Abstract
Compared to idiopathic pulmonary arterial hypertension (IPAH), patients with portopulmonary hypertension (POPH) have worse survival. Health disparities may contribute to these differences but have not been studied. We sought to compare socioeconomic factors in patients with POPH and IPAH and to determine whether socioeconomic status and/or POPH diagnosis were associated with treatment and health-care utilization. We performed a cross-sectional study of adults enrolled in the Pulmonary Hypertension Association Registry. Patients with IPAH (n = 344) and POPH (n = 57) were compared. Compared with IPAH, patients with POPH were less likely to be college graduates (19.6% vs. 34.9%, p = 0.02) and more likely to be unemployed (54.7% vs. 30.5%, p < 0.001) and have an annual household income below poverty level (45.7% vs. 19.0%, p < 0.001). Patients with POPH had similar functional class, quality of life, 6-min walk distance, and mean pulmonary arterial pressure with a higher cardiac index. Compared with IPAH, patients with POPH were less likely to receive combination therapy (46.4% vs. 62.2%, p = 0.03) and endothelin receptor antagonists (28.6% vs. 55.1%, p < 0.001) at enrollment with similar treatment at follow-up. Patients with POPH had more emergency department visits (1.7 ± 2.1 vs. 0.9 ± 1.2, p = 0.009) and hospitalizations in the six months preceding enrollment (1.5 ± 2.1 vs. 0.8 ± 1.1, p = 0.02). Both POPH diagnosis and lower education level were independently associated with a higher number of emergency department visits. Compared to IPAH, patients with POPH have lower socioeconomic status, are less likely to receive initial combination therapy and endothelin receptor antagonists but have similar treatment at follow-up, and have increased health-care utilization.
Keywords: pulmonary hypertension, socioeconomic status, health-care utilization
Portopulmonary hypertension (POPH) is defined as pulmonary arterial hypertension (PAH) that develops in the setting of portal hypertension, often related to chronic liver disease. 1 It is characterized by an elevated mean pulmonary arterial pressure due to increased pulmonary vascular resistance and is classified as Group 1 PAH by the Sixth World Symposium on Pulmonary Hypertension. 2 POPH affects 5%–6% of patients with liver disease and is the third most common cause of associated PAH. 3 ,4 A diagnosis of POPH has important therapeutic and prognostic implications. Similar to other forms of PAH, POPH is typically treated with PAH-targeted therapy. 4 Liver transplantation can also be beneficial, but severe POPH precludes liver transplantation due to increased perioperative mortality. 5 ,8 Without medical therapy or liver transplantation, five-year survival rates for patients with POPH are dismal at 14%, significantly worse than other forms of PAH. 6 Even in patients treated with medical therapy, long-term survival of POPH remains poor.28
POPH is pathologically indistinct from idiopathic PAH (IPAH).3,7 Compared to IPAH, however, patients with POPH are less likely to receive PAH-targeted therapy and have worse survival despite a higher cardiac output. 8 The reasons for differences in treatment and survival in POPH versus IPAH are not known. Possible explanations include differences in disease biology, morbidity/mortality related to the underlying liver disease, comorbidities, access to medical care, socioeconomic status (SES), or other factors. Health disparities are defined as significant differences in health that are closely linked to racial, social, economic, or environmental differences. 9 Health disparities can impact quality of life as well as disease treatment and outcomes.10,11 The American Thoracic Society recently encouraged research to identify health disparities in pulmonary hypertension in order to achieve more equitable care.9,12 Health disparities may contribute to differences in treatment and survival in POPH, but have not been previously studied. In this study, we sought to (1) compare socioeconomic factors in patients with POPH and IPAH and (2) determine whether socioeconomic factors and/or POPH diagnosis were independently associated with PAH treatment and health-care utilization.
Methods
Study design and study sample
We performed a cross-sectional study of adult patients (≥18 years old) enrolled in the multicenter Pulmonary Hypertension Association Registry (PHAR). The PHAR began enrollment in 2015, with the main goal of assessing the quality of care and outcomes of patients at accredited Pulmonary Hypertension Care Centers (PHCC). Inclusion criteria have been previously reported. 13 Patients were enrolled in the registry within six months of establishing care at an accredited PHCC. Incident patients were diagnosed within six months of registry entry, while treated patients refer to patients who were treated with a PAH-targeted therapy for more than six months prior to registry entry. Patients with IPAH (n = 344) and POPH (n = 57) who enrolled at 1 of 40 participating PHCCs throughout the United States between September 2015 and September 2019 were included. The current data set from PHAR was locked on 4 September 2019. All study participants provided informed consent at enrollment. All centers relied on the University of Pennsylvania, which serves as the single institutional review board for the PHAR.
