Abstract
Background
Mycobacterium species, specifically M. abscessus and M. chelonae (MABs), are known to contaminate water systems and are uncommon causes of health care–associated infection, but morbidity can be significant and treatment complex.
Methods
Odontogenic MAB infections occurred in patients following pulpotomy procedures at dental clinic A from 1 January to 6 September 2016. We identified confirmed and probable cases using culture data, imaging, pathology results, and surgical findings. Epidemiologic and clinical data including demographics, symptoms, laboratory findings, treatment regimens, and outcomes were extracted.
Results
Of 1082 at-risk patients, 71 case patients (22 confirmed; 49 probable) were identified. Median age was 6 years. Median symptom onset was 85 days postpulpotomy. Pain and/or swelling on admission occurred in 79%. On imaging, 49 of 70 had abnormalities of the mandible or maxilla, 13 of 70 had lymphadenopathy, and 19 of 68 had pulmonary nodules. Seventy were hospitalized (average of 8.5 days). Intravenous antibiotics were administered to 32 cases for a median length of 137 days. Clofazimine was administered to 29 patients as part of their multidrug regimen. Antibiotic treatment was associated with many adverse effects. Treated children showed evidence of jaw healing with resolved/improving pulmonary nodules at 1-year follow-up.
Conclusions
This is the largest outbreak of invasive MAB infections associated with a pediatric dental practice. While infections were indolent, patients suffered medical and surgical consequences of treatment, including permanent tooth loss. Identification of this outbreak led to a change in water standards for pediatric dental procedures in California. Enhanced national dental water quality standards are needed to prevent future outbreaks.
Keywords: Mycobacterium abscessus, nontuberculous mycobacteria, odontogenic infections, pediatric infections, pulpotomy
We describe 71 pediatric cases of invasive M. abscessus infections associated with a dental practice. While infections were indolent, patients suffered medical and surgical consequences. This led to a change in water standards for pediatric dental procedures in California.
Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and regularly inhabit human drinking water distribution systems [1]. They are uncommon causes of health care–associated infection, but their morbidity can be significant and their treatment complex. Mycobacterium abscessus (MAB) is a rapid-growing, multidrug-resistant NTM species that is resistant to many disinfectants and has been recognized to cause postprocedural infections [2]. Previous MAB outbreaks have been reported from acupuncture clinics [3, 4] and following procedures such as liposuction [5, 6], laparoscopy [7], and brain biopsy [8]. MAB odontogenic infections have been identified previously in both adults and children following dental procedures [9, 10]. In Venezuela a 2020 report described 3 adults with MAB infections following recent dental procedures performed at multiple facilities in Caracas [9], and a 2017 report from Georgia, United States described 24 children with MAB-associated odontogenic infections following pulpotomy procedures performed at a single dental facility in Atlanta [10]. Both reports identified that water used for dental procedures was drawn from improperly maintained clinic water systems and was the likely source of each outbreak.
Pulpotomy procedures in children are similar to root canals in adults, but unlike a root canal procedure, a pulpotomy is not considered a surgical procedure and does not require the use of sterile water [11–13]. Thus, untreated municipal water is often used for drilling and irrigation during pulpotomy procedures. Here we describe an investigation and clinical report of children affected by the largest outbreak yet described of MAB odontogenic infections related to a specific pediatric dental practice in Orange County, California.
Between July and September 2016, 3 patients were admitted to the same children’s hospital in Orange County, California, with atypical oral maxillofacial infections. All patients had some combination of facial cellulitis, dental abscess, and/or cervical adenitis that had been present for weeks. The infections were unresponsive to drainage and multiple courses of standard antibiotic therapy. Mycobacterium abscessus subspecies abscessus with inducible macrolide resistance was isolated from a fine needle aspiration of an affected lymph node in 1 patient. Pediatric infectious disease physicians reviewed the patient histories and noted that all 3 patients had received pulpotomy procedures from dental clinic A. The Orange County Health Care Agency (OCHCA) was contacted to investigate this common source and an epidemiologic investigation was initiated in September 2016. Public health response was directed by OCHCA and conducted in collaboration with community pediatric infectious disease physicians and the California Department of Public Health.
