assessing the efficacy of rapid acting antidepressants. For example, the MADRS, as with many other standard depression rating scales, includes several items that are physiologically unlikely, e.g., appetite changes, or unable, e.g., sleep quality, to change quickly in response to a rapid-acting antidepressant. This may be rectified by the development, validation and adoption of a depression rating scale that is more sensitive to rapid symptom improvement. 61 Moreover, much of the literature derives from small samples from only a few laboratories, which, due to selection biases and systematic differences in data collection and processing, respectively, may further hamper replicability. The inability to replicate may also be due to the fact that linear correlations are too simplistic in a complex, heterogenous disorder like MDD/TRD, and more sophisticated, multivariate modeling and/or machine learning approaches are required. Next, several studies presented here investigated the response to a single subanesthetic dose ketamine infusion, and, in clinical practice, an acute index series is typically administered. 62 Repeated-dose trials have demonstrated additive antidepressant response, 63 so those that do not respond immediately may ultimately convert after several treatments.
Nevertheless, there remains room for optimism in ketamine response biomarker research. As a single example, with the recent FDA approval of intranasal esketamine for the adjunctive treatment of TRD and MDD with suicidality, biomarker identification and replication may now be attempted in larger, real-world populations in the context of a reimbursable and more widely disseminated treatment.
Acknowledgements
The authors would like to acknowledge Teresa Ruggle and the University of Iowa Hospitals and Clinics Design Center for their assistance with the figures in this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge funding from the National Institutes of Alcohol Abuse and Alcoholism.
ORCID iDs: Alexandra A. Alario https://orcid.org/0000-0002-0395-7137
Mark J. Niciu https://orcid.org/0000-0002-5612-3021
References
- 1.Ritchie H. and Roser M.. Our World in Data: Mental Health; https://ourworldindata.org/mental-health
- 2.National Institute of Mental Health (2019) Major Depression; https://www.nimh.nih.gov/health/statistics/major-depression.shtml
- 3.Carvalho AF Cavalcante JL Castelo MS and Lima MC. Augmentation strategies for treatment-resistant depression: a literature review. J Clin Pharm Ther. 2007; 32: 415–428. [DOI] [PubMed] [Google Scholar]
- 4.Mrazek DA Hornberger JC Altar CA and Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014; 65: 977–987. [DOI] [PubMed] [Google Scholar]
- 5.Delfino RS, Del-Porto JA, Surjan J, et al. Comparative effectiveness of esketamine in the treatment of anhedonia in bipolar and unipolar depression. J Affect Disord. 2020; 278: 515–518. [DOI] [PubMed] [Google Scholar]
- 6.Marcantoni WS, Akoumba BS, Wassef M, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 – January 2019. J Affect Disord. 2020; 277: 831–841. [DOI] [PubMed] [Google Scholar]
- 7.Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013; 74: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 9.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012; 338: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003; 112: 257–269. [DOI] [PubMed] [Google Scholar]
- 11.Liu RJ Lee FS Li XY, Bambico F Duman RS and Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012; 71: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laje G, Lally N, Mathews D, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012; 72: e27–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su TP, Chen MH, Li CT, et al. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017; 42: 2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petryshen TL, Sabeti PC, Aldinger KA, et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 2010; 15: 810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MH, Lin WC, Wu HJ, et al. Antisuicidal effect, BDNF Val66Met polymorphism, and low-dose ketamine infusion: reanalysis of adjunctive ketamine study of Taiwanese patients with treatment-resistant depression (AKSTP-TRD). J Affect Disord. 2019; 251: 162–169. [DOI] [PubMed] [Google Scholar]
- 16.Guo W, Machado-Vieira R, Mathew S, et al. Exploratory genome-wide association analysis of response to ketamine and a polygenic analysis of response to scopolamine in depression. Transl Psychiatry. 2018; 8: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niciu MJ, Iadarola ND, Banerjee D, et al. The antidepressant efficacy of subanesthetic-dose ketamine does not correlate with baseline subcortical volumes in a replication sample with major depressive disorder. J Psychopharmacol. 2017; 31: 1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv Q, Yang L, Li G, et al. Large-scale persistent network reconfiguration induced by ketamine in anesthetized monkeys: relevance to mood disorders. Biol Psychiatry. 2016; 79: 765–775. [DOI] [PubMed] [Google Scholar]
