Abstract
Adult rats that experienced neonatal limited bedding (NLB), a form of early-life stress, experience persistent muscle mechanical hyperalgesia. Since there is a growing recognition that the gut microbiome regulates pain and nociception, and that early-life stress produces a long-lasting impact on the gut microbiome, we tested the hypothesis that persistent muscle hyperalgesia seen in adult NLB rats could be ameliorated by interventions that modify the gut microbiome. Adult NLB rats received probiotics, either Lactobacillus rhamnosus GG (10 billion CFU/150 ml) or De Simone Formulation (DSF) (112.5 billion CFU/150 ml mixture of 8 bacterial species), in their drinking water, or non-absorbable antibiotics, rifaximin or neomycin, admixed with cookie dough, to provide 50 mg/kg. Mechanical nociceptive threshold in the gastrocnemius muscle was evaluated before and at several time points after administration of probiotics or antibiotics. Adult NLB rats fed probiotics L. Rhamnosus or DSF, antibiotics, as well as rats fed non-absorbable antibiotics rifaximin or neomycin, had markedly attenuated muscle mechanical hyperalgesia. We hypothesize that persistent skeletal muscle hyperalgesia produced by NLB stress may be, at least in part, due to a contribution of the gut microbiome, and that modulation of gut microbiome using probiotics or non-absorbable antibiotics, may be novel therapeutic approaches for the treatment of chronic musculoskeletal pain.
Keywords: Neonatal limited bedding, myalgia, nociceptors, probiotics, antibiotics, microbiome
Introduction
There is an increasing appreciation that the gastrointestinal (gut) microbiome affects the course and severity of many biological processes and diseases, including pain.1–6 Most studies evaluating the role of the gut microbiome in pain have focused on modulation of visceral pain and hypersensitivity (e.g. preclinical models, as well as in inflammatory bowel disease and colitis),7–10 and others have shown that the gut microbiome may affect chemotherapy-induced neuropathic pain.11,12 And, while some clinical evidence shows that there may be a relationship between gut dysbiosis and fibromyalgia symptoms,13,14 no studies have directly investigated whether manipulation of the gut microbiome affect musculoskeletal pain. In this study we tested the hypothesis that persistent muscle pain in adult rats (produced by early-life stress) is affected by modulating gut microbiome. Using a model of early life stress-induced adult muscle pain, based on the disruption of maternal care by limiting bedding/nesting material (neonatal limited bedding, NLB).15,16
Methods
Animals
Primiparous timed-pregnant female Sprague Dawley rats were obtained from Charles River (Hollister, CA). Dams were housed with their litter in standard cages on postnatal days 0 – 1. On postnatal day 2, litters were assigned to limited bedding (NLB) or standard care (control) conditions, or received corticosterone. Behavioral experiments were performed on 220–280 g adult female rats.
Animals were housed in the Laboratory Animal Resource Center of the University of California, San Francisco, under a 12 h light/dark cycle (lights on 7 am–7 pm) and environmentally controlled conditions; ambient room temperature (21–23°C), with food and water available ad libitum. Their care and use in experiments conformed to National Institutes of Health guidelines and measures were taken to minimize pain and discomfort. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
NLB stress
We used the NLB protocol, a well-established model of early-life stress. 17 Dams and their pups were housed in standard cages on postnatal days 0 and 1, and beginning on postnatal day 2, dams and their pups were placed in cages fitted with a custom stainless steel mesh grid bottom (Ancare, Bellmore, NY), raised ∼2.5 cm from the floor of the home cage, to provide space for collection of urine and feces. 18 The nesting/bedding material provided consisted of one sheet of paper towel (∼112 × 22 cm), and no environmental enrichment. Litters were left undisturbed during postnatal days 2 – 9. From postnatal day 10 until weaning, dams and pups were again housed in standard cages with normal bedding (Paperchip® animal bedding, Shepherd Specialty Papers, Watertown, TN), and standard enrichment. On postnatal day 21 pups were weaned and same sex rats housed 3 per cage, in standard housing conditions.