METHODS
Setting and Epidemiologic Investigation
Dental clinic A is a pediatric dental clinic located in Southern California, serving primarily families of low socioeconomic status, seeing between 50 and 70 patients per day and performing approximately 30 pulpotomies per week. OCHCA conducted an on-site inspection of the clinic and reviewed infection control practices. Water samples and environmental swabs were taken from multiple sites along the dental clinic’s patient-care water system. Dental unit water lines, source taps, and treatment systems were all tested for bacterial heterotrophic plate counts (HPC) and cultured for mycobacteria.
Upon review of patient records, the clinic reported that 13 additional patients had developed illness that was similar to the 3 previously identified patients. All patients had developed indolent, progressive dental infections following pulpotomies. These infections had not responded to treatment with antibiotics (amoxicillin, amoxicillin-clavulanic acid, and/or clindamycin) or to incision and drainage procedures. Dental clinic A suspended pulpotomy procedures on 6 September 2016 per OCHCA recommendation until a source of infection could be identified and patient safety could be ensured.
Multiple advisories were sent to community providers, including dentists, oral surgeons, pediatricians, infectious disease physicians, and hospital infection preventionists describing the outbreak. All patients who received a pulpotomy at Dental Clinic A from 1 January to 6 September 2016 were referred for monthly dental exams for the first 6 months after pulpotomy, with follow-up bimonthly exams for the next 6 months. As most patients came to medical attention in the summer of 2016, 1 January 2016 was chosen to be inclusive of a potentially long incubation period. Patients with concern for infection were referred either to a local oral surgeon or to local hospitals where a protocol was put in place to obtain a face, neck, and chest computed tomography (CT) scan. Local providers were instructed to report any suspected case patients to OCHCA for follow-up.
Definitions
A case definition was established to systematically identify suspected or probable case patients. All case patients received a pulpotomy procedure at dental clinic A from 1 January to 6 September 2016. Probable cases presented with 1 or more of the following: multiple (3 or more) pulmonary nodules on chest CT scan, pathology-confirmed granulomatous changes or evidence of chronic osteomyelitis, operative report describing necrotic bone and/or osteomyelitis, and/or a laboratory-confirmed positive acid-fast bacillus (AFB) smear of infected tissue. Confirmed cases had MABs isolated by culture.
All suspected cases had a medical record review to assign case status, which was conducted by a committee consisting of a public health epidemiologist, the medical director of epidemiology at OCHCA, and a pediatric infectious disease physician from a local children’s hospital. Data were extracted from medical records, including clinical and surgical notes, radiographic and pathologic reports, microbiologic results, antibiotic susceptibility results, and treatment regimens and outcomes.
Patient Consent
The Children’s Hospital of Orange County (CHOC) obtained the following institutional review board (IRB) approvals and patient consents:
1.“Clofazimine for the Treatment of Nontuberculosis Mycobacteria (single patient investigational new drug [IND])” was an IRB-approved project to allow for multiple patients to receive access to emergency use clofazimine due to the active outbreak. Individual emergency INDs were sought through the US Food and Drug Administration (FDA) for each patient. All patients were consented for use of clofazimine on an IRB-approved informed consent. This project was initially approved by the CHOC Industry-Track IRB on 23 September 2016.
2.“Outbreak of M. abscessus Infections Related to a Dental Clinic-Reviewing Diagnosis, Treatment and Outcome” was IRB approved under 45 CFR 46.404, research/clinical investigational not involving greater than minimal risk. This was a retrospective medical record review on patients due to the dental abscess outbreak. Protected health information was not collected or shared on these patients, so per IRB approval/risk category, no informed consent was required. This project was initially approved by the CHOC In-House IRB on 24 April 2017.
For cases not cared for at CHOC, the OCHCA collected necessary data to assess the scope and disease burden of the outbreak in order to guide an appropriate public health response. No patient consent was required for data collected in relation to public health outbreak response actions.
