Abstract
Background
Although cytomegalovirus (CMV)-seropositive solid organ transplant recipients have a relatively lower risk of CMV infection than CMV-seronegative recipients who receive allograft from CMV-seropositive donors, some patients remain at risk of CMV infection after transplant. We investigated the pretransplant CMV-specific humoral immunity (CHI) and other CMV infection predictors in CMV-seropositive kidney transplant (KT) recipients.
Methods
This retrospective study was conducted on adult CMV-seropositive KT recipients during 2017 and 2018. The cumulative incidence of CMV infection was estimated using the Kaplan-Meier method. CHI, measured with an enzyme-linked fluorescent immunoassay and other predictors for CMV infection, was analyzed using Cox proportional hazards models.
Results
Of the 340 CMV-seropositive KT recipients (37% female; mean ± SD age, 43 ± 11 years), 69% received deceased-donor allograft and 64% received induction therapy. During a mean follow-up of 14 months, the cumulative incidence of CMV infection was 14.8%. In multivariate analysis, low pretransplant CHI (defined as anti-CMV immunoglobulin [IgG] titer <20 AU/mL) was significantly associated with CMV infection (hazard ratio [HR], 2.98; 95% CI, 1.31–6.77; P = .009). Other significant predictors of CMV infection included older donor age (HR, 1.03; 95% CI, 1.01–1.06; P = .005), antithymocyte induction therapy (HR, 2.90; 95% CI, 1.09–7.74; P = .033), and prolonged cold ischemic time (HR, 1.06; 95% CI, 1.02–1.10; P = .002).
Conclusions
A low pretransplant CHI is independently associated with post-transplant CMV infection in CMV-seropositive KT recipients. A quantitative anti-CMV IgG assay could potentially stratify CMV-seropositive patients at risk of CMV infection after KT.
Keywords: anti-CMV immunoglobulin G titer, CMV infection, humoral immunity, kidney transplantation, viral-specific immunity
Kidney transplantation (KT) is a well-established strategy to improve the quality of life and survival of patients with end-stage renal disease [1]. However, these patients are at increased risk of infectious complications due to an immunocompromised state acquired from immunosuppressive therapy. Among the many pathogens that commonly infect KT recipients, cytomegalovirus (CMV) is a leading cause of substantial morbidity [2, 3]. Previous retrospective studies conducted among CMV-seropositive KT recipients revealed a prevalence rate of CMV infection ranging from 4% to 25% [4, 5]. CMV infection was found to be associated with kidney allograft failure after adjusting for other risk factors. Thus, to reduce allograft failure and CMV-associated morbidity, it is critical to take steps before organ transplantation to prevent the occurrence of CMV infection. Pretransplant qualitative CMV-specific humoral immunity (CHI), defined by anti-CMV immunoglobulin G (IgG), is universally recommended to stratify the risk of infection after transplant [2]. Although CMV-seropositive KT recipients are considered to have a relatively lower risk of post-transplant CMV infection than those with CMV seronegativity, a subgroup of these patients remains at risk of CMV infection after transplant [6]. The independent risk factors identified in the aforementioned cohort study are older donor age and the occurrence of acute cellular rejection, especially those requiring antithymocyte globulin therapy [4, 5]. Lately, immunological factors have been investigated as markers to predict post-transplant CMV infection. Candidate markers have included components of both nonspecific and viral-specific immunity [7]. To date, the research and associated clinical studies on CMV-specific cellular immunity (CMI) have mostly focused on its potential role to guide management in solid organ transplant (SOT) recipients [8]. However, the financial incompatibility of utilizing these tests in a resource-limited setting remains a barrier to their implementation [9]. Instead, the anti-CMV immunoglobulin G (IgG) titer has been reported to have a potential role in predicting CMV infection among CMV-seropositive liver transplant recipients, especially those with severe CMV infection [10, 11]. The guidelines for prophylaxis and treatment of CMV infection in SOT recipients recommended by the Study Group on Infection in Transplantation (GESITRA) of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC) suggest that pretransplant CMI may be used together with CHI to better stratify the risk of CMV infection after transplantation in CMV-seropositive liver transplant recipients [12]. A previous study of CMV-seropositive heart transplant recipients reported a low pretransplant anti-CMV IgG titer associated with the risk of CMV infection after transplantation [13]. However, the possibility of a similar association in CMV-seropositive KT recipients has not been explored. Therefore, we aimed to assess the association between the anti-CMV IgG titer and the risk of post-transplant CMV infection among CMV-seropositive KT recipients.