Probiotic and antibiotic feeding
Probiotics
Rats receiving probiotics were divided into 3 groups: the control group received only tap water for drinking, in another group the drinking water contained Lactobacillus rhamnosus GG (10 billion CFU/150 ml L. rhamnosus, Culturelle®, Amerifit, Inc, Cromwell, CT), and in the third group the drinking water contained De Simone Formulation (DSF) (112.5 billion CFU/150 ml mixture of L. acidophilus, L. plantarum, L. casei, L. delbrueckii subspecies bulgaricus, Bifidobacterium breve, B. longum, B. infantis, and Streptococcus salivarius subspecies thermophilus, VSL Pharmaceuticals, Inc, Towson, MD). Drinking water containing probiotics was made fresh each day. Water or probiotic-containing water was provided to rats ad libitum for 8 days.
Non-absorbable antibiotics
Typically, administration of non-absorbable antibiotics to rats is accomplished by gavage feeding. However, in order to eliminate stress associated with gavage feeding (stress would affect nociceptive threshold), we employed an established method of voluntary oral administration, 19 in which rifaximin or neomycin was admixed with 4 g of cookie dough mix (Pillsbury™ Sugar Cookie dough); this method of feeding has been shown to have an ingestion reliability of 99.9–100% in Sprague Dawley rats. To acclimate rats, they were fed cookie dough for 3 days prior to being fed the cookie dough-antibiotic mix. Rats receiving antibiotics were divided into 3 groups: the control group fed cookie dough, another group, cookie dough contained rifaximin (to provide 50 mg/kg; Xifaxan, Salix Pharmaceuticals, Inc.), and a third group, cookie dough containing neomycin sulfate (to provide 50 mg/kg; MilliporeSigma, Burlington, MA). Rats were fed cookie dough (plain, or with non-absorbable antibiotics) daily for 10 days.
Mechanical nociceptive threshold in skeletal muscle
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a Chatillon digital force transducer (model DFI2, Amtek Inc., Largo, FL). 16 Rats were placed in cylindrical acrylic restrainers designed to minimize restraint stress and allow extension of their hind legs from lateral ports. To acclimatize rats to the testing procedure, they were placed in restrainers and exposed to the testing procedure, daily for 3 days, prior to starting experiments. On the day of the experiment, rats were placed in a restrainer for 30 minutes before experimental manipulations. To determine nociceptive threshold, a 7-mm diameter probe attached to the force transducer was applied to the gastrocnemius muscle to deliver an increasing compression force. The nociceptive threshold was defined as the force, in Newtons, at which the rat withdrew its hind leg. Mechanical nociceptive thresholds was determined by measuring the mean of 3 withdrawal thresholds taken at 5-min intervals; one hind limb of each rat was used. All behavioral testing was done between 10 am and 4 pm (no differences in baseline nociceptive threshold was observed over this time period), and was performed blind to treatment condition.
Statistical analyses
Group data are expressed as mean ± SEM of n independent observations. Statistical analysis of experimental data, conducted using Prism 9 (GraphPad Software, San Diego, CA), employed two-way analysis of variance (ANOVA) for groups with equal numbers (Figure 1), or a mixed-effect analysis (restricted maximum likelihood) for groups with unequal numbers (Figure 2). Where there was a significant main difference between treatment groups, Dunnett’s post-hoc test was used. The accepted level for significance was P < 0.05.
Figure 1.
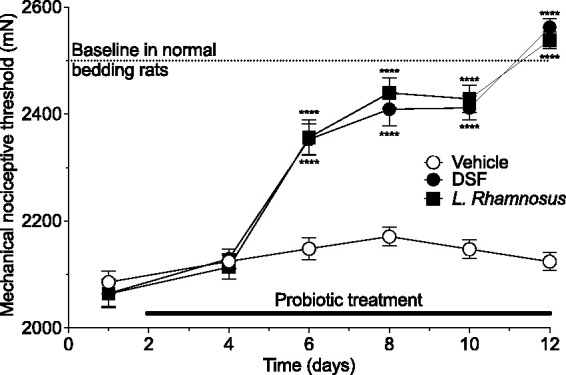
Probiotics DSF and L. Rhamnosus reverse NLB-induced muscle mechanical hyperalgesia. Adult NLB rats received tap water (vehicle), or tap water containing DSF (112.5 billion CFU/150 ml) or L. Rhamnosus (10 billion CFU/150 ml). NLB rats were hyperalgesic (dashed lines indicate muscle mechanical nociceptive threshold in rats raised on standard bedding) prior to probiotics. While rats receiving tap water showed no change in nociceptive threshold, both probiotics significantly increased nociceptive threshold in males (2- way ANOVA, interaction F8,132 = 21.67, P < 0.0001, Tukey’s multiple comparison test showed significant differences for both probiotics from vehicle control rats on days 6 – 12 ****P < 0.0001); all groups n = 12.