RESULTS
Epidemiologic and Diagnostic Investigation
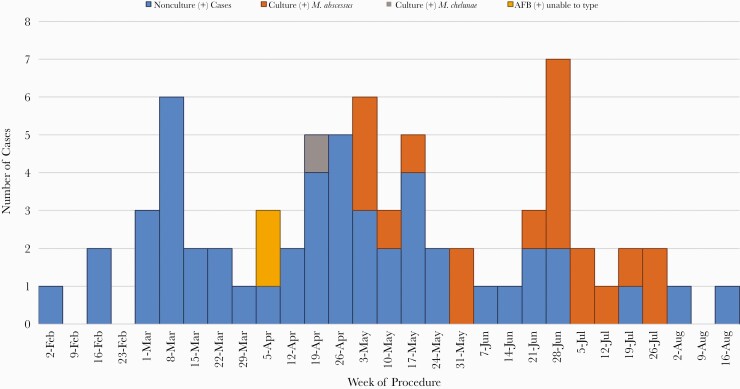
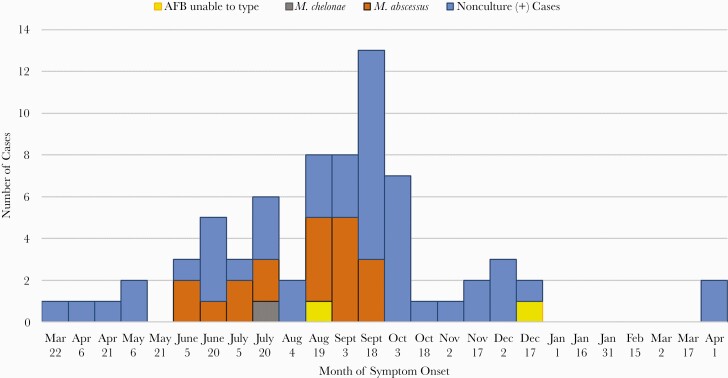
Dental clinic A performed pulpotomy procedures on 1082 patients between 1 January and 6 September 2016. OCHCA was able to contact the families of 1072 patients (99%), and 1029 (95%) were documented to have had at least 1 follow-up clinic visit for assessment. A single local oral surgeon’s outpatient office conducted additional evaluation for 221 patients who were suspected to have infection on initial assessment, and local hospitals admitted an additional 124 patients for evaluation. A total of 71 case patients (22 confirmed and 49 probable) were identified as part of this outbreak (Figure 1 and Figure 2), yielding an attack rate of 6.6%.
Figure 1.
Epidemic curve for dental–clinic associated nontuberculous mycobacterial infections according to month of pulpotomy procedure, February–August 2016. Abbreviation: AFB, acid-fast bacilli.
Figure 2.
Epidemic curve for dental clinic–associated nontuberculous mycobacterial infections according to date of symptom onset, March 2016–April 2017. Abbreviation: AFB, acid-fast bacilli.
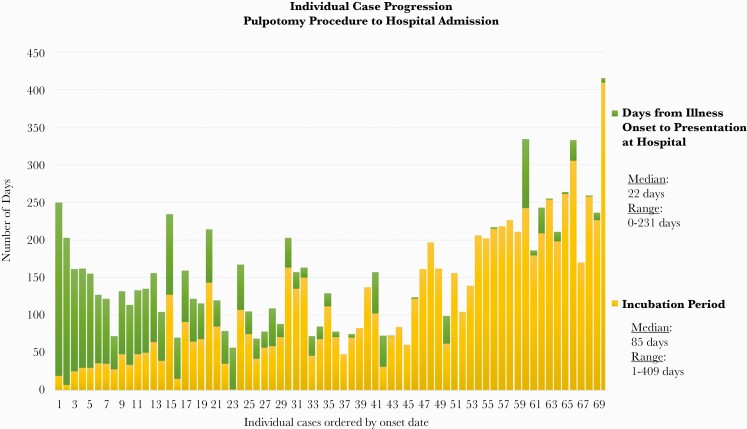
The median case patient age was 6 years (interquartile range [IQR], 4–7.5 years), and 51% of case patients were female (Table 1). Symptoms began a median of 85 days (IQR, 48–174.5 days) after pulpotomy (Figure 3). Seventy of 71 case patients were hospitalized, with 65 (92%) managed at 1 local children’s hospital. The single nonhospitalized case patient was identified through a local oral surgeon’s outpatient office with biopsy-confirmed granulomatous inflammation on specimens collected through debridement of the mandible. In 79% of case patients, pain and/or swelling was reported on admission; however, symptoms were insidious and generally mild, and patients were not systemically ill. Imaging studies of the face were completed on 70 case patients and revealed 49 of 70 with abnormalities of the mandible or maxilla, where the majority (84%) of abnormalities were mandibular, and 13 of 70 cases had lymphadenopathy (Figure 4). None of the patients had respiratory symptoms, but CT scans of the chest revealed that 19 of 68 case patients had 3 or more pulmonary nodules. The vast majority of those with pulmonary nodules presented with innumerable nodules in a miliary pattern.
