Abstract
trans-Cyclooctenes and trans-cycloheptenes have long been the subject of physical organic study, but the broader application had been limited by synthetic accessibility. This account describes the development of a general, flow photochemical method for the preparative synthesis of trans-cycloalkene derivatives. Here, photoisom erization takes place in a closed-loop flow reactor where the reaction mixture is continuously cycled through Ag(I) on silica gel. Selective complexation of the trans-isomer by Ag(I) during flow drives an otherwise unfavorable isomeric ratio toward the trans-isomer. Analogous photoreactions under batch-conditions are low yielding, and flow chemistry is necessary in order to obtain trans-cycloalkenes in preparatively useful yields. The applications of the method to bioorthogonal chemistry and stereospecific transannulation chemistry are described.
Keywords: trans-cyclooctene, trans-cycloheptene, flow chemistry, photochemistry, bioorthogonal chemistry
The unusual reactivity and well-defined chiral structure of trans-cycloalkenes has made them attractive targets for synthesis for nearly 70 years.[1–3] For example, trans-cyclooctene possesses planar chirality and displays a high barrier to racemization (Ea = 35.6 kcal/mol),[4] and the most stable “crown” conformer has an alternating sequence of equatorial and axial hydrogens that is akin to chair cyclohexane.[5–6] The double bond of trans-cyclooctene is twisted severely in the crown conformation,[7] and as a consequence the HOMO of trans-cyclooctene is relatively high in energy.[8] trans-Cycloheptene also has a rigid structure with a distorted alkene.[7] The double bonds of medium-ring trans-alkenes are twisted.[7] trans-Cycloalkenes display unusual reactivity in HOMO-alkene controlled cycloaddition reactions with dienes,[9] 1,3-dipoles[8] and ketenes.[10] Strained trans-cycloalkenes also serve as ligands for transition metals.[11–16] This reactivity profile has made trans-cycloalkanes interesting targets for applications in synthesis and biology.
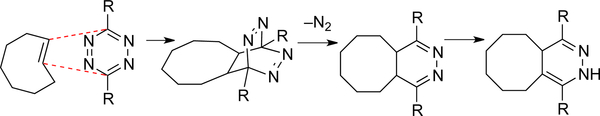
The broadest applications of trans-cycloalkenes are in the field of bioorthogonal chemistry. The inverse-electron demand Diels–Alder reaction between tetrazines and strained alkenes has a rich history in physical organic and synthetic chemistry (Figure 1).[17–19] In 2008, three groups described the bioorthogonal reactions of tetrazines with strained alkenes.[20–22] The variant introduced by our group that used trans-cyclooctene is marked by exceptionally rapid kinetics, with rate constants that can exceed 106 M–1s–1 in the fastest cases.[23–24] With the advances including the development of fluorogenic tetrazines[25] and reactions in live cells[26] and animals[27], the tetrazine ligation has become a widely used tool for applications that span chemical biology, biomedical imaging, and materials science.[28–37]
Figure 1.
Tetrazine ligation with trans-cycloalkenes has become
a broadly used tool for chemical biology, medicine and materials science.
This account describes the development of general, flow photochemical methods for the preparative synthesis of trans-cycloalkene derivatives and enabled applications in synthesis and bioorthogonal chemistry. Selective complexation of the trans-isomer by Ag(I) during flow drives an otherwise unfavorable isomeric ratio toward the trans-isomer. For the synthesis of trans-cycloalkenes in preparatively useful yields, flow chemistry is necessary as analogous photoreactions under batch-conditions are low yielding.
Synthesis of trans-Cyclooctenes and trans-cycloheptenes
While there are established routes to the parent trans-cycloalkenes, there were relatively few methods for preparing functionalized derivatives.[8, 16, 38–41] trans-Cyclooctene, which is the most studied, was first prepared in 1950 as a mixture with cis-cyclooctene via Hoffman elimination of trimethylcyclooctyl ammonium iodide.[1] In 1953, trans-cyclooctene was separated from cis-cyclooctene through formation of the water soluble trans-cyclooctene•AgNO3 complex, which was subsequently decomplexed by aq. NH4OH to provide the pure trans-alkene.[2] Several stereospecific methods for preparing trans-cyclooctene from cis-cyclooctene have also been described,[42–45] but a consideration for these protocols is that multistep synthesis is required to invert the alkene stereochemistry. First demonstrated by Hsung, trans-cycloalkenes can also be prepared by 4-π electrocyclic ring opening.[46–48] Recent progress has resulted in a number of new routes to heteroatom containing trans-cycloalkenes. Woerpel[49–57] and Tomooka[58–63] have described a number of methods for preparing oxasila-trans-cycloalkenes as well as the synthesis and chemistry of oxa, aza and sulfa (E,Z)-nonadienes.