Figure 2.
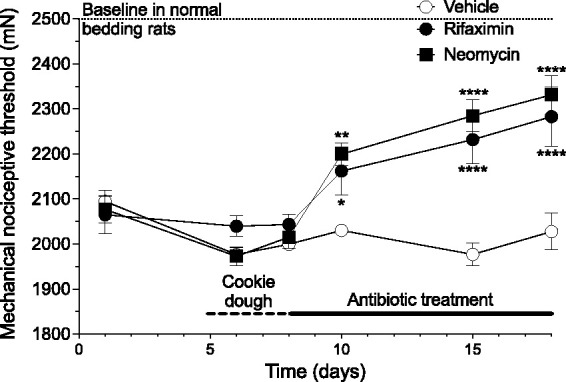
Antibiotics, rifaximin and neomycin, attenuate NLB-induced muscle mechanical hyperalgesia. Adult NLB rats were fed 4 g cookie dough (vehicle), or cookie dough containing rifaximin (50 mg/kg in 4 g cookie dough) or neomycin sulfate (50 mg/kg in 4 g cookie dough). The muscle mechanical nociceptive threshold of NLB rats was lower (i.e., they were hyperalgesic) compared to rats raised on standard bedding (threshold indicated by dashed lines) prior to antibiotic feeding. While rats receiving vehicle (cookie dough) showed no change in nociceptive threshold, both antibiotics significantly increased nociceptive threshold (2-way ANOVA, Time x Antibiotic treatment interaction F16,171 = 5.78, P < 0.0001). Dunnett’s multiple comparison test showed significant differences for both antibiotics from vehicle control rats on days 10 – 22, *P,0.05, **P < - 0.005, ****P < 0.0001); Control n = 6, both rifaximin and neomycin n = 10.
Results
Administration of probiotics, DSF and L. Rhamnosus, in adult rats attenuates NLB-induced muscle hyperalgesia
Adult NLB rats treated with a probiotic, either DSF or L. Rhamnosus, showed markedly higher mechanical nociceptive threshold in the gastrocnemius muscle compared to control (vehicle-fed) rats (2-way repeated measures ANOVA, time × antibiotic treatment, F8,132 = 21.67, P < 0.0001, Figure 1). And, by day 10 of probiotic feeding, nociceptive threshold was similar to that seen in rats raised on standard bedding during postnatal days 2–9.
Administration of rifaximin and neomycin, in adult rats, attenuates NLB-induced muscle hyperalgesia
Adult rats that had been exposed to the limited bedding protocol during postnatal days 2–9 (NLB), have a lower mechanical nociceptive threshold compared to rats that had standard bedding during the same postnatal period. When rats were fed non-absorbable antibiotics, rifaximin or neomycin, muscle mechanical hyperalgesia in NLB rats was markedly attenuated compared to control (vehicle-fed) rats (2-way repeated measures ANOVA, time × antibiotic treatment, F8,79 = 7.211, P < 0.0001; Figure 2). Antibiotic feeding was stopped after 10 days, and by the seventh day of normal diet, nociceptive threshold decreased to the level seen in vehicle-fed rats.
Discussion
In this study, we found that persistent skeletal muscle hyperalgesia seen in adult rats that had been exposed to early-life stress was markedly attenuated by administration of non-absorbable antibiotics (rifaximin, neomycin), or of probiotics (DSF, L. Rhamnosus). It is likely that the marked attenuation of the persistent muscle hyperalgesia by these treatments is due to the effect of changes in the gut microbiome, since both the antibiotics20–23 and probiotics have been shown to affect gut microbiome diversity.