Table 1.
Demographic and Clinical Characteristics of All Confirmed or Probable Case Patients With Mycobacterium abscessus Infections
| Characteristic | No. (%)a |
|---|---|
| Age, y, median (IQR) | 6 (4–7.5) |
| Sex | |
| Male | 35 (49) |
| Female | 36 (51) |
| Diagnostic evaluation | |
| Hospitalized | 70 (99) |
| Outpatient oral surgeon’s office | 1 (1) |
| Face/neck CT | 70 (99) |
| Chest CT | 68 (96) |
| Chest radiograph only | 2 (3) |
| Signs and symptoms | |
| Swelling | 47 (66) |
| Pain | 45 (63) |
| Osteomyelitis on CT | 49/70 (69) |
| Pulmonary nodules | 19/70 (27) |
| Lymphadenopathy | 13 (18) |
| Fever | 3 (4) |
| Treatment | |
| Surgery | 71 (100) |
| Extractions | 71 (100) |
| Hospital | 67 (94) |
| Outpatient oral surgeon’s office | 11 (15) |
| Dental clinic A | 18 (25) |
| Debridement | 71 (100) |
| IV antibiotics by PICC | 32 (45) |
| Clofazimine | 29/32 (91) |
| Laboratory results | |
| Granulomatous on pathology | 29 (41) |
| AFB stain positive | 12 (17) |
| Chronic osteomyelitis on pathology | 33 (46) |
| Culture-positive AFB, not further identified | 2 (3) |
| Culture-positive Mycobacterium abscessus | 19 (27) |
| Culture-positive Mycobacterium chelonae | 1 (1) |
| Incubation, d, mean (IQR) | 85 (48–174.5) |
| Asymptomatic at hospital admission, No.; median (range) | 13; 197 (84–261) |
| Length of hospitalization, d, average (SD) | 8.5 (11.5) |
| No. of pulpotomies, mean (range) | 3 (1–11) |
| Abx therapy length, median, d (IQR) | 137 (122.8–162.3) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: Abx, antibiotic; AFB, acid-fast bacilli; CT, computed tomography; IQR, interquartile range; IV, intravenous; PICC, peripherally inserted central catheter; SD, standard deviation.
aDenominator is 71 unless otherwise specified.
Figure 3.
Individual case progression of dental clinic–associated nontuberculous mycobacterial infections. Time from procedure to illness onset to presentation at an acute care hospital.
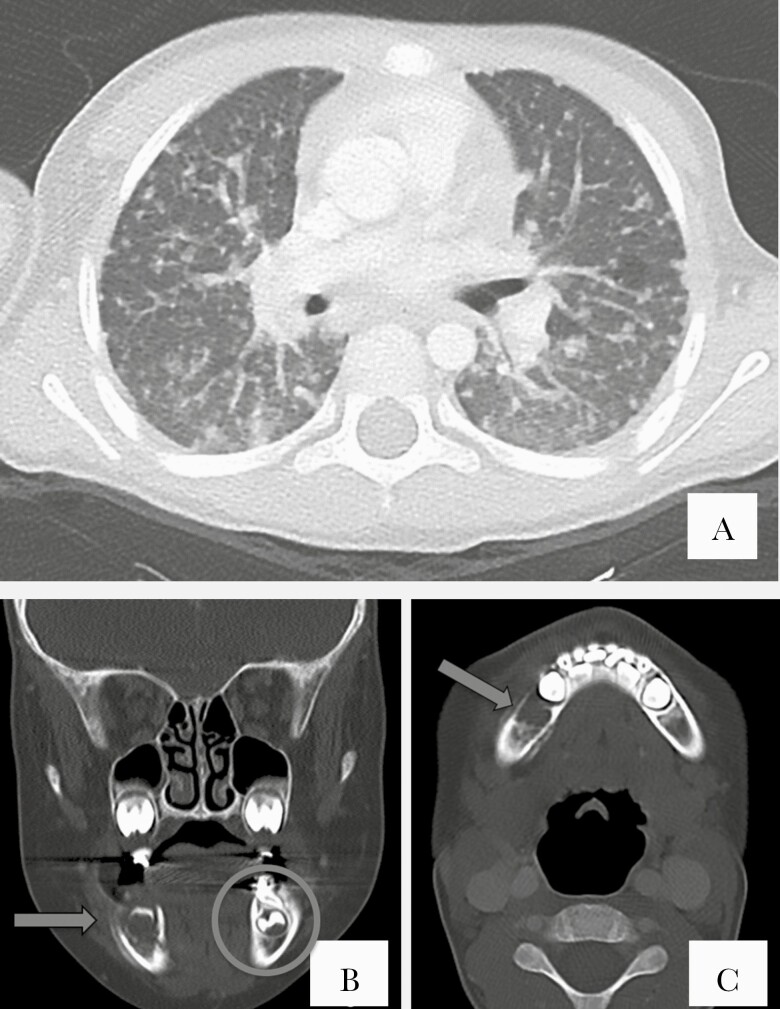
Figure 4.
