Abstract
Background
More than 75% of neonatal deaths occurred in the first weeks of life as a result of adverse birth outcomes. Low birth weight, preterm births are associated with a variety of acute and long-term complications. In Sub-Saharan Africa, there is insufficient evidence of adverse birth outcomes. Hence, this study aimed to determine the pooled prevalence and determinants of adverse birth outcomes in Sub-Saharan Africa.
Method
Data of this study were obtained from a cross-sectional survey of the most recent Demographic and Health Surveys (DHS) of ten Sub-African (SSA) countries. A total of 76,853 children born five years preceding the survey were included in the final analysis. A Generalized Linear Mixed Models (GLMM) were fitted and an adjusted odds ratio (AOR) with a 95% Confidence Interval (CI) was computed to declare statistically significant determinants of adverse birth outcomes.
Result
The pooled prevalence of adverse birth outcomes were 29.7% (95% CI: 29.4 to 30.03). Female child (AOR = 0.94, 95%CI: 0.91 0.97), women attended secondary level of education (AOR = 0.87, 95%CI: 0.82 0.92), middle (AOR = 0.94,95%CI: 0.90 0.98) and rich socioeconomic status (AOR = 0.94, 95%CI: 0.90 0.99), intimate-partner physical violence (beating) (AOR = 1.18, 95%CI: 1.14 1.22), big problems of long-distance travel (AOR = 1.08, 95%CI: 1.04 1.11), antenatal care follow-ups (AOR = 0.86, 95%CI: 0.83 0.86), multiparty (AOR = 0.88, 95%CI: 0.84 0.91), twin births (AOR = 2.89, 95%CI: 2.67 3.14), and lack of women involvement in healthcare decision-making process (AOR = 1.10, 95%CI: 1.06 1.13) were determinants of adverse birth outcomes.
Conclusion
This study showed that the magnitude of adverse birth outcomes was high, abnormal baby size and preterm births were the most common adverse birth outcomes. This finding suggests that encouraging antenatal care follow-ups and socio-economic conditions of women are essential. Moreover, special attention should be given to multiple pregnancies, improving healthcare accessibilities to rural areas, and women’s involvement in healthcare decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-021-11113-z.
Keywords: Adverse birth outcomes, Determinants, Sub-Saharan Africa
Background
According to the global report, about 2.9 million babies die in the first month of life, of which preterm births, complications during pregnancy, and sepsis are the leading causes of death [1–3]. Particularly, adverse birth outcomes contributed to more than 75% of neonatal deaths occurred in the first weeks of life [1]. Adverse birth outcomes are defined by the World Health Organization as events of low birth weight, preterm birth, stillbirth, or perinatal deaths [4–7]. In particular, low birth weight (LBW) is often defined as a birth weight of below 2500 g, which might be resulted from intrauterine growth retardations or shorter gestational age. Globally, about 15 to 20% of births weighted below 2500 g and associated with various neonatal health complications like hypothermia, hypoglycemia, and early deaths. Moreover, neurocognitive problems and developmental delays are the long-term complications of LBW that determine child survival and future health [4–12].
On top of that, about 15 million babies are born too preterm (often before 37 completed weeks of gestation) each year, of whom more than one million died immediately after birth due to complications and lack of appropriate treatment [13]. Many of the survivors face a lifetime of disability, including learning disabilities and visual and hearing problems [12]. Meanwhile, experiencing a stillbirth during pregnancy or childbirth is a tragedy insufficiently addressed in global agendas, policies, and funded programs.
Approximately, 2.6 million stillbirths occurred each year, of which about 50% of the incidents occurred just after the onset of labor [7]. About 84% of all stillbirths occur in low-and-middle-income countries including SSA where maternal health service coverage is low. Furthermore, stillbirth has psychological costs to women and their families, such as maternal depression, financial and economic repercussions, as well as stigma and taboo.
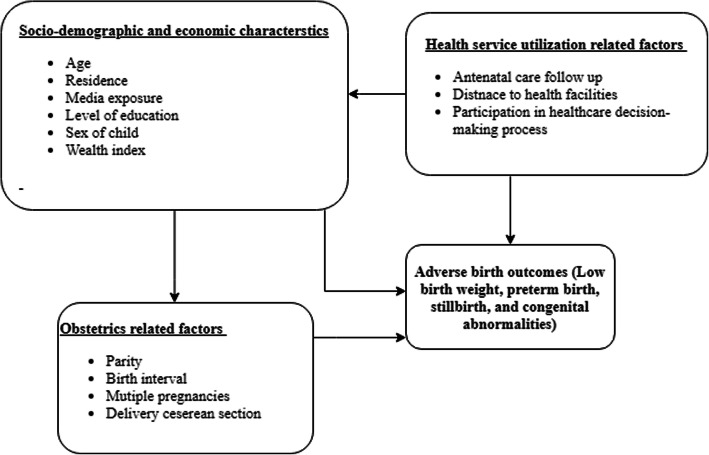
A variety of intertwined maternal, nutritional, environmental, and healthcare system factors contributed to the occurrence of adverse birth outcomes. Thus, advanced maternal age, level of education, antenatal care, home delivery, and healthcare access problems, and economic conditions, maternal clinical conditions like anemia, malaria, chronic illnesses, and HIV were determinants of adverse birth outcomes (Fig. 1) [13–20].
Fig. 1.