It is well-established that early-life stress affects gut microbiome,24–29 a dysbiosis that persists into adulthood.24,25,29 Importantly, in addition to local effects, such as visceral pain30,31 and gut permeability,26,27 gut dysbiosis has been implicated in several systemic pathologies, including altered behavior (e.g. depression and anxiety),24,32,33 Parkinson’s disease, 34 increased hypothalamic pituitary adrenal axis (HPA) activity, 35 systemic lupus erythematosus 36 and systemic inflammation.37,38 And, there is a growing appreciation that the gut microbiome may contribute to chronic extra-abdominal pain states,1,3–6 for example in cutaneous inflammatory hyperalgesia 39 and paclitaxel-induced cutaneous thermal and mechanical nociception. 11 However, to the best of our knowledge evaluation of the role of the gut microbiome on skeletal muscle mechanical hyperalgesia has not previously been evaluated.
The mechanism by which early-life stress produces muscle hyperalgesia the persists in to adulthood has yet to be determined. However, it is known that early-life stress (maternal separation), produces a change in beta diversity (i.e. a change in microbial composition, or dysbiosis), for example a deficiency in Lactobacillus,26,40 Staphylococcus and Mucispirillum, 29 Clostridium, Bilophia 30 and increases in Bacteroides, 29 Alloprevotella and Acetivibrio. 30 Other studies have observed additional changes in gut microbiome following early-life stress and with 5,000 – 10,000 bacterial species it is going to be challenging to determine which bacterial species contribute to local or systemic hyperalgesia. However, altering the gut microbiome population by administration of locally-acting antibiotics or by probiotics has been shown in preclinical and clinical studies to attenuate stress-induced visceral hyperalgesia,22,23,30,41 as well as attenuate neuropathic cutaneous mechanical allodynia and thermal hyperalgesia.42,43 How probiotic and antibiotic administration can ameliorate hyperalgesia, which is currently unknown, may depend on more than one mechanism. One mechanism may depend on modifying stress-induced gut dysbiosis. For example, early-life stress-induced gut dysbiosis and gut permeability, is ameliorated by probiotics,18,26,30,44 as well as by the antibiotic, rifaximin.22,45 Stress-induced increase in gut permeability results in increased levels of lipopolysaccharide (LPS) and inflammatory cytokines, an effect that is reversed by probiotics. 46 Importantly, in addition to affect the gut microbiome, early-life stress produces a decreased expression of tight junction expression in the gut,47,48 leading to persistent increase gut permeability,26,49 which allows for systemic leakage of bacteria products, such as LPS, 45 proinflammatory cytokines,50,51 and bacterial translocation from the gut. 52 Since LPS, inflammatory cytokines and bacteria act directly on nociceptors to decrease nociceptive threshold and increase neuronal excitability,53–58 since early-life stress-induced increased gut permeability persists in to adulthood, 59 leakage of LPS, cytokines and/or bacteria could contribute to hyperalgesia seen in adult NLB rats. While NLB-induced stress increase in gut permeability could affect the pharmacokinetics of the poorly absorbable antibiotics used in this study, rifaximin reverses stress-induced gut permeability to normal, non-stressed levels,22,45 and neomycin also reduces gut permeability. 60 Given these effects of the antibiotics, it is unlikely that the increased gut permeability seen in NLB adult rats would significantly affect antibiotic pharmacokinetics. In addition to leakage of mediators or bacteria, the gut microbiome may affect nociception via direct action on the vagus nerve. We61,62 and others63,64 have shown that activity of vagal visceral afferents have a marked effect on somatic nociceptor sensitivity. And, since afferent vagus neurons innervating the gut sense resident bacteria and their mediators and metabolites2,65,66 this could be another mechanism whereby the gut microbiome influences nociceptive threshold. While our data indicate that the probiotics and antibiotics we used produce an antihyperalgesic effect on NLB-induced hyperalgesia, it is possible that these agents could have independent analgesic effects. However, to the best of our knowledge, none of these agents has been reported to be analgesic following oral administration.
