Abstract
Background
The risk of progression of latent tuberculosis infection (LTBI) to active disease increases with pregnancy. This study determined the prevalence and risk factors associated with LTBI among pregnant women in Uganda.
Methods
We enrolled 261 pregnant women, irrespective of gestational age. Participants who had known or suspected active tuberculosis (TB) on the basis of clinical evaluation or who had recently received treatment for TB were excluded. LTBI was defined as an interferon-γ concentration ≥0.35 IU/mL (calculated as either TB1 [eliciting CD4+ T-cell responses] or TB2 [eliciting CD8+ T-cell responses] antigen minus nil) using QuantiFERON TB Gold-Plus (QFT-plus) assay.
Results
LTBI prevalence was 37.9% (n = 99) (95% confidence interval [CI], 32.3–44.0). However, 24 (9.2%) subjects had indeterminate QFT-plus results. Among participants with LTBI, TB1 and TB2 alone were positive in 11 (11.1%) and 18 (18.2%) participants, respectively. In multivariable analysis, human immunodeficiency virus (HIV) infection (adjusted odds ratio [aOR], 4.4 [95% confidence interval {CI}, 1.1–18.0]; P = .04) and age 30–39 years (aOR, 4.0 [95% CI, 1.2–12.7]; P = .02) were independently associated with LTBI. Meanwhile, smoking status, alcohol use, nature of residence, crowding index, and TB contact were not associated with LTBI.
Conclusions
Our findings are in keeping with the evidence that HIV infection and advancing age are important risk factors for LTBI in pregnancy. In our setting, we recommend routine screening for LTBI and TB preventive therapy among eligible pregnant women.
Keywords: CD4+ T-cell and CD8+ T-cell TB responses, latent tuberculosis infection, pregnancy, risk factors, Uganda
About one-third of pregnant women in Uganda had latent tuberculosis (TB) infection, especially those with HIV infection and those 30 years or older. We recommend routine screening for latent TB and TB preventive therapy among eligible pregnant women.
The World Health Organization (WHO) estimates that close to 2 billion people worldwide are latently infected with Mycobacterium tuberculosis complex [1, 2]. Latent tuberculosis infection (LTBI) manifests as a specific immune response in the absence of clinical and radiological disease but with capacity to reactivate and cause clinical disease at a later time [1, 2]. In fact, an estimated 5%–10% of human immunodeficiency virus (HIV)–negative individuals with LTBI progress to active disease at some point in their life, translating into approximately 10 million cases progressing to active tuberculosis (TB) cases and about 1.4 million deaths every year [3].
Women in their reproductive years, during pregnancy or the early postpartum period, are disproportionately affected by TB, and TB remains an important cause of death in this group [3]. In 2019 alone, 450 000 of the estimated 3.2 million women who fell ill with TB died of the disease [3]. The WHO has no specific report on active TB in pregnancy. However, a study estimated that >200 000 annual cases of active TB disease occur during pregnancy globally [4].
TB in pregnancy poses a substantial risk of morbidity to both the pregnant woman and the fetus if not diagnosed and treated in a timely manner [5]. Immune dysregulation in pregnancy is associated with a more insidious onset of active TB, increased risk of LTBI, and progression of LTBI to active TB disease [4, 6]. Globally, an estimated 900 million women have latent LTBI [4, 6]. These women have a considerably increased risk of reactivation to active disease during pregnancy or in puerperium [4, 6].
In a recent systematic review, among pregnant women in the United States, the prevalence of LTBI ranged from 14% to 48% [6]. In this study, the diagnostic performance of both tuberculin skin test (TST) and interferon-gamma (IFN-γ) release assay (IGRA) was comparable and was unaffected by pregnancy [6]. However, there are limited data and understanding of factors associated with LTBI among pregnant women in Africa, a region with a high TB and HIV burden. One study from Tanzania found a prevalence of LTBI among pregnant women as high as 37.4% [7]. Two studies from Ethiopia showed a prevalence of LTBI of 31.9% [8] and 33% [9] among pregnant women using IGRA.
IGRAs, such as the QuantiFERON TB Gold-Plus (QFT-plus) assay, have very high sensitivities and are not influenced by prior BCG vaccination [10]. QFT-plus has TB antigens that elicit CD4+ T-cell IFN-γ response reflecting a recall from reexposure and antigens that elicit CD8+ T-cell IFN-γ induced from viable or recent exposure [11].
Uganda is a high-TB-burden country with >30% of reported TB cases affecting women [12]. It was estimated that 1400–4400 pregnant women in Uganda had active TB in 2011 [4], but the TB epidemiology has changed considerably since Uganda achieved the 2015 TB-related Millennium Development Goals. The burden of both active TB and LTBI among pregnant women is largely unknown. Therefore, we aimed at determining the prevalence of and risk factors for LTBI among pregnant women in Uganda.
METHODS
Study Design
This was a single-center, antenatal care–based, cross-sectional study conducted among pregnant women attending a routine antenatal care clinic at Kawempe National Referral Hospital (KNRH), Kampala, Uganda. The study was conducted between September 2020 and December 2020.
Study Setting
KNRH is an obstetrics and gynecology hospital, and a teaching and research center affiliated to the Makerere University College of Health Science. It is a 170-bed national referral hospital located along the Kampala-Gulu Highway that receives referrals mainly from lower health centers in Kampala and neighboring districts. The antenatal care clinic at KNRH runs on Tuesday through Thursday every week, offering antenatal care services to about 50–60 new mothers every clinic day.
Study Population
We enrolled pregnant women who were willing and competent to provide informed written consent, regardless of gestational age or gravidity. We excluded from the study patients who had known or suspected active TB based on the Uganda National Intensified TB Case Finding Guide [13] followed by a routine physical examination, or who had recently (past 6 months) received treatment for TB. Trained study nurses consecutively enrolled eligible participants from the daily antenatal care attendance register until the sample size was reached.
Sample Size
Using formula for a single population, we calculated a sample size of 260 participants based on an estimated prevalence of LTBI of 16.1% as reported in a previous study in the general population in Uganda [14], a margin of error of 5%, 20% incomplete data or withdrawal of consent, and a z statistic at a 95% confidence interval (CI).
Demographic and Obstetric Data
A study assistant administered a semistructured study questionnaire through a face-to-face interview to collect information on maternal characteristics such as age, gravidity, education level, occupation, marital status, HIV status and antiretroviral therapy (ART), TB contact, gestational age, history of abortion, smoking and alcohol usage, and the number of antenatal care visits in the current pregnancy. Gestational age was estimated using the date of the last normal menstrual period.
Anthropometric Data
Body mass index (kg/m2) and waist-hip ratio (cm) were calculated following anthropometric measurements. Specifically, weight was measured with minimal clothing and without shoes using a digital bathroom weighing scale (SECA-Germany) and height was measured with a stadiometer (Fazzini S208 height rod). The weighing scale was calibrated on a daily basis. The waist and hip circumferences were measured using a tailor’s measuring tape. The brachial blood pressure (BP) was measured on both arms using a MEDQUIP arm-type fully automatic digital blood pressure monitor (model BP-2400) with an appropriate adult cuff size and the participant seated upright in a comfortable position after resting. BPs were taken 5 minutes apart and the average of the 2 measurements was considered as the participant’s BP.
IGRA
A study nurse drew blood for IGRA directly into the IGRA tubes. The IGRA assay, QuantiFERON-TB Gold-plus (Qiagen, Hilden, Germany) was performed according to the manufacturer’s instructions [11]. All samples were processed and ran in a clinical laboratory at the Department of Immunology and Molecular Biology, School of Biomedical Sciences, Makerere University, which is 30 minutes away from KNRH. In brief, 1 mL of blood was drawn directly into 4 separate heparinized tubes: the nil control (containing only heparin), the mitogen control (containing phytohemagglutinin), TB1 (containing M. tuberculosis–specific antigens ESAT-6 and CFP-10 modified for eliciting CD4+ T-cell responses), and TB2 (containing M. tuberculosis–specific antigens ESAT-6 and CFP-10 modified for eliciting CD8+ T-cell responses). Immediately after filling each tube, the tube was inverted at least 10 times to allow the blood to coat the entire wall. Within 2 hours of venipuncture, the tubes were remixed by inverting them again 10 times before immediately being placed in an incubator set at 37°C. After 24 hours of incubation, the tubes were centrifuged at 3000g and the plasma was collected using single wrapped 3-mL Pasteur pipettes. The amount of IFN-γ in the plasma was measured by enzyme-linked immunosorbent assay (ELISA) with the reagents included in the test kit according to the manufacturer’s recommendations. In brief, 50 µL of working-strength conjugate was added to each well of the QFT-Plus ELISA plate, followed by 50 µL of the plasma and standards to the appropriate wells. The plate was incubated for 2 hours in the biosafety cabinet at room temperature. The plates were washed at least 6 times and an allowance of 5 seconds of soak time with 400 µL of 1X wash buffer using an ELISA washer. Next, 100 µL of the substrate solution was added and the plate was incubated at room temperature for 30 minutes. Last, 50 µL of substrate solution was added and the plate was immediately read using an ELISA reader at 450 nm with a reference wavelength of 620 nm to obtain optical densities. Results were calculated using QFT-Plus analysis software version 2.71.2 [11].
Definitions
LTBI was defined as an IFN-γ concentration ≥0.35 IU/mL (calculated as either TB1 or TB2 antigen minus nil) per the manufacturer’s guideline [11]. If antigen-nil was <0.35 IU/mL or <25% of the nil value, when the mitogen was ≥0.5 IU/mL, the result was considered negative. If (1) nil was >8 IU/mL or (2) antigen-nil ≥0.35 IU/mL and <25% of the nil value when the nil was ≤8.0 IU/mL and the mitogen was <0.5 IU/mL, the results were considered indeterminate.
Statistical Methods
We used Stata version 16 software (StataCorp, College Station, Texas) to perform all statistical analyses. Categorical variables were expressed as frequencies and percentages. For all numerical variables, Shapiro-Wilk normality test was completed to select an appropriate test. Normally distributed data were summarized as mean and standard deviations and nonnormally distributed data as median and interquartile range (IQR). The χ 2 or Fisher exact test was used to assess for associations between LTBI and categorical variables while Mann–Whitney U/Student t tests and Wilcoxon-signed rank/analysis of variance were used to assess for associations between LTBI and continuous variables (age, blood pressure, weight, age, gestational age, and height, waist, and hip circumferences). All variables with P < .2 in the bivariate analyses were fitted into a multivariate logistic regression model to adjust for potential confounders such as age, parity, gestational age, and HIV status. Multivariable logistic regression model was used to assess for independent predictors of LTBI among the study participants. Results were presented as odds ratios (ORs) and 95% CIs. All analyses were 2-tailed and P < .05 was considered significant at 95% CI. Graphs were prepared using GraphPad Prism version 8.0.2 software (GraphPad, La Jolla, California)
Patient Consent Statement
All participants provided informed written consent after the study procedure, risks, and benefits were explained to them. The study protocol was approved by the Makerere University School of Medicine Ethics and Research Committee (reference number 2020–113). All principles of research involving human subjects outlined in the Declaration of Helsinki were adhered to.
RESULTS
Baseline Characteristics
A total of 261 pregnant women met the inclusion criteria. Table 1 summarizes the characteristics of the recruited participants. The median age of the participants was 26 (IQR, 23.0–30.0) years. The majority of mothers were married (86.2%) and had reported for their first antenatal care visit (75.9%). Up to 99% (n = 258) of the participants were in the second trimester with a median gestational age at enrollment of 26 (IQR, 20–31) weeks. About 11.1% had history of TB contact with family members. Thirteen (5%) participants reported that they were HIV positive. All HIV-positive women were on ART and had not received TB preventive therapy.
Table 1.
Baseline Characteristics of Participants (N = 261)
| Participant Characteristics | No. (%) |
|---|---|
| Antenatal visit at enrollment | |
| First | 198 (75.9) |
| Second | 36 (13.8) |
| Third | 7 (2.7) |
| Fourth or more | 20 (7.7) |
| Age, y, median (IQR) | 26 (23.0–30.0) |
| Age group | |
| 15–19 | 20 (7.7) |
| 20–29 | 161 (61.7) |
| 30–39 | 77 (29.5) |
| 40–49 | 3 (1.2) |
| Marital status | |
| Married | 225 (86.2) |
| Single | 36 (13.8) |
| Education level | |
| Informal | 4 (1.5) |
| Primary | 59 (22.6) |
| Secondary | 151 (57.9) |
| Tertiary | 47 (18.0) |
| Occupational status | |
| Unemployed | 119 (45.6) |
| Business | 80 (30.7) |
| Professional | 34 (13.0) |
| Skilled worker | 24 (9.2) |
| Unskilled worker | 4 (1.5) |
| Smoking status | |
| Former | 1 (0.4) |
| Never | 260 (99.6) |
| Alcohol usage | |
| Current | 29 (11.1) |
| Former | 38 (14.6) |
| Never | 194 (74.3) |
| Family history of diabetes | |
| No | 218 (83.5) |
| Yes | 43 (16.5) |
| Residence | |
| Urban | 217 (84.4) |
| Rural | 40 (15.6) |
| HIV status | |
| Negative | 248 (95.0) |
| Positive | 13 (5.0) |
| BCG scar | |
| Yes | 177 (67.8) |
| No | 84 (32.2) |
| Family history of TB | |
| No | 232 (88.9) |
| Yes | 29 (11.1) |
| TB contact | |
| No | 244 (93.5) |
| Yes | 17 (6.5) |
| Crowding index | |
| ≤4 household occupants | 196 (75.1) |
| ≥5 household occupants | 65 (24.9) |
| Gravidity | |
| Primigravida | 89 (34.1) |
| Multigravida | 154 (59.0) |
| Grand multigravida | 18 (6.9) |
| Previous abortion | |
| No | 217 (83.1) |
| Yes | 44 (16.9) |
| Gestational age at enrollment, wk, median (IQR) | 26 (20–31) |
| Trimester at enrollment | |
| 1 | 1 (0.4) |
| 2 | 258 (98.9) |
| 3 | 2 (0.8) |
| Anthropometry, median (IQR) | |
| BMI, kg/m2 | 27.2 (23.7–31.4) |
| Waist-hip ratio, cm | 0.91 (0.86–0.96) |
| Blood pressure at enrollment, median (IQR) | |
| Systolic, mm Hg | 122.5 (114.0–131.0) |
| Diastolic, mm Hg | 76.5 (71.5–84.0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
QuantiFERON-TB Gold-Plus Results
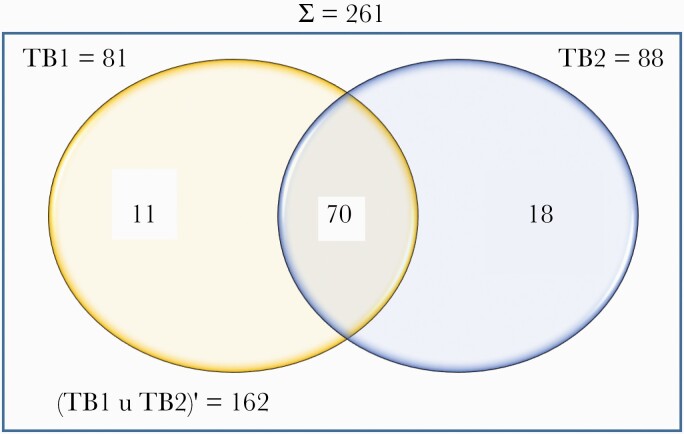
Overall, 99 (37.9%) participants had a positive QFT-plus result. Of these, TB1 and TB2 alone were positive in 11 (11.1%) and 18 (18.2%) participants, respectively (Figure 1). Twenty-four (9.2%) and 138 (52.9%) participants had indeterminate and negative QFT-plus results, respectively. Considering indeterminate results as negative, the overall prevalence of LTBI prevalence was 37.9% (n = 99) (95% CI, 32.3%–44.0%).
Figure 1.
QuantiFERON-TB Gold-plus results of the participants. Abbreviations: TB1, CD4+ T-cell tuberculosis response; TB2, CD8+ T-cell tuberculosis response.
Overall, the interreliability of QFT-Plus assay for TB1 vs TB2 was moderate (agreement = 88.9%, κ = .75; P < .0001). In subanalysis, there was also moderate interreliability agreement between TB1 and TB2 among HIV-negative pregnant mothers (agreement = 89.5%, κ = .75; P < .0001), but this was weak for HIV-positive clients (agreement = 76.9%, κ = .49; P = .036).
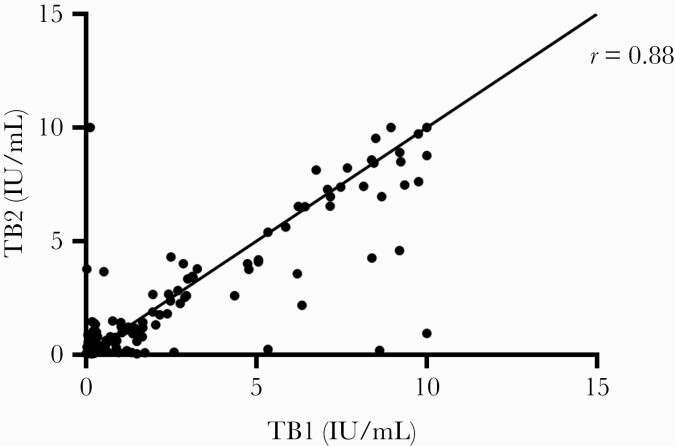
There was a strong positive correlation between IFN-γ value in TB1 and TB2 (Pearson coefficient = 0.88; P < .0001; Figure 2).
Figure 2.
Correlation of quantitative interferon-γ values in TB1 and TB2 tubes. Abbreviations: TB1, CD4+ T-cell tuberculosis response; TB2, CD8+ T-cell tuberculosis response.
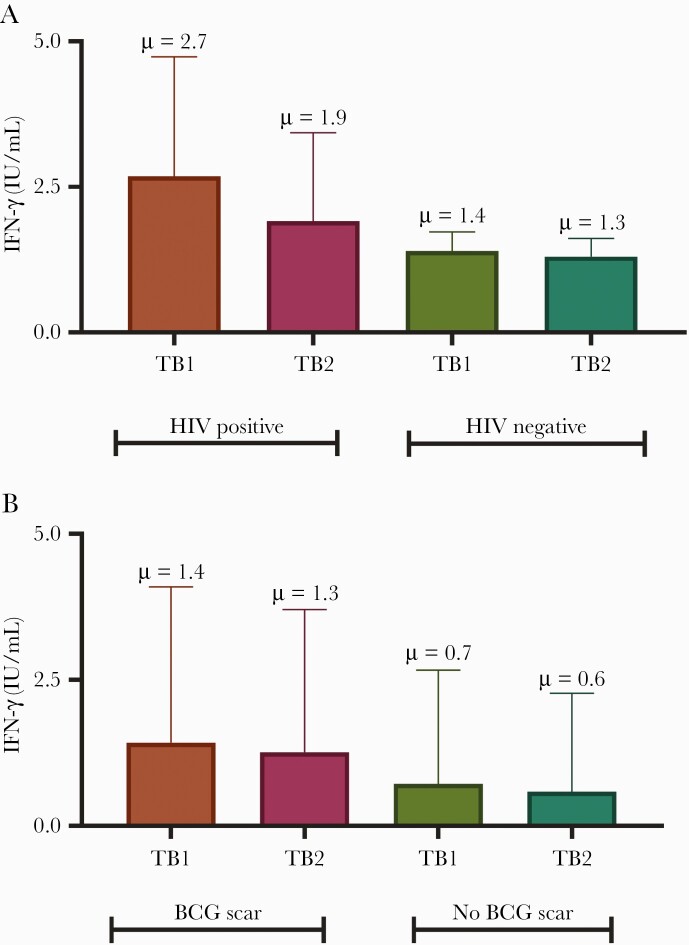
The quantitative IFN-γ value in TB2 was similar to that in TB1 irrespective of HIV status (TB1: 2.7 ± 3.4 [HIV positive, n = 13] vs 1.4 ± 2.7 IU/mL [HIV negative, n = 248], P = .2; TB2: 1.9 ± 2.5 [HIV positive, n = 13] vs 1.3 ± 2.5 IU/mL [HIV negative, n = 248], P = .4 (Figure 3A). However, participants with BCG scars had higher values compared to those without (TB1: 1.4 ± 2.7 [BCG scar, n = 177] vs 0.7 ± 1.9 IU/mL [no BCG scar, n = 84], P = .003; TB2: 1.3 ± 2.4 [BCG scar, n = 177] vs 0.6 ± 1.7 IU/mL [no BCG scar, n = 84], P = .002) (Figure 3B). There was no statistically significant difference between mitogen and nil levels across HIV status (mitogen: 9.4 IU/mL [HIV positive] vs 7.3 IU/mL [HIV negative], P = .85; nil: 0.24 IU/mL [HIV positive] vs 0.15 IU/mL [HIV negative], P = .26).
Figure 3.
Quantitative interferon-γ value in TB1 and TB2 tubes stratified by HIV status (A) and presence or absence of a BCG scar (B). Abbreviations: HIV, human immunodeficiency virus; IFN-γ, interferon gamma; TB1, CD4+ T-cell tuberculosis response; TB2, CD8+ T-cell tuberculosis response.
Risk Factors for LTBI Among Participants
On bivariate analysis (Table 2), LTBI was associated with advancing age (P < .001) and HIV status (P = .006). BCG scar (P = .259), smoking status (P = .433), nature of residence (P = .707), crowding index (P = .433), and TB contact (P = .302) were not significantly associated with LTBI status.
Table 2.
Factors Associated With Latent Tuberculosis Infection Among Study Participants
| Participant Characteristics | LTBI | No LTBI | P Value |
|---|---|---|---|
| Antenatal care visit at enrollment | |||
| First | 74 (74.8) | 124 (76.5) | .919 |
| Second | 15 (15.2) | 21 (13) | |
| Third | 2 (2) | 5 (3.1) | |
| Fourth or more | 8 (8.1) | 12 (7.4) | |
| Age group, y | |||
| 15–19 | 5 (5.1) | 15 (9.3) | <.001 |
| 20–29 | 49 (49.5) | 112 (69.1) | |
| 30–39 | 42 (42.4) | 35 (21.6) | |
| 40–49 | 3 (3) | 0 (0) | |
| Marital status | |||
| Married | 86 (86.9) | 139 (85.8) | .808 |
| Single | 13 (13.1) | 23 (14.2) | |
| Education level | |||
| Informal | 3 (3) | 1 (0.6) | .164 |
| Primary | 27 (27.3) | 32 (19.8) | |
| Secondary | 51 (51.5) | 100 (61.7) | |
| Tertiary | 18 (18.2) | 29 (17.9) | |
| Occupational status | |||
| Unemployed | 39 (39.4) | 80 (49.4) | .335 |
| Business | 34 (34.3) | 46 (28.4) | |
| Professional | 13 (13.1) | 21 (13) | |
| Skilled worker | 10 (10.1) | 14 (8.6) | |
| Unskilled worker | 3 (3) | 1 (0.6) | |
| Smoking status | |||
| Former | 0 (0) | 1 (0.6) | .433 |
| Never | 99 (100) | 161 (99.4) | |
| Alcohol usage | |||
| Current | 9 (9.1) | 20 (12.4) | .113 |
| Former | 20 (20.2) | 18 (11.1) | |
| Never | 70 (70.7) | 124 (76.5) | |
| Residence | |||
| Rural | 16 (16.7) | 24 (14.9) | .707 |
| Urban | 80 (83.3) | 137 (85.1) | |
| HIV status | |||
| Negative | 89 (89.9) | 159 (98.2) | .006 |
| Positive | 10 (10.1) | 3 (1.9) | |
| BCG scar | |||
| No | 36 (36.4) | 48 (29.6) | .259 |
| Yes | 63 (63.6) | 114 (70.4) | |
| Family history of TB | |||
| No | 90 (90.9) | 142 (87.7) | .417 |
| Yes | 9 (9.1) | 20 (12.4) | |
| TB contact | |||
| No | 95 (96) | 149 (92) | .302 |
| Yes | 4 (4) | 13 (8) | |
| Crowding index | |||
| ≤4 household members | 77 (77.8) | 119 (73.5) | .433 |
| ≥5 household members | 22 (22.2) | 43 (26.5) | |
| Gravidity | |||
| Primigravida | 39 (39.4) | 50 (30.9) | .193 |
| Multigravida | 56 (56.6) | 98 (60.5) | |
| Grand multigravida | 4 (4) | 14 (8.6) | |
| Previous abortion | |||
| No | 86 (86.9) | 131 (80.9) | .209 |
| Yes | 13 (13.1) | 31 (19.1) | |
| Gestational age at enrollment, wk, median (IQR) | 26 (20–32) | 25.9 (19.7–30.6) | .962 |
| Trimester at enrollment | |||
| 1 | 1 (1) | 0 (0) | .054 |
| 2 | 96 (97) | 162 (100) | |
| 3 | 2 (2) | 0 (0) | |
| Anthropometry, median (IQR) | |||
| BMI, kg/m2 | 27.1 (23.2–30.9) | 27.5 (24.0–31.8) | .174 |
| Waist-hip ratio, cm | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | .238 |
| Blood pressure at enrollment, median (IQR) | |||
| Systolic, mm Hg | 123 (114–131) | 122 (115–131) | .589 |
| Diastolic, mm Hg | 77 (70.5–84.5) | 76 (72–84) | .896 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; LTBI, latent tuberculosis infection; TB, tuberculosis.
On multivariable logistic regression (Table 3), pregnant women in their third decade of life (30–39 years of age) were 4 times more likely to have LTBI compared to teenagers (adjusted OR [aOR], 3.96 [95% CI, 1.23–12.73]; P = .021). In addition, participants with HIV were also 4 times more likely to have LTBI (aOR, 4.37 [95% CI, 1.06–18.04]; P = .041) than HIV-negative women.
Table 3.
Multivariable Logistic Regression Model Showing Factors Associated With Latent Tuberculosis Among Study Participants
| Variables | Adjusted OR | (95% CI) | P Value |
|---|---|---|---|
| Age category, y | |||
| 15–19 | Reference | ||
| 20–29 | 1.4 | (.5–4.1) | .580 |
| 30–39 | 4.0 | (1.2–12.7) | .021 |
| Alcohol usage | |||
| Never | Reference | ||
| Former | 1.0 | (.5–2.3) | .928 |
| Current | 0.8 | (.3–1.9) | .595 |
| HIV status | |||
| Negative | Reference | ||
| Positive | 4.4 | (1.1–18.0) | .041 |
| Gravidity | |||
| Primigravida | Reference | ||
| Multigravida | 0.9 | (.47–1.57) | .632 |
| Grand multigravida | 0.4 | (.11–1.5) | .173 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
DISCUSSION
Routine antenatal care visit provides a unique opportunity to identify pregnant women with LTBI and facilitate further evaluation and follow-up as needed. In the present study, we found that about 38% of pregnant women attending antenatal care at a tertiary hospital in Uganda had LTBI, using IGRA. Our findings suggest that pregnant women who had HIV infection and those aged 30 years or older were 4-fold more likely to have LTBI. Our finding is congruent with similar studies from Tanzania [7] and Ethiopia [9] which found a prevalence of LTBI of 37.4% and 33% among pregnant women, respectively. In 2 systematic reviews, LTBI was reported in about 33% of women in the Middle East and North Africa [15] and among 14%–48% of pregnant women in the United States [6]. Interesting, we showed that participants with BCG scars had much higher IFN-γ concentration, reflecting the immunomodulatory effect of BCG in LTBI. BCG revaccination may be further investigated in this population.
A few prior studies looked into LTBI prevalence in the general and special populations in Uganda. Two observational studies conducted in Uganda found the prevalence of LTBI to be 16.1% among adolescents and 49.0% among the general adult population [14, 16]. Both studies used TST to demonstrate LTBI status, which has a relatively lower diagnostic accuracy than IGRA in populations that have high BCG vaccination coverage [10]. Another study investigated household contacts of TB patients in Uganda [17]. The prevalence of LTBI in this study was found to be as high as 65%. Among persons living with HIV in western Uganda who are engaged in hazardous alcohol use, the prevalence of LTBI based on TST positivity was reported at 35% [18]. In this study, the prevalence of LTBI among women was 33.9%.
Pregnancy is characterized by immunological changes that may predispose or increase TB reactivation [6]. Immune dysregulation in pregnancy, mainly driven by a surge in serum progesterone levels, is marked by an anti-inflammatory milieu characterized by quantitative and qualitative defects in circulating CD4+ and CD8+ T cells with a corresponding increase in the number of circulating regulatory T cells [19]. This dysregulation dampens the proinflammatory immune response, often by producing interleukin 10 (IL-10). Furthermore, progesterone also induces the placenta to produce IL-10, [20] which suppresses cell-mediated Th1 cytokines (IFN-γ, IL-2, tumor necrosis factor–α) [21]. Consequently, pregnant women are at higher risk of acquiring both primary TB disease (from recent TB infection) and progression of LTBI to active disease compared to nonpregnant women [22].
Active TB is more likely during pregnancy and the early postpartum period than any other time in a woman’s life [23]. Therefore, women of reproductive age are suffering disproportionate morbidity and mortality due to TB. TB in pregnancy is associated with poor outcomes, including increased mortality in both the neonate and the pregnant woman [4]. In our study, HIV infection was found in 13 participants, of whom 10 (77%) had LTBI, and HIV infection was independently associated with LTBI. This finding is consistent with published case series, which shows that the risk of TB in HIV-infected pregnant women appears to be higher, with 1%–11% developing active TB during pregnancy or the early postpartum period [22, 24]. However, recent work has shown that despite the high TB incidence among HIV-positive individuals, detection of LTBI among these individuals is lower [8]. In Africa, WHO estimates revealed that TB rates are up to 10 times higher in pregnant women living with HIV than in pregnant women without HIV infection [3].
Similar to our study, Sheriff et al [7] in Tanzania reported that parity, body mass index, gestational age, and HIV serostatus were not associated with LTBI among pregnant women. However, in contrast, we found a significant association between age and HIV infection with LTBI among our participants. Notably, Sheriff et al used TST for the diagnosis of LTBI and 3 different cutoffs were considered in classifying participants as having LTBI, whereas we used IGRA. Additionally, almost 30% of the study participants (significantly younger women) were lost to follow-up. This could explain the differences with our study.
Currently, there is no gold-standard diagnostic tool for the diagnosis of LTBI. TST or IGRA, both of which screen for immunological memory, are the most common screening tools in clinical practice [25]. IGRA is specific for M. tuberculosis while there is cross-reactivity with nontuberculous mycobacterial infections when using TST or in those who previously received BCG vaccine, hence a false positive [25]. QFT-plus, an IGRA platform used in the present study, has M. tuberculosis–specific antigen that triggers both CD4+ and CD8+ T-cell responses [11]. Whereas positivity of both TB1-nil and TB2-nil indicates LTBI, TB2 tube response can be associated with active or subclinical TB disease and/or recent TB infection [26]. In our study, up to 18.2% of the participants had an isolated positive response to TB2 antigens, suggestive of possible acquisition of M. tuberculosis infection during pregnancy or subclinical TB disease.
Our study has limitations. Participants were derived from a single center, so our findings may not be generalizable. Second, we had a high number of patients who had indeterminate QFT-plus, and it is likely we may have underestimated the true burden of LTBI in this population. Moreover, 21 of 24 (88%) indeterminate results were due to low mitogen, indicating a dampened immune response. Third, the cross-sectional design did not allow us to prospectively look for whether CD8+ women were more likely to develop active TB disease (which would support subclinical disease) and we were unable assess for prior exposure (which would support recent exposure). Fourth, we were unable to obtain a complete detail of ART regimens, including duration, current viral load, and CD4+ T-cell count for HIV-positive participants. However, we used the QFT-plus assay, which is a more specific test for M. tuberculosis infection than the TST. To the best of our knowledge, this is the first study to report on the prevalence of LTBI among pregnant women in Uganda. We recommend future studies to prospectively evaluate maternal and fetal outcomes in a population of LTBI vs non-LTBI mothers. Also, studies looking at determinants of other important risk factors for LTBI progression such as diabetes mellitus and chronic kidney disease among pregnant women are welcome. Last but not least, the high burden of LTBI and TB2 in this population merits active TB investigation and TB preventive therapy.
In conclusion, we report a high prevalence of LTBI among pregnant women in Uganda, especially those with HIV infection and those with advanced age. These findings suggest a need for strategies to scale up LTBI screening among high-risk pregnant women and TB preventive treatment in this population to advance progress toward TB elimination.
Notes
Author contributions. I. A. B., J. B. B., and F. B. designed the study. G. N., P. S., S. N., A. P. K., W. N., R. K., S. C., D. K., C. B., M. K., B. J. K., and F. B. collected the data. O. R., with oversight from F. B., I. A. A., and J. B. B., performed the statistical analyses. F. B., R. O., P. S., G. N., J. B. B., M. K., and I. A. B. wrote the manuscript with input from all the authors. All of the authors reviewed the manuscript and approved the final version.
Data availability. Data are available upon request from the corresponding author.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Research reported in this publication was supported by the Forgarty International Centre of the National Institute of Health (award number D43 TW011401). Also, this work was supported by the Crick African Network, which receives its funding from the United Kingdom’s Global Challenges Research Fund (MR/P028071/1).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Latent tuberculosis infection. updated and consolidated guidelines for programatic management. Geneva, Switzerland, Switzerland: WHO, 2018. [PubMed] [Google Scholar]
- 2. Nuermberger E, Bishai WR, Grosset JH. Latent tuberculosis infection. Semin Respir Crit Care Med 2004; 25:317–36. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Global tuberculosis report 2020. Geneva, Switzerland: WHO,2020. [Google Scholar]
- 4. Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in pregnancy: an estimate of the global burden of disease. Lancet Global Health 2014; 2:e710–6. [DOI] [PubMed] [Google Scholar]
- 5. Miele K, Bamrah Morris S, Tepper NK. Tuberculosis in pregnancy. Obstet Gynecol 2020; 135:1444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malhamé I, Cormier M, Sugarman J, Schwartzman K. Latent tuberculosis in pregnancy: a systematic review. PLoS One 2016; 11:e0154825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheriff FG, Manji KP, Manji MP, et al. . Latent tuberculosis among pregnant mothers in a resource poor setting in northern Tanzania: a cross-sectional study. BMC Infect Dis 2010; 10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birku M, Desalegn G, Kassa G, et al. . Pregnancy suppresses Mycobacterium tuberculosis-specific Th1, but not Th2, cell-mediated functional immune responses during HIV/latent TB co-infection. Clin Immunol 2020; 218:108523. [DOI] [PubMed] [Google Scholar]
- 9. König Walles J, Tesfaye F, Jansson M, et al. . Performance of QuantiFERON-TB Gold Plus for detection of latent tuberculosis infection in pregnant women living in a tuberculosis- and HIV-endemic setting. PLoS One 2018; 13:e0193589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diel R, Goletti D, Ferrara G, et al. . Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 2011; 37:88–99. [DOI] [PubMed] [Google Scholar]
- 11. Qiagen. QuantiFERON®-TB Gold Plus (QFT®-Plus) ELISA package insert. Hilden, Germany, 2017. [Google Scholar]
- 12. World Health Organization. Uganda tuberculosis profile. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 13. Ministry of Health of Uganda. The Uganda national tuberculosis prevalence survey, 2014–2015 survey report. Available at: http://health.go.ug/sites/default/files/Uganda National TB Prevalence Survey 2014-2015_final 23rd Aug17.pdf. Accessed 2 March 2021.
- 14. Mumpe-Mwanja D, Verver S, Yeka A, et al. . Prevalence and risk factors of latent tuberculosis among adolescents in rural Eastern Uganda. Afr Health Sci 2015; 15:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barry M. Prevalence of latent tuberculosis infection in the Middle East and North Africa: a systematic review. Pulm Med 2021; 2021:6680651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kizza FN, List J, Nkwata AK, et al. . Prevalence of latent tuberculosis infection and associated risk factors in an urban African setting. BMC Infect Dis 2015; 15:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biraro IA, Egesa M, Toulza F, et al. . Impact of co-infections and BCG immunisation on immune responses among household contacts of tuberculosis patients in a Ugandan cohort. PLoS One 2014; 9:e111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puryear SB, Fatch R, Beesiga B, et al. . Higher levels of alcohol use are associated with latent tuberculosis infection in adults living with human immunodeficiency virus. Clin Infect Dis 2020; 72:865–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 2012; 62:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polanczyk MJ, Carson BD, Subramanian S, et al. . Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol 2004; 173:2227–30. [DOI] [PubMed] [Google Scholar]
- 21. Piccinni MP, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol 2000; 109:30–3. [DOI] [PubMed] [Google Scholar]
- 22. Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012; 55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 2012; 185:779–84. [DOI] [PubMed] [Google Scholar]
- 24. Gounder CR, Wada NI, Kensler C, et al. . Active tuberculosis case-finding among pregnant women presenting to antenatal clinics in Soweto, South Africa. J Acquir Immune Defic Syndr 2011; 57:e77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sauzullo I, Mengoni F, Mascia C, et al. . Diagnostic performance in active TB of QFT-Plus assay and co-expression of CD25/CD134 in response to new antigens of Mycobacterium tuberculosis. Med Microbiol Immunol 2019; 208:171–83. [DOI] [PubMed] [Google Scholar]