Abstract
In China, baicalin is the main active component of Scutellaria baicalensis, which has been used in the treatment of inflammation-related diseases, such as inflammation-induced acute lung injury. However, its specific mechanism remains unclear. This study examined the protective effect of baicalin on LPS-induced inflammation injury of alveolar epithelial cell line A549 and explored its protective mechanism. Compared with the LPS-induced group, the proliferation inhibition rates of alveolar type II epithelial cell line A549 intervened by different concentrations of baicalin decreased significantly, as did the levels of inflammatory factors IL-6, IL-1β, prostaglandin 2 and TNF-α in the supernatant. The expression levels of inflammatory proteins inducible NO synthase (iNOS), NF-κB65, phosphorylated ERK (p-ERK1/2), and phosphorylated c-Jun N-terminal kinase (p-JNK1) significantly decreased, as did the protein expression of follistatin-like protein 1 (FSTL1). In contrast, expression of miR-200b-3p significantly increased in a dose-dependent manner. These results suggested that baicalin could significantly inhibit the expression of inflammation-related proteins and improve LPS-induced inflammatory injury in alveolar type II epithelial cells. The mechanism may be related to the inhibition of ERK/JNK inflammatory pathway activation by increasing the expression of miR-200b-3p. Thus, FSTL1 is the regulatory target of miR-200b-3p.
Keywords: Baicalin, alveolar type II epithelial cells, inflammatory response, miR-200b-3p, FSTL1
Introduction
Sepsis is a fatal organ dysfunction caused by infection-induced host reaction disorder. 1 About 31.5 million sepsis cases and 19.4 million severe sepsis cases have been reported worldwide, resulting in a death toll as high as 5.3 million every year. 2 The lungs are the most vulnerable target organ of sepsis. About 25–50% of sepsis cases might develop into acute lung injury (ALI) or even acute respiratory distress syndrome. 3 In complicated organ dysfunction, ALI has the earliest occurrence and the highest incidence rate. 4 The pathological basis of ALI includes excessive persistent alveolar inflammation accompanied with alveolar epithelial cell damage or even apoptosis. At present, ALI caused by sepsis is still treated with anti-infection, respiratory support, immune regulation, and symptomatic support; its death rate can reach as high as 70%. 5 , 6 Therefore, searching for new therapeutic targets and effective drugs may be helpful to reduce the death rate of sepsis-induced ALI.
Follistatin-like protein 1 (FSTL1), also known as TGF-pi-stimulated clone 36 (TSC-36) or follistatin-related protein (FRP), is an extracellular matrix protein and a secretory glycoprotein with molecular mass of about 35,000 Da. 7 FSTL1 is involved in the occurrence and development of many diseases and is closely related to autoimmune diseases, tumor occurrence and metastasis, and cardiovascular diseases such as heart development, myocardial ischemia, cardiac hypertrophy, angiogenesis, and mitral valve disease.8–11 Studies have found that FSTL1 is highly expressed in the serum of patients with rheumatoid arthritis, osteoarthritis, and Sjogren’s syndrome.12–14 In rheumatoid arthritis, FSTL1 can promote the secretion of pro-inflammatory cytokines IFN-γ, TNF-α, IL-1β, and IL-6 and induce synovitis and inflammatory cell infiltration in synovium and surrounding tissues. 15 The overexpression of FSTL1 in monocytes and macrophages cultured in vitro and septic shock in mice can induce the expression of caspase-1 and NLRP3, thereby confirming that FSTL1 can mediate the occurrence of pro-inflammatory events. 16 FSTL1 can promote the inflammatory response through signaling pathways such as ERK1/2, IKKβ, IκB-a, JNK, and NF-κB. 17
Scutellaria baicalensis is a traditional Chinese medicine with a long history. Its effects include clearing away heat and drying dampness, purging fire for removing toxins, and preventing hemostasis and miscarriage. Baicalin is one of the main effective components of S. baicalensis, which features good anti-inflammatory, antioxidant, and anti-apoptosis activities. Baicalin can attenuate myocardial ischemia or reperfusion injury through multiple signaling pathways after inhibiting 12/15-lipoxygenase. 18 It can also attenuate hepatic and renal injury caused by myocardial ischemia/reperfusion injury. 19 , 20 Baicalin can prevent cisplatin-induced acute renal injury by down-regulating the activities of MAPKs and the NF-κB pathway and increasing the production of antioxidants. 21 Recent studies have found that baicalin can alleviate the inflammatory response by inhibiting the TLR4-mediated NF-κB and MAPK signaling pathways in an LPS-induced mice mastitis model. 22 Baicalin can attenuate oxLDL-induced oxidative stress and inflammatory response by regulating the expression of AMPK-α. 23
MicroRNA (miRNA) is a series of endogenous noncoding small RNAs with a length of 19–22 nucleic acids. It is cut from the double-stranded RNA precursor with hairpin structures. It has high conservation, expression sequence, and tissue specificity. By binding with the 3'-untranslated region (3'-UTR) of the Zen gene miRNA, miRNA inhibits OPG translation at the post-transcriptional level to achieve negative regulation of gene expression. miRNA has a wide range of influence on different molecular signaling pathways; it is closely related to cell proliferation, differentiation, and apoptosis, making it important for the growth and development of animals and plants, tissue differentiation, and disease occurrence. 24 An increasing number of studies have adopted miRNAs as targets or therapeutic targets for disease detection. 25 In the inflammatory response, different miRNAs may promote or inhibit inflammation. 26 In the differentiation process from monocyte into macrophages, miR-223, miR-15a, and miR-16 jointly regulate IKKα. When their expression levels decrease, IKKα phosphorylation increases and activates NF-κB. 27 , 28 In the peripheral blood mononuclear cells of patients with type 1 diabetes, overexpression of miR-885-3p can inhibit the inflammatory response of mononuclear cells by regulating the activity of the TLR4/NF-κB signaling pathway. 29 However, the inflammation regulating mechanism of miRNAs remains unclear.
MiR-200b-3p is a member of the miR-200 family, which is involved in a variety of biological events, such as angiogenesis, cell migration, organ fibrosis, and regulation of inflammatory factor secretion.30–33 Studies have shown that the expression of miR-200b significantly decreases in mucosal lesions at the acute stage of ulcerative colitis. Overexpression of miR-200b in vitro can promote IEC proliferation, reduce the IEC inflammatory response, inhibit epithelial–mesenchymal transition (EMT) of IEC, and improve intestinal epithelial barrier function through the transepithelial cell pathway. 34 The expression of miR-200b-3p in HCMV-infected tissues decreases and is negatively correlated with inflammation and viral load of the lesion. 35 However, the specific mechanism is unclear. The ERK/JNK signaling pathway is an important inflammation regulation pathway. 29 In this experiment, we predicted that FSTL1 was the regulatory target of miR-200b-3p using TargetScan software. Therefore, we hypothesized that miR-200b-3p played an important role in the occurrence and development of endotoxin-induced ALI. We further explored whether baicalin can improve endotoxin-induced ALI by increasing the expression of miR-200b-3p and decreasing the activity of FSTL1 and the ERK/JNK signaling pathway.
Material and methods
Materials and reagents
Baicalin was obtained from Nanjing Jingzhu Biotechnology Co., Ltd. (batch no.: 21967-41-9, purity 95%). Alveolar type II epithelial cells (A549 cell line) were provided by Shanghai Sixin Technology Biological Co., Ltd. (sourced from US ATCC Cell Bank). FBS and 1640 medium were purchased from US Gibco Company. Trypsin was purchased from US Thermo Scientific Company. CCK8 kits were purchased from Beijing Zhijie Fangyuan Technology Co., Ltd. LPS was from Abcam Company (UK). ELISA kits of IL-6, IL-1β, prostaglandin 2 (PGE2), and TNF-α were purchased from Wuhan Huamei Biological Co., Ltd. Rabbit anti-mouse β-actin, NF-κB65, p-IκB-α, IκB-α, FSTL1, ERK1/2, and JNK1 primary Abs were all purchased from UK Abcam Company. HRP-labeled IgG secondary Ab was purchased from Wuhan Guge Biology Co., Ltd.
A549 cell culture and group
In this study, 1640 medium containing 10% FBS was used to culture A549 cells. When A549 cells grew and fused to 70%, they were seeded into the culture dish at the density of 1 × 104/ml per well plate and cultured in a 5% CO2 incubator at 37°C. They were digested with 0.25% trypsin and passaged at 1:2 after 7 d of culture. The cells were inoculated in a 25 cm2 culture flask with a density of 1 × 105 cells/ml. When the fusion rate reached 60%, the cells were synchronized to grow for 24 h and then divided into different groups. To observe the effect of LPS on the activity and proliferation of A549 cells, according to a previously described technique, 22 we added different concentrations of LPS (0.5, 1.0, 1.5, and 2.0 mg/l) for 24 h. To observe the effect of baicalin on endotoxin-induced A549 cell injury, the cells were divided into the control group, LPS-induced group, LPS + 10 μmol/l baicalin group, LPS + 25 μmol/l baicalin group, LPS + 50 μmol/l baicalin group, and LPS + 75 μmol/l baicalin group. As previously described, 19 we first added different concentrations of baicalin (10–75 μM) and cultured the cells for 2 h. Second, we added LPS (1.0 μM) solution and cultured the cells for 24 h. Finally, we determined the indicators.