In summary, persistent mechanical hyperalgesia in adult rats exposed to early-life stress (NLB) is attenuated by interventions that modify the gut microbiome, either through administration of probiotics or non-absorbable antibiotics. We hypothesize that the gut microbiome and/or gut permeability is altered by early-life stress, which results in enhanced nociceptive excitability, either by action of gut microbiome-derived pronociceptive mediators and/or via modulation of vagal afferent activity. Our results suggest novel therapeutic approaches for the treatment of chronic musculoskeletal pain involving modulation of gut microbiome using probiotics or non-absorbable antibiotics.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH grant AR063312.
ORCID iD: Paul G Green https://orcid.org/0000-0001-7648-6826
References
- 1.Defaye M, Gervason S, Altier C, Berthon JY, Ardid D, Filaire E, Carvalho FA. Microbiota: a novel regulator of pain. J Neural Transm (Vienna) 2020; 127: 445–465. [DOI] [PubMed] [Google Scholar]
- 2.Dworsky-Fried Z, Kerr BJ, Taylor AMW. Microbes, microglia, and pain. Neurobiol Pain 2020; 7: 100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo R, Chen LH, Xing C, Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth 2019; 123: 637–654. [DOI] [PubMed] [Google Scholar]
- 4.Lagomarsino VN, Kostic AD, Chiu IM. Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol Pain 2021; 9: 100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin B, Wang Y, Zhang P, Yuan Y, Zhang Y, Chen G. Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain 2020; 21: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoni M, Miccini F, Battelli N. Gut microbiota, immunity and pain. Immunol Lett 2021; 229: 44–47. [DOI] [PubMed] [Google Scholar]
- 7.Dai C, Guandalini S, Zhao DH, Jiang M. Antinociceptive effect of VSL#3 on visceral hypersensitivity in a rat model of irritable bowel syndrome: a possible action through nitric oxide pathway and enhance barrier function. Mol Cell Biochem 2012; 362: 43–53. [DOI] [PubMed] [Google Scholar]
- 8.Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One 2013; 8: e63893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannampalli P, Pochiraju S, Chichlowski M, Berg BM, Rudolph C, Bruckert M, Miranda A, Sengupta JN. Probiotic Lactobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol Motil 2014; 26: 1694–1704. [DOI] [PubMed] [Google Scholar]
- 10.Theodorou V, Ait Belgnaoui A, Agostini S, Eutamene H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes 2014; 5: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakrishna C, Corleto J, Ruegger PM, Logan GD, Peacock BB, Mendonca S, Yamaki S, Adamson T, Ermel R, McKemy D, Borneman J, Cantin EM. Dominant role of the gut microbiota in chemotherapy induced neuropathic pain. Sci Rep 2019; 9: 20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen S, Lim G, You Z, Ding W, Huang P, Ran C, Doheny J, Caravan P, Tate S, Hu K, Kim H, McCabe M, Huang B, Xie Z, Kwon D, Chen L, Mao J. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci 2017; 20: 1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crock LW, Baldridge MT. A role for the microbiota in complex regional pain syndrome. Neurobiol Pain 2020; 8: 100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdrich S, Hawrelak JA, Myers SP, Harnett JE. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: a systematic review. BMC Musculoskelet Disord 2020; 21: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez P, Green PG, Levine JD. Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biol Psychiatry 2013; 74: 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain 2011; 152: 2549–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol 1996; 15: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukui H, Oshima T, Tanaka Y, Oikawa Y, Makizaki Y, Ohno H, Tomita T, Watari J, Miwa H. Effect of probiotic bifidobacterium bifidum G9-1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Sci Rep 2018; 8: 12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbett A, McGowin A, Sieber S, Flannery T, Sibbitt B. A method for reliable voluntary oral administration of a fixed dosage (mg/kg) of chronic daily medication to rats. Lab Anim 2012; 46: 318–324. [DOI] [PubMed] [Google Scholar]