Photochemical Syntheses of trans-Cycloalkenes
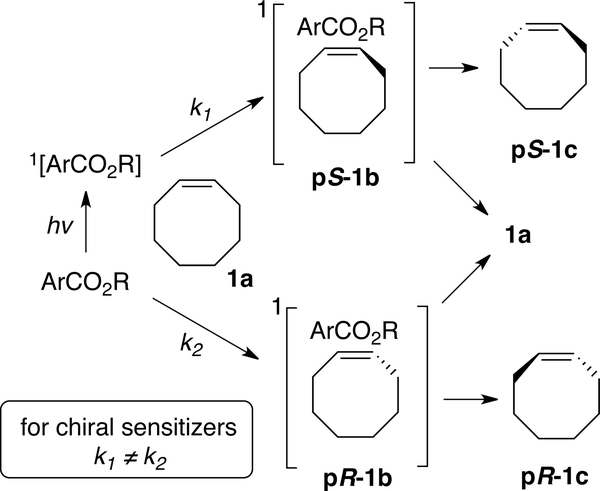
The singlet sensitized photoisomerization of cis-cyclooctene, pioneered by Inoue over 40 years ago,[64–76] is a direct method for the synthesis of trans-cycloalkenes from their cis-isomers. In 1978,[76] Inoue published the first in an series of papers on the enantioselective photoisomerization of cyclooctene, cycloheptene, and 1,3-cyclooctadiene to their trans-isomers.[64–75] Chiral aromatic esters function most efficiently for this process, the proposed mechanism of which is summarized in Fig 2.[74] Upon irradiation at 254 nm the aromatic ester forms a singlet excited state, which combines with cis-cyclooctene 1a to form diastereomeric exciplexes pS-1b and pR-1b. These “twisted singlet”[74] exciplexes subsequently partition into the corresponding trans-cyclooctene (pS-1c or pR-1c) and cis-cyclooctene (1a). With chiral sensitizers, the relative rates for the formation of the “twisted singlet” exciplexes are not equal, providing the basis for enantioselectivity.
Figure 2.
Enantioselective photoisomerization based on chiral exciplex formation.
Flow Photochemical Syntheses of trans-Cyclooctenes
Photochemical syntheses of functionalized trans-cyclooctenes had been limited by low yields and by the photodegradation of the trans-cyclooctene. With time course monitoring, cis-cyclooct-4-enol irradiation produces diastereomers of trans-cyclooct-4-enol with a maximum of 23% conversion.[77] On prolonged irradiation (18 h), the yield drops to <5% trans-cyclooct-4-enol.[78]
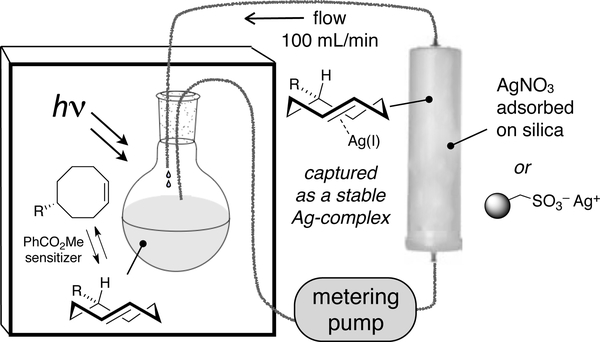
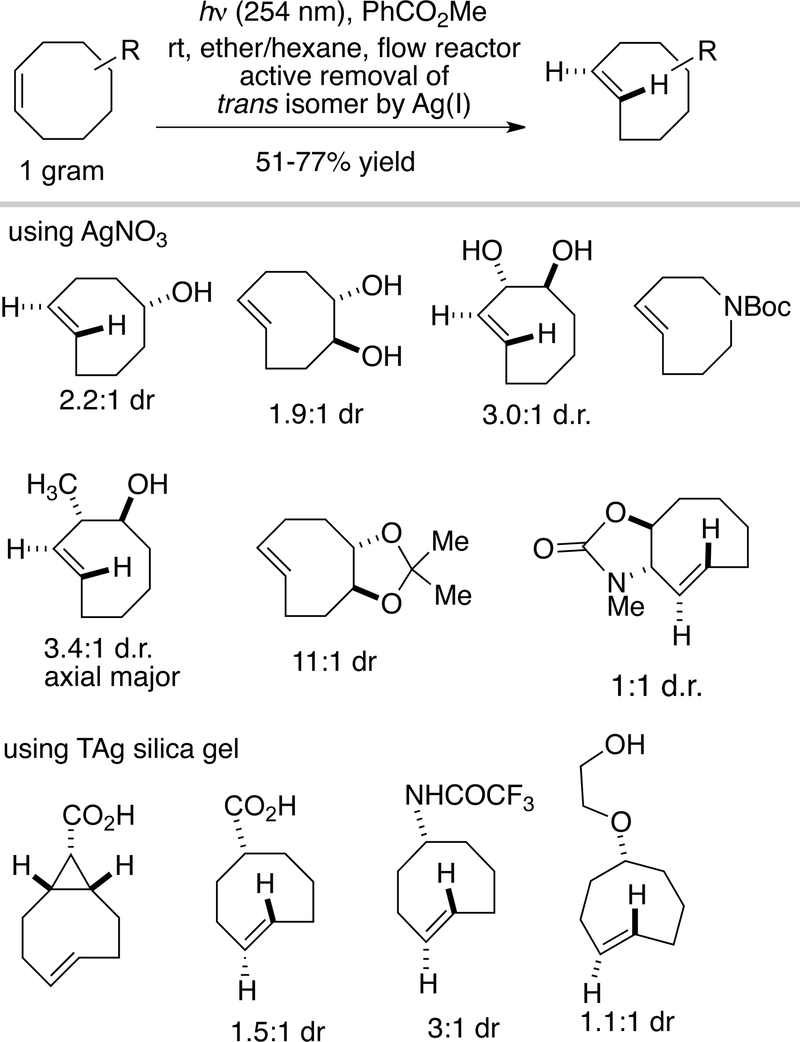
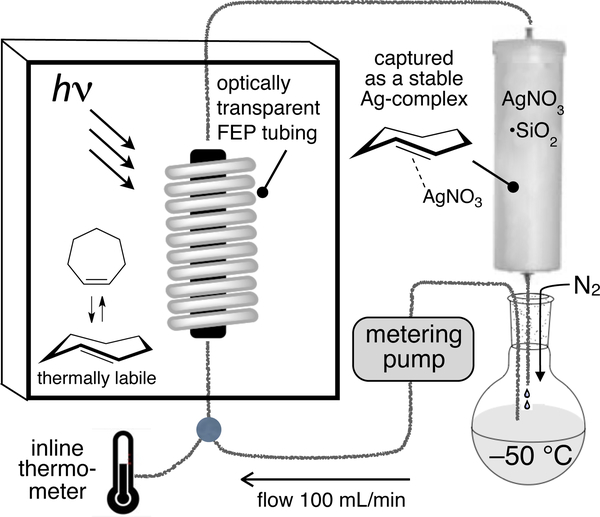