Analysis of cell viability
The proliferation activities of A549 cells in different groups were measured by using a CCK-8 kit (CCK-8, Qi Hai Biology). A549 cells were seeded on a 96-well plate. After cells were fused to 70%, they were moved to serum-free medium. After treatment with different conditions, 10 μl of CCK-8 solution was added into each well and incubated in the dark for 2–4 h. The absorbance values (A450 nm) were measured at 450 nm by using an enzyme-labeled instrument.
Cell transfection
An appropriate amount of A549 cells at the logarithmic phase was inoculated into a six-well plate. When the cells grew overnight and adhered to the wall until 60%, miR-200b-3p mimic, miR-200b-3p inhibitor, mimic control, and inhibitor control were transfected into A549 cells following the operation instructions of Lipofectamine 2000 transfection reagent. The transfection concentration was 50 nmol/l. After transfecting for 6 h, the cells were moved to fresh medium and cultured for another 24 h. The cells were then collected for RT-PCR and Western blot analyses.
Determination of cell survival
The liquid in each well of the 96-well plate was removed. About 270 μl of DMEM and 30 μl of 5g/l MTT working solution were added and mixed. After incubation at 37°C for 4 h, the culture medium in the well was discarded, and 270 μl of DMSO was added into each well. The solution was fully dissolved after shaking for 10 min. The OD values of each well were measured at 490 nm by using an enzyme-labeled instrument. Compared with the OD value of the control group, the cell survival rate of each well (%) = OD value of each well/mean OD value of the control group × 100 was calculated. 5
Cell morphology
The cell morphology of A549 cells in different groups was detected by using the AO/EB method. A549 cells were seeded on a 96-well plate. After cells were fused to 70%, they were moved to serum-free medium. After treatment with different conditions, a working solution of AO solution: EB solution = 1:1 was prepared. About 20 μl of working solution was added to each well and left at room temperature (37°C) for 5 min. Finally, the cells were observed under a fluorescent inverted microscope, and images were obtained.
Flow cytometric detection of the apoptotic rate of A549 cells
The A549 cells were washed with pre-cooled PBS and suspended with 300 µl of buffer solution. The cell concentration was adjusted to 1 × 106/ml, and 100 μl was placed in a flow tube. About 5 µl of ANNEXIN V-FITC (BD Pharmingen, San Diego, CA, USA) and 5 µl of PI (Abcam, Cambridge, MA, USA) were added to the tube, incubated at room temperature for 15 min, and added with 400 µl of PBS to detect cell apoptosis through flow cytometry. Apoptotic cells were quantified as the percentage of cells stained with Annexin V.
Target gene prediction
Target gene prediction software such as miR-Base (http://www.mirbase.org/), TargetScan (http://www.targetscan.org/), and PicTar (http://pictar.m dc-berlin.de/) were used to predict the target gene of miR-200b-3p, which may be FSTL1. Wild type (WT)-FSTL1 and mutant (MUT)-FSTL1 luciferase gene vectors were constructed by conventional methods. The two plasmids were mixed with miR-200b-3p mimics, miR-200b-3p inhibitor, mimic control, and inhibitor control and then co-transfected into A549 cells, which were recorded as Wt+miR-200b-3p mimics, Mut+miR-200b-3p, Wt+negative control, and Mut+negative control, respectively. After incubation in a 5% CO2 incubator at 37°C for 48 h, the activities of dual luciferase were measured by dual luciferase reporter gene kits. The level of FSTL1 in A549 cells with overexpression of miR-200b-3p was detected by RT-PCR and Western blot.
Real-time quantitative PCR
A549 cells in the treatment group were collected. The cell RNA was extracted by a total RNA extraction kit and reverse transcribed into cDNA following the PrimeScript™ RT reagent kit. The expression levels of FSTL1, ERK1, JNK1mRNA, and miR-200b-3p were measured by real-time PCR. The reaction system was as follows: SYBR Premix Ex Taq™ II (10 μl), PCR primer (2 μl), DNA template (2 μl), DEPC water (6 μl), and total reaction system (20 μl). Each sample was set with three parallel multiple pores, and the reaction system was completed by a PCR instrument. To detect the level of miR-200b-3p, we used a TaqMan MicroRNA Assay Kit (Applied Biosystems, Foster City, CA, USA) to determine the expression level of miR-200b-3p by the 2−ΔΔCt method. Reaction conditions were as follows: pre-denaturation at 95°C for 30 s, reaction at 95°C for 5 s, and annealing at 60°C for 30 s for 40 cycles. The primer series are shown in Table 1.
Table 1.
Primer sequences of RT-PCR analysis.
| Gene | Primer | Product(bp) |
|---|---|---|
| miR-200b-3p | F-5′-GCGGGCTAATACTGCCTGG -3′ | 218 bp |
| R-5′-ATCCAGTCCAGGGTCCGAAA-3′ | ||
| U6 | F-5′-CTCGCTTCGGCAGCACA-3′ | 194 bp |
| R-5′-AACGCTTCACGAATTTGCGT-3′ | ||
| FSTL1mRNA | F-5′-TTATGATGGGCAGGCAAAGAA-3′ | 183 bp |
| R-5′-ACTGCCTTTAGAGAACCAGCC-3′ | ||
| ERK1mRNA | F-5′-AGGCATCCGAGACATCCTC -3′ | 217 bp |
| R-5′-CGGTCAGAAAGCCAGTGTG-3′ | ||
| JNK1mRNA | F-5′-GCCCGATGAAACCTCGCAGAT -3′ | 218 bp |
| R-5′-ACGCAGGCAATCCTACTGGA-3′ | ||
| β-Actin | F-5′-ATGGTGAAGGTCGGTGTG-3′ | 154 bp |
| R-5′-AACTTGCCGTGGGTAGAG-3′ |
U6 was used as the internal reference for miR-200b-3p, and β-actin was used as the internal reference for the expression of the FSTL1, ERK1, and JNK1 genes. After the reaction, the cycle threshold (CT) of each gene was calculated and compared with the reference gene U6 to determine the expression level.
Western blot analysis
The cell lysis solution, protease inhibitor, and phosphatase inhibitor were mixed at the ratio of 8:1:1 to produce cell lysis buffer. About 150 μl of RIPA cell lysis buffer was added into each well, and the cells were lysed for 30 min in an ice bath to extract the total cellular protein of each group. After centrifugation at 4°C for 15 min, the purity of protein was detected by using a BCA protein quantitative kit. About 50 μg of protein extraction solution was electrophoretically separated by 10% SDS-PAGE and then incubated with 5% defatted milk for 1.5 h. NF-κB65, p-IκB-α, IκB-α, β-actin, FSTL1, and ERK1/2 primary Ab (1:1000) were correspondingly added at 4°C for the whole night. The next day, the membrane was washed with TBST three times, and HRP-labeled IgG (volume dilution ratio at 1:3000) was added. After reacting at room temperature for 1 h, the membrane was washed with TBST three times again for 10 min each time. ECL chemiluminescence solution was added for color development. The gray value of the strip was analyzed by Image lab software, and β-actin was used as an internal reference.
Determination of inflammatory factors of A549
Cells in the logarithmic growth phase were digested, re-suspended, and inoculated on six plates. After cells grew by adhering to the wall, the old culture medium was removed. After culturing cells of different groups for 24 h, the cells and cell supernatant were collected. The inflammatory factors PGE2, IL-6, IL-1β, and TNF-α were detected by using an ELISA kit. The operation steps were strictly in accordance with the instruction book.
Statistical analysis
GraphPad Prism 6 was used for statistical analysis. The experimental data was represented as mean ± standard deviation. One-way ANOVA was used for comparison among groups. The LSD method was used for pairwise significant comparison between groups. P < 0.05 was considered statistically significant.
Results
LPS attenuated the cell viability and survival of alveolar type II A549 cells
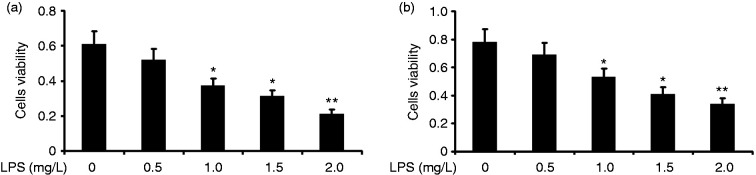
To observe the effect of LPS on the cell viability and survival rate of alveolar A549 cells, we incubated the cells with various concentrations of LPS (0.5, 1.0, 1.5, and 2.0 mg/l) for 24 h. A549 cell viability was measured using CCK-8, and the proliferation rate was measured by the MTT assay. LPS attenuated the cell viability and survival rate of A549 cells in a concentration-dependent manner (Figure 1). Concentrations as low as 1.0 mg/l LPS were effective in decreasing the cell viability and survival rate of A549 cells.
Figure 1.
Effect of LPS on the cell viability and survival rate of alveolar type II epithelial cells. After A549 cells were incubated with various concentrations of LPS (0.5, 1.0, 1.5, and 2.0 mg/l) for 24 h, their cell viability was measured using CCK-8. The survival rate was then measured by the MTT assay. The data from three independent experiments are presented as the mean ± SEM. *P < 0.05, **P < 0.01 versus the control group. P < 0.05, the LPS (1.5 mg/l) group versus the LPS (1.5 mg/l) group and the LPS (2.0 mg/l) group. P > 0.05, the LPS (1.5 mg/l) group versus the LPS (2.0 mg/l) group.