METHODS
We conducted a retrospective study of all adult (ie, aged ≥18 years) KT recipients with CMV seropositivity during 2017–2018 at a single transplant center. Clinical characteristics, risk factors, and outcomes were extracted from patient medical records. The majority of recipients were monitored for CMV infection, and plasma CMV quantitative polymerase chain reaction (qPCR) was measured when clinically indicated. Only those receiving antithymocyte globulin (ATG) for induction therapy or steroid-refractory rejection were provided intravenous ganciclovir or oral valganciclovir for anti-CMV prophylaxis for a total of 3 months or switched to preemptive CMV monitoring by plasma CMV qPCR if clinically indicated (if they could not complete the course of therapy). Trimethoprim/sulfamethoxazole (1 year) for Pneumocystis jirovecii prophylaxis, acyclovir (6 months) for herpes simplex virus prophylaxis, and isoniazid (9 months) for latent tuberculous infection therapy were prescribed to all KT recipients.
CMV-Specific Humoral Immunity
CMV-specific humoral immunity was assessed by anti-CMV IgG titer. Pretransplant anti-CMV IgG antibody titers were measured with a semiquantitative enzyme-linked fluorescent immunoassay performed on the VIDAS (bioMérieux, Durham, NC, USA), reported as numeric values, and interpreted as follows: negative (<4 arbitrary units [AU]/mL), equivocal (4–5 AU/mL), or positive (≥6 AU/mL). Low CHI and high CHI were defined as anti-CMV IgG titer <20 AU/mL and ≥20 AU/mL, respectively.
CMV Infection
CMV infection was defined as the presence of CMV DNA in plasma regardless of symptoms. Plasma CMV DNA load was measured via quantitative real-time polymerase chain reaction performed on a Roche COBAS AmpliPrep/COBAS Taqman (Branchburg, NJ, USA). The DNA load was reported in IU/mL. The lower limit of quantification was <137 IU/mL. All patients with CMV infection were classified as follows: asymptomatic CMV infection (CMV infection without signs and symptoms) or CMV disease (CMV infection accompanied by compatible clinical signs and symptoms). CMV disease was further categorized as CMV syndrome (eg, fever and/or malaise, leukopenia, or thrombocytopenia) or tissue-invasive CMV disease (eg, gastrointestinal disease, pneumonitis, and hepatitis) [2].
Statistical Analyses
The cumulative incidence of CMV infection after transplant was estimated using the Kaplan-Meier method. Descriptive analysis was used for reporting baseline characteristics. Continuous variables were summarized as the mean and SD and compared by the Student t test or Mann-Whitney U test. Categorical variables were summarized as frequencies and percentages and were compared by the χ 2 test or Fisher exact test. Multivariate Cox proportional hazards models were used to analyze for independent predictors of CMV infection including anti-CMV IgG titer by cutoff value. P values <.05 were considered significant. Statistical analyses were performed with Stata statistical software, version 15 (StataCorp, LLC, College Station, TX, USA). A dot plot of pretransplant anti-CMV IgG titer distributions between KT recipients with and without CMV infection was performed with GraphPad Prism 6.0 (GraphPad Software, Inc, San Diego, CA, USA).