To improve the practicality of photoisomerization, we designed a closed-loop flow reactor that drives the transformation through selective metal complexation of the trans-isomer.[78] Our experiments were based on the well-known observation that trans-cyclooctene, but not cis-cyclooctene, forms a complex with AgNO3.[79] Cyclic olefins such as trans-cyclooctene interact strongly with metals due to strain relief with relatively minimal energetic cost associated with the reorganization of the hydrocarbon framework.[80] Our strategy was also influenced by classic studies on photoprotonation of cyclic alkenes by Marshall, Kropp and Beauchenmin.[81–83] A schematic of the apparatus for preparing trans-cyclooctenes is shown in Fig 3. A quartz reaction flask containing methyl benzoate, a singlet sensitizer, and a solution of a cis-cyclooctene derivative is irradiated at 254 nm. During irradiation, the reaction mixture is continuously flowed through column containing AgNO3 adsorbed on silica gel.[84] The trans-cyclooctene derivative is selectively retained by the AgNO3•silica, but the cis-isomer elutes back to the reaction flask, where it is photoisomerized and recirculated through the column. After consumption of the cis-cyclooctene, the silica is removed and stirred with NH4OH, which liberated the trans-cyclooctene from the AgNO3. For base sensitive substrates, NaCl can be used can be used to decomplex AgNO3. The trans-cyclooctene derivative is then recovered by extraction. As an alternative to AgNO3, Ag(I) immobilized on tosic silica gel can be used to capture trans-cyclooctene products at higher silver loadings without leaching, which can be especially beneficial for polar substrates or large scale photoisomerizations.[85] However, the low cost of AgNO3•silica still makes it beneficial for routine (gram scale) isomerizations. Examples of trans-cyclooctenes that have been prepared by flow photoisomerization are given in Figure 4.