LPS increased the inflammation of alveolar type II epithelial cells
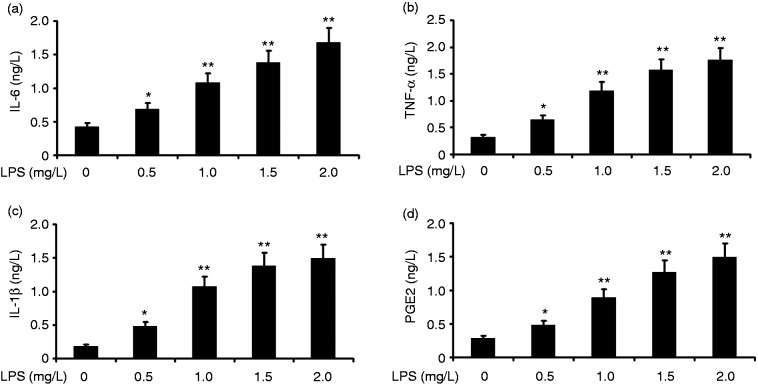
To investigate the effect of LPS on the inflammation of alveolar type II epithelial cells, we incubated A549 cells with various concentrations of LPS (0.25, 0.5, 1.0, 1.5, and 2.0 mg/l) for 24 h. IL-6, PGE2, IL-1β, and TNF-α in A549 cells were measured by ELISA. LPS increased the levels of IL-6, IL-1β, and TNF-α in A549 cells in a concentration-dependent manner (Figure 2). LPS at concentrations as low as 1.0 μM was effective in increasing IL-6, IL-1β, and TNF-α levels in A549 cells.
Figure 2.
Effect of LPS on the inflammation of alveolar type II epithelial cells. A549 cells were incubated with various concentrations of LPS (0.5, 1.0, 1.5, and 2.0 mg/l) for 24 h. IL-6, IL-1β, PGE2, and TNF-α in A549 cells were measured by ELISA. The data from three independent experiments is presented as the mean ± SEM. *P < 0.05, **P < 0.01 versus the control group. P < 0.05, the LPS (1.5 mg/l) group versus the LPS (1.5 mg/l) group and the LPS (2.0 mg/l) group. P > 0.05, the LPS (1.5 mg/l) group versus the LPS (2.0 mg/l) group.
LPS increased the activity of the FSTL1 and ERK/JNK signaling pathway in alveolar type II epithelial cells
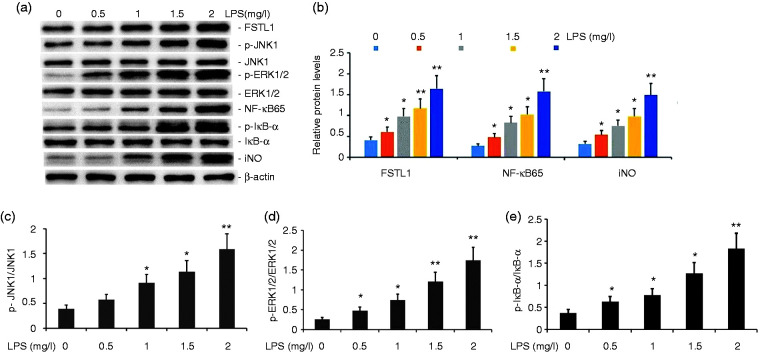
To evaluate the effect of LPS on the FSTL1 and ERK/JNK signaling pathway in A549 cells, we incubated A549 cells with various concentrations of LPS (0.5, 1.0, 1.5, and 2.0 μM) for 24 h. The expression levels of FSTL1, p-JNK1, p-ERK1/2, NF-κB65, p-IκB-α, iNOS, and COX-2 proteins were measured by Western blot analysis. LPS increased the expression levels of FSTL1, p-JNK1, p-ERK1/2, NF-κB65, p-IκB-α, iNOS, and COX-2 proteins in A549 cells in a concentration-dependent manner (Figure 3). LPS at concentrations as low as 1.0 μM was effective in increasing the activity of the FSTL1 and ERK/JNK signaling pathway in A549 cells.
Figure 3.
Effect of LPS on the activity of the FSTL1 and ERK/JNK signaling pathway in A549 cells. A549 cells were incubated with various concentrations of LPS (0.5, 1.0, 1.5, and 2.0 mg/l) for 24 h. FSTL1, p-JNK1, p-ERK1/2, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression levels were measured by Western blot. The data from three independent experiments is presented as the mean ± SEM. *P < 0.05, **P < 0.01 versus the control group. P < 0.05, the LPS (1.5 mg/l) group versus the LPS (1.5 mg/l) group and the LPS (2.0 mg/l) group. P > 0.05, the LPS (1.5 mg/l) group versus the LPS (2.0 mg/l) group.
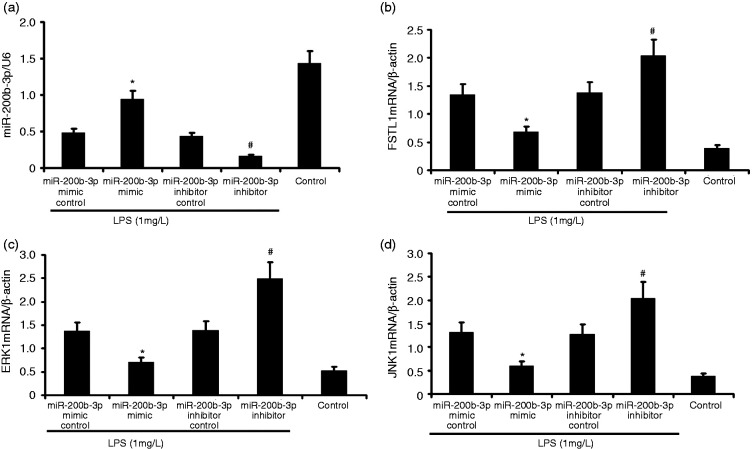
Effect of miR-200b-3p mimic on miR-200b-3p, FSTL1, ERK, and JNK1 mRNA in LPS-induced alveolar type II epithelial cells
The A549 cells were transfected with miR-200b-3p mimic and miR-200b-3p inhibitor in the presence of 1 mg/l LPS for 24 h. miR-200b-3p, FSTL1, ERK, and JNK1 mRNA expression in LPS-induced alveolar type II epithelial cells was measured by RT-PCR. As shown in Figure 4, miR-200b-3p mimic increased miR-200b-3p expression and decreased FSTL1, ERK, and JNK1 mRNA expression. miR-200b-3p inhibitor attenuated miR-200b-3p expression and increased FSTL1, ERK, and JNK1 mRNA expression in LPS-induced A549 cells.
Figure 4.
miR-200b-3p mimic increased miR-200b-3p expression and attenuated FSTL1, ERK, and JNK1 mRNA expression in LPS-induced alveolar type II epithelial cells. The A549 cells were transfected with miR-200b-3p mimic and miR-200b-3p inhibitor in the presence of 1 mg/l LPS for 24 h. miR-200b-3p, FSTL1, ERK, and JNK1 mRNA expression in LPS-induced alveolar type II epithelial cells was measured by RT-PCR. The data from three independent experiments is presented as the mean ± SD. *P < 0.05 versus the miR-200b-3p mimic control group. #P < 0.05 versus the miR-200b-3p inhibitor control group.
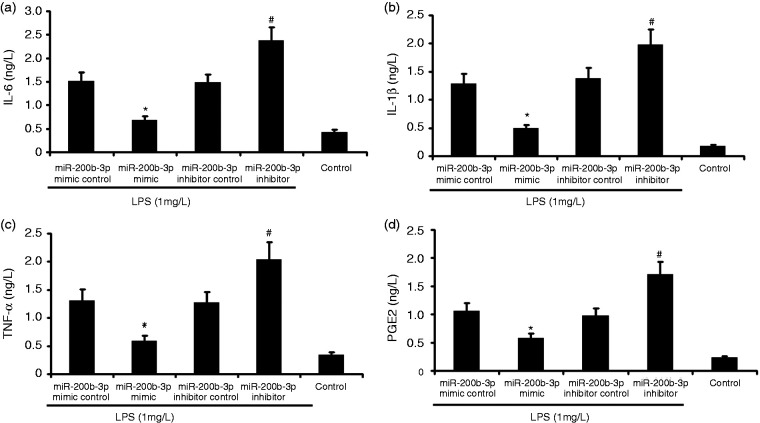
miR-200b-3p overexpression attenuated the inflammation of LPS-induced alveolar type II epithelial cells
The A549 cells were transfected with miR-200b-3p mimic and miR-200b-3p inhibitor in the presence of 1 mg/l LPS for 24 h. PGE2, IL-6, IL-1β, and TNF-α in A549 cells were measured by ELISA. A549 cell viability was measured by CCK-8. As shown in Figure 5, miR-200b-3p mimic decreased the secretion of PGE2, IL-6, IL-1β, and TNF-α in A549 cells and increased A549 cell viability. miR-200b-3p inhibitor increased the secretion of PGE2, IL-6, IL-1β, and TNF-α in LPS-induced A549 cells and increased LPS-induced A549 cell viability.
Figure 5.
Effect of miR-200b-3p overexpression on the inflammation of LPS-induced alveolar type II epithelial cells. The A549 cells were transfected with miR-200b-3p mimic and miR-200b-3p inhibitor in the presence of 1 mg/l LPS for 24 h. PGE2, IL-6, IL-1β, and TNF-α in A549 cells were measured by ELISA. The data from three independent experiments is presented as the mean ± SD. *P < 0.05 versus the miR-200b-3p mimic control group. #P < 0.05 versus the miR-200b-3p inhibitor control group.