- 20.Kaji K, Saikawa S, Takaya H, Fujinaga Y, Furukawa M, Kitagawa K, Ozutsumi T, Kaya D, Tsuji Y, Sawada Y, Kawaratani H, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin alleviates endotoxemia with decreased serum levels of soluble CD163 and mannose receptor and partial modification of gut microbiota in cirrhotic patients. Antibiotics (Basel) 2020; 9: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray P, Chakraborty S, Ghosh A, Aich P. Effects of treatment with three antibiotics, vancomycin, neomycin, and AVNM on gut microbiome in C57BL/6 mice. bioRxiv 2021. [Google Scholar]
- 22.Xu D, Gao J, Gillilland M, Wu X, Song I, Kao JY, Owyang C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014; 146: 484–496.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CQ, Guo XS, Ji- Li, Wei ZB, Zhao L, Zhao GT, Sheng ST. Rifaximin improves visceral hyperalgesia via TRPV1 by modulating intestinal flora in the water avoidance stressed rat. Gastroenterol Res Pract 2020; 2020: 4078681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, Denou E, Silva MA, Santacruz A, Sanz Y, Surette MG, Verdu EF, Collins SM, Bercik P. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun 2015; 6: 7735. [DOI] [PubMed] [Google Scholar]
- 25.Enqi W, Jingzhu S, Lingpeng P, Yaqin L. Comparison of the gut microbiota disturbance in rat models of irritable bowel syndrome induced by maternal separation and multiple early-life adversity. Front Cell Infect Microbiol 2020; 10: 581974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007; 56: 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussaoui N, Jacobs JP, Larauche M, Biraud M, Million M, Mayer E, Taché Y. Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: influence of sex. J Neurogastroenterol Motil 2017; 23: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 2009; 65: 263–267. [DOI] [PubMed] [Google Scholar]
- 29.Park HJ, Kim SA, Kang WS, Kim JW. Early-life stress modulates gut microbiota and peripheral and central inflammation in a sex-dependent manner. Int J Mol Sci 2021; 22: 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McVey Neufeld KA, Strain CR, Pusceddu MM, Waworuntu RV, Manurung S, Gross G, M Moloney G, Hoban AE, Murphy K, Stanton C, Dinan TG, Cryan JF, O’Mahony SM. Lactobacillus rhamnosus GG soluble mediators ameliorate early life stress-induced visceral hypersensitivity and changes in spinal cord gene expression. Neuron Signal 2020; 4: NS20200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, Woznicki J, Hyland NP, Shanahan F, Quigley EM, Marchesi JR, O’Toole PW, Dinan TG, Cryan JF. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014; 277: 885–901. [DOI] [PubMed] [Google Scholar]
- 32.Cowan CSM, Stylianakis AA, Richardson R. Early-life stress, microbiota, and brain development: probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev Cogn Neurosci 2019; 37: 100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress 2017; 7: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baizabal-Carvallo JF, Alonso-Juarez M. The link between gut dysbiosis and neuroinflammation in Parkinson’s disease. Neuroscience 2020; 432: 160–173. [DOI] [PubMed] [Google Scholar]
- 35.Wu Q, Xu Z, Song S, Zhang H, Zhang W, Liu L, Chen Y, Sun J. Gut microbiota modulates stress-induced hypertension through the HPA axis. Brain Res Bull 2020; 162: 49–58. [DOI] [PubMed] [Google Scholar]
- 36.Katz-Agranov N, Zandman-Goddard G. The microbiome and systemic lupus erythematosus. Immunol Res 2017; 65: 432–437. [DOI] [PubMed] [Google Scholar]
- 37.Rizzetto L, Fava F, Tuohy KM, Selmi C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun 2018; 92: 12–34. [DOI] [PubMed] [Google Scholar]
- 38.Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol 2018; 84: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM, Ferreira SH, Cunha FQ, Silva TA, Nicoli JR, Vieira LQ, Souza DG, Teixeira MM. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A 2008; 105: 2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol 1999; 35: 146–155. [PubMed] [Google Scholar]
- 41.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 1547–1561; quiz 1546–1562. [DOI] [PubMed] [Google Scholar]