RESULTS
Patient Population
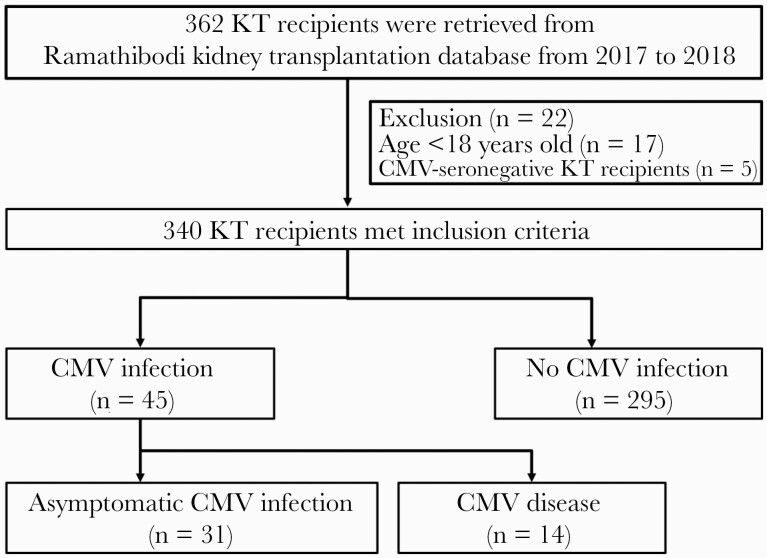
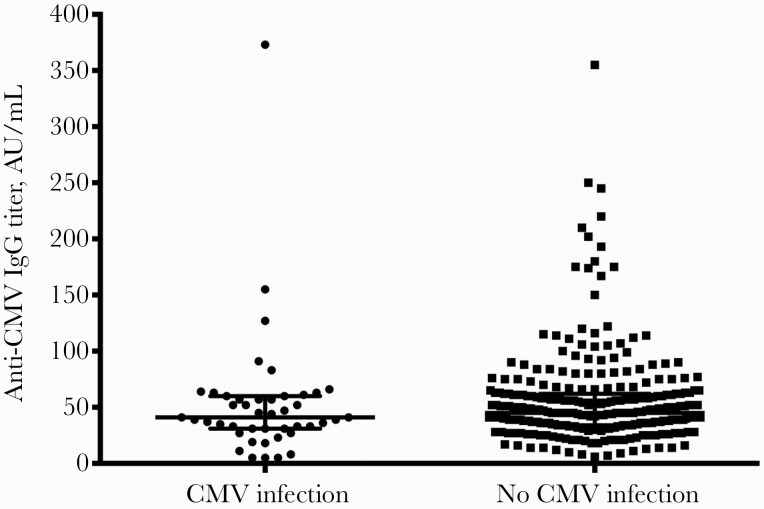
The medical records of a total of 362 KT patients whose surgeries occurred during 2017–2018 were retrieved; 22 of these patients were excluded from our analysis because they were younger than 18 years old (17 patients) or were CMV seronegative (5 patients) (Figure 1). Our study included 340 CMV-seropositive KT recipients, 37% of whom were female. Their mean ± SD age was 43 ± 11 years. Among these, 69% received deceased-donor allograft, and 64% received induction therapy. Pretransplant anti-CMV IgG titer distributions in KT recipients with and without CMV infection are shown in Figure 2. There were 7.1% patients classified as having low pretransplant CHI, while the remaining 92.9% had high pretransplant CHI. The baseline characteristics of the 340 patients (45 of whom developed post-transplant CMV infection) are compared in Table 1. Recipient age, sex, body mass index (BMI), and surgical time were not significantly different between patients with or without post-transplant CMV infection. The following variables were statistically different between KT recipients with or without post-transplant CMV infection: mean ± SD donor age (45 ± 12 vs 39 ± 14 years, respectively), receipt of an allograft from a deceased donor (41/45 [91.1%] vs 191/295 [64.7%], respectively), and mean ± SD cold ischemic time (16.41 ± 5.95 hours vs 11.38 ± 8.86 hours, respectively). Additionally, a low pretransplant CHI was significantly associated with post-transplant CMV infection: 7/45 (15.6%) vs 17/295 (5.8%).
Figure 1.
Study flowchart. Abbreviations: CMV, cytomegalovirus; KT, kidney transplant.
Figure 2.
Anti-CMV IgG titer distributions between kidney transplant recipients with and without CMV infection. Abbreviations: AU, arbitrary units; CMV, cytomegalovirus; IgG, immunoglobulin G.
Table 1.