Clinical variables
Participants enrolled in the registry completed a tablet-based survey that included demographics, self-identified race/ethnicity, SES, including annual household income, social history, anthropomorphic data, pulmonary hypertension history, symptoms, current PAH-specific therapy, and health-related quality of life (HRQoL) questionnaires (Medical Outcome Study Short Form-12 (SF-12) and the emPHasis-10). Hemodynamics from the initial diagnostic right heart catheterization were collected. Participants underwent repeat study assessments approximately every six months, with some variability in the interval. At each follow-up visit, repeated measures of symptoms, PAH therapy, HRQoL questionnaires, and clinical outcomes, including interval hospitalizations and emergency department (ED) visits within the preceding six months, were collected. 13
For the purposes of this study, we defined combination therapy as the use of two or more PAH disease-specific medications. We did not consider calcium channel blockers as PAH disease-specific therapies since they can be used for other indications and are generally contraindicated in POPH. 4 Parenteral therapy was defined as use of intravenous or subcutaneous treprostinil or intravenous epoprostenol. Liver transplant centers were defined as centers which performed more than five liver transplants per year according to the Scientific Registry of Transplant Recipients (srtr.org). As defined by the United States Department of Health and Human Services, we considered an annual household income below poverty level if the self-reported annual household income range was less than 2019 poverty guideline thresholds for the number of family members in the household. 14 We calculated prognostic REVEAL 2.0 scores at enrollment according to the REVEAL 2.0 risk calculator. 15 The number of French Registry non-invasive low-risk criteria (6-min walk distance (6MWD) >440 m, World Health Organization (WHO) functional class I/II, and N-terminal-pro B-type natriuretic peptide (NT-proBNP) <300 pg/ml or B-type natriuretic peptide (BNP) < 50 pg/ml) and invasive low-risk criteria (WHO functional class I or II, 6MWD >440 m, right atrial pressure <8 mmHg, and cardiac index ≥2.5 L/min/m2) at enrollment was also determined. 16
Statistical analysis
Patients with IPAH and POPH were compared using a Student’s t-test or Wilcoxon rank-sum test for continuous variables and a chi-square or Fisher’s exact test for categorical variables with Cochran Armitage chi-square test for trends, as appropriate. We used univariate and multivariate logistic regression to identify whether socioeconomic factors (age, sex, race (Non-Hispanic white, Hispanic, Black or African-American, Asian/Pacific Islander or other), education level (college graduate), annual household income (<$50,000 vs. ≥$50,000 per year), employment, and marital status) and POPH diagnosis were associated with use of combination therapy or parenteral therapy. We adjusted these models for a priori determined prognostic variables that could potentially impact treatment approach: age, functional class, cardiac index, right atrial pressure, and 6MWD. We performed a sensitivity analysis where we adjusted for REVEAL 2.0 score rather than age, functional class, cardiac index, right atrial pressure, and 6MWD, as these variables are all included in the REVEAL 2.0 score. 15 As exploratory analyses, we used univariate and multivariate logistic and linear regression to identify whether socioeconomic factors and/or POPH diagnosis were associated with ED visits, hospitalizations, and nights spent in the hospital within the six months prior to enrollment. We adjusted these models for the same a priori determined prognostic variables as above: age, functional class, cardiac index, right atrial pressure, and 6MWD. Socioeconomic factors and POPH diagnosis were included in the multivariate model if the p value was <0.05. Models included only complete cases with all relevant variables available; we did not impute missing variables or outcomes. For all analyses, a p value <0.05 was considered statistically significant. Statistical analyses were performed using SAS Version 9.4.
Results
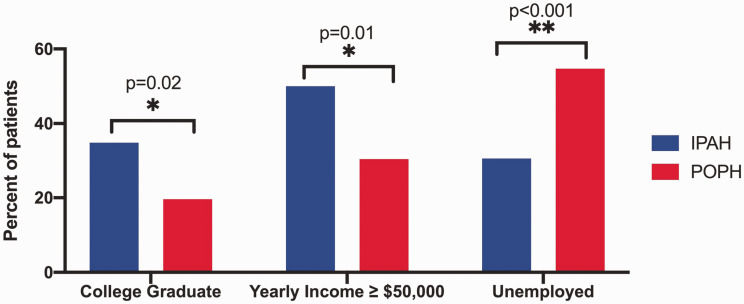
Patient characteristics are detailed in Table 1. Compared with IPAH, patients with POPH were more likely to be male and were similarly aged with similar racial and geographic distribution. There were significant differences between the groups in socioeconomic factors. Patients with POPH were less likely to have graduated from college, less likely to have an annual household income ≥$50,000 per year, and more likely to have an annual household income below poverty level (Fig. 1). There were also significant differences in employment and insurance status: patients with POPH were more likely to be unemployed (Fig. 1), less likely to have private insurance, and more likely to have insurance through Medicaid or other government programs. Current alcohol use was lower in patients with POPH, and history of tobacco use and methamphetamine use was similar (Table 1). Patients with POPH had a lower body mass index and higher oxygen saturation with otherwise similar physical examination characteristics (Table 1).
Table 1.
Patient characteristics and socioeconomic factors.