- 42.Cuozzo M, Castelli V, Avagliano C, Cimini A, d’Angelo M, Cristiano C, Russo R. Effects of chronic oral probiotic treatment in paclitaxel-induced neuropathic pain. Biomedicines 2021; 9: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding W, You Z, Chen Q, Yang L, Doheny J, Zhou X, Li N, Wang S, Hu K, Chen L, Xia S, Wu X, Wang C, Zhang C, Chen L, Ritchie C, Huang P, Mao J, Shen S. Gut microbiota influences neuropathic pain through modulating proinflammatory and anti-inflammatory T cells. Anesth Analg 2021; 132: 1146–1155. [DOI] [PubMed] [Google Scholar]
- 44.Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol 2021; 12: 578386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuti D, Winkler Z, Horváth K, Juhász B, Paholcsek M, Stágel A, Gulyás G, Czeglédi L, Ferenczi S, Kovács KJ. Gastrointestinal (non-systemic) antibiotic rifaximin differentially affects chronic stress-induced changes in colon microbiome and gut permeability without effect on behavior. Brain Behav Immun 2020; 84: 218–228. [DOI] [PubMed] [Google Scholar]
- 46.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012; 37: 1885–1895. [DOI] [PubMed] [Google Scholar]
- 47.Karailiev P, Hlavacova N, Chmelova M, Homer NZM, Jezova D. Tight junction proteins in the small intestine and prefrontal cortex of female rats exposed to stress of chronic isolation starting early in life. Neurogastroenterol Motil 2021; 33: e14084. [DOI] [PubMed] [Google Scholar]
- 48.Seifi M, Rodaway S, Rudolph U, Swinny JD. GABAA receptor subtypes regulate stress-induced colon inflammation in mice. Gastroenterology 2018; 155: 852–864.e3. [DOI] [PubMed] [Google Scholar]
- 49.Lennon EM, Maharshak N, Elloumi H, Borst L, Plevy SE, Moeser AJ. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10-/- mice. Inflamm Bowel Dis 2013; 19: 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011; 25: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu YW, Liu WH, Wu CC, Juan YC, Wu YC, Tsai HP, Wang S, Tsai YC. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res 2016; 1631: 1–12. [DOI] [PubMed] [Google Scholar]
- 52.Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut 2004; 53: 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013; 501: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol 2018; 39: 240–255. [DOI] [PubMed] [Google Scholar]
- 55.Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain 2011; 15: 796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 2019; 33: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernández-Peña C, Talavera A, Kichko T, Navia B, Sánchez A, Señarís R, Reeh P, Pérez-García MT, López-López JR, Voets T, Belmonte C, Talavera K, Viana F. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun 2014; 5: 3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang NJ, Chiu IM. Bacterial signaling to the nervous system through toxins and metabolites. J Mol Biol 2017; 429: 587–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rincel M, Olier M, Minni A, Monchaux de Oliveira C, Matime Y, Gaultier E, Grit I, Helbling JC, Costa AM, Lépinay A, Moisan MP, Layé S, Ferrier L, Parnet P, Theodorou V, Darnaudéry M. Pharmacological restoration of gut barrier function in stressed neonates partially reverses long-term alterations associated with maternal separation. Psychopharmacology (Berl) 2019; 236: 1583–1596. [DOI] [PubMed] [Google Scholar]
- 60.Nevado R, Forcén R, Layunta E, Murillo MD, Grasa L. Neomycin and bacitracin reduce the intestinal permeability in mice and increase the expression of some tight-junction proteins. Rev Esp Enferm Dig 2015; 107: 672–676. [DOI] [PubMed] [Google Scholar]
- 61.Jänig W, Khasar SG, Levine JD, Miao FJ. The role of vagal visceral afferents in the control of nociception. Prog Brain Res 2000; 122: 273–287. [DOI] [PubMed] [Google Scholar]
- 62.Khasar SG, Isenberg WM, Miao FJ, Gear RW, Green PG, Levine JD. Gender and gonadal hormone effects on vagal modulation of tonic nociception. J Pain 2001; 2: 91–100. [DOI] [PubMed] [Google Scholar]
- 63.Chakravarthy K, Chaudhry H, Williams K, Christo PJ. Review of the uses of vagal nerve stimulation in chronic pain management. Curr Pain Headache Rep 2015; 19: 54. [DOI] [PubMed] [Google Scholar]
- 64.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part III. Headache 2016; 56: 479–490. [DOI] [PubMed] [Google Scholar]
- 65.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 2018; 12: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fülling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus…. Neuron 2019; 101: 998–1002. [DOI] [PubMed] [Google Scholar]