- 19.Gärtner M, Aust S, Bajbouj M, et al. Functional connectivity between prefrontal cortex and subgenual cingulate predicts antidepressant effects of ketamine. Eur Neuropsychopharmacol. 2019; 29: 501–508. [DOI] [PubMed] [Google Scholar]
- 20.Downey D, Dutta A, McKie S, et al. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur Neuropsychopharmacol. 2016; 26: 994–1003. [DOI] [PubMed] [Google Scholar]
- 21.Nugent AC Farmer C Evans JW, Snider SL Banerjee D and Zarate CA. Multimodal imaging reveals a complex pattern of dysfunction in corticolimbic pathways in major depressive disorder. Hum Brain Mapp. 2019; 40: 3940–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillan R, Sumner R, Forsyth A, et al. Simultaneous EEG/fMRI recorded during ketamine infusion in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2020; 99: 109838. [DOI] [PubMed] [Google Scholar]
- 23.Evans JW Szczepanik J Brutsché N, Park LT Nugent ACand Zarate CA. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry. 2018; 84: 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Cui Y, Sang K, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018; 554: 317–322. [DOI] [PubMed] [Google Scholar]
- 25.Rivas-Grajales AM Salas R Robinson ME, Qi K Murrough JWand Matthew SJ. Habenula connectivity and intravenous ketamine in treatment-resistant depression [published online ahead of print November 30, 2020]. Int J Neuropsychopharmacol. doi:10.1093/ijnp/pyaa089 [DOI] [PMC free article] [PubMed]
- 26.Chen MH, Lin WC, Tu PC, et al. Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: a double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. J Affect Disord. 2019; 259: 15–20. [DOI] [PubMed] [Google Scholar]
- 27.Mkrtchian A, Evans JW, Kraus C, et al. Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals [published online ahead of print September 16, 2020]. Mol Psychiatry. doi:10.1038/s41380-020-00878-1 [DOI] [PMC free article] [PubMed]
- 28.Vasavada MM, Loureiro J, Kubicki A, et al. Effects of serial ketamine infusions on corticolimbic functional connectivity in major depression [published online ahead of print September 10, 2020]. Biol Psychiatry Cogn Neurosci Neuroimaging. doi:10.1016/j.bpsc.2020.06.015 [DOI] [PMC free article] [PubMed]
- 29.Roy AV, Thai M, Klimes-Dougan B, et al. Brain entropy and neurotrophic molecular markers accompanying clinical improvement after ketamine: preliminary evidence in adolescents with treatment-resistant depression [published online ahead of print July 10, 2020]. J Psychopharmacol. 2020: 269881120928203. doi:10.1177/0269881120928203 [DOI] [PMC free article] [PubMed]
- 30.Chen MH, Chang WC, Lin WC, et al. Functional dysconnectivity of frontal cortex to striatum predicts ketamine infusion response in treatment-resistant depression [published online ahead of print July 30, 2020]. Int J Neuropsychopharmacol. doi:10.1093/ijnp/pyaa056 [DOI] [PMC free article] [PubMed]
- 31.Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017; 42: 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuo C, Ji F, Tian H, et al. Transient effects of multi-infusion ketamine augmentation on treatment-resistant depressive symptoms in patients with treatment-resistant bipolar depression - An open-label three-week pilot study. Brain Behav. 2020; 10: e01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdallah CG, Averill CL, Salas R, et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017; 2: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients [published online ahead of print September 29, 2018]. Chronic Stress (Thousand Oaks). doi:10.1177/2470547018796102 [DOI] [PMC free article] [PubMed]
- 35.Kraus C Mkrtchian A Kadriu B, Nugent AC Zarate CA and Evans JW. Evaluating global brain connectivity as an imaging marker for depression: influence of preprocessing strategies and placebo-controlled ketamine treatment. Neuropsychopharmacology. 2020; 45: 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahib AK, Loureiro JR, Vasavada MM, et al. Modulation of inhibitory control networks relate to clinical response following ketamine therapy in major depression. Transl Psychiatry. 2020; 10: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murrough JW, Collins KA, Fields J, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. 2015; 5: e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed JL Nugent AC Furey ML, Szczepanik JE Evans JWand Zarate CA. Ketamine normalizes brain activity during emotionally valenced attentional processing in depression. Neuroimage Clin. 2018; 20: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loureiro JRA, Leaver A, Vasavada M, et al. Modulation of amygdala reactivity following rapidly acting interventions for major depression. Hum Brain Mapp. 2020; 41: 1699–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thai M, Başgöze Z, Klimes-Dougan B, et al. Neural and behavioral correlates of clinical improvement to ketamine in adolescents with treatment resistant depression. Front Psychiatry. 2020; 11: 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris LS Costi S Tan A, Stern ER Charney DSand Murrough JW. Ketamine normalizes subgenual cingulate cortex hyper-activity in depression. Neuropsychopharmacology. 2020; 45: 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leuchter AF, Cook IA, Gilmer WS, et al. Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in major depressive disorder. Psychiatry Res. 2009; 169: 132–138. [DOI] [PubMed] [Google Scholar]