| Characteristic | n | IPAH | n | POPH | p |
|---|---|---|---|---|---|
| Age, years | 344 | 55.2 ± 16.8 | 57 | 53.4 ± 12.7 | 0.34 |
| Sex, female, n (%) | 343 | 259 (75.5) | 57 | 29 (50.9) | <0.001 |
| Race/ethnicity | 341 | 57 | 0.15 | ||
| Non-Hispanic White | 244 (71.6) | 38 (66.7) | |||
| Hispanic | 20 (5.9) | 2 (3.5) | |||
| Black or African-American | 41 (12.0) | 5 (8.8) | |||
| Asian/Pacific Islander | 7 (2.1) | 4 (7.0) | |||
| Other/Mixed race | 29 (8.5) | 8 (14.0) | |||
| United States region | 344 | 57 | 0.20 | ||
| Northeast | 60 (17.4) | 12 (21.1) | |||
| Midwest | 66 (19.2) | 10 (17.5) | |||
| South | 128 (37.2) | 14 (24.6) | |||
| West | 90 (26.2) | 21 (36.8) | |||
| College graduate | 338 | 118 (34.9) | 56 | 11 (19.6) | 0.02 |
| Yearly household income ≥$50,000 | 274 | 137 (50.0) | 46 | 14 (30.4) | 0.01 |
| Yearly household income below poverty levela | 274 | 52 (19.0) | 46 | 21 (45.7) | <0.001 |
| Occupation | 335 | 53 | 0.009 | ||
| Unemployed | 102 (30.5) | 29 (54.7) | |||
| Employed | 124 (37.0) | 13 (24.5) | |||
| Retired | 100 (29.9) | 11 (20.8) | |||
| Student | 9 (2.7) | 0 (0.0) | |||
| Marital status, n (%) | 333 | 57 | 0.89 | ||
| Married or living with partner | 201 (60.4) | 33 (57.9) | |||
| Widowed, divorced, or separated | 76 (22.9) | 13 (22.8) | |||
| Single | 56 (16.8) | 11 (19.3) | |||
| Household number | 342 | 56 | 0.77 | ||
| 0 | 62 (18.1) | 8 (14.3) | |||
| 1 | 138 (40.4) | 23 (41.1) | |||
| 2 | 142 (41.5) | 25 (44.6) | |||
| Heath insurance | 334 | 57 | 0.02 | ||
| Medicare | 60 (18.0) | 12 (21.1) | |||
| Medicaid | 21 (6.3) | 8 (14.0) | |||
| Private insurance | 202 (60.5) | 23 (40.4) | |||
| Other government programs | 47 (14.1) | 13 (22.8) | |||
| Uninsured | 4 (1.2) | 1 (1.8) | |||
| Current alcohol useb | 338 | 126 (37.3) | 56 | 5 (8.9) | <0.001 |
| Current or past smoker | 339 | 162 (47.8) | 56 | 28 (50.0) | 0.76 |
| History of methamphetamine useb | 339 | 23 (6.8) | 56 | 6 (10.7) | 0.30 |
| Physical examination | |||||
| Body mass index, kg/m2 | 335 | 31.0 ± 7.7 | 54 | 28.9 ± 5.2 | 0.001 |
| Heart rate, beats per minute | 225 | 78.9 ± 15.9 | 44 | 73.9 ± 15.0 | 0.06 |
| Systolic blood pressure, mmHg | 209 | 130.3 ± 25.8 | 38 | 129.6 ± 16.8 | 0.82 |
| Diastolic blood pressure, mmHg | 208 | 76.8 ± 12.8 | 38 | 74.9 ± 14.8 | 0.39 |
| Oxygen saturation, % | 227 | 90.9 ± 9.8 | 44 | 94.8 ± 3.5 | <0.001 |
IPAH: idiopathic pulmonary arterial hypertension; POPH: portopulmonary hypertension.
aAs defined by United States Department of Human and Health Services. Statistically significant p-values (<0.05) are denoted in bold.
bCurrent alcohol use defined by a “yes” response to the question, “Do you presently drink alcoholic beverages?” and history of methamphetamine use defined as a “yes” response to the question, “Have you ever used methamphetamines, even once?”
Fig. 1.
Socioeconomic factors in patients with IPAH and POPH. Compared with IPAH, patients with POPH are significantly less likely to be college graduates, less likely to have an annual household income ≥ $50,000 per year and are more likely to have an annual household income below poverty level and be unemployed.
There was a similar proportion of incident and prevalent IPAH and POPH patients (Table 2). Compared with IPAH, patients with POPH had similar functional class, quality of life, and 6MWD (Table 2). Patients with POPH had a similar right atrial pressure, mean pulmonary artery pressure, and pulmonary artery wedge pressure. POPH was associated with a higher cardiac output and cardiac index, a lower pulmonary vascular resistance, and a higher pulmonary artery compliance (Table 2). Patients with POPH had higher REVEAL 2.0 risk scores which were primarily driven by POPH diagnosis; REVEAL 2.0 risk scores without POPH diagnosis were similar. The number of French registry non-invasive and invasive low-risk criteria fulfilled was also similar (Table 2).
Table 2.
Pulmonary hypertension disease severity and treatment.
| Characteristic | n | IPAH | n | POPH | p |
|---|---|---|---|---|---|
| Incident cases (diagnosed within 6 months) | 344 | 166 (48.3) | 57 | 31 (54.4) | 0.39 |
| Prevalent cases (diagnosed more than 6 months prior to registry entry) | 344 | 178 (51.7) | 57 | 26 (45.6) | 0.39 |
| Treated more than 6 months | 344 | 94 (27.3) | 57 | 12 (21.1) | 0.32 |
| Treated <6 months | 344 | 84 (24.4) | 57 | 14 (24.6) | 0.98 |
| World Health Organization functional class | 315 | 53 | 0.84 | ||
| 1 | 31 (9.8) | 2 (3.8) | |||
| 2 | 109 (34.6) | 24 (45.3) | |||
| 3 | 157 (49.8) | 26 (49.1) | |||
| 4 | 18 (5.7) | 1 (1.9) | |||
| Quality of life | 340 | 55 | |||
| SF-36 physical component summary | 33.6 ± 6.6 | 34.9 ± 5.9 | 0.2 | ||