Figure 3.
Schematic of Apparatus for trans-Cyclooctene Synthesis
Figure 4.
Examples of trans-cyclooctene derivatives that have been prepared using flow photoisomerization
Since our original description of the flow photoisomerization protocol, it has been employed by a number of groups for trans-cyclooctene synthesis with applications that include radiochemistry, cellular imaging, drug delivery and materials science.[55, 86–120] There have also been a number of modifications to the flow procedure that have been introduced. To avoid the capital cost of the flow equipment, several groups have described protocols where the flow photoisomerization is mimicked by periodically stopping irradiation, capturing the trans-cyclooctene by filtering through AgNO3 on silica, and resubjecting the filtrate to photoisomerization (254 nm). [26, 121] These procedures are more labor intensive and lower yielding than the flow chemistry protocols.
A modification of the flow system utilized a quartz tube in conjunction with a UV light.[122] In this setup, the bulk of the reaction solution resided in a reservoir flask and was continually pumped through the quartz tube where irradiation (254 nm) occurred. This setup rendered the reaction scalable without the expense of purchasing multiple quartz flasks for different reaction scales. A microflow system for cyclooctene photoisomerization has been described which utilizes two microreactors coiled around a UV lamp and several beds of AgNO3-impregnated silica that are exchangeable during irradiation.[123] This system was employed for small scale synthesis of 5-hydroxy-trans-cyclooctene and other trans-cyclooctene derivatives.
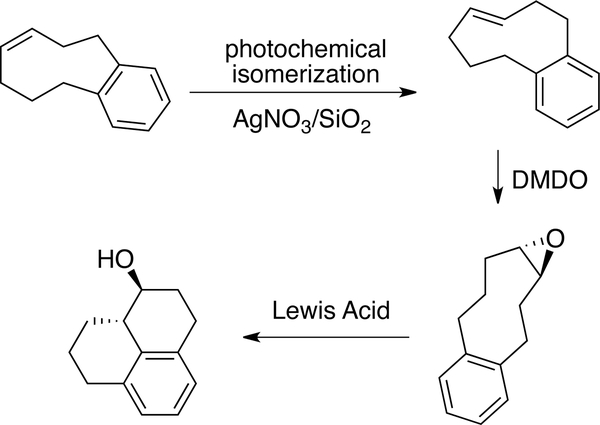
Tomooka has prepared (E)-4-[7]orthocyclophene without flow chemistry by directly adding AgNO3/SiO2 to a photoisomerization reaction in pentane.[63, 124] The heterogeneous solution was irradiated for 42 hours. After workup in NH4OH, the product was isolated in 76% yield (Figure 5). The enantiomers of (E)-4-[7]orthocyclophene could be separated by chiral HPLC and subjected to epoxidation and Lewis-acid catalyzed, stereoselective cyclization as shown in Figure 5.
Figure 5.
Synthesis of (E)-4-[7]orthocyclophene without flow chemistry by directly adding AgNO3 /SiO2
In a recent development, a liquid-liquid extraction method was developed by Rutjes. The apparatus is comprised of UV lamp and a continuously flowing heptane solution containing cis-cyclooctene overtop an aqueous AgNO3 solution.[77] The organic phase flows through UV-permeable FEP tubing (fluorinated ethylene propylene) wrapped around a UV lamp (254 nm) and into an AgNO3 aqueous solution where trans-cyclooctene is trapped and cis-cyclooctene returns to the organic phase. This system is scalable due to the ability to maintain a consistent concentration of cis-cyclooctene in heptane via external addition of substrate. This method allotted up to 2.2 g/h of TCO to be produced and was employed on several of the commonly utilized trans-cyclooctenes.