Conceptual framework of factors associated with adverse birth outcomes
The government and stakeholders made tremendous efforts to curb the magnitude and impacts of adverse birth outcomes. Furthermore, the reduction of adverse birth outcomes like LBW, stillbirth, and preterm births are the parts of the Sustainable Development Goal (SDG) targeted under goals 3.1 and 3.2 [21]. Focused antenatal care, institutional delivery, nutritional counseling, and improving healthcare services availability and accessibility were some of the interventions used to reduce unfavorable birth outcomes in low-income settings. For instance, continuity of maternal healthcare services led by midwifery contributed to the reduction of preterm births by 24% witnessed by previous study results [22].
Most of the studies conducted previously had been single-centered and facility-based which was less likely to be representative. In addition, there is a need for quality, large, and population based-studies from SSA countries where a large share of adverse birth outcomes occurred. Hence, this study aimed to assess the pooled prevalence and determinants of adverse birth outcomes in SSA. A better understanding of the adverse birth outcomes could provide more generalizable evidence to justify the better quality of maternal healthcare and improving accessibility of health services in low-income countries. Moreover, evidence from this study could be helpful to design and integrated efforts at the regional level to hasten favorable birth outcomes.
Method
The most recent Demographic and Health Surveys (DHS) of ten Sub-African (SSA) countries (Angola, Congo, Cote d’Ivoire, Gambia, Lesotho, Liberia, Madagascar, Nigeria, Rwanda, Togo) data were used to make analysis of this study. The DHS is a part of the measure DHS programs that collect national information on basic health measures such as mortality, morbidity, and maternal and child health service utilization. Using the Kids Record (KR file) dataset, all births in the preceding five years before the survey were the study population. In the selected enumeration areas (EAs) births that had data about birth weight, gestational age at birth, and perinatal death records were included in the study. During the measure DHS survey, a multi-stage (two-stage) stratified sampling technique was used to select study participants; children were nested within the enumeration areas. After the dataset was appended, the weighted sample size became 76,853 children and women who had given birth five years preceding the survey. The methodology section of the DHS report goes into great detail about the study participant selection and data collection [23].
Measurements
The main outcome variable of this study was adverse birth outcomes, which is defined as the presence of at least one or more of the following conditions in recent pregnancy (low birth weight, macrosomia, preterm birth, or stillbirth) [13, 19]. The outcome variable was generated by composite low birth weight, macrosomia, stillbirth, and gestational age less than 37 weeks of pregnancy. Finally, the variable takes 1 if at least one of adverse birth outcomes reported which was labeled as “adverse birth outcome”, and 0 otherwise.
Independent variables
Socio-demographic characteristics (residence, maternal education, husband education, maternal age, mother marital status, sex of the child, media exposure, household wealth index, and maternal working status), health service utilization and accessibility (women healthcare decision-making autonomy, ANC follow up, and distance to health facility), and obstetrics related characteristics (preceding birth interval, parity, type of birth, and delivery by CS) were explanatory variables identified after thorough review of literatures [13, 24–31] .
Short birth interval is defined as the time between two births which is less than 24 months [32]. Also, women’s healthcare decision-making autonomy is the ability of the women to make decisions to use health care services and treatment options [25]. Finally, media exposure was defined as when a woman reads a newspaper or listens to the radio, or watches television at least three times per week.
Data management and analysis
Before any statistical analysis, the data were weighted using sampling weight based on primary sampling unit, and strata to restore the representativeness of the survey and take sampling design when calculating standard errors and reliable estimates. Cross-tabulations and summary statistics were done using STATA software version 14 (StataCorp.2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). The pooled prevalence of adverse birth outcomes with a 95% Confidence Interval (CI) was reported using a forest plot. The DHS dataset has a hierarchical structure that failed to meet the standard logistic regression model assumptions of independent observation and equal variance. Meanwhile, the children were nested within a cluster household, and children from the same cluster were more similar than from other clusters. Therefore, a mixed effect logistic regression model (both fixed and random effect) was fitted to account for cluster variability by using the advanced models. The outcome variable of the study was binary, a standard logistic regression and Generalized Linear Mixed Models (GLMM) were fitted step by step. Because the models were nested, model fitness was compared using the Intra-class Correlation Coefficient (ICC), Likelihood Ratio (LR), Median Odds Ratio (MOR), and deviance (−2LLR) values. As a result, the mixed-effect logistic regression model with the lowest deviance value was selected as the most parsimonious model. (Shown on Supplementary file Table 1). In the bivariable analysis, variables with less than 0.2 p-values were selected and entered into the multivariable mixed-effect logistic regression model. Adjusted Odds Ratios (AOR) with a 95% CI were calculated in the multivariable model to see the strength of association between independent variables and adverse birth outcomes. Variables with a 0.05 p-value in the final model being used as a statistically significant determinant of adverse birth outcomes.
Ethical consideration
Permission for data access was obtained from measure demographic and health survey through an online request from http://www.dhsprogram.com. The data used for this study were publicly available with no personal identifier.
Result
Socio-demographic characteristics
The median age of the women in this sample was 28 years, with an interquartile range of 24 to 28 years, and half of them (51.1%) were between the ages of 20 and 29 years. Nearly two-thirds of mothers (62.5%) lived in rural areas, 19.7% had no formal schooling, and 83.8% were married at the time of data collection. Whilst more than one-third (34.3%) of women experienced physical violence (beating) from the intimate partner due to the reason of refusals for sex, neglect of a child, and goes out of home without telling to the husband (Table 1).
Table 1.