| SF-36 mental component summary | 49.0 ± 8.3 | 48.7 ± 9.0 | 0.82 | ||
| EMPHASIS-10 score | 24.1 ± 12.4 | 24.2 ± 11.2 | 0.96 | ||
| Laboratory data | |||||
| Creatinine | 332 | 1.0 ± 0.5 | 55 | 1.0 ± 0.7 | 0.93 |
| BNP | 198 | 329.5 ± 684.6 | 29 | 231.8 ± 362.1 | 0.24 |
| N-terminal pro BNP (NTproBNP) | 147 | 1811.7 ± 3597.8 | 22 | 2001.6 ± 3463.0 | 0.82 |
| 6-min walk distance, m | 287 | 344.6 ± 141.8 | 52 | 351.2 ± 82.0 | 0.64 |
| Hemodynamics | |||||
| Right atrial pressure, mmHg | 323 | 10.1 ± 5.9 | 56 | 10.3 ± 5.0 | 0.87 |
| Pulmonary artery systolic pressure | 332 | 81.0 ± 21.0 | 56 | 82.2 ± 15.7 | 0.61 |
| Pulmonary artery diastolic pressure | 332 | 33.7 ± 11.7 | 56 | 33.1 ± 8.5 | 0.62 |
| Mean pulmonary arterial pressure, mmHg | 327 | 51.2 ± 14.1 | 56 | 50.9 ± 10.4 | 0.84 |
| Pulmonary artery wedge pressure, mmHg | 320 | 11.6 ± 6.0 | 54 | 11.4 ± 5.0 | 0.82 |
| Cardiac output, L/min | 311 | 4.23 ± 1.37 | 56 | 5.50 ± 2.24 | <0.001 |
| Cardiac index, L/min/m2 | 306 | 2.20 ± 0.67 | 54 | 2.84 ± 1.22 | <0.001 |
| Pulmonary vascular resistance, Wood units | 295 | 10.5 ± 5.5 | 54 | 8.2 ± 3.8 | <0.001 |
| Stroke volume, mL | 218 | 55.3 ± 21.0 | 44 | 79.3 ± 27.5 | <0.001 |
| Stroke volume index, ml/m2 | 213 | 28.9 ± 10.2 | 42 | 40.5 ± 13.9 | <0.001 |
| Pulmonary artery compliance, mL/mmHg | 218 | 1.33 ± 0.94 | 44 | 1.78 ± 0.91 | 0.004 |
| Risk stratification at enrollment | |||||
| REVEAL 2.0 score | 344 | 7.2 ± 2.7 | 57 | 9.8 ± 2.7 | <0.001 |
| REVEAL 2.0 score (without POPH diagnosis) | 344 | 7.2 ± 2.7 | 57 | 6.8 ± 2.7 | 0.24 |
| French Registry # low-risk criteria (non-invasive) | 344 | 57 | 0.81 | ||
| 0 | 140 (40.7) | 21 (36.8) | |||
| 1 | 127 (36.9) | 22 (38.6) | |||
| 2 | 56 (16.3) | 12 (21.1) | |||
| 3 | 21 (6.1) | 2 (3.5) | |||
| French registry # low-risk criteria (invasive) | 344 | 57 | 0.14 | ||
| 0 | 101 (29.4) | 13 (22.8) | |||
| 1 | 121 (35.2) | 16 (28.1) | |||
| 2 | 75 (21.8) | 17 (29.8) | |||
| 3 | 38 (11.1) | 11 (19.3) | |||
| 4 | 9 (2.6) | 0 (0.0) | |||
| PAH therapy at enrollment | |||||
| Number of PAH medications | 341 | 1.7 ± 0.9 | 56 | 1.4 ± 0.8 | 0.01 |
| Combination therapy | 341 | 212 (62.2) | 56 | 26 (46.4) | 0.03 |
| Phosphodiesterase type 5 inhibitor | 343 | 240 (70.0) | 56 | 43 (76.8) | 0.3 |
| Riociguat | 343 | 12 (3.5) | 56 | 1 (1.8) | 1 |
| Endothelin receptor antagonist | 341 | 189 (55.4)a | 56 | 16 (28.6) | <0.001 |
| Bosentan | 7 (2.1) | 0 (0.0) | 0.6 | ||
| Ambrisentan | 98 (28.7) | 11 (19.6) | 0.16 | ||
| Macitentan | 85 (24.9) | 5 (8.9) | 0.008 | ||
| Parenteral prostacyclin | 344 | 86 (25.0) | 57 | 10 (17.5) | 0.22 |
| Inhaled prostacyclin | 341 | 20 (5.9) | 56 | 2 (3.6) | 0.75 |
| Selexipag | 341 | 17 (5.0) | 56 | 4 (7.1) | 0.52 |
| Oral treprostinil | 341 | 7 (2.1) | 56 | 0 (0.0) | 0.6 |
| First follow-up visit | |||||
| Number of outpatient visits with PH doctor or nurse in clinic since enrollment | 221 | 1.9 ± 1.6 | 34 | 2.3 ± 1.5 | 0.26 |
| PAH therapy at first follow-up visit | |||||
| Number of PH medications | 234 | 1.0 ± 0.8 | 38 | 1.8 ± 0.7 | 0.49 |
| Combination therapy | 234 | 170 (72.7) | 38 | 25 (65.8) | 0.38 |
| Phosphodiesterase type 5 inhibitor | 235 | 173 (73.6) | 40 | 36 (90.0) | 0.03 |
| Riociguat | 234 | 19 (8.1) | 38 | 0 (0.0) | 0.08 |
| Endothelin receptor antagonist | 234 | 147 (62.8) | 38 | 19 (50.0) | 0.13 |
| Parenteral prostacyclin | 234 | 59 (25.2) | 39 | 8 (20.5) | 0.53 |
| Inhaled prostacyclin | 234 | 15 (6.4) | 38 | 2 (5.3) | 1 |
| Selexipag | 234 | 21 (9.0) | 38 | 3 (7.9) | 1 |
| Oral treprostinil | 234 | 8 (3.4) | 38 | 0 (0.0) | 0.61 |
| Health-care utilization | |||||
| ED visit within last 6 months | 343 | 179 (52.2) | 56 | 39 (69.6) | 0.02 |
| Number of ED visits within last 6 months | 343 | 0.9 ± 1.2 | 56 | 1.7 ± 2.1 | 0.009 |
| Hospitalized within last 6 months | 343 | 169 (49.3) | 56 | 33 (58.9) | 0.18 |
| Number of hospitalizations within last 6 months | 343 | 0.8 ± 1.1 | 56 | 1.5 ± 2.1 | 0.02 |
| Number of nights in hospital within last 6 months | 343 | 6.3 ± 11.5 | 56 | 10.1 ± 12.2 | 0.02 |
BNP: B-type natriuretic peptide; ED: emergency department; NTproBNP: N-terminal pro B-type natriuretic peptide; PAH: pulmonary arterial hypertension; POPH: portopulmonary hypertension; SF-36: Short Form 36; IPAH: idiopathic pulmonary arterial hypertension. Statistically significant p-values (<0.05) are denoted in bold.
aNumber of IPAH patients treated with an endothelin receptor antagonist does not equal number of patients treated with individual endothelin receptor antagonists since one patient was recorded as being on both ambrisentan and macitentan at time of enrollment.