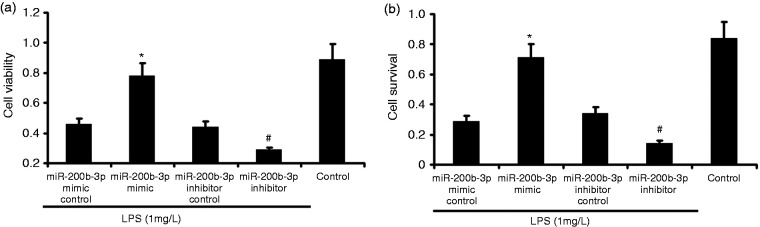
miR-200b-3p overexpression increased cell viability and survival of LPS-induced alveolar type II epithelial cells
The A549 cells were transfected with miR-200b-3p mimic and miR-200b-3p inhibitor in the presence of 1 mg/l LPS for 24 h. A549 cell survival was measured by MTT, and A549 cell viability was measured by CCK-8. As shown in Figure 6, miR-200b-3p mimic increased A549 cell viability and survival. miR-200b-3p inhibitor decreased LPS-induced A549 cell viability and survival.
Figure 6.
Effect of miR-200b-3p overexpression on the cell survival and viability of LPS-induced alveolar type II epithelial cells. The A549 cells were transfected with miR-200b-3p mimic and miR-200b-3p inhibitor in the presence of 1 mg/l LPS for 24 h. A549 cell survival was measured by MTT. A549 cell viability was measured by CCK-8. The data from three independent experiments is presented as the mean ± SD. *P < 0.05 versus the miR-200b-3p mimic control group. #P < 0.05 versus the miR-200b-3p inhibitor control group.
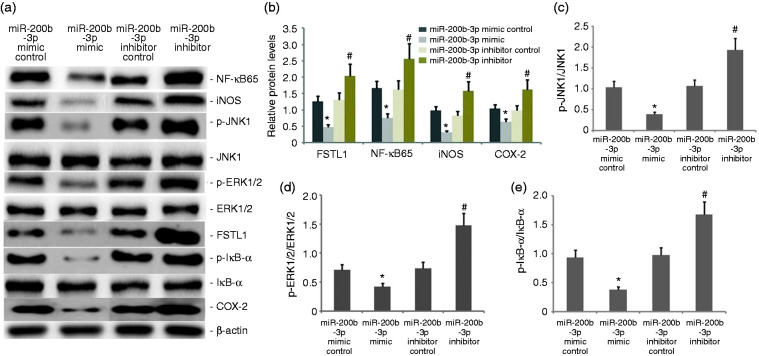
miR-200b-3p overexpression blocked the FSTL1 and ERK/JNK signaling pathway and inflammation of LPS-induced alveolar type II epithelial cells
The A549 cells were transfected with miR-200b-3p mimic and miR-200b-3p inhibitor in the presence of 1 mg/l LPS for 24 h. FSTL1, p-JNK1, p-ERK1/2, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression levels were measured by Western blot. As shown in Figure 7, miR-200b-3p mimic decreased FSTL1, p-JNK1, p-ERK1/2, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression. miR-200b-3p inhibitor increased FSTL1, p-JNK1, p-ERK1/2, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression in LPS-induced A549 cells.
Figure 7.
Effect of miR-200b-3p overexpression on the FSTL1 and ERK/JNK signaling pathway in the inflammation of LPS-induced alveolar type II epithelial cells. The A549 cells were transfected with miR-200b-3p mimic and miR-200b-3p inhibitor in the presence of 1 mg/l LPS for 24 h. FSTL1, p-JNK1, p-ERK1/2, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression levels were measured by Western blot. The data from three independent experiments is presented as the mean ± SD. *P < 0.05 versus the miR-200b-3p mimic control group. #P < 0.05 versus the miR-200b-3p inhibitor control group.
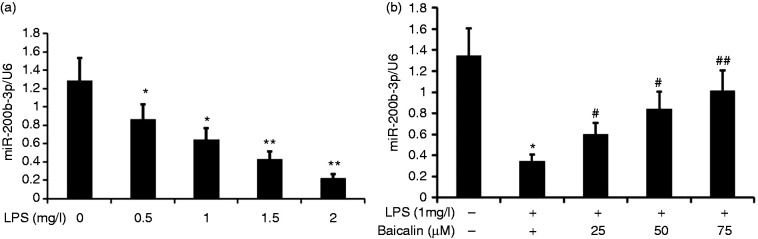
Baicalin increased the expression of miR-200b-3p in LPS-induced alveolar type II epithelial cells
To analyze the effect of LPS on miR-200b-3p expression in A549 cells, we incubated A549 cells with various concentrations of LPS (0.5, 1.0, 1.5, and 2.0 mg/l) for 24 h. miR-200b-3p expression was measured by RT-PCR. LPS decreased miR-200b-3p expression in A549 cells in a concentration-dependent manner (Figure 8).
Figure 8.
Effect of baicalin on miR-200b-3p expression in LPS-induced A549 cells. A549 cells were incubated with various concentrations of LPS (0.5, 1.0, 1.5, and 2.0 mg/l) or LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. miR-200b-3p expression in A549 cells was measured by RT-PCR. The data from three independent experiments is presented as the mean ± SD. a: *P < 0.05, **P < 0.01 versus the control group. b: *P < 0.05 versus the control group. #P < 0.05; ##P < 0.01 versus only LPS group. P < 0.05 versus the baicalin (25 μM) group versus the baicalin (50 μM) group and the baicalin (75 μM) group. P > 0.05, the baicalin (50 μM) group versus the baicalin (75 μM) group.
LPS at concentrations as low as 1.0 μM LPS was effective in decreasing miR-200b-3p expression in A549 cells. To further analyze the effect of baicalin on the decrease in miR-200b-3p expression by LPS induction in A549 cells, we incubated A549 cells with LPS (1.0 μM) and various concentrations of baicalin (12.5, 25, 37.5, 50, and 75 μM) for 24 h. We measured miR-200b-3p expression by RT-PCR and found that baicalin increased miR-200b-3p expression via LPS-induced A549 cells in a concentration-dependent manner. Baicalin at concentrations as low as 50 μM was effective in increasing miR-200b-3p expression in LPS-induced A549 cells.
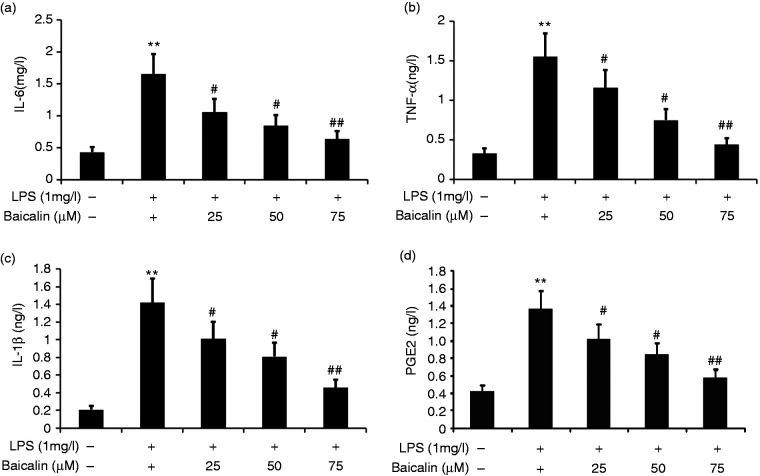
Baicalin decreased A549 LPS-induced cell inflammation
To observe the effect of baicalin on A549 cell inflammation, we incubated A549 cells with LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. IL-6, PGE2, IL-1β, and TNF-α in A549 cells were measured by ELISA. Baicalin decreased the secretion of IL-6, PGE2, IL-1β, and TNF-α in LPS-induced A549 cells in a concentration-dependent manner (Figure 9). Baicalin at concentrations as low as 50 μM was effective in decreasing IL-6, IL-1β, PGE2, and TNF-α levels in LPS-induced A549 cells.
Figure 9.
Effect of baicalin on LPS-induced A549 cell inflammation. A549 cells were incubated with LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. IL-6, IL-1β, PGE2, and TNF-α in A549 cells were measured by ELISA. The data from three independent experiments is presented as the mean ± SD. **P < 0.01 versus the control group. #P < 0.05; ##P <0.01 versus only LPS group. P < 0.05 versus the baicalin (25 μM) group versus the baicalin (50 μM) group and the baicalin (75 μM) group. P > 0.05, the baicalin (50 μM) group versus the baicalin (75 μM) group.
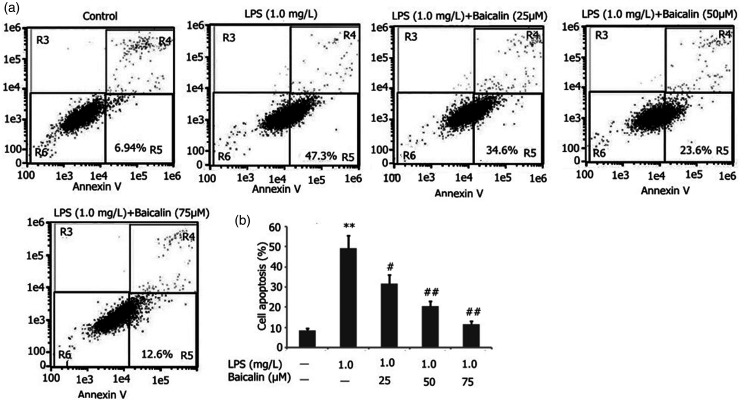
Baicalin decreased A549 LPS-induced cell apoptosis
To observe the effect of baicalin on A549 cell inflammation, we incubated A549 cells with LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. Baicalin decreased A549 cell apoptosis in a concentration-dependent manner (Figure 10). Baicalin at concentrations as low as 50 μM was effective in decreasing A549 cell apoptosis LPS-induced A549 cells.