A, Axial chest computed tomography (CT) lung window image at the subcarinal location shows both a tree-in-bud pattern and random nodular pattern. Some of the nodules are calcified. B, Coronal face CT bone window image at the maxillary sinus location. Periapical lucency reflects periapical tooth abscess (arrow) compared to the normal contralateral left tooth (circle). C, Axial face CT bone window image at the mandibular symphysis location. There is cancellous and cortical bony destruction of the right mandibular body with buccal and lingual periosteal reaction consistent with osteomyelitis (arrow).
Treatment
The 70 hospitalized case patients were admitted for an average of 8.5 inpatient days (standard deviation, 11.5 days) and 23 patients had >1 hospitalization. All case patients required surgical intervention and 26 required >1 inpatient surgery. Surgical procedures included mandible/maxilla debridement, cervical lymph node dissection and excision, neck dissection, and tooth extraction. Permanent teeth were lost in 45 of 65 case patients (range, 1–6 teeth lost). Pathology results showed 29 case patients with granulomatous changes, 33 case patients with chronic osteomyelitis, and 12 case patients with infected tissue that was AFB stain positive. Tissue specimens from 22 patients had M. abscessus (19), M. chelonae (1), or AFB organisms unable to be identified from culture (2). Pulsed-field gel electrophoresis (PFGE) patterns of all M. abscessus isolates were indistinguishable, suggesting a common source.
Due to incomplete surgical excisions or the presence of pulmonary nodules, intravenous antibiotics were administered to 32 case patients for a median length of 137 days (IQR, 122.8–162.3 days). Clofazimine was obtained from the FDA through individual IND applications for 29 of 32 case patients as part of their regimen due to the presence of inducible macrolide resistance found on testing of the original patient isolate. At CHOC, 27 case patients were treated with antibiotic therapy comprised of intravenous (IV) amikacin (13–32 mg/kg/dose 3 times weekly with the dose adjusted by therapeutic drug monitoring) and imipenem (100 mg/kg/day divided every 8 hours) or cefoxitin (150 mg/kg/day divided every 8 hours) plus oral azithromycin (10 mg/kg/day every 24 hours) and oral clofazimine (7 mg/kg/week). Because clofazimine is not approved for this purpose, an IND application was submitted to and approved by the FDA for each child who received the drug. Clofazimine dosing was assigned by the FDA at 1 mg/kg per day. Clofazimine is manufactured into a soft gelatin capsule that contains 50 mg of micronized clofazimine suspended in an oil–wax base, and the capsules must be swallowed whole; patients were given the dose once daily 2–6 days/week depending on their weight. Amikacin dose was reduced (10–19 mg/kg/dose 3 times a week) after approximately 1 month of clofazimine treatment based on reported synergistic activity of the 2 agents in combination. Amikacin levels were obtained at 2 and 6 hours postdose to target peaks of approximately 60 mg/L initially and 30 mg/L after dose reduction. Two case patients also received a course (10–14 days) of tigecycline (1 mg/kg/dose every 12 hours).