Baseline Characteristics of Kidney Transplant Recipients With and Without Post-transplant CMV Infection
| Baseline Characteristics, No. (%) | CMV Infection (n = 45) | No CMV Infection (n = 295) | P Value |
|---|---|---|---|
| Recipient variables | |||
| Age, mean ± SD, y | 44 ± 10 | 43 ± 11 | .794 |
| Sex | |||
| Male | 27 (60) | 189 (64.1) | .597 |
| Female | 18 (40) | 106 (35.9) | |
| BMI, mean ± SD, kg/m2 | 23.18 ± 3.92 | 22.66 ± 3.93 | .412 |
| Pretransplant anti-CMV IgG titer | |||
| Low (<20 AU/mL) | 7 (15.6) | 17 (5.8) | .027 |
| High (≥20 AU/mL) | 38 (84.4) | 278 (94.2) | |
| Donor variables | |||
| Age, mean (SD), y | 45 ± 12 | 39 ± 14 | .005 |
| Donor status | |||
| Living donor | 4 (8.9) | 104 (35.3) | <.001 |
| Deceased donor | 41 (91.1) | 191 (64.7) | |
| Transplant variables | |||
| Cold ischemic time, mean ± SD, h | 16.41 ± 5.95 | 11.38 ± 8.86 | <.001 |
| Surgical time, mean ± SD, h | 5.03 ± 1.80 | 4.68 ± 1.29 | .215 |
| No. of KTs | |||
| First KT | 45 (100) | 290 (98.3) | >.999 |
| Second KT | 0 (0) | 5 (1.7) | |
| HLA mismatch | |||
| ≥3 | 16 (35.6) | 111 (37.6) | .789 |
| PRA | |||
| ≥51 | 5 (11.1) | 24 (8.1) | .564 |
| Pretransplant DFPP | 2 (4.4) | 2 (0.7) | .086 |
| Pretransplant IVIG | 1 (2.2) | 2 (0.7) | .348 |
| Induction therapy | |||
| No | 15 (33.4) | 107 (36.3) | .052 |
| ATG | 6 (13.3) | 13 (4.4) | |
| Anti-IL-2 receptor antagonist | 24 (53.3) | 175 (59.3) | |
| Post-transplant variables | |||
| Maintenance therapy | |||
| Prednisolone | 45 (100) | 295 (100) | >.999 |
| Tacrolimus | 29 (64.4) | 230 (78) | .047 |
| Cyclosporin A | 16 (35.6) | 64 (21.7) | .041 |
| Mycophenolate mofetil | 38 (84.4) | 243 (82.4) | .732 |
| Mycophenolate sodium | 7 (15.6) | 50 (16.9) | .816 |
| Reoperation | 0 (0) | 11 (3.7) | .371 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ATG, antithymocyte globulin; AU, arbitrary unit; BMI, body mass index; CMV, cytomegalovirus; DFPP, double-filtration plasmapheresis; HLA, human leukocyte antigen; IgG, immunoglobulin G; IL, interleukin; IVIG, intravenous immunoglobulin; KT, kidney transplant; PRA, panel reactive antibody.
CMV Infection
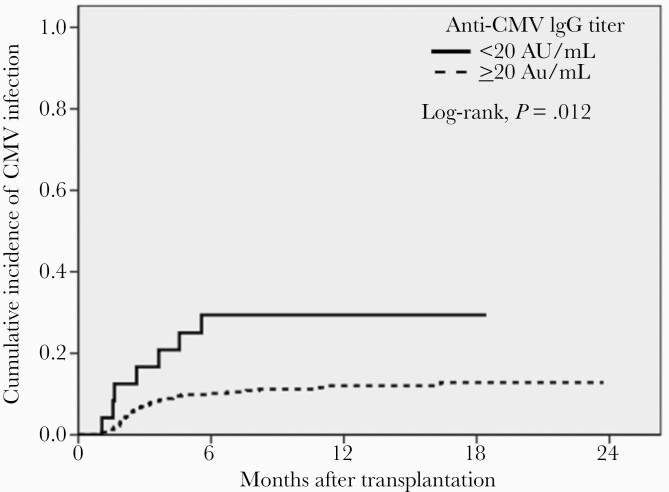
During a mean follow-up of 14 months, the cumulative incidence of CMV infection was 14.8%. Of the KT recipients with post-transplant CMV, 31 (69%) had asymptomatic CMV infection and 14 (31%) had tissue-invasive disease, including gastrointestinal disease (15.5%) and pneumonia (15.5%). The time to CMV infection stratified by CHI up to 1 year after transplant is presented in Figure 3 by a Kaplan-Meier curve.
Figure 3.
Kaplan-Meier plot for cumulative incidence of CMV infection after kidney transplantation. Abbreviations: AU, arbitrary units; CMV, cytomegalovirus; IgG, immunoglobulin G.