Figure 10.
Effect of baicalin on LPS-induced A549 cell apoptosis. A549 cells were incubated with LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. A549 cell apoptosis was measured by flow cytometry. The data from three independent experiments is presented as the mean ± SD. **P < 0.01 versus the control group. #P < 0.05; ##P < 0.01 versus only LPS group. P < 0.05 versus the baicalin (25 μM) group versus the baicalin (50 μM) group and the baicalin (75 μM) group. P > 0.05, the baicalin (50 μM) group versus the baicalin (75 μM) group.
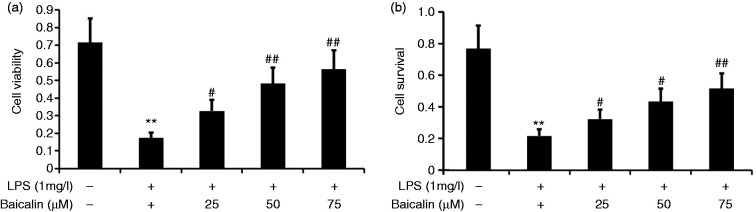
Baicalin increased LPS-induced A549 cell viability and survival rate
To observe the effect of baicalin on A549 cell viability and survival rate, we incubated A549 cells with LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. A549 cell viability was measured using CCK-8, and the proliferation rate was measured by MTT assay. Baicalin increased the cell viability and proliferation rate in LPS-induced A549 cells in a concentration-dependent manner (Figure 11). Baicalin at concentrations as low as 50 μM was effective in increasing the cell viability and proliferation rate in LPS-induced A549 cells.
Figure 11.
Effect of baicalin on A549 cell viability and survival rate. A549 cells were incubated with LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. A549 cell viability was measured using CCK-8, and the proliferation rate was measured by MTT. The data from three independent experiments is presented as the mean ± SD. **P < 0.01 versus the control group. #P < 0.05; ##P < 0.01 versus only LPS group. P < 0.05 versus the baicalin (25 μM) group versus the baicalin (50 μM) group and the baicalin (75 μM) group. P > 0.05, the baicalin (50 μM) group versus the baicalin (75 μM) group.
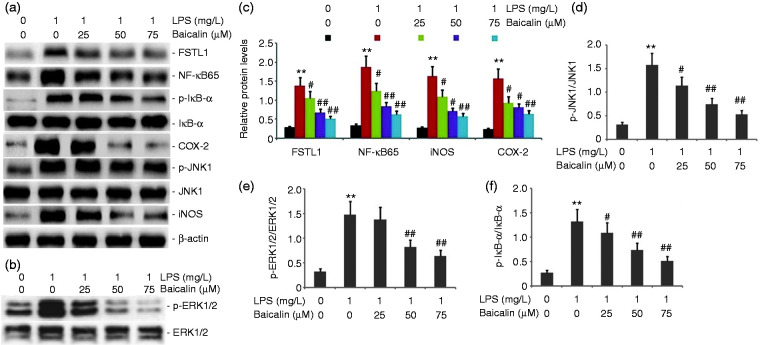
Baicalin decreased the activity of the FSTL1 and ERK/JNK signaling pathway in LPS-induced alveolar type II epithelial cells
To observe the effect of baicalin on the activity of the FSTL1 and ERK/JNK signaling pathway in LPS-induced A549 cells, we incubated A549 cells with LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. FSTL1, p-ERK1/2, p-JNK1, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression levels were measured by Western blot. Baicalin decreased FSTL1, p-ERK1/2, p-JNK1, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression in LPS-induced A549 cells in a concentration-dependent manner (Figure 12). Baicalin at concentrations as low as 50 μM was effective in blocking the activity of the FSTL1 and ERK/JNK signaling pathway in LPS-induced A549 cells.
Figure 12.
Effect of baicalin on the activity of the FSTL1 and ERK/JNK signaling pathway in LPS-induced alveolar type II epithelial cells. A549 cells were incubated with LPS (1.0 mg/l) and various concentrations of baicalin (25, 50, and 75 μM) for 24 h. FSTL1, p-ERK1/2, p-JNK1, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression levels were measured by Western blot. The data from three independent experiments is presented as the mean ± SEM. **P < 0.01 versus the control group. #P < 0.05; ##P < 0.01 versus only LPS group. P < 0.05 versus the baicalin (25 μM) group versus the baicalin (50 μM) group and the baicalin (75 μM) group. P > 0.05, the baicalin (50 μM) group versus the baicalin (75 μM) group. a and b: Representative Western blots of FSTL1, p-ERK1/2, p-JNK1, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression in LPS-induced alveolar type II epithelial cells. c–f: Statistical summary of the densitometric analysis of FSTL1, p-ERK1/2, p-JNK1, NF-κB65, p-IκB-α, iNOS, and COX-2 protein expression in LPS-induced alveolar type II epithelial cells.
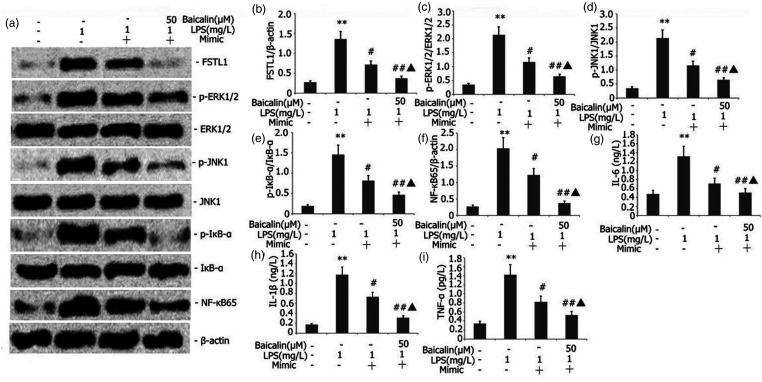
Baicalin combined with miR-200b-3p mimic inhibited the FSTL1 signaling pathway and inflammation in LPS-induced alveolar type II epithelial cells
The A549 cells were transfected with miR-200b-3p mimic in the presence of 1 mg/l LPS and 50 μM baicalin for 24 h. FSTL1, p-ERK1/2, p-JNK1, NF-κB65, and p-IκB-α protein expression levels were measured by Western blot. Baicalin decreased FSTL1, p-ERK1/2, NF-κB65, p-JNK1, and p-IκB-α protein expression in LPS-induced A549 cells in a concentration-dependent manner. IL-6, IL-1β, and TNF-α in A549 cells were measured by ELISA. Baicalin combined with miR-200b-3p mimic decreased FSTL1, p-ERK1/2, NF-κB65, p-JNK1, and p-IκB-α protein expression in LPS-induced A549 cells (Figure 13).
Figure 13.
Effect of baicalin combined with miR-200b-3p mimic on the FSTL1 signaling pathway and inflammation in LPS-induced alveolar type II epithelial cells. The A549 cells were transfected with miR-200b-3p mimic in the presence of 1 mg/l LPS and 50 μM baicalin for 24 h. FSTL1, p-ERK1/2, p-JNK1, NF-κB65, and p-IκB-α protein expression levels were measured by Western blot. IL-6, IL-1β, and TNF-α in A549 cells were measured by ELISA. The data from three independent experiments is presented as the mean ± SEM. *P < 0.05 versus the control group. #P < 0.05 versus the only LPS group. ▲P < 0.05 versus the mimic group.
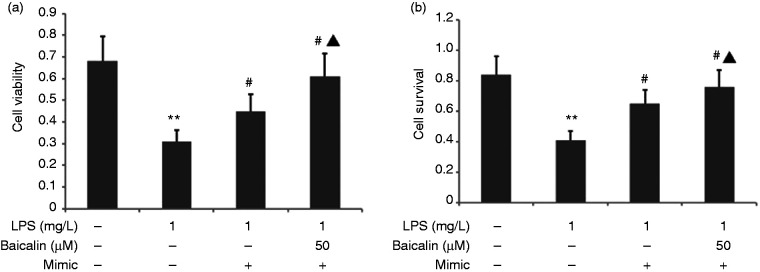
Baicalin combined with miR-200b-3p inhibitor increased LPS-induced A549 cell viability and the survival rate
The A549 cells were transfected with miR-200b-3p inhibitor in the presence of 1 mg/l LPS and 50 μM baicalin for 24 h. A549 cell viability was measured using CCK-8, and the proliferation rate was measured by MTT assay. Baicalin combined with miR-200b-3p mimic increased LPS-induced A549 cell viability and the survival rate in LPS-induced A549 cells (Figure 14).
Figure 14.