- 43.Hunter AM Nghiem TX Cook IA, Krantz DE Minzenberg MJand Leuchter AF. Change in quantitative EEG theta cordance as a potential predictor of repetitive transcranial magnetic stimulation clinical outcome in major depressive disorder. Clin EEG Neurosci. 2018; 49: 306–315. [DOI] [PubMed] [Google Scholar]
- 44.Zehong C Chin-Teng L Weiping D, Chen MH Li CTand Su TP. Identifying ketamine responses in treatment-resistant depression using a wearable forehead EEG. IEEE Trans Biomed Eng. 2019; 66: 1668–1679. [DOI] [PubMed] [Google Scholar]
- 45.Duncan WC, Sarasso S, Ferrarelli F, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013; 16: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan WC Jr Selter J Brutsche N, Sarasso Sand Zarate CA. Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J Affect Disord. 2013; 145: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumner RL, McMillan R, Spriggs MJ, et al. Ketamine improves short-term plasticity in depression by enhancing sensitivity to prediction errors. Eur Neuropsychopharmacol. 2020; 38: 73–85. [DOI] [PubMed] [Google Scholar]
- 48.Sumner RL, McMillan R, Spriggs MJ, et al. Ketamine enhances visual sensory evoked potential long-term potentiation in patients with major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020; 5: 45–55. [DOI] [PubMed] [Google Scholar]
- 49.Nugent AC, Ballard ED, Gould TD, et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. 2019; 24: 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farmer CA, Gilbert JR, Moaddel R, et al. Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacology. 2020; 45: 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nugent AC Robinson SE Coppola R, and Zarate CA. Preliminary differences in resting state MEG functional connectivity pre- and post-ketamine in major depressive disorder. Psychiatry Res Neuroimaging. 2016; 254: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nugent AC Ballard ED Gilbert JR, Tewarie PK Brookes MJand Zarate CA. The effect of ketamine on electrophysiological connectivity in major depressive disorder. Front Psychiatry. 2020; 11: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilbert JR Ballard ED Galiano CS, Nugent ACand Zarate AC. Magnetoencephalographic correlates of suicidal ideation in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020; 5: 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lundin NB, Gilbert JR, Carver FW, Furey ML, Zarate CA, Nugent AC. Ketamine alters electrophysiological responses to emotional faces in major depressive disorder. J Affect Disord. 2021; 279: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salvadore G, Cornwell BR, Colon-Rosario V, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009; 65: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salvadore G, Cornwell BR, Sambataro F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010; 35: 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nugent AC Wills KE Gilbert JR, and Zarate CA. Synaptic potentiation and rapid antidepressant response to ketamine in treatment-resistant major depression: a replication study. Psychiatry Res Neuroimaging. 2019; 283: 64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cornwell BR, Salvadore G, Furey M, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012; 72: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbert JR Yarrington JS Wills KE, Nugent ACand Zarate CA. Glutamatergic signaling drives ketamine-mediated response in depression: evidence from dynamic causal modeling. Int J Neuropsychopharmacol. 2018; 21: 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niciu MJ Grunschel BD Corlett PR, Pittenger Cand Bloch MH. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol. 2013; 27: 651–654. [DOI] [PubMed] [Google Scholar]
- 61.McIntyre RS, Rodrigues NB, Lipsitz O, et al. Validation of the McIntyre And Rosenblat Rapid Response Scale (MARRRS) in adults with treatment-resistant depression receiving intravenous ketamine treatment. J Affect Disord. 2021; In press: doi:10.1016/j.jad.2021.03.053 [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson ST Toprak M Turner MS, Levine SP Katz RBand Sanacora G, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017; 174: 695–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips JL, Norris S, Talbot J, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019; 176: 401–409. [DOI] [PubMed] [Google Scholar]