Stereospecific Transannulation of trans-Cyclooctenes
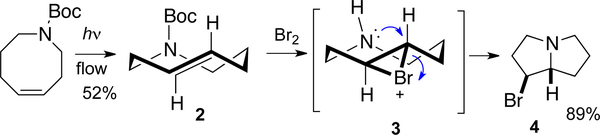
Flow photoisomerization has provided access to functionalized trans-cyclooctenes capable of stereospecific, transannular cyclization reactions. While stereospecific, transannular cyclizations of (E)-cycloalkenes have been studied with larger ring systems,[125–128] few studies had been carried out on trans-cyclooctene derivatives. (E)-Thiacyclooct-4-ene has been shown to undergo acid catalyzed transannular cyclization,[129] as does the anion of (E)-4,5-epoxy-1-thiacyclooctane-1,1–dioxide.[130–131] As shown in Figure 6, transannular hydrobromination of 4-aza-trans-cyclooctene provides the pyrrolizidine framework that is common to a range of natural products.[78] Thus, treatment of 2 with bromine provides pyrrolizidine 4 in >90% isomeric purity (crude 1H NMR analysis).[78] 4-Aza-cis-cyclooctene gives the opposite diastereomer of 2, demonstrating that the stereochemistry of the alkene and putative bromonium ion intermediate 3 controls the diasteoselectivity of the cyclization.
Figure 6.
Stereospecific, transannular bromoamination
Access to the pyrrolizidine alkaloids can also be realized through transannular hydroamination of a 5-aza-cyclooctene.[132] While intermolecular hydroamination of trans-cycloalkenes had been described by Beauchemin,[83] the transannular hydroamination of an 5-aza-trans-cyclooctene was previously unknown.
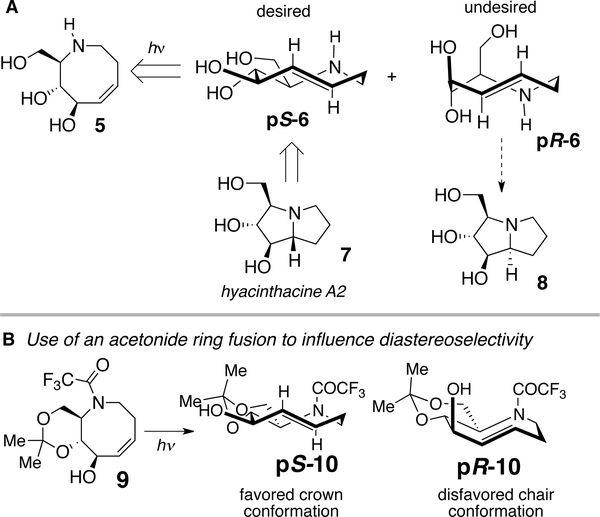
An initial retrosynthetic analysis for the total synthesis of hyacinthacine A2 (7) is displayed in Figure 7A. It was considered that 7 could arise from the hydroamination of a 5-aza-trans-cyclooctene pS-6, which would in turn arise from 5-aza-cis-cyclooctene 5. A key consideration was stereocontrol in the photoisomerization step, as trans-cyclooctenes possess planar chirality and are configurationally stable. Only the pS isomer of 6 would lead to the natural product, whereas the pR isomer would lead to diastereomer 8. Generally, the photoisomerization reactions of cis-cyclooctenes bearing a stereogenic center are poorly diastereoselective.[78] To address the issue, it was predicted that a trans-ring fusion could influence diastereocontrol in the synthesis of hyacinthacine A2. Thus, acetonide 9 was expected to photoisomerize to pS-10 (Figure 7B). The 8-membered ring of pS-10 can adopt a crown conformation, which is the lowest energy conformer of trans-cyclooctene. The eight-membered ring of diastereomer pR-10 is unable to adopt a crown conformation, and instead be forced to adopt the much higher energy chair conformation. The considerable difference in conformational energy between pS-10 and pR-10 was predicted to provide a basis for stereocontrol in the photochemical step.