Effect of baicalin combined with miR-200b-3p mimic on LPS-induced A549 cell viability and the survival rate. The A549 cells were transfected with miR-200b-3p inhibitor in the presence of 1 mg/l LPS and 50 μM baicalin for 24 h. A549 cell viability was measured using CCK-8, and the proliferation rate was measured by MTT assay. The data from three independent experiments is presented as the mean ± SEM. *P < 0.05 versus the control group. #P < 0.05 versus the only LPS group. ▲P < 0.05 versus the mimic group.
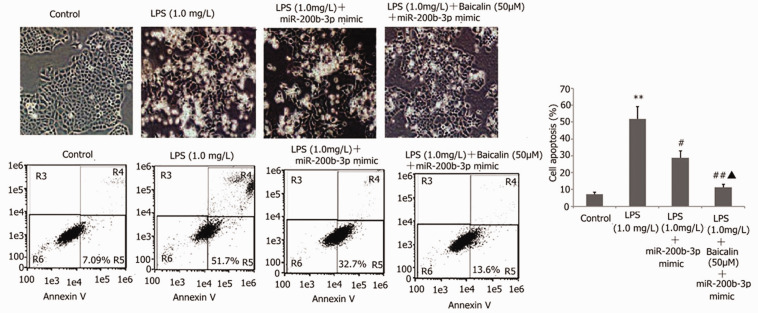
Baicalin combined with miR-200b-3p inhibitor decreased LPS-induced A549 cell apoptosis and improved LPS-induced A549 cell morphology
The A549 cells were transfected with miR-200b-3p inhibitor in the presence of 1 mg/l LPS and 50 μM baicalin for 24 h. The A549 cell morphology was detected via the AO/EB method. A549 cell apoptosis was measured by flow cytometry. Baicalin combined with miR-200b-3p mimic decreased LPS-induced A549 cell apoptosis. Compared with the control group, the number of adherent cells was significantly reduced, the cells became rounded or branched, some cells were granular with intact cell membranes but fragile nuclei, and white granular sloughed cells could be observed in the supernatant (Figure 15). Compared with the mimic group, the baicalin combined with miR-200b-3p mimic group had relatively more adherent cells and fewer white granular shed cells (Figure 15).
Figure 15.
Effect of baicalin combined with miR-200b-3p mimic on LPS-induced A549 cell apoptosis and morphology. The A549 cells were transfected with miR-200b-3p inhibitor in the presence of 1 mg/l LPS and 50 μM baicalin for 24 h. The A549 cell morphology was detected via the AO/EB method. A549 cell apoptosis was measured by flow cytometry. The data from three independent experiments is presented as the mean ± SEM. *P < 0.05 versus the control group. #P < 0.05 versus the only LPS group. ▲P < 0.05 versus the mimic group.
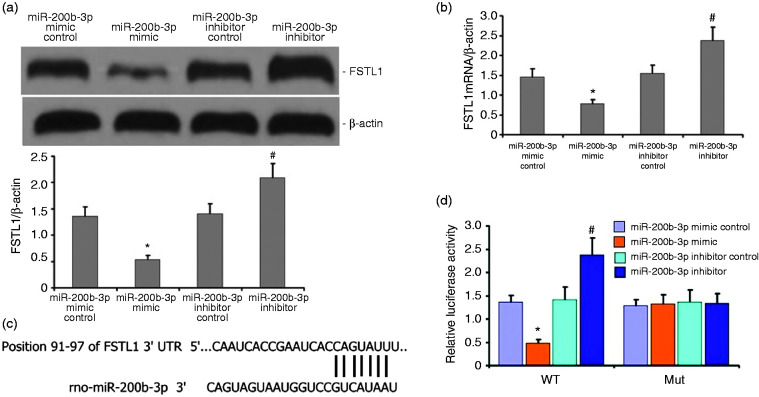
FSTL1 is a regulatory target of miR-200b-3p
We found that FSTL1 and miR-200b-3p are important regulators of LPS-induced A549 cell inflammation. To determine how miR-200b-3p regulated LPS-induced A549 cell inflammation, we used TargetScan to predict likely targets of miR-200b-3p. The results revealed that the FSTL1 mRNA and protein levels dramatically decreased after miR-200b-3p overexpression, whereas the FSTL1 mRNA and protein levels increased after miR-200b-3p down-regulation (Figures 16a and 16b). To validate the interaction between miR-200b-3p and FSTL1, we constructed wild type and mutant FSTL1 for a dual luciferase reporter assay (Figures 16c and 16d). As hypothesized, miR-200b-3p bound to the WT FSTL1 3′-UTR but not to the mutant FSTL1 3′-UTR (Figures 16c and 16d).
Figure 16.
FSTL1 is a downstream target of miR-200b-3p. a and b: FSTL1 decreased in LPS-induced A549 cells with miR-200b-3p overexpression relative to the empty vector control (P < 0.05). FSTL1 significantly increased in A549 cells with miR-200b-3p down-regulation. c and d: Decreased mRNA levels of FSTL1 in LPS-induced A549 cells after miR-200b-3p overexpression (P < 0.05). Levels were enhanced after miR-200b-3p up-regulation (P < 0.05). E, miR-200b-3p was bound to the 3'-UTR regions of FSTL1. Binding was interrupted in mutant FSTL1. F, Dual luciferase reporter assay indicated that the miR-200b-3p mimic was bound to the 3'-UTR region of wild type FSTL1 rather than to FSTL1 mutants (P < 0.05).
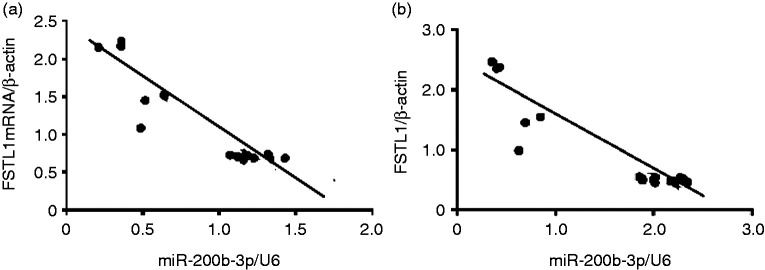
Correlation between FSTL1 and miR-200b-3p in LPS-induced alveolar type II epithelial cells
We found that FSTL1 mRNA expression decreased in LPS-induced A549 cells transfected with an miR-200b-3p overexpression vector compared with changes from an empty vector control (P < 0.05; Figure 17). FSTL1 protein expression increased in LPS-induced A549 cells via miR-200b-3p down-regulation (Figure 17).
Figure 17.
Correlation between FSTL1 and miR-200b-3p in LPS-induced alveolar type II epithelial cells. a and b: Statistical summary of the correlation between mRNA and protein expression of FSTL1 and miR-200b-3p. A negative correlation existed between FSTL1 and miR-200b-3p, as well as between FSTL1 mRNA and miR-200b-3p, in LPS-induced alveolar type II epithelial cells (r = −0.4588, P < 0.05 and r = −0.539, P < 0.05, respectively).
We further analyzed the correlation between FSTL1 and miR-200b-3p in LPS-induced A549 cells. We found a negative correlation between FSTL1 protein and miR-200b-3p, as well as between FSTL1 mRNA and miR-200b-3p, in LPS-induced A549 cells (Figures 17a and 17b).
Discussion
Alveolar epithelial cells (AECs) are the primary target cells attacked by pathogenic endotoxins, as well as inflammatory activated cells and effector cells. They are activated by LPS and the release of inflammatory mediators (such as IL-6, IL-8, TNF-α, intercellular adhesion molecule 1 [ICAM-1], and PGE2), resulting in structural damage, dysfunction, apoptosis, and necrosis of cells.36–40 A549 cells are widely used in studies on the injury protection mechanism of AECs. 41 Therefore, in this study, we also found that the treatment of A549 cells with endotoxins could cause A549 cells to secrete inflammatory mediators such as IL-6, IL-8, TNF-α, and PGE2. With the increase in endotoxin dosage, the secretion of inflammatory mediators such as IL-6, IL-8, TNF-a, and PGE2 also increased, and the cell activity and cell proliferation of A549 cell decreased. After treatment with baicalin, the secretion of inflammatory mediators such as IL-6, IL-8, TNF-α, and PGE2 decreased, and the cell activity and cell proliferation of A549 cells increased.
Transfection of FSTL1 into macrophages and fibroblasts can lead to the up-regulation of pro-inflammatory cytokines (including IL-1β, TNF-α, and IL-6). 14 Overexpression of FSTL1 in mouse paws through gene transfer can lead to severe paw swelling and arthritis. 42 Miyamae et al. 42 transfected FSTL1 into U937 and COS-7 cells, and a large number of pro-inflammatory factors such as IL-1β, IL-6, and TNF-α were produced after stimulating with endotoxin and phorbol ester. In addition, injection of adenovirus carrying FSTL1 protein can aggravate joint swelling and cartilage injury in mice, which might be related to the IFN-γ signaling pathways. 42 FSTL1 can activate peripheral blood macrophages to secrete a large number of inflammatory factors. 42 Recent studies have shown that FSTL1 plays an important role in the pathogenesis of bronchial asthma, which can promote airway remodeling and airway inflammation in patients with asthma. 41 The level of FSTL1 in plasma and BALF of patients with asthma was higher than that of the control group, and the increasing degree was positively correlated with airway smooth muscle bundles and thickened reticular basement membrane. 43 Proteomic analysis of sputum from patients with asthma showed that FSTL1 was one of the high expression proteins. FSTL1 can also promote airway remodeling in patients with asthma by promoting the autophagy of airway epithelial cells and inducing EMT. 44 In this study, we further found that the expression of FSTL1 and the secretion of inflammatory mediators increased in A549 cells by inducing A549 cells with endotoxin. By using baicalin to treat LPS-induced A549 cells, we found that the expression of FSTL1 decreased, the inflammation of A549 cells was significantly attenuated, and the activity of A549 cells increased. All these changes suggested that baicalin could reduce the expression of FSTL1 in A540 cells and improve LPS-induced A549 cell inflammation.