Treatment-Related Adverse Events
Of the 27 case patients who received IV antibiotic therapy at CHOC, 13 (48%) experienced peripherally inserted central catheter malfunction, and 8 (29.6%) required line replacement. Two patients developed severe urticarial rash to β-lactams (1 imipenem, 1 cefoxitin) requiring desensitization and omalizumab prior to continuing their treatment course. Twelve (44.4%) patients experienced neutropenia (<1000 cells/µL) while on antibiotics with 3 (11.1%) experiencing severe neutropenia (ANC <500 cells/µL). The median time to onset and resolution of neutropenia was 33 days (IQR, 23–50 days) and 10 days (IQR, 7–24 days), respectively. Of the 26 of 27 case patients who received imipenem treatment, 11 (42.3%) developed yellowing of teeth that was reversible once the drug was stopped. The median time to onset and resolution of teeth yellowing was 59 and 21 days, respectively. Mildly elevated liver enzyme (<100 U/L, grade 1) and serum creatinine levels (50% from baseline, Kidney Disease—Improving Global Outcomes [KDIGO] stage 1) were observed in 3 (11.1%) and 7 (25.9%) patients, respectively; none required a modification in treatment regimen. The increase in serum creatinine was transient and levels returned to baseline at end of treatment in all 7 patients. Six patients (22%) experienced increased skin pigmentation during treatment with clofazimine and all resolved after completion of therapy. Gastrointestinal symptoms including abdominal pain, nausea/vomiting, and diarrhea occurred in 12 (44%) children; none required a change in treatment course. Three (11%) children experienced mild to moderate hearing loss, which resolved at end of treatment without change of regimen. All children were monitored at outpatient visits every 2 weeks while on treatment. All treated case patients showed evidence of jaw healing with resolved or improving lung nodules at 1-year follow up.
Environmental Investigation
The public health investigation of dental clinic A revealed that the facility used municipal water that was stored in a pressurized bladder holding tank. Water was drawn from this tank to fill 750-mL bottles, which were attached to water line systems at each clinic chair and fed water through plastic tubing into the dental instruments. Disinfecting treatments were not applied to the water system and water quality testing was not performed regularly.
Public health staff collected water samples from the facility’s water system to test for HPC and culture for mycobacteria. Mycobacterial culture testing was also performed on 43 environmental swabs taken from all 6 treatment rooms used for pulpotomies. HPC counts were performed for water samples taken from clinical syringes from all 6 treatment rooms; all were greater than the 500 colony-forming units (CFU)/mL cutoff recommended by Centers for Disease Control and Prevention (CDC) guidelines for water used for nonsurgical dental procedures, ranging from 610 to 16 000 CFU/mL. Syringe water samples identified MAB growth of species including M. abscessus, M. chelonae, and M. franklinii in 5 of the 6 treatment rooms. HPC counts from tap water obtained at multiple sites in the clinic ranged from 71 to 74 CFU/mL, much lower than the counts found inside the clinic, suggesting bacterial contamination inside the clinic’s own water system such as the tubing, water bottles, etc. Patient and environmental isolates were forwarded to CDC for PFGE. Testing of patient isolates identified 4 different PFGE clusters of M. abscessus. The patient PFGE clusters did not match any environmental samples.
Dental clinic A was mandated by health order to cease the use of the facility’s water system for patient care until the system had been replaced and appropriate water quality safeguards put in place. The public health investigation yielded no potential sources aside from the clinic’s water supply. Since reopening, no additional suspect or confirmed cases have been identified.
DISCUSSION
In 2016, our community experienced the largest outbreak described of MAB odontogenic infections from a single pediatric dental clinic. Proper maintenance of dental facility water lines is critical to prevent overgrowth of microorganisms such as MAB. These organisms display tolerance to commonly used disinfectants and have been identified in the plumbing of health care facilities and dental office water distribution systems [14, 15]. Culturing of the water system yielded elevated heterotrophic plate counts and multiple mycobacterial species, which differed from clinical species identified. This dichotomy has been reported previously [16]. There are currently no standards for quantifying NTM load in water.
In children, pulpotomy procedures are considered nonsurgical; thus, current guidance does not require sterile water be used for drilling and irrigation. This is the second outbreak associated with performance of pulpotomies using nonsterile water. Both events occurred in the setting of grossly contaminated clinical water systems. An anecdotal survey of local providers revealed that many area dental clinics were not appropriately treating their water systems according to American Dental Association and CDC guidelines [17], including not regularly checking HPCs. MAB infections have been made reportable in several states. Whether or not they are, any individual mycobacterial postoperative infection should be considered a sentinel event that should be followed up with active case finding and an environmental investigation, particularly of the water supply. Following this outbreak and advocacy from the OCHCA, the California Conference of Local Health Officers, and the California Dental Association to the California State Senate, in September 2018 the Governor signed Senate Bill 1491 into law. Senate Bill 1491 specified as unprofessional conduct the use of water that is not sterile or that does not contain recognized disinfecting or antibacterial properties when performing dental procedures on exposed dental pulp.