Figure 7.
(A) Retrosynthetic analysis of hyacinthacine A2 (7) using transannular hydroamination and diastereoselective photoisomerization as key steps. Poor diastereoselectivity in the photoisomerization step would lead to undesired isomers pR-6 and 8. (B) An acetonide ring fusion would force the minor diastereomer pR-10 to adopt a high energy chair conformation, favoring the formation of pS-10 in the photoisomerization 5-aza-cyclooctene 9.
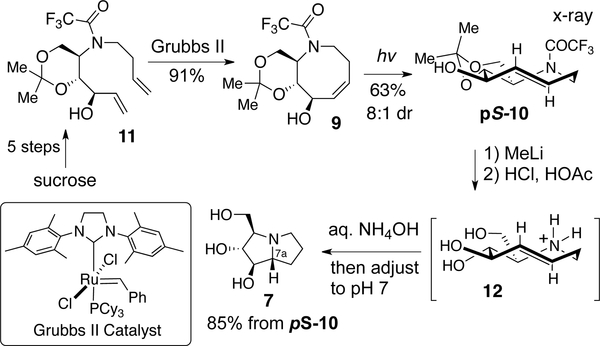
The total synthesis of hyacinthacine A2 was completed as shown in Figure 8. Diene 11 was prepared in 5 steps from sucrose by a modification of a method developed by Lauritsen and Madsen.[133] Ring closing metathesis using the 2nd Generation Grubbs catalyst (Grubbs II) gave 9 in 91% yield. Flow photoisomerization (254 nm) provided 10 with 8:1 dr, favoring the pS-isomer. X-ray crystallography confirmed that the eight-membered ring of pS-10 adopts a crown structure in the solid state (Figure 2a). The total synthesis of hyacinthacine A2 (7) was completed by trifluoroacetyl removal with MeLi followed by acidic treatment to give triol 12 as the ammonium salt. 5-Aza-trans-cyclooctene 12 smoothly underwent transannular hydroamination to give hyacinthacine A2 (7) upon treatment with aq. NH4OH and adjustment to pH 7. In the hydroamination, the absolute planar chirality of 12 was transferred with excellent fidelity providing a single diastereomer of 7 in the transannular reaction.
Figure 8.
Total synthesis of hyacinthacine A2 via photoisomerization and stereospecific transannular hydroamination.
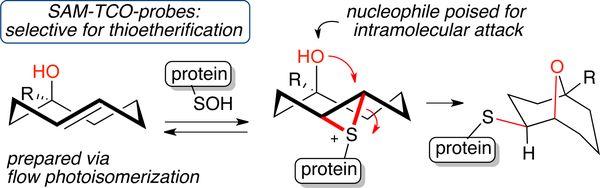
trans-Annulation for Sulfenic Acid Detection in Live Cells
Sulfenylation (RSH –>RSOH) is a post-translational protein modification associated with cellular mechanisms for signal transduction and regulating reactive oxygen species. Flow photochemistry was used to prepare specialized sulfenic acid modifying trans-cycloocten-5-ol (SAM-TCO) probes for labeling sulfenic acid functionality in live cells.[134] It was reasoned that the olefinic strain of SAM-TCO’s would make them particularly capable of forming thiiranium ions, and that the ring system would position a hydroxyl nucleophile for subsequent transannular attack (Fig 9). The probes enabled a new method of capturing sulfenic acids via transannular thioetherification, whereas ‘ordinary’ trans-cyclooctenes react only slowly with sulfenic acids. Bioorthogonal quenching of excess unreacted SAM-TCOs with tetrazines in live cells provided temporal control and prevented artifacts caused by cellular-lysis. A cell-based proteomic study showed that SAM-TCO probes could be used to identify and quantify known sulfenic acid redox proteins as well as targets not captured by previously established probes.
Figure 9.
SAM-TCO probes are specialized trans-cyclooctenes that react with sulfenic acids in cellular context via transannulation. The probes are small, cell permeant, selective, irreversible, and can be quenched in vivo to enable cellular reporting temporal precision.
trans-Cyclooctenes in Bioorthogonal Chemistry