MAPKs, including ERK, JNK, and p38MAPK, belong to the serine/threonine protein kinase family; they play an important role in regulating cell proliferation, differentiation, migration, invasion, inflammation, apoptosis, and other biological processes. 45 ERK1/2 is widely expressed in mammalian cells, and it plays an important role in the regulation of cell mitosis, meiosis, and cell differentiation. After LPS stimulates inflammatory cells, it activates tyrosine protein kinase and induces ERK1/2 phosphorylation through Raf-1, which leads to a large amount of secreted inflammatory factors (such as IL-1, IL-6, and TNF-α) and increases the expression of iNOS and NO. 46 JNK is closely related to the inflammatory response. The surface receptors of inflammatory cells recognize external irritants such as endotoxins. After JNK is phosphorylated and activated, two serine residues at the terminals of c-Jun amino, namely, the sites ser63 and ser73, are also phosphorylated. Homologous or heterodimers are recruited to combine with cis-acting elements of the AP-1 gene site to start gene expression of inflammatory cytokines. 47 In this study, LPS promoted the phosphorylation of ERK1/2 and JNK, resulting in increased ERK/JNK pathway activity and increased A549 cell inflammation. After baicalin treatment, the phosphorylation expression levels of ERK1/2 and JNK decreased, and the ERK/JNK pathway activities were inhibited. Moreover, the endotoxin-induced A549 cell inflammatory response was attenuated. This study also found that FSTL1 could regulate cell and tissue inflammation by regulating ERK/JNK pathway activity. In this study, baicalin could reduce FSTL1, inhibit ERK/JNK pathway activity, and improve LPS-induced A549 cell inflammation.
The miR-200 family, including miR-200a, miR-200b, miR-200c, miR-141, and miR-429, is a family of epithelial cell markers. Among them, miR-200b is an important regulatory factor in EMT progression, which is closely related to the invasion, metastasis, and chemotherapy resistance of tumor cells. 48 In human lung adenocarcinoma cells, artificial overexpression of miR-200b can inhibit the expression levels of VEGF, Fit-1, and KDR. 49 In human umbilical vein endothelial cells, overexpression of miR-200b can inhibit VEGF-induced ERK1/2 phosphorylation and the ability of cells to form capillaries in the matrix glue. 50 Recent studies found that the overexpression of miR-200a can increase nuclear factor erythroid 2-related factor 2 and reduce fructose-induced liver inflammation and oxidative stress by regulating Kelch-like ECH-associated protein 1. miR-200b mimic transfection of high Glc-induced human aortic endothelial cells (HAECs) could reduce the gene expression of ICAM-1, vascular cellular adhesion molecule 1, and E-selectin, as well as decrease the inflammation of high Glc-induced HAECs. In this experiment, in miR-200b-3p mimic-transfected A549 cells, the expression of miR-200b-3p increased; the expression of FSTL1 decreased; the secretion of IL-6, IL-8, TNF-α, PGE2, and other inflammatory mediators in A549 cells decreased; and inflammation was alleviated. The expression of miR-200b-3p increased after treatment with baicalin, thereby inhibiting the expression of FSTL1 and reducing the inflammation of A549 cells.
NF-κB is an important pleiotropic nuclear transcription factor, which can specifically bind to the κB site of various gene promoters to promote transcription expression. 51 Under normal conditions, NF-κB and IκB exist in the cytoplasm in the form of a complex. When stimulated by external irritants (such as pathogen invasion), IκB phosphorylates and then ubiquitinates. Subsequently, it is degraded by proteasome, releasing NF-κB from the cytoplasm into the nucleus, which binds to DNA. Transcription of binding promoter genes induces the release of inflammatory mediators. 52 In this experiment, after treatment with baicalin, the activity of NF-κB/IκB decreased, and LPS-induced A549 cell inflammation was inhibited.
In this experiment, both miR-200b-3p and FSTL1 could regulate LPS-induced A549 cell inflammation. To clarify the relationship between miR-200b-3p and FSTL1, we predicted that FSTL was the regulatory target of miR-200b-3p by TargetScan and confirmed that FSTL was the regulatory target of miR-200b-3p through the luciferase reporter assay. These results suggested that miR-200b-3p regulated LPS-induced A549 cell inflammation by regulating the expression of FSTL1.
Baicalin can effectively reduce the proliferation, migration, and apoptosis resistance of pulmonary artery smooth muscle cells induced by hypoxia by up-regulating A2AR and down-regulating the stromal cell-derived factor-1/CXC chemokine receptor 4 axis. 53 Baicalin may reduce the level of pro-inflammatory cytokines by regulating the SIRT1 NF-κB pathway and improve the depression induced by olfactory bulb resection. 54 Baicalin can also inhibit the NF-κB and p38 MAPK signaling pathways to achieve anti-fat, antioxidant, and anti-inflammatory effects in a dose-dependent manner; thus, baicalin can improve arteriosclerosis, 55 inhibit Akt/NF-κB activation, prevent inflammatory cytokines, and effectively alleviate chronic gastritis. 56 In this experiment, we found that baicalin could increase the expression of miR-200b-3p, regulate the expression of FSTL1, inhibit the activation of the pro-inflammatory ERK/JNK inflammatory pathway and A549 cell apoptosis, and attenuate LPS-induced A549 cell injury.
In conclusion, this study indicated that miR-200b-3p played an important role in the occurrence and development of endotoxin-induced ALI. Overexpression of miR-200b-3p could regulate the expression of FSTL1, reduce the phosphorylation of ERK1/2 and JNK, inhibit the pathway activity of ERK/JNK, and attenuate the inflammatory response of endotoxin-induced A549 cells. FSTL1 was the regulatory target of miR-200b-3p. Further studies showed that baicalin can increase the expression of miR-200b-3p, regulate the expression of FSTL1, inhibit the activation of the pro-inflammatory ERK/JNK inflammatory pathway and A549 cell apoptosis, and attenuate the LPS-induced injury of alveolar type II epithelial cells. This experiment revealed the mechanism of baicalin in the treatment of endotoxin-induced ALI and provided the basic theoretical basis for baicalin in treating endotoxin-induced ALI. The mechanism of ALI induced by endotoxin is complicated and unclear, and further research is needed.
Acknowledgements
The authors are grateful to Enpaper for editing the English text of a draft of this manuscript.
Footnotes
Ethics approval and consent to participate: Not applicable.
Patient consent for publication: Not applicable.
Availability of data and materials: The datasets used and/or analyzed in the study are available from the corresponding author upon reasonable request.
Declaration of conflicting interests: The authors(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: a grant provided by the National Natural Science Foundation (Grant No. 81960350).