The socio-demographic and economic characteristics of the study population in Sub-Saharan Africa
| Variables | Weighted frequency | Percentage (%) |
|---|---|---|
| Country | ||
| Angola | 7154 | 9.3 |
| Congo | 10,663 | 13.9 |
| Côte d’Ivoire | 2105 | 2.7 |
| Gambia | 14,605 | 19 |
| Lesetho | 10,415 | 13.6 |
| Liberia | 7783 | 10.1 |
| Madagascar | 5201 | 6.8 |
| Nigeria | 3081 | 4 |
| Rwanda | 8156 | 10.6 |
| Togo | 7690 | 10 |
| Residence | ||
| Urban | 28,825 | 37.5 |
| Rural | 48,028 | 62.5 |
| Maternal age | ||
| 15–19 | 4385 | 5.7 |
| 20–29 | 39,255 | 51.1 |
| 30–39 | 27,325 | 35.5 |
| 40–49 | 5878 | 7.7 |
| Mothers education status | ||
| No | 15,158 | 19.7 |
| Primary | 31,832 | 41.4 |
| Secondary and above | 29,863 | 38.9 |
| Husband education status | ||
| No | 9921 | 12.9 |
| Primary | 22,977 | 29.9 |
| Secondary and above | 43,955 | 57.2 |
| Marital status | ||
| Married | 64,416 | 83.8 |
| Divorced/widowed/not living together | 12,437 | 16.2 |
| Wealth status | ||
| Poor | 27,538 | 35.8 |
| Middle | 15,470 | 20.1 |
| Rich | 33,845 | 44.1 |
| Woman in paid employment | ||
| No | 24,789 | 32.3 |
| Yes | 52,064 | 67.7 |
| Women involvement in healthcare decision-making | ||
| No | 45,304 | 58.9 |
| Yes | 31,549 | 41.1 |
| Media exposure | ||
| No | 51,947 | 67.6 |
| Yes | 24,906 | 32.4 |
| Experienced intimate partner beating | ||
| Yes | 26,391 | 34.3 |
| No | 50,462 | 65.7 |
Obstetrics characteristics of women in SSA
About 71.4% of women had antenatal care follow up for the recent pregnancies, 94.2% of women gave birth in health institutions, of whom 7.6% births were by cesarean section mode of delivery. The majority (87.7%) women had greater than 24 birth months’ of the interval from the preceding births and 15.2% were on the birth order of 6th and above (Table 2).
Table 2.
Maternal obstetrics characteristics of the study population in Sub-Saharan Africa
| Characteristics | Frequency | Percentage |
|---|---|---|
| Number of ANC visits | ||
| No visit | 21,948 | 28.6 |
| 1–3 visits | 18,134 | 23.6 |
| 4+ visits | 36,771 | 47.8 |
| Place of delivery | ||
| Home | 4467 | 5.8 |
| Health institution | 72,386 | 94.2 |
| Mode of delivery | ||
| Vaginal | 70,770 | 92.4 |
| Cesarean section | 5797 | 7.6 |
| Iron supplementation | ||
| Yes | 48,165 | 85.3 |
| No | 8309 | 14.7 |
| Sex of child | ||
| Male | 38,964 | 50.7 |
| Female | 37,889 | 49.3 |
| Type of pregnancy | ||
| Single | 74,179 | 96.5 |
| Twin | 2674 | 3.5 |
| Birth order | ||
| 1–2 | 35,618 | 46.4 |
| 3–5 | 29,518 | 38.4 |
| ≥ 6 | 11,717 | 15.2 |
| Preceding birth intervals | ||
| < 24 | 9411 | 12.3 |
| ≥ 24 | 67,442 | 87.7 |
| Ever termination of pregnancy | ||
| Yes | 9813 | 12.8 |
| No | 67,040 | 87.2 |
The pooled prevalence of the adverse birth outcome in SSA
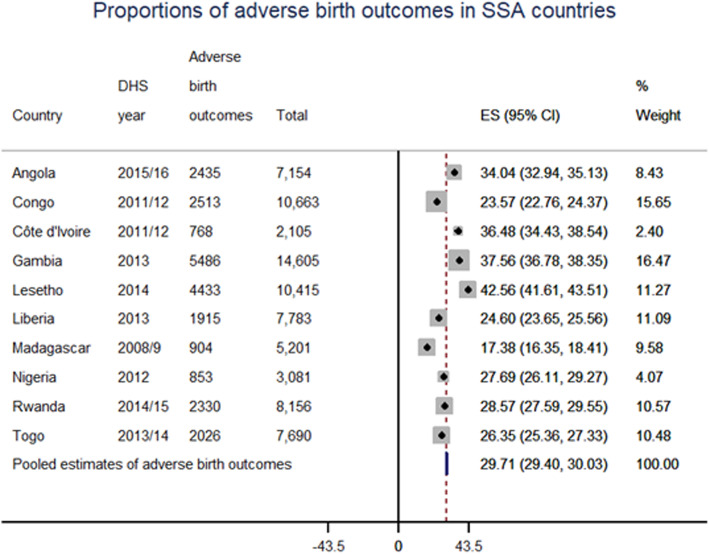
The prevalence of adverse birth outcomes in Sub-Saharan countries was 29.7% with a 95% CI of 29.4 to 30.03%, variations among countries also observed ranged from 17.4% in Madagascar to 42.6% in Lesotho (Fig. 2). More specifically, the rate of stillbirth was 11.5 per 1000 births; with preterm birth, fetal low birth weight, and macrosomia accounted for 7.6, 14.7, and 25.1% of all births, respectively. Of the reported adverse birth outcomes; macrosomia, low birth weight, preterm birth, and stillbirth accounted for 44, 31%, 22, and 3% of cases, respectively, and 3.24% had more than one adverse birth outcome.