This event highlighted the challenging nature of the clinical and public health responses to MAB outbreaks. Patients frequently experienced prolonged incubation periods and/or insidious illness onsets; both contributed to a delay in identification of the outbreak until a large number of patients had been exposed. Providers may not suspect or culture for MAB; when performed, culture is relatively insensitive. Even when MAB was already suspected in this outbreak, most cultures were negative. Of the 27 patients who received antibiotic therapy after a debridement procedure at CHOC, only 14 had positive cultures all with MAB, and all with the same susceptibility pattern. In addition, children with significant disease often had relatively mild symptoms. Therefore, aggressive local public health outreach was needed to assure long-term clinical follow-up for all exposed patients, which was labor-intensive but crucial. Due to prolonged incubation periods, maintaining follow-ups for 1 year after the procedure was also required.
Treatment of infected patients was made more complex by the relative paucity of national guidance for treatment of MAB infections in normal hosts. In this way our article adds to the experience published by Hatzenbueler et al on their dental outbreak in Atlanta [10], in that our patients did well despite evidence of disseminated pulmonary involvement and need for extensive jaw debridements [18]. In contrast, we were aware early on that the isolate with which our patients were infected had inducible macrolide resistance as well as intermediate susceptibility to most first-line agents including imipenem and cefoxitin. This led us to use oral clofazimine successfully and safely in these patients, the largest series of children to ever receive clofazimine outside of treatment for leprosy.
Clofazimine was chosen as earlier studies in patients with pulmonary MAB infection found that the addition of clofazimine to the antimicrobial regimen may improve treatment outcomes [19, 20]. Unfortunately, clofazimine was difficult to obtain when needed. We had to apply for and receive an investigational new drug through the FDA for each patient, which was time consuming. Other challenges included lack of clear dosing guidelines and an undefined toxicity profile in children [21].
In addition, the group in Atlanta reported a higher incidence of hearing loss in their subjects. Due to the slow-growing nature of mycobacteria, we used amikacin 3 times a week, aiming initially for a peak concentration (Cmax) of 60 mg/L. After clofazimine was introduced, taking advantage of laboratory-reported synergy between these 2 agents, we decreased the amikacin dose, aiming for a new target Cmax of 30 mg/L. While other researchers have described in vitro synergy between clofazimine and amikacin for MAB isolates [22], the clinical implications are less clear. Additional studies are needed to determine amikacin effectiveness at lower doses when synergy is present. Considering the risk of hearing loss associated with aminoglycosides and macrolides, we monitored for hearing loss every 2 weeks. No dose adjustment was necessary [21]. We hypothesize that our subjects’ lower amikacin exposure may have led to a lower incidence of hearing loss with no obvious sacrifice in efficacy.
One limitation to this case series is that patients may have been missed due to the dates chosen. However, these dates were chosen to account for a 6-month incubation period. Due to lack of guidance on treatment of MAB with inducible macrolide resistance, patients remained on azithromycin possibly unnecessarily. This is because macrolide therapy has been the mainstay of therapy for nontuberculous mycobacterial infections in the past, is relatively safe, and has anti-inflammatory properties. Future studies should address optimal treatment regimens.
In conclusion, children treated with pulpotomies are at risk for serious infections leading to prolonged treatments and resulting morbidities, including permanent tooth loss. We report the largest group of children treated safely and successfully with oral clofazimine combination therapy for MAB infection, for which there are no established guidelines. The authors believe the measure adopted in California for the use of sterile water for all pulpotomies is an appropriate standard which we would like to see embraced by the American Dental Association and state dental boards around the country.