ORCID iD: Ming-wei Liu https://orcid.org/0000-0002-3728-2350
References
- 1.Jain S. Sepsis: An update on current practices in diagnosis and management. Am J Med Sci 2018; 356: 277–286. [DOI] [PubMed] [Google Scholar]
- 2.Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet 2018; 392: 75–87. [DOI] [PubMed] [Google Scholar]
- 3.Bajwa RPS, Mahadeo KM, Taragin BH, et al. Consensus report by pediatric acute lung injury and sepsis investigators and pediatric blood and marrow transplantation consortium joint working committees: supportive care guidelines for management of veno-occlusive disease in children and adolescents, part 1: focus on investigations, prophylaxis, and specific treatment. Biol Blood Marrow Transplant 2017; 23: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 4.Zheng H, Liang W, He W, et al. Ghrelin attenuates sepsis-induced acute lung injury by inhibiting the NF-κB, iNOS, and Akt signaling in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 2019; 317: L381–L391. [DOI] [PubMed] [Google Scholar]
- 5.Gouda MM, Bhandary YP. Acute lung injury: IL-17A-mediated inflammatory pathway and its regulation by curcumin. Inflammation 2019; 42: 1160–1169. d [DOI] [PubMed] [Google Scholar]
- 6.D'Alessio FR. Mouse models of acute lung injury and ARDS. Methods Mol Biol 2018; 1809: 341–350. [DOI] [PubMed] [Google Scholar]
- 7.Peters MMC, Meijs TA, Gathier W, et al. Follistatin-like 1 in cardiovascular disease and inflammation. Mini Rev Med Chem 2019; 19: 1379–1389. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Duan Q, Zhu X, et al. Follistatin-like 1 attenuates ischemia/reperfusion injury in cardiomyocytes via regulation of autophagy. Biomed Res Int 2019; 2019: 9537382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters MMC, Meijs TA, Gathier W, et al. Follistatin-like 1 in cardiovascular disease and inflammation. Mini Rev Med Chem 2019; 19: 1379–1389. [DOI] [PubMed] [Google Scholar]
- 10.Prakash S, Mattiotti A, Sylva M, et al. Identifying pathogenic variants in the Follistatin-like 1 gene (FSTL1) in patients with skeletal and atrioventricular valve disorders. Mol Genet Genomic Med 2019; 7: e00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oshima Y, Ouchi N, Sato K, et al. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 2008; 117: 3099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawabata D, Tanaka M, Fujii T, et al. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum 2004; 50: 660–8. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Wang Y, Xu N, et al. Follistatin-like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res Ther 2011; 13: R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Wang Y, Xu N, et al. Follistatin-like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res Ther 2011; 13: R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson DC, Marinov AD, Blair HC, et al. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum 2010; 62: 2510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaly Y, Fu Y, Marinov A, et al. Follistatin-like protein 1 enhances NLRP3 inflammasome-mediated IL-1β secretion from monocytes and macrophages Eur J Immunol 2014; 44: 1467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni S, Li C, Xu N, et al. Follistatin-like protein 1 induction of matrix metalloproteinase 1, 3 and 13 gene expression in rheumatoid arthritis synoviocytes requires MAPK, JAK/STAT3 and NF-κB pathways. J Cell Physiol 2018; 234: 454–463. [DOI] [PubMed] [Google Scholar]
- 18.Song L, Yang H, Wang HX, et al. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis 2014; 19: 567–580. [DOI] [PubMed] [Google Scholar]
- 19.Lai CC, Huang PH, Yang AH, et al. Baicalein reduces liver injury induced by myocardial ischemia and reperfusion. Am J Chinese med 2016; 44: 531–550. [DOI] [PubMed] [Google Scholar]
- 20.Lai CC, Huang PH, Yang AH, et al. Baicalein, a component of Scutellaria baicalensis, attenuates kidney injury induced by myocardial ischemia and reperfusion. Planta medica 2016; 82: 181–189. [DOI] [PubMed] [Google Scholar]
- 21.Sahu BD, Mahesh Kumar J, Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-kappa B pathways. PloS one 2015; 10: e0134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabian M R, Sonenberg N, Filipowicz W: Regulation of mRNA translation and stability by micro RNAs, Annu Rev Biochem 2010; 79: 351–379. [DOI] [PubMed] [Google Scholar]
- 23.van Leyen K, Kim HY, Lee SR, et al. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 2006; 37: 3014–3018. [DOI] [PubMed] [Google Scholar]
- 24.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, et al. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol 2019; 234: 5451–5465. [DOI] [PubMed] [Google Scholar]
- 25.Zendjabil M, Favard S, Tse C, et al. The microRNAs as biomarkers: what prospects? C R Biol 2017; 340: 114–131. [DOI] [PubMed] [Google Scholar]
- 26.Zou L, Xiong X, Wang K, et al. MicroRNAs in the intestine: role in renewal, homeostasis, and inflammation. Curr Mol Med 2018; 18: 190–198. [DOI] [PubMed] [Google Scholar]
- 27.Danaii S, Shiri S, Dolati S, et al. The association between inflammatory cytokines and miRNAs with slow coronary flow phenomenon. Iran J Allergy Asthma Immunol 2020; 19: 56–64. [DOI] [PubMed] [Google Scholar]
- 28.Möhnle P, Hirschberger S, Hinske LC, et al. MicroRNAs 143 and 150 in whole blood enable detection of T-cell immunoparalysis in sepsis. Mol Med 2018; 24: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Gu H, Wang L, et al. MiR-885-3p is down-regulated in peripheral blood mononuclear cells from T1D patients and regulates the inflammatory response via targeting TLR4/NF-κB signaling. J Gene Med 2020; 22: e3145. [DOI] [PubMed] [Google Scholar]
- 30.Lin T, Yu CC, Hsieh PL, et al. Down-regulation of miR-200b-targeting Slug axis by cyclosporine A in human gingival fibroblasts. J Formos Med Assoc 2018; 117: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 31.Ladak SS, Roebuck E, Powell J, et al. The role of miR-200b-3p in modulating TGF-β1-induced injury in human bronchial epithelial cells. Transplantation 2019; 103: 2275–2286 [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Wang L, Li X. HMGB3 promotes the proliferation and metastasis of glioblastoma and is negatively regulated by miR-200b-3p and miR-200c-3p. Cell Biochem Funct 2018; 36: 357–365. [DOI] [PubMed] [Google Scholar]
- 33.Cao Y, Liu Y, Ping F, et al. miR-200b/c attenuates lipopolysaccharide-induced early pulmonary fibrosis by targeting ZEB1/2 via p38 MAPK and TGF-β/smad3 signaling pathways. Lab Invest 2018; 98: 339–359. [DOI] [PubMed] [Google Scholar]
- 34.Chen WX, Ren LH, Shi RH. Implication of miRNAs for inflammatory bowel disease treatment: systematic review. World J Gastrointest Pathophysiol 2014; 5: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KH, Lim BJ, Ferreira VH, et al. Expression of Human miR-200b-3p and -200c-3p in Cytomegalovirus-Infected Tissues. Biosci Rep 2018; 38: BSR20180961. https://pubmed.ncbi.nlm.nih.gov/30366960/?from_single_result=miR-200b+and+HCMV&expanded_search_query=miR-200b+and+HCMV - affiliation-3, [DOI] [PMC free article] [PubMed]
- 36.Yi X, Wei X, Lv H, et al. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res 2019; 383: 111454. [DOI] [PubMed] [Google Scholar]
- 37.Shen B, Zhao C, Chen C, et al.Picroside II protects rat lung and A549 cell against LPS-induced inflammation by the NF-κB pathway. Inflammation 2017; 40: 752–761. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Cong Z, Yang C, et al. Inhibition of LPS-induced Nox2 activation by VAS2870 protects alveolar epithelial cells through eliminating ROS and restoring tight junctions. Biochem Biophys Res Commun 2020; 524: 575–581. [DOI] [PubMed] [Google Scholar]
- 39.Grazioli S, Dunn-Siegrist I, Pauchard LA, et al. Mitochondrial alarmins are tissue mediators of ventilator-induced lung injury and ARDS. PLoS One 2019; 14: e0225468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng X, Li Q, Tian H, et al. HIP/PAP protects against bleomycin-induced lung injury and inflammation and subsequent fibrosis in mice. J Cell Mol Med 2020; 24: 6804–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen B, Zhao C, Chen C, et al. Picroside II protects rat lung and A549 cell against LPS-induced inflammation by the NF-κB pathway. Inflammation 2017; 40: 752–761. [DOI] [PubMed] [Google Scholar]
- 42.Miyamae T, Marinov AD, Sowders D, et al. Follistatin-like protein-1 is a novel proinflammatory molecule J Immunol 2006; 177: 4758–62. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Liu T, Wu J, et al. The correlation between FSTL1 expression and airway remodeling in asthmatics. Mediators Inflamm 2017; 2017: 7918472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu T, Liu Y, Miller M, et al. Autophagy plays a role in FSTL1-induced epithelial mesenchymal transition and airway remodeling in asthma. Am J Physiol Lung Cell Mol Physiol 2017; 313: L27–L40. [DOI] [PubMed] [Google Scholar]
- 45.Zehorai E, Seger R. Beta-like importins mediate the nuclear translocation of MAPKs. Cell Physiol Biochem 2019; 52: 802–821. [DOI] [PubMed] [Google Scholar]
- 46.Zhou LF, Chen QZ, Yang CT, et al. TRPC6 contributes to LPS-induced inflammation through ERK1/2 and p38 pathways in bronchial epithelial cells. Am J Physiol Cell Physiol 2018; 314: C278–C288. [DOI] [PubMed] [Google Scholar]
- 47.Guo M, Härtlova A, Gierliński M, et al. Triggering MSR1 promotes JNK-mediated inflammation in IL-4-activated macrophages. Embo J 2019; 38: e100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou W, Ye XL, Xu J, et al. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200b/c and let-7b. Sci Signal 2017; 10: eaak9557. [DOI] [PubMed] [Google Scholar]
- 49.Tang Q, Li M, Chen L, et al. miR-200b/c targets the expression of RhoE and inhibits the proliferation and invasion of non-small cell lung cancer cells. Int J Oncol 2018; 53: 1732–1742. [DOI] [PubMed] [Google Scholar]
- 50.Yang MC, You FL, Wang Z, et al. Salvianolic acid B improves the disruption of high glucose-mediated brain microvascular endothelial cells via the ROS/HIF-1α/VEGF and miR-200b/VEGF signaling pathways. Neurosci Lett 2016; 630: 233–240. [DOI] [PubMed] [Google Scholar]
- 51.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev 2012; 246: 379–400. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Zhao J, Gurkar A, et al. Methods to quantify the NF-κB pathway during senescence. Methods Mol Biol 2019; 1896: 231–250. [DOI] [PubMed] [Google Scholar]
- 53.Huang X, Mao W, Zhang T, et al. Baicalin promotes apoptosis and inhibits proliferation and migration of hypoxia-induced pulmonary artery smooth muscle cells by up-regulating A2a receptor via the SDF-1/CXCR4 signaling pathway. BMC Complement Altern Med 2018; 18: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu H, Zhang F, Guan X. Baicalin reverse depressive-like behaviors through regulation SIRT1-NF-kB signaling pathway in olfactory bulbectomized rats. Phytother Res 2019; 33: 1480–1489. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y, Wang F, Fan L, et al. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-κB and p38 MAPK signaling pathways. Biomed Pharmacother 2018; 97: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 56.Ji W, Liang K, An R, et al. Baicalin protects against ethanol-induced chronic gastritis in rats by inhibiting Akt/NF-κB pathway. Life Sci 2019; 239: 117064. [DOI] [PubMed] [Google Scholar]