At enrollment, patients with POPH were less likely to be treated with combination therapy and were prescribed less PAH-targeted therapies (Table 2). Patients with POPH were less likely to receive endothelin receptor antagonists (ERAs). Among the ERAs, macitentan use was lower in POPH as compared to IPAH (8.9% vs. 24.9%, p = 0.008). Overall use of ambrisentan and bosentan was statistically similar (p > 0.05 for both), but no patients with POPH were treated with bosentan. Use of other PAH therapeutic classes were similar (Table 2). Among patients with POPH, those who received care at liver transplant centers were less likely to receive combination therapy at enrollment (17/46, 37% vs. 9/10, 90%, p = 0.004). At the first follow-up visit, ERA use in POPH patients increased from 28.6% to 50.0%. Treatment differences no longer existed, and patients with POPH and IPAH had a similar likelihood of being treated with combination therapy and ERAs (Table 2). After adjusting for age, functional class, 6MWD, cardiac index and right atrial pressure, POPH diagnosis was associated with a lower likelihood of receiving combination therapy at enrollment, but the results were no longer statistically significant (OR 0.52, 95% CI 0.26–1.04, p = 0.06). Results were similar when we adjusted for REVEAL 2.0 score rather than variables above (OR: 0.61, 95% CI: 0.34–1.11, p = 0.11). Increased age was associated with lower use of combination therapy, but other socioeconomic factors were not (p > 0.05 for all). In unadjusted analysis, younger age (p < 0.001), race (p = 0.001), and marital status (p = 0.02) were associated with use of parenteral therapy (Supplemental Table 1), while education, household income, employment status, insurance, and POPH diagnosis were not. After adjusting for age, functional class, 6MWD, cardiac index, and right atrial pressure, associations between parenteral therapy and race (p = 0.13) and marital status (p = 0.69) were no longer significant.
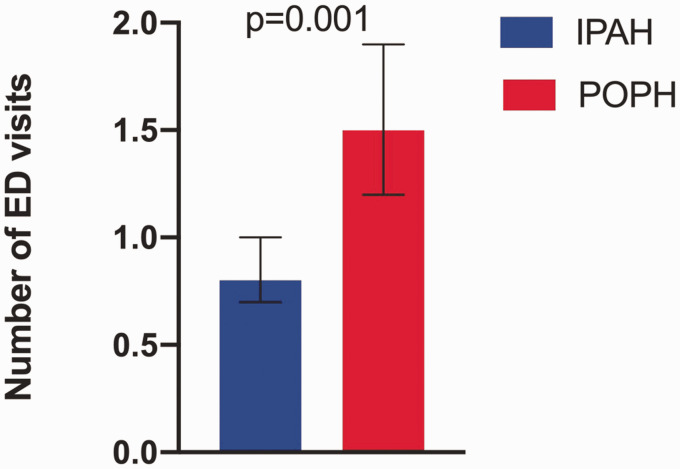
Compared with IPAH, patients with POPH were more likely to have visited the ED in the six months preceding enrollment (Table 2). Patients with POPH also had an increased number of hospitalizations and spent more total nights in the hospital (Table 2). POPH diagnosis and several socioeconomic factors (race, education, household income, employment, and marital status) (p < 0.05 for all) were associated with an ED visit within the preceding six months, but only POPH diagnosis and education level remained significantly associated with ED visits in multivariate analysis (Table 3). After adjusting for age, functional class, 6MWD, cardiac index, right atrial pressure and education, patients with POPH had significantly more ED visits compared to patients with IPAH (least square means 1.5, 95% CI: 1.2–1.9 vs. 0.8, 95% CI: 0.7–1.0, p = 0.001) (Fig. 2), while college graduates had significantly less ED visits after adjusting for the above factors and POPH diagnosis (least square means 0.6, 95% CI: 0.4–0.9 vs. 1.1, 95% CI: 0.9–1.2, p = 0.007). Only POPH diagnosis (p = 0.02) and employment (p = 0.02) were associated with number of nights spent in the hospital, but neither variable remained associated after adjusting for age, functional class, 6MWD, cardiac index, and right atrial pressure.
Table 3.
Variables associated with an emergency department visit in the 6 months prior to enrollment in multivariate analysis.
| Variable | Odds ratio | 95% CI | p |
|---|---|---|---|
| Age, per year | 0.97 | 0.95–0.99 | 0.001 |
| Portopulmonary hypertension diagnosis | 2.22 | 1.07–4.59 | 0.03 |
| College graduate | 0.49 | 0.29–0.84 | 0.01 |
| WHO functional class, per unit increase | 1.41 | 0.93–2.15 | 0.11 |
| 6-min walk distance, per m | 1.00 | 1.00–1.00 | 0.06 |
| Right atrial pressure, per mm Hg | 0.99 | 0.94–1.04 | 0.59 |
| Cardiac index, per L/min/m2 | 1.04 | 0.73–1.48 | 0.84 |
Statistically significant p-values (<0.05) are denoted in bold. CI: confidence interval; WHO: World Health Organization.