Notes
Acknowledgments. We thank the whole team who worked very hard to evaluate and treat the affected children: Aaron Sassoon, MD, Wendi Gornick, Heather Huszti, PhD, Cathy Flores, and Raj Vyas, MD. We also thank CHOC’s hospitalists, clinical support staff, pathology department, infection prevention team, psychology team, social workers, microbiology laboratory, and dentists; and also thank Healthy Smiles, OC Supervisors, and Providence Speech and Hearing.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Falkinham JO 3rd. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol 2009; 107:356–67. [DOI] [PubMed] [Google Scholar]
- 2. Lee MR, Sheng WH, Hung CC, et al. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 2015; 21:1638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang P, Walsh S, Murray C, et al. Outbreak of acupuncture-associated cutaneous Mycobacterium abscessus infections. J Cutan Med Surg 2006; 10:166–9. [DOI] [PubMed] [Google Scholar]
- 4. Koh SJ, Song T, Kang YA, et al. An outbreak of skin and soft tissue infection caused by Mycobacterium abscessus following acupuncture. Clin Microbiol Infect 2010; 16:895–901. [DOI] [PubMed] [Google Scholar]
- 5. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among “lipotourists” from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis 2008; 46:1181–8. [DOI] [PubMed] [Google Scholar]
- 6. Engdahl R, Cohen L, Pouch S, Rohde C. Management of Mycobacterium abscessus post abdominoplasty. Aesthetic Plast Surg 2014; 38:1138–42. [DOI] [PubMed] [Google Scholar]
- 7. Baruque Villar G, de Mello Freitas FT, Pais Ramos J, et al. Risk factors for Mycobacterium abscessus subsp. bolletii infection after laparoscopic surgery during an outbreak in Brazil. Infect Control Hosp Epidemiol 2015; 36: 81–6. [DOI] [PubMed] [Google Scholar]
- 8. Martin JS, Zagzag D, Egan M, et al. Intracranial Mycobacterium abscessus infection in a healthy toddler. Pediatr Infect Dis J 2015; 34:223–4. [DOI] [PubMed] [Google Scholar]
- 9. Pérez-Alfonzo R, Poleo Brito LE, Vergara MS, et al. Odontogenic cutaneous sinus tracts due to infection with nontuberculous mycobacteria: a report of three cases. BMC Infect Dis 2020; 20:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hatzenbuehler LA, Tobin-D’Angelo M, Drenzek C, et al. Pediatric dental clinic-associated outbreak of Mycobacterium abscessus infection. J Pediatric Infect Dis Soc 2017; 6:e116–22. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Atlanta, GA: CDC; 2016. [Google Scholar]
- 12. American Academy of Pediatric Dental Care. Clinical practice guidelines: guideline on pulp therapy for primary and immature permanent teeth. 2016. https://www.aapd.org/research/oral-health-policies--recommendations/pulp-therapy-for-primary-and-immature-permanent-teeth/. Accessed 20 August 2019.
- 13. American Association of Endodontists. Endodontics colleagues for excellence: root canal irrigates and disinfectants.2016. http://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/rootcanalirrigantsdisinfectants.pdf. Accessed 06 November 2019.
- 14. Castellano Realpe OJ, Gutiérrez JC, Sierra DA, et al. Dental unit waterlines in Quito and Caracas contaminated with nontuberculous mycobacteria: a potential health risk in dental practice. Int J Environ Res Public Health 2020; 17:2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams MM, Chen TH, Keane T, et al. Point-of-use membrane filtration and hyperchlorination to prevent patient exposure to rapidly growing mycobacteria in the potable water supply of a skilled nursing facility. Infect Control Hosp Epidemiol 2011; 32:837–44. [DOI] [PubMed] [Google Scholar]
- 16. D’Antonio S, Rogliani P, Paone G, et al. An unusual outbreak of nontuberculous mycobacteria in hospital respiratory wards: association with nontuberculous mycobacterial colonization of hospital water supply network. Int J Mycobacteriol 2016; 5:244–7. [DOI] [PubMed] [Google Scholar]
- 17. American Dental Association. Oral health topics, dental unit waterlines.2019. Available at: https://www.ada.org/en/member-center/oral-health-topics/dental-unit-waterlines. Accessed 15 November 2020.
- 18. Mueller MA, Kanack MD, Singh J, et al. Pediatric mandible reconstruction for osteomyelitis during largest reported Mycobacterium abscessus outbreak. J Craniofac Surg 2020; 31:274–7. [DOI] [PubMed] [Google Scholar]
- 19. Yang B, Juhn BW, Moon SM, et al. Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 2017; 61:e2052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martiniano SL, Wagner BD, Levin A, et al. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest 2017; 152:800–9. [DOI] [PubMed] [Google Scholar]
- 21. Adler-Shohet FC, Singh J, Nieves D, et al. Safety and tolerability of clofazimine in a cohort of children with odontogenic Mycobacterium abscessus infection. J Pediatric Infect Dis Soc 2020; 9:483–5. [DOI] [PubMed] [Google Scholar]
- 22. van Ingen J, Totten SE, Helstrom NK, et al. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother 2012; 56:6324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]