Fig. 2.
Forest plot of adverse birth outcomes in Sub-Saharan Africa countries
Determinants of adverse birth outcomes in SSA
In the multivariable mixed-effect logistic regression analysis, sex of a child, maternal level of education, socioeconomic status, intimate partner violence (beating), women-autonomy, distance to the health facility, parity, and twin birth were associated with adverse birth outcomes. Thus, the odds of adverse birth outcomes decreased among middle (AOR = 0.94, 95%CI: 0.90 0.98) and rich socioeconomic status (AOR = 0.90, 95%CI: 0.90 0.99) compared to poorer women. Similarly, for women who attended secondary and above levels of education, the odds of adverse birth outcomes decreased by 13% (AOR = 0.87, 95%CI: 0.82 0.92) than those who had no formal education. Likewise, antenatal care visits and multiparity birth associated with 14% (AOR = 0.86, 95%CI: 0.83 0.89) and 12% (AOR = 0.88, 95%CI: 0.84 0.91) lower odds of adverse birth outcomes compared to those who had no follow-ups and those primiparous women, respectively. In addition, female child associated with 6% (AOR = 0.94, 95%CI: 0.91 0.97) lower odds of adverse birth outcomes than males. Whilst, mothers who experienced intimate partner violence (beating) had 1.18 (AOR = 1.18, 95%CI: 1.14 1.22) times higher odds of adverse birth outcomes than women with no such history. In the same way, for women who didn’t participate in healthcare decision-making, the odds of adverse birth outcomes were 1.10 (AOR = 1.10, 95%CI: 1.06 1.13) times higher than participated in decision-making. Similarly, women who perceived distance to health facilities as big problems had 1.08 (AOR = 1.08, 95%CI: 1.04 1.11) times higher compared to the counterpart. Furthermore, twin birth was associated with 2.89 (AOR = 2.89, 95%CI: 2.67 3.14) times more likely to had adverse birth outcomes than singleton birth (Table 3).
Table 3.
Multivariable multilevel logistic regression analysis to identify factors associated with low adverse birth outcomes in sub-Saharan Africa
| Characteristics | Adverse birth outcome | Odds Ratio (OR) | ||
|---|---|---|---|---|
| Yes | No | Crude OR | Adjusted OR | |
| Maternal age | ||||
| 15–19 | 1478 | 2917 | 1 | 1 |
| 20–29 | 12,181 | 27,074 | 0.91 (0.85 0.98) | 0.95 (0.89 1.02) |
| 30–39 | 8217 | 19,108 | 0.91 (0.85 0.98) | 0.98 (0.91 1.07) |
| 40–49 | 1787 | 4091 | 0.86 (0.86 1.02) | 0.99 (0.89 1.09) |
| Sex of child | ||||
| Male | 12,215 | 26,749 | 1 | 1 |
| Female | 11,448 | 26,441 | 0.94 (0.91 0.97) | 0.94 (0.91 0.97)* |
| Residence | ||||
| Urban | 8204 | 20,621 | 1 | 1 |
| Rural | 15,459 | 32,569 | 1.15 (1.11 1.19) | 1.02 (0.98 1.06) |
| Maternal level of education | ||||
| No formal education | 4669 | 10,489 | 1 | 1 |
| Primary education | 10,956 | 20,876 | 1.00 (0.96 1.05) | 1.04 (0.99 1.09) |
| Secondary and above | 8038 | 21,825 | 0.80 (0.76 0.84) | 0.87 (0.82 0.92)* |
| Media exposure | ||||
| Yes | 7949 | 16,957 | 0.89 (0.86 0.92) | 0.97 (0.94 1.01) |
| No | 15,714 | 36,233 | 1 | 1 |
| Wealth index | ||||
| Poor | 9190 | 18,348 | 1 | 1 |
| Middle | 4790 | 10,680 | 0.90 (0.86 0.94) | 0.94 (0.90 0.98)* |
| Rich | 9683 | 24,162 | 0.82 (0.80 0.85) | 0.92 (0.90 0.99)* |
| Women didn’t participate in healthcare decision making | ||||
| Yes | 13,396 | 31,908 | 1 | 1 |
| No | 10,267 | 21,282 | 1.12 (1.09 1.16) | 1.10 (1.06 1.13)* |
| Distance to a health facility | ||||
| Big problems | 9215 | 17,979 | 1.13 (1.09 1.17) | 1.08 (1.04 1.11)* |
| No big problem | 14,448 | 35,211 | 1 | 1 |
| ANC follow up | ||||
| Yes | 7488 | 14,460 | 0.80 (0.77 0.82) | 0.86 (0.83 0.89)* |
| No | 16,175 | 38,730 | 1 | 1 |
| Parity | ||||
| 1–2 births | 11,030 | 24,588 | 1 | 1 |
| 3–5 births | 8737 | 20,781 | 093 (0.90 0.96 | 0.88 (0.84 0.91)* |
| > =6 births | 3896 | 7821 | 1.07 (1.02 1.12) | 0.97 (0.88 1.006) |
| Birth interval | ||||
| < 24 months | 3014 | 6397 | 1 | 1 |
| 24 and above months | 20,649 | 46,973 | 0.96 (0.91 1.00) | 0.95 (0.90 1.00) |
| Experienced intimate partner beating | ||||
| Yes | 8617 | 17,774 | 1.23 (1.19 1.27) | 1.18 (1.14 1.22)* |
| No | 15,046 | 35,416 | 1 | 1 |
| Type of birth | ||||
| Single | 22,198 | 51,981 | 1 | |
| Twin | 1465 | 1209 | 2.95 (2.95 3.19) | 2.89 (2.67 3.14)* |
* Shows a statistical significance of at 0.05 p-value
Discussion
According to this report, the pooled prevalence of adverse birth outcomes was 29.7%, with (95% CI: 29.4 to 30.03%). Stillbirth rate was 11.5 per 1000 births, with preterm birth, fetal low birth weight, and macrosomia accounted for 7.6, 14.7, and 25.1% of all births, respectively. A woman’s level of education, socioeconomic status, intimate partner physical violence (beating), sex of child, women autonomy for health care decision-making, distance travel, twin birth, multiparity, and ANC follow-up were determinants of adverse birth outcomes. The rate of stillbirth in this study was lower than the results of 48.5 per 1000 births in Southeast Asia [33]. This could be because most of the stillbirths in the community are under-reported and is a common problem in SSA [34]. However, the magnitude of adverse birth outcomes in this study was higher than the study finding 18.3% in Ethiopia [19]. However, this study finding of low birth weight (14.7%%) and prematurity (7.6%) were lower than the findings of LBW (19.6%) and prematurity (17.7%) among HIV-infected women in Uganda [20]. This could be because of chronic diseases and HIV/AIDS, in which highly active antiretroviral therapy (HAART) is associated with small gestational age and preterm labor among pregnant women [35]. Similarly, this study finding of low birth weight (7.99%) and prematurity (7.60%) was lower than the previous study finds of 12.36 and 8.28% among teenagers in the US [36]. This could be due to study population differences in teen ages associated with a higher risk of adverse birth outcomes.
Various maternal and contextual factors are associated with adverse birth outcomes, thus distance travel to health facilities associated with higher odds of adverse birth outcomes. This finding was consistent with those of studies in Africa [19, 24, 32, 37, 38]. This could be due to the fact long-distance travel restricts the utilization of basic maternal health services like ANC checkups and institutional delivery. Meanwhile, long-distance travel to health facilities also directly responsible for perinatal deaths from fetal distresses [39]. Some countries like Ethiopia established maternal waiting areas (MWA) for mothers who came from hard-to-reach areas [40]. Moreover, integrating emergency obstetrics transportation with complementary maternal health services avert adverse pregnancy outcomes and improves access to skilled obstetric services for women in LMICs [41]. Likewise, women experienced intimate partner physical violence (beating) associated with increased occurrence of adverse birth outcomes. This finding was consistent with previous study findings [42].
In contrast, birth interval above 24 months is associated with a decreased risk of adverse birth outcomes. This finding was consistent with those of studies SSA [15, 35, 43–45]. This could be due to the fact associated with gestational diabetes mellitus, and a narrow inter-pregnancy interval related to lower weight losses. In addition, female child associated with lower odds of adverse birth outcomes than males. This could be due to the differential effects of stress on female and male pregnancies which are supported by previous evidence [46].
This study also revealed that twin pregnancies were associated with increased risks of adverse birth outcomes. This finding was in agreement with previous studies [31, 47]. This could be due to the reason for the high rate of pre-eclampsia and antepartum hemorrhages during pregnancies that lead to unfavorable birth outcomes. Thus, a twin pregnancy is defined as a high-risk pregnancy with special attention, and birth preparedness and complication readiness counseling should be given to pregnant mothers. Furthermore, the study’s findings point to the value of affordable imaging technology in low-income countries.
Likewise, women who didn’t participate in healthcare decision-making were more likely to had adverse birth outcomes than participated in decision makings [27]. This might be due to the reason that women declined and less involvement in healthcare decision-making was associated with decreased utilization of antennal care and institutional delivery. Moreover, a woman decreased involvement in decision-making may indicate that she is exposed to intimate partner violence [27].
On the other hand, women who had antenatal care visits for the recent birth were associated with lower odds of adverse birth outcomes than those who had no follow-up visits. This finding was consistent with those of studies in SSA [18, 19, 27, 32, 37, 45]. Antenatal care checkups help to identify most at-risk pregnancies like intrauterine growth retardation, nutritional counseling, and supplementation of nutrient fortified foods. In addition, antenatal care visits allowed identifying diseases like HIV/AIDS, syphilis, malaria, and intestinal helminthiasis infection that could affect fetal outcomes. Therefore, further improvement of quality of antenatal care and mobilization of pregnant women to WHO recommended focused care would halt adverse birth outcomes and achievement of Sustainable development goals.
Similarly, better socio-economic attributes like a better level of education and wealthy economic conditions associated with a lower risk of adverse birth outcomes. This finding was consist of previous results [15, 35, 48]. Better socio-economic attributes might hasten health-seeking behaviors and maternal nutrition. In addition, it might be associated with good knowledge of danger signs of pregnancies and early initiation of antenatal follow-ups.
This research has implications for mothers, healthcare planners, and maternal health program coordinators who are working to develop evidence-based approaches to help achieve the Sustainable Development Goals (SDG). Furthermore, increasing healthcare access to rural and hard-to-reach areas and encouraging women’s involvement in healthcare decision-making could help to minimize the extent of adverse birth outcomes. Furthermore, healthcare providers should pay more time and attention to women who had a history of partner physical violence, multiple pregnancies and have difficult labors.
This study has the strengths of a large sample size from multiple countries that would help to assess the regional situation of adverse birth outcomes. In addition, factors identified from these pooled studies might be used areas interventions for stakeholders. However, due to reasons of secondary data sources, some important clinical parameters like gestational diabetes and chronic disease conditions were not assessed. In addition, this study included only a few countries from the Sub-Saharan region that leads to under representations. Moreover, adverse birth outcomes reported in this study were based on the recent pregnancies from the Demography and health surveys.
Conclusion
This study showed that the magnitude of adverse birth outcomes was high, abnormal baby size and preterm births were the most common types. Problems of adverse birth outcomes were more common among women who experienced intimate partner physical violence (beating), distance travel, multiple pregnancies, and lack of involvement in the healthcare decision-making process. According to the findings, improving maternal health services such as antenatal care is critical in reducing adverse birth outcomes. Furthermore, this research emphasizes the importance of paying particular attention to women who have had multiple births, expanding healthcare coverage to rural areas, and promoting women’s participation in the healthcare decision-making process.
Supplementary Information
Additional file 1: Supplementary file Table 1: Model comparison and random effect results.
Acknowledgments
We would like to thank the Ethiopian Central Statistics Agency for providing us with all the relevant secondary data used in this study. Finally, we would like to thank all who directly or indirectly supported us.
Abbreviations
- ABO
Adverse Birth Outcomes
- ANC
Antenatal Care
- AOR
Adjusted Odds Ratio
- CI
Confidence Interval
- DHS
Demographic and Health Survey
- EA
Enumeration Area
- LBW
Low Birth Weight
- LLR
Likelihood Ratio
- SD
Standard Deviation
- SGD
Sustainable Development Goal
- SSA
Sub-Saharan Africa
- USA
United States of America
Authors’ contributions
KST, MMS, GAT, and ZTT conceived the study, data analysis, drafted the manuscript, and critically reviewed the manuscript. Read and approved the final version of the manuscript.
Funding
We didn’t receive external funds for this research.
Availability of data and materials
The datasets used in this analysis are publicly available data from the DHS program, which can be accessed after filling out a data request form at http://www.dhsprogram.com.
Declarations
Ethics approval and consent to participate
Ethical clearance was obtained from measure DHS through filling requesting a form for accessing data. The data used in this study are publicly available, aggregated secondary data that hasn’t any personal identifying information that can be linked to study participants. The confidentiality of data was maintained anonymously.
Consent for publication
Not applicable as there are no image or other confidentiality-related issues.
Competing interests
The authors declared that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Koku Sisay Tamirat, Email: kokusisay23@gmail.com.
Malede Mequanent Sisay, Email: maledecsa@gmail.com.
Getayeneh Antehunegn Tesema, Email: getayenehantehunegn@gmail.com.
Zemenu Tadesse Tessema, Email: zemenut1979@gmail.com.
References
- 1.Ballot DE, Chirwa T, Ramdin T, Chirwa L, Mare I, Davies VA, Cooper PA. Comparison of morbidity and mortality of very low birth weight infants in a central Hospital in Johannesburg between 2006/2007 and 2013. BMC Pediatr. 2015;15(1):20. doi: 10.1186/s12887-015-0337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, Borghi E, Hayashi C, Estevez D, Cegolon L, Shiekh S, Ponce Hardy V, Lawn JE, Cousens S. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019;7(7):e849–e860. doi: 10.1016/S2214-109X(18)30565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You D, et al. Estimates developed by the UN Inter-agency Group for Child Mortality Estimation. 2011. Levels and trends in child mortality. Report 2015. [Google Scholar]
- 4.Agbozo F, Abubakari A, der J, Jahn A. Prevalence of low birth weight, macrosomia and stillbirth and their relationship to associated maternal risk factors in Hohoe municipality, Ghana. Midwifery. 2016;40:200–206. doi: 10.1016/j.midw.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Amhara E. A review of low birth weight in Ethiopia: socio-demographic and obstetric risk factors. Global J Res Rev. 2018;5(1):4. [Google Scholar]
- 6.Bansal P, Garg S, Upadhyay HP. Prevalence of low birth weight babies and its association with socio-cultural and maternal risk factors among the institutional deliveries in Bharatpur, Nepal. Asian J Med Sci. 2019;10(1):77–85. doi: 10.3126/ajms.v10i1.21665. [DOI] [Google Scholar]
- 7.Blencowe H. Counting the smallest: data to estimate global stillbirth, preterm birth and low birthweight rates (Doctoral dissertation, London School of Hygiene & Tropical Medicine).
- 8.Class QA, Rickert ME, Lichtenstein P, D'Onofrio BM. Birth weight, physical morbidity, and mortality: a population-based sibling-comparison study. Am J Epidemiol. 2014;179(5):550–558. doi: 10.1093/aje/kwt304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlui M, Azahar N, Oche OM, Aziz NA. Risk factors for low birth weight in Nigeria: evidence from the 2013 Nigeria demographic and health survey. Glob Health Action. 2016;9(1):28822. doi: 10.3402/gha.v9.28822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes MM, Black RE, Katz J. 2500-g low birth weight cutoff: history and implications for future research and policy. Matern Child Health J. 2017;21(2):283–289. doi: 10.1007/s10995-016-2131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baran M, Celikkalkan K, Cagan Appak Y, Karakoyun M, Bozkurt M, Kocyigit C, Kanik A, Dundar BN. Body fat mass is better indicator than indirect measurement methods in obese children for fatty liver and metabolic syndrome. Sci Med J. 2019;1(4):168–175. doi: 10.28991/SciMedJ-2019-0104-2. [DOI] [Google Scholar]
- 12.Swierczynski A. Pathogenicity of endocrine dysregulation in autism: the role of the melanin-concentrating hormone system. SciMed J. 2019;1(2):74–111. doi: 10.28991/SciMedJ-2019-0102-5. [DOI] [Google Scholar]
- 13.Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, Ota E, Gilmour S, Shibuya K. Maternal anemia and risk of adverse birth and health outcomes in low-and middle-income countries: systematic review and meta-analysis, 2. Am J Clin Nutr. 2016;103(2):495–504. doi: 10.3945/ajcn.115.107896. [DOI] [PubMed] [Google Scholar]
- 14.Chibwesha CJ, Zanolini A, Smid M, Vwalika B, Phiri Kasaro M, Mwanahamuntu M, Stringer JSA, Stringer EM. Predictors and outcomes of low birth weight in Lusaka, Zambia. Int J Gynecol Obstet. 2016;134(3):309–314. doi: 10.1016/j.ijgo.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebremeskel F, et al. Determinants of adverse birth outcome among mothers who gave birth at hospitals in Gamo Gofa Zone, southern Ethiopia: a facility based case control study. Qual Prim Care. 2017;25(5):259–266. [Google Scholar]
- 16.Kader M, Perera NKP. Socio-economic and nutritional determinants of low birth weight in India. N Am J Med Sci. 2014;6(7):302–308. doi: 10.4103/1947-2714.136902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kebede AS, Muche AA, Alene AG. Factors associated with adverse pregnancy outcome in Debre Tabor town, Northwest Ethiopia: a case control study. BMC Res Notes. 2018;11(1):820. doi: 10.1186/s13104-018-3932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siza J. Risk factors associated with low birth weight of neonates among pregnant women attending a referral hospital in northern Tanzania. Tanzania J Health Res. 2008;10(1):1–8. doi: 10.4314/thrb.v10i1.14334. [DOI] [PubMed] [Google Scholar]
- 19.Tsegaye B, Kassa A. Prevalence of adverse birth outcome and associated factors among women who delivered in Hawassa town governmental health institutions, South Ethiopia, in 2017. Reprod Health. 2018;15(1):193. doi: 10.1186/s12978-018-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young S, Murray K, Mwesigwa J, Natureeba P, Osterbauer B, Achan J, Arinaitwe E, Clark T, Ades V, Plenty A, Charlebois E, Ruel T, Kamya M, Havlir D, Cohan D. Maternal nutritional status predicts adverse birth outcomes among HIV-infected rural Ugandan women receiving combination antiretroviral therapy. PLoS One. 2012;7(8):e41934. doi: 10.1371/journal.pone.0041934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahl R, et al. Setting research priorities to reduce global mortality from preterm birth and low birth weight by 2015. J Global Health. 2012;2(1). [DOI] [PMC free article] [PubMed]
- 22.Tolefac PN, et al. Ten years analysis of stillbirth in a tertiary hospital in sub-Sahara Africa: a case control study. BMC Res Notes. 2017;10(1):1–6. doi: 10.1186/s13104-016-2345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selftreported VODO, Malawi HSI. DHS Methodological reports 10. 2014. [Google Scholar]
- 24.Adane AA, Ayele TA, Ararsa LG, Bitew BD, Zeleke BM. Adverse birth outcomes among deliveries at Gondar University hospital, Northwest Ethiopia. BMC Pregnancy Childbirth. 2014;14(1):90. doi: 10.1186/1471-2393-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adokiya MN, Boah M, Adampah T. Women’s autonomy and modern contraceptive use in Ghana: a secondary analysis of data from the 2014 Ghana Demographic and Health Survey. Eur J Contracept Reprod Health Care. 2021:1–7. [DOI] [PubMed]
- 26.Althabe F, et al. Adverse maternal and perinatal outcomes in adolescent pregnancies: the global Network’s maternal newborn health registry study. Reprod Health. 2015;12(2):1–9. doi: 10.1186/1742-4755-12-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill A, Pallitto C, McCleary-Sills J, Garcia-Moreno C. A systematic review and meta-analysis of intimate partner violence during pregnancy and selected birth outcomes. Int J Gynecol Obstet. 2016;133(3):269–276. doi: 10.1016/j.ijgo.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Kozuki N, Katz J, Khatry SK, Tielsch JM, LeClerq SC, Mullany LC. Risk and burden of adverse intrapartum-related outcomes associated with non-cephalic and multiple birth in rural Nepal: a prospective cohort study. BMJ Open. 2017;7(4):e013099. doi: 10.1136/bmjopen-2016-013099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Said AS, Manji KP. Risk factors and outcomes of fetal macrosomia in a tertiary Centre in Tanzania: a case-control study. BMC Pregnancy Childbirth. 2016;16(1):1–8. doi: 10.1186/s12884-016-1044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanardi DM, et al. Adverse perinatal outcomes are associated with severe maternal morbidity and mortality: evidence from a national multicentre cross-sectional study. Arch Gynecol Obstet. 2019;299(3):645–654. doi: 10.1007/s00404-018-5004-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhu C, Wang M, Niu G, Yang J, Wang Z. Obstetric outcomes of twin pregnancies at advanced maternal age: a retrospective study. Taiwanese J Obstetrics Gynecol. 2018;57(1):64–67. doi: 10.1016/j.tjog.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Muchemi OM, Echoka E, Makokha A. Factors associated with low birth weight among neonates born at Olkalou District Hospital, Central Region, Kenya. Pan Afr Med J. 2015;20(1). [DOI] [PMC free article] [PubMed]
- 33.Ali SA, et al. Hemoglobin concentrations and adverse birth outcomes in south Asian pregnant women: findings from a prospective maternal and neonatal health registry. Reprod Health. 2020;17(2):1–13. doi: 10.1186/s12978-020-01006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiguli J, Namusoko S, Kerber K, Peterson S, Waiswa P. Weeping in silence: community experiences of stillbirths in rural eastern Uganda. Glob Health Action. 2015;8(1):24011. doi: 10.3402/gha.v8.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kebede B, Andargie G, Gebeyehu A. Birth outcome and correlates of low birth weight and preterm delivery among infants born to HIV-infected women in public hospitals of Northwest Ethiopia. 2013. [Google Scholar]
- 36.Chen X-K, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol. 2007;36(2):368–373. doi: 10.1093/ije/dyl284. [DOI] [PubMed] [Google Scholar]
- 37.Niyitegeka J, Nshimirimana G, Silverstein A, Odhiambo J, Lin Y, Nkurunziza T, Riviello R, Rulisa S, Banguti P, Magge H, Macharia M, Habimana R, Hedt-Gauthier B. Longer travel time to district hospital worsens neonatal outcomes: a retrospective cross-sectional study of the effect of delays in receiving emergency cesarean section in Rwanda. BMC Pregnancy Childbirth. 2017;17(1):242. doi: 10.1186/s12884-017-1426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sebayang SK, Dibley MJ, Kelly PJ, Shankar AV, Shankar AH, on behalf of the SUMMIT Study Group Determinants of low birthweight, small-for-gestational-age and preterm birth in Lombok, Indonesia: analyses of the birthweight cohort of the SUMMIT trial. Tropical Med Int Health. 2012;17(8):938–950. doi: 10.1111/j.1365-3156.2012.03039.x. [DOI] [PubMed] [Google Scholar]
- 39.Wariri O, Onuwabuchi E, Alhassan JAK, Dase E, Jalo I, Laima CH, Farouk HU, el-Nafaty AU, Okomo U, Dotse-Gborgbortsi W. The influence of travel time to health facilities on stillbirths: a geospatial case-control analysis of facility-based data in Gombe, Nigeria. Plos one. 2021;16(1):e0245297. doi: 10.1371/journal.pone.0245297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly J, Kohls E, Poovan P, Schiffer R, Redito A, Winter H, MacArthur C. The role of a maternity waiting area (MWA) in reducing maternal mortality and stillbirths in high-risk women in rural Ethiopia. BJOG Int J Obstet Gynaecol. 2010;117(11):1377–1383. doi: 10.1111/j.1471-0528.2010.02669.x. [DOI] [PubMed] [Google Scholar]
- 41.Alaofe H, et al. Emergency Transportation Interventions for Reducing Adverse Pregnancy Outcomes in Low-and Middle-Income Countries: A Systematic Review. Ann Global Health. 2020;86(1). [DOI] [PMC free article] [PubMed]
- 42.Laelago T, Belachew T, Tamrat M. Effect of intimate partner violence on birth outcomes. Afr Health Sci. 2017;17(3):681–689. doi: 10.4314/ahs.v17i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regan AK, Gissler M, Magnus MC, Håberg SE, Ball S, Malacova E, Nassar N, Leonard H, Pereira G. Association between interpregnancy interval and adverse birth outcomes in women with a previous stillbirth: an international cohort study. Lancet. 2019;393(10180):1527–1535. doi: 10.1016/S0140-6736(18)32266-9. [DOI] [PubMed] [Google Scholar]
- 44.Laptook AR, O'Shea TM, Shankaran S, Bhaskar B, NICHD Neonatal Network Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115(3):673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 45.Lema D. Determinants ofLlow Birth Weight in Debre Berehan Referal Hospital, North Shoa Zone, Amhara Regional State, Ethiopia (A Case–Control Study) AAU; 2015. [Google Scholar]
- 46.Wainstock T, Shoham-Vardi I, Glasser S, Anteby E, Lerner-Geva L. Fetal sex modifies effects of prenatal stress exposure and adverse birth outcomes. Stress. 2015;18(1):49–56. doi: 10.3109/10253890.2014.974153. [DOI] [PubMed] [Google Scholar]
- 47.Francisco C, Wright D, Benkő Z, Syngelaki A, Nicolaides KH. Hidden high rate of pre-eclampsia in twin compared with singleton pregnancy. Ultrasound Obstet Gynecol. 2017;50(1):88–92. doi: 10.1002/uog.17470. [DOI] [PubMed] [Google Scholar]
- 48.Gedefaw G, Alemnew B, Demis A. Adverse fetal outcomes and its associated factors in Ethiopia: a systematic review and meta-analysis. BMC Pediatr. 2020;20:1–12. doi: 10.1186/s12887-020-02176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary file Table 1: Model comparison and random effect results.
Data Availability Statement
The datasets used in this analysis are publicly available data from the DHS program, which can be accessed after filling out a data request form at http://www.dhsprogram.com.