Fig. 2.
Emergency department visits in IPAH and POPH. Least square means for emergency department visits in IPAH and POPH after adjustment for age, functional class, 6-min walk distance, cardiac index, right atrial pressure, and education level. Compared with IPAH, patients with POPH had a higher number of emergency department visits in the six months preceding enrollment in the Pulmonary Hypertension Association Registry (1.5, 95% CI: 1.2–1.9 visits vs. 0.8, 95% CI: 0.7–1.0 visits, p = 0.001).
Discussion
In this analysis of patients enrolled in PHAR, we identified significant health disparities between patients with POPH and IPAH. Patients with POPH had worse SES, were less likely to be treated with combination therapy at enrollment, and had increased health-care utilization.
Patient characteristics
In this multicenter registry, there were important differences between patients with POPH and IPAH. Similar to findings in the REVEAL Registry, patients with POPH were more likely to be male, had a lower body mass index, and had a higher cardiac output.8,17 Compared with IPAH, POPH patients had similar mean pulmonary arterial and right atrial pressures with a lower pulmonary vascular resistance and higher stroke volume index and pulmonary artery compliance. Reduced pulmonary artery compliance has been associated with worse survival in IPAH, but findings in POPH versus IPAH have not been previously described. 18 We also found that patients with POPH had higher oxygen saturations, although the reason for this finding is not clear. Despite the comorbid liver disease, HRQoL in patients with POPH and IPAH were similar.
Socioeconomic factors
Although there were no significant differences in age, race, or geographic region, we found that patients with POPH had significantly worse SES compared to patients with IPAH. Patients with POPH were less likely to have graduated from college and more likely to be unemployed and have an annual household income below poverty level; patients with POPH also had less private insurance coverage. These factors are important, as they may impact access to medical care and PAH-targeted therapies. Differences in SES could also lead to diagnostic delays and impact outcomes. Although studies regarding the impact of socioeconomic factors in PAH have been limited, one prior study found that lower SES was associated with worse functional class at the time of diagnosis, suggesting delays in diagnosis. 19 These differences in socioeconomic factors suggest that there should be improved advocacy efforts for patients with POPH in order to ensure they have equitable access to care. As data regarding etiology of liver disease are not included in this registry, we cannot determine whether confounders, such as history of alcohol use or other factors, contribute to differences in SES. Patients with POPH, however, were significantly less likely to report current alcohol use, potentially related to avoidance in the setting of known liver disease or liver transplant evaluation, so it is unlikely that ongoing alcohol use contributed to differences in SES.
Treatment approaches
Patients with POPH were less likely to receive combination therapy and were less likely to be treated with ERAs at enrollment. Although there were significant differences in SES between patients with POPH and IPAH, treatment differences were not related to socioeconomic factors and were no longer significant when adjusted for a priori determined prognostic factors. Potential reasons for treatment differences include less severe hemodynamic impairment in POPH patients, prescriber concern regarding medication clearance, or adverse effects in the setting of liver disease or other factors, such as limited data on efficacy of PAH therapy in POPH.
Patients with POPH were less likely to be treated with ERAs at enrollment, and we hypothesize that this could be related to concern regarding hepatotoxicity or adverse effects, such as edema or anemia. Other potential reasons for decreased ERA utilization at enrollment include medication cost and need for enrollment in Risk Evaluation and Mitigation monitoring programs. Although bosentan has been associated with liver toxicity, ambrisentan and macitentan have not. 20 A recently completed multicenter phase 4 study of macitentan in POPH (PORTICO) also demonstrated efficacy with a 35% reduction in PVR with macitentan versus placebo and no hepatic safety concerns. 21 A post hoc analysis also showed that macitentan significantly improved risk categorization for liver transplantation mortality in 47% of patients. 22 Other recent studies have also demonstrated safety of ambrisentan in POPH. 23 Thus, POPH diagnosis should not be considered a contraindication to use of this important class of therapeutic agents. Our findings suggest that ERAs may initially be underutilized in POPH; accordingly, there may be opportunity for education regarding the safety of ERAs, specifically macitentan and ambrisentan, in POPH.
Current PAH treatment guidelines recommend upfront combination therapy in most patients and the use of risk stratification to guide treatment. 24 Some factors that adversely impact prognosis, however, such as POPH diagnosis, do not change with additional PAH therapy and thus do not necessarily warrant a more aggressive treatment approach. In our study, patients with POPH had higher REVEAL 2.0 risk scores (primarily driven by the POPH diagnosis), but were less likely to receive combination therapy. Although the guidelines do not differentiate treatment recommendations based on disease etiology, they do acknowledge that data regarding combination therapy in POPH is limited. 24 Clinical trials that evaluated the safety and efficacy of upfront combination therapy, such as the AMBITION study, excluded patients with POPH. 25 Thus, little is known regarding the safety, efficacy, and appropriateness of upfront versus sequential combination therapy in POPH.
Recent studies have shown improved PAH outcomes with management at a PHCC. 26 Similarly, we found that treatment differences between IPAH and POPH no longer existed at the first follow-up visit at a PHCC. Thus, decreased ERA use at enrollment may be more reflective of community-based treatment of POPH, while increased use of ERAs at follow-up may be more reflective of treatment approaches at PHCCs. Since the overall number of POPH patients treated with combination therapy did not significantly change at follow-up, the increased proportion treated with combination therapy at follow-up may have also been due to closer follow-up of patients being treated with combination therapy compared to monotherapy. Surprisingly, we also found that patients with POPH who were evaluated at liver transplant centers were less likely to be treated with combination therapy at enrollment. The reason for this is not known, but we hypothesize that it could be due to concerns regarding hepatic safety, concerns related to meeting specific criteria for liver transplantation waitlist priority upgrades, or other reasons for treatment disparities as discussed above.
Health-care utilization
Compared to IPAH, patients with POPH had increased health-care utilization. POPH patients had more ED visits and spent more nights in the hospital in the six months preceding enrollment despite improved hemodynamics. The REVEAL registry similarly found that patients with POPH had more all-cause hospitalizations compared with IPAH and also found that patients with POPH who were hospitalized had greater mortality. 8 A recent study also found that a diagnosis of pulmonary hypertension among patients with portal hypertension was associated with increased health-care utilization (hospitalization and emergency room visits) and costs. 27 Since reasons for ED visit and hospitalization were not included in PHAR, we do not know if increased health-care utilization was related to POPH, liver disease, or other factors. Socioeconomic factors, specifically education level, were also associated with ED visits. College graduates were significantly less likely to have an ED visit in the six months preceding enrollment, even after adjustment for POPH diagnosis and other prognostic factors. Whether this finding is due to differences in access to primary and preventive care or differences in chronic disease self-management or health literacy is not known but deserves further study.
Limitations
Our study had several limitations. These include the retrospective nature of the study and the inclusion of patients only seen at accredited PHCCs. Thus, our findings may not be generalizable to patients who are evaluated and treated at community centers where access to PH expertise is limited. Additionally, the PHAR registry is designed to study PH and does not include information related to an individual’s liver disease etiology or severity as assessed by the Model for End Stage Liver Disease Sodium score or Child-Pugh score, or other factors such as eligibility and consideration for liver transplantation. Since most POPH patients were treated at liver transplant centers, there may be a bias toward including patients with POPH with adequate social support to be considered for liver transplantation. This important information regarding liver disease etiology, severity, and transplant eligibility may impact both treatment and outcomes in POPH but cannot be ascertained from the database. Lastly, given the small sample size and limited follow-up at the time of data analysis, we were underpowered to detect racial/ethnic disparities and differences in survival.
Conclusions
Compared with IPAH, patients with POPH have lower SES, are less likely to receive initial combination therapy and ERAs but have similar treatment approaches at follow-up, and have increased health-care utilization. Awareness and understanding of health disparities in POPH and their impact on outcomes could help improve quality and equity of care for this high-risk group of patients. Future studies are needed to better understand the impact of health disparities on treatment and outcomes in POPH as well as other types of PAH.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_20458940211020913 for Health disparities and treatment approaches in portopulmonary hypertension and idiopathic pulmonary arterial hypertension: an analysis of the Pulmonary Hypertension Association Registry by Hilary M. DuBrock, Charles D. Burger, Sonja D. Bartolome, Jeremy P. Feldman, D. Dunbar Ivy, Erika B. Rosenzweig, Jeffrey S. Sager, Kenneth W. Presberg, Stephen C. Mathai, Matthew R. Lammi, James R. Klinger, Michael Eggert, Teresa De Marco, Jean M. Elwing, David Badesch, Todd M. Bull, Linda M. Cadaret, Gautam Ramani, Thenappan Thenappan, H. James Ford, Nadine Al-Naamani, Marc A. Simon, Sula Mazimba, James R. Runo, Murali Chakinala, Evelyn M. Horn, John J. Ryan, Robert P. Frantz and Michael J. Krowka; on behalf of the PHAR Investigators in Pulmonary Circulation
Acknowledgements
The Pulmonary Hypertension Association Registry (PHAR) is supported by Pulmonary Hypertension Care Centers, Inc., a supporting organization of the Pulmonary Hypertension Association. The authors thank the other investigators, the staff, and particularly the participants of the PHAR for their valuable contributions. A full list of participating PHAR sites and institutions can be found at www.PHAssociation.org/PHAR. The authors would like to acknowledge the PHAR site principal investigators: Roblee P. Allen, MD, Jeffrey Fineman, MD, Roham T. Zamanian, MD, Raymond Foley, MD, Paul Boyce, MD, MPH, Michael Duncan, MD, Timothy Williamson, MD, Wesley McConnell, MD, Stacy Mandras, MD, Paul Hassoun, MD, Rana Awdish, MD, R. James White, MD, PhD, Kishan Parikh, MD, Terry Fortin, MD, MS, Russel Hirsch, MD, Jeffrey C. Robinson, MD, Steven M. Kawut, MD, Amresh Raina, MD, Stephen Chan, MD, PhD, Corey Ventetuolo, MD, MS, Abhijit Raval, MD, John Swisher, MD, PhD, Eric D. Austin, MD, Anna R. Hemnes, MD, Nidhy Varghese, MD, Sahil Bakshi, MD, Oksana A. Shlobin, MD, Steven Nathan, MD, Daniel Grinnan, MD, Peter Leary, MD, PhD, and Dianne L. Zwicke, MD.
Footnotes
Conflict of interest: Consultant for Janssen and have served on advisory boards for Janssen and United Therapeutics.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors’ contributions: H. M. D. was responsible for study conception and design, data analysis, and drafting of the manuscript. All authors made contributions to data acquisition and interpretation as well as drafting and critical revision of the manuscript for important intellectual content. All authors were responsible for ensuring the accuracy and integrity of the work and have given final approval for its publication.Consent to participate
Informed consent was obtained from all participants.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HMD reports consulting fees from Janssen and has served on advisory boards for Janssen and United Therapuetics. JPF is a consultant for Actelion, Gilead, Bayer, United Therapeutics, Altavant, Gossamer and Acceleron. SCM reports consulting fees from Actelion, Liquidia and United Therapeutics. HJF reports grants and personal fees from United Therapeutics/Lung Biotechnology, grants and personal fees from Actelion/Janssen, grants from Liquidia, personal fees from Bayer, and personal fees from Altavant outside the submitted work.
Ethical approval: Research was approved by the institutional review board and conducted according to the World Medical Association Declaration of Helsinki.
Guarantor: H. M. D. is the guarantor of this manuscript.
ORCID iDs: Hilary M. DuBrock https://orcid.org/0000-0001-8410-4429
Erika B. Rosenzweig https://orcid.org/0000-0003-4849-214X
James R. Klinger https://orcid.org/0000-0003-2997-5955
References
- 1.Rodriguez-Roisin R, Krowka MJ, Herve P, et al. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004; 24: 861–880. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 4.Krowka MJ, Fallon MB, Kawut SM, et al. International liver transplant society practice guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation 2016; 100: 1440–1452. [DOI] [PubMed] [Google Scholar]
- 5.Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl 2000; 6: 443–450. [DOI] [PubMed] [Google Scholar]
- 6.Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant 2008; 8: 2445–2453. [DOI] [PubMed] [Google Scholar]
- 7.Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transpl 2000; 6: 241–242. [DOI] [PubMed] [Google Scholar]
- 8.Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest 2012; 141: 906–915. [DOI] [PubMed] [Google Scholar]
- 9.Talwar A, Garcia JGN, Tsai H, et al. Health disparities in patients with pulmonary arterial hypertension: a blueprint for action. An official American Thoracic Society statement. Am J Respir Crit Care Med 2017; 196: e32–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khariton Y, Nassif ME, Thomas L, et al. Health status disparities by sex, race/ethnicity, and socioeconomic status in outpatients with heart failure. JACC Heart Fail 2018; 6: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belachew AA, Reyes ME, Ye Y, et al. Patterns of racial/ethnic disparities in baseline health-related quality of life and relationship with overall survival in patients with colorectal cancer. Qual Life Res 2020; 29: 2977–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celedon JC, Burchard EG, Schraufnagel D, et al. An American Thoracic Society/National Heart, Lung, and Blood Institute workshop report: addressing respiratory health equality in the United States. Ann Am Thorac Soc 2017; 14: 814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray MPKSM. The Pulmonary Hypertension Association Registry: rationale, design, and role in quality improvement. Adv Pulm Hypertens 2018; 16: 185–188. [Google Scholar]
- 14.U.S. Department of Health & Human Services. HHS poverty guidelines for 2021, https://aspe.hhs.gov/poverty-guidelines (accessed 4 January 2021).
- 15.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 16.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. [DOI] [PubMed] [Google Scholar]
- 17.Burger CD, Foreman AJ, Miller DP, et al. Comparison of body habitus in patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term PAH disease management with normative values from the National Health and Nutrition Examination Survey. Mayo Clin Proc 2011; 86: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahapatra S, Nishimura RA, Sorajja P, et al. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2006; 47: 799–803. [DOI] [PubMed] [Google Scholar]
- 19.Talwar A, Sahni S, Talwar A, et al. Socioeconomic status affects pulmonary hypertension disease severity at time of first evaluation. Pulm Circ 2016; 6: 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei A, Gu Z, Li J, et al. Clinical adverse effects of endothelin receptor antagonists: insights from the meta-analysis of 4894 patients from 24 randomized double-blind placebo-controlled clinical trials. J Am Heart Assoc 2016; 5: e003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitbon O, Bosch J, Cottreel E, et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med 2019; 7: 594–604. [DOI] [PubMed] [Google Scholar]
- 22.Krowka M, Cottreel E, Hoeper MM, et al. Macitentan Improves Risk Categorization for Liver Transplant Mortality in Patients With Portopulmonary Hypertension: A PORTICO Study Post Hoc Analysis. Liver Transpl 2020; 26: 935–940. 2020/03/10. DOI: 10.1002/lt.25747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston IR, Burger CD, Bartolome S, et al. Ambrisentan in portopulmonary hypertension: a multicenter, open-label trial. J Heart Lung Transplant 2020; 39: 464–472. [DOI] [PubMed] [Google Scholar]
- 24.Galie N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 26.Pi H, Kosanovich CM, Handen A, et al. Outcomes of pulmonary arterial hypertension are improved in a specialty care center. Chest 2020; 158: 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahay S, Tsang Y, Flynn M, et al. Burden of pulmonary hypertension in patients with portal hypertension in the United States: a retrospective database study. Pulm Circulat 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sithamparanathan S, Nair A, Thirugnanasothy L, Coghlan JG, Condliffe R, Dimopoulos K, et al. Survival in portopulmonary hypertension: Outcomes of the United Kingdom National Pulmonary Arterial Hypertension Registry. J Heart Lung Transplant 2017; 36: 770–779. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_20458940211020913 for Health disparities and treatment approaches in portopulmonary hypertension and idiopathic pulmonary arterial hypertension: an analysis of the Pulmonary Hypertension Association Registry by Hilary M. DuBrock, Charles D. Burger, Sonja D. Bartolome, Jeremy P. Feldman, D. Dunbar Ivy, Erika B. Rosenzweig, Jeffrey S. Sager, Kenneth W. Presberg, Stephen C. Mathai, Matthew R. Lammi, James R. Klinger, Michael Eggert, Teresa De Marco, Jean M. Elwing, David Badesch, Todd M. Bull, Linda M. Cadaret, Gautam Ramani, Thenappan Thenappan, H. James Ford, Nadine Al-Naamani, Marc A. Simon, Sula Mazimba, James R. Runo, Murali Chakinala, Evelyn M. Horn, John J. Ryan, Robert P. Frantz and Michael J. Krowka; on behalf of the PHAR Investigators in Pulmonary Circulation