Risk Factors of CMV Infection
The variables potentially related to CMV infection are described in Table 2. In our univariate analysis, a low pretransplant CHI was significantly associated with post-transplant CMV infection (HR, 2.70; 95% CI, 1.21–6.05; P = .02). Other significant risk factors of post-transplant CMV infection included older donor age per 1-year increase, (HR, 1.03; 95% CI, 1.01–1.06; P = .008), deceased donor (HR, 5.17; 95% CI, 1.85–14.45; P = .002), prolonged cold ischemic time per 1-hour increase (HR, 1.07; 95% CI, 1.03–1.12; P = .001), pretransplant double filtration plasmapheresis (DFPP; HR, 5.30; 95% CI, 1.28–21.91; P = .021), antithymocyte globulin (ATG) induction therapy (HR, 3.08; 95% CI, 1.20–7.95; P = .020), and cyclosporin A maintenance therapy (HR, 1.84; 95% CI, 1.00–3.40; P = .049).
Table 2.
Univariate and Multivariate Analysis Cox Proportional Hazard Models for Risk Factors of Post-transplant CMV Infection
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Risk Factors | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Recipient age (per year) | 1.00 (0.98–1.03) | .798 | ||
| Male | 0.85 (0.47–1.53) | .580 | ||
| BMI (per unit), kg/m2 | 1.03 (0.96–1.10) | .401 | ||
| Low pretransplant CMV-specific humoral immunity (anti-CMV IgG titer <20 AU/mL) | 2.70 (1.21–6.05) | .016 | 2.98 (1.31–6.77) | .009 |
| Donor age (per year) | 1.03 (1.01–1.06) | .008 | 1.03 (1.01–1.06) | .005 |
| Deceased donor | 5.17 (1.85–14.45) | .002 | ||
| Cold ischemic time (per hour) | 1.07 (1.03–1.12) | .001 | 1.06 (1.02–1.10) | .002 |
| Surgical time (per hour) | 1.14 (0.97–1.33) | .104 | ||
| Second KT | 0.05 (<0.001–1734) | .572 | ||
| HLA mismatch of ≥3 | 0.92 (0.50–1.70) | .800 | ||
| PRA of ≥51% | 1.40 (0.55–3.54) | .482 | ||
| Pretransplant DFPP | 5.30 (1.28–21.91) | .021 | ||
| Pretransplant IVIG | 3.48 (0.48–25.27) | .218 | ||
| Induction therapy | ||||
| ATG | 3.08 (1.20–7.95) | .020 | 2.90 (1.09–7.74) | .033 |
| Anti-IL-2 receptor antagonist | 0.99 (0.52–1.88) | .970 | ||
| Maintenance therapy | ||||
| Tacrolimus | 0.55 (0.30–1.02) | .056 | ||
| Cyclosporin A | 1.84 (1.00–3.40) | .049 | ||
| Mycophenolate mofetil | 1.45 (0.51–2.56) | .742 | ||
| Reoperation | 0.47 (<0.001–65.39) | .408 | ||
Abbreviations: ATG, antithymocyte globulin; AU, arbitrary unit; BMI, body mass index; CMV, cytomegalovirus; DFPP, double-filtration plasmapheresis; HLA, human leukocyte antigen; IgG, immunoglobulin G; IL, interleukin; IVIG, intravenous immunoglobulin; KT, kidney transplant; PRA, panel reactive antibody.
In multivariate analysis, a pretransplant CHI remained significantly associated with post-transplant CMV infection (HR, 2.98; 95% CI, 1.31–6.77; P = .009). Other significant risk factors of post-transplant CMV infection included older donor age per 1-year increase (HR, 1.03; 95% CI, 1.0–1.06; P = .005), ATG induction therapy (HR, 2.90; 95% CI, 1.09–7.74; P = .033), and prolonged cold ischemic time per 1-hour increase (HR, 1.06; 95% CI, 1.02–1.10; P = .002).
OUTCOME
The outcomes of KT recipients with and without CMV infection were compared (Table 3). All the patients without a post-transplant CMV infection survived. The numbers of patients with graft loss were 6 (13.3%) and 5 (1.7%) in the CMV infection and non-CMV infection groups, respectively (P = .001).
Table 3.
Outcome of Kidney Transplant Recipients With and Without Post-transplant CMV Infection
| Outcome | CMV Infection (n = 45), No. (%) | Non-CMV Infection (n = 295), No. (%) | P Value |
|---|---|---|---|
| Mortality | 3 (6.7) | 0 (0) | .002 |
| Graft failure | 6 (13.3) | 5 (1.7) | .001 |
Abbreviation: CMV, cytomegalovirus.
DISCUSSION
Here, we report the first study investigating a potential role for quantitative measurement of CHI as a predictor of post-transplant CMV infection in CMV-seropositive KT recipients. We observed that a lower pretransplant anti-CMV IgG titer is associated with an increased risk of post-transplant CMV infection among CMV-seropositive KT recipients. This association remained significant after adjustments for other variables. We further identified other independent risk factors for post-transplant CMV infection, such as older donor age, prolonged cold ischemic time, and use of ATG for induction therapy.
Global nonspecific and CMV-specific immunity is essential in controlling viral replication. Lack of either global innate or CMV-specific adaptive immunity has been described as a poor prognostic factor for CMV reactivation after SOT [14]. The restoration of viral-specific cell-mediated immunity is associated with viral clearance, and, conversely, the failure of this immunity is associated with uncontrolled infection by viruses such as adenovirus, BK polyomavirus, and CMV [8, 15, 16]. However, the measurement of viral-specific cell-mediated immunity is not universally available, and its accessibility is low compared with the measurement of anti-CMV IgG titer. Previous studies have indicated that measuring the anti-CMV IgG titer has promise as a predictive tool for post-transplant CMV infection in liver and heart transplant recipients [10, 11, 17]. This universally available and relatively affordable test could be used as a simple tool to better classify those at risk of infection among CMV-seropositive SOT recipients. We confirmed this association in CMV-seropositive KT recipients. It is hypothesized that the low IgG titer may reflect weaker pretransplant immunity, which could then be aggravated by pharmacologic immunosuppression, thereby leading to a higher post-transplant CMV risk. A lower pretransplant non-CMV (BK) virus IgG titer is also affirmed to be associated with early BK viremia in pediatric KT recipients, especially in those paired with high BK virus IgG titer in donors [18]. Additionally, a pretransplant BK virus antibody level was significantly higher in KT recipients who did not develop BK viremia than those who developed BK viremia [19].
In addition to extending the association of CMV infection and antibody titers in KT recipients, our study also confirmed several identified risk factors of post-transplant CMV infection among CMV-seropositive KT recipients. Older donor age, prolonged cold ischemic time, and use of ATG for induction therapy were also described as independent risk factors in 2 retrospective studies conducted in transplant centers with a high prevalence of CMV seropositivity [4, 5].
Due to the nature of retrospective studies, some data may be affected by recall bias. Furthermore, the lack of a standardized protocol for preemptive monitoring of CMV in our center may have underestimated the true prevalence of CMV infection, especially in patients without symptoms. Additionally, there is a lack of standardization among semiquantitative and quantitative CMV serologic assays. This precludes accurate direct comparison because of inter- and sometimes intralaboratory test variations in cutoffs. While our study was conducted using a single CMV serologic assay with a single cutoff value, which offered a standardized assessment in our study, we suggest caution in comparing studies using different assays. We also encourage further studies using more standardized serologic tests to better generalize this potential predictor in clinical practice.
In summary, a low level of pretransplant CHI is independently associated with post-transplant CMV infection in CMV-seropositive KT recipients. The universally available test for anti-CMV IgG titer could potentially stratify individuals at risk and target them to receive a more specific preventive strategy.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The patient’s written consent was obtained. The study protocol was reviewed and approved by the Institutional Review Board of the Faculty of Medicine at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (approval number: ID 06-61-34).
Author contributions. Conceptualization: Similan Kirisri, Raymund R. Razonable, Jackrapong Bruminhent. Data collation: Similan Kirisri, Apirom Vongsakulyanon, Jackrapong Bruminhent. Data analysis: Similan Kirisri, Jackrapong Bruminhent. Manuscript writing (original draft): Similan Kirisri, Jackrapong Bruminhent. Manuscript reviewing and editing: Similan Kirisri, Apirom Vongsakulyanon, Surasak Kantachuvesiri, Raymund R. Razonable, Jackrapong Bruminhent.
References
- 1. Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: a national kidney foundation/kidney disease outcomes quality initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol 2008; 3:471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American Society of Transplantation Infectious Diseases community of practice. Clin Transplant 2019; 33:e13512. [DOI] [PubMed] [Google Scholar]
- 3. Bruminhent J, Razonable RR. Advances in drug therapies for cytomegalovirus in transplantation: a focus on maribavir and letermovir. Exp Opin Orphan Drugs 2020; 8:393–401. [Google Scholar]
- 4. Watcharananan SP, Louhapanswat S, Chantratita W, et al. Cytomegalovirus viremia after kidney transplantation in Thailand: predictors of symptomatic infection and outcome. Transplant Proc 2012; 44:701–5. [DOI] [PubMed] [Google Scholar]
- 5. Chiasakul T, Townamchai N, Jutivorakool K, et al. Risk factors of cytomegalovirus disease in kidney transplant recipients: a single-center study in Thailand. Transplant Proc 2015; 47:2460–4. [DOI] [PubMed] [Google Scholar]
- 6. Bruminhent J, Dajsakdipon T, Ingsathit A, et al. Impact of cytomegalovirus serostatus on allograft loss and mortality within the first year after kidney transplantation: an analysis of the national transplant registry. Transplant Proc 2020; 52:829–35. [DOI] [PubMed] [Google Scholar]
- 7. Meesing A, Razonable RR. Absolute lymphocyte count thresholds: a simple, readily available tool to predict the risk of cytomegalovirus infection after transplantation. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meesing A, Abraham RS, Razonable RR. Clinical correlation of cytomegalovirus infection with CMV-specific CD8+ T-cell immune competence score and lymphocyte subsets in solid organ transplant recipients. Transplantation 2019; 103:832–8. [DOI] [PubMed] [Google Scholar]
- 9. Bruminhent J, Worawichawong S, Tongsook C, et al. Epidemiology and outcomes of early-onset and late-onset adenovirus infections in kidney transplant recipients. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruminhent J, Thongprayoon C, Dierkhising RA, et al. Risk factors for cytomegalovirus reactivation after liver transplantation: can pre-transplant cytomegalovirus antibody titers predict outcome? Liver Transpl 2015; 21:539–46. [DOI] [PubMed] [Google Scholar]
- 11. Gupta E, Pamecha V, Verma Y, et al. Pre-transplant cytomegalovirus immunoglobulin G antibody levels could prevent severe cytomegalovirus infections post-transplant in liver transplant recipients: experience from a tertiary care liver centre. Indian J Med Microbiol 2017; 35:499–503. [DOI] [PubMed] [Google Scholar]
- 12. Torre-Cisneros J, Aguado JM, Caston JJ, et al. ; Spanish Society of Transplantation (SET); Group for Study of Infection in Transplantation of the Spanish Society of Infectious Diseases and Clinical Microbiology (GESITRA-SEIMC); Spanish Network for Research in Infectious Diseases (REIPI) . Management of cytomegalovirus infection in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant Rev (Orlando) 2016; 30: 119–43. [DOI] [PubMed] [Google Scholar]
- 13. Carbone J, Lanio N, Gallego A, et al. Simultaneous monitoring of cytomegalovirus-specific antibody and T-cell levels in seropositive heart transplant recipients. J Clin Immunol 2012; 32:809–19. [DOI] [PubMed] [Google Scholar]
- 14. Carbone J. The immunology of posttransplant CMV infection: potential effect of CMV immunoglobulins on distinct components of the immune response to CMV. Transplantation 2016; 100(Suppl 3):S11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruminhent J, Apiwattanakul N, Hongeng S, et al. Absolute lymphocyte count and human adenovirus-specific T-cell immune restoration of human adenovirus infection after kidney transplantation. J Med Virol 2019; 91:1432–9. [DOI] [PubMed] [Google Scholar]
- 16. Bruminhent J, Srisala S, Klinmalai C, et al. BK polyomavirus-specific T cell immune responses in kidney transplant recipients diagnosed with BK polyomavirus-associated nephropathy. BMC Infect Dis 2019; 19:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarmiento E, Lanio N, Gallego A, et al. Immune monitoring of anti cytomegalovirus antibodies and risk of cytomegalovirus disease in heart transplantation. Int Immunopharmacol 2009; 9:649–52. [DOI] [PubMed] [Google Scholar]
- 18. Ali AM, Gibson IW, Birk P, Blydt-Hansen TD. Pretransplant serologic testing to identify the risk of polyoma BK viremia in pediatric kidney transplant recipients. Pediatr Transplant 2011; 15:827–34. [DOI] [PubMed] [Google Scholar]
- 19. Bohl DL, Brennan DC, Ryschkewitsch C, et al. BK virus antibody titers and intensity of infections after renal transplantation. J Clin Virol 2008; 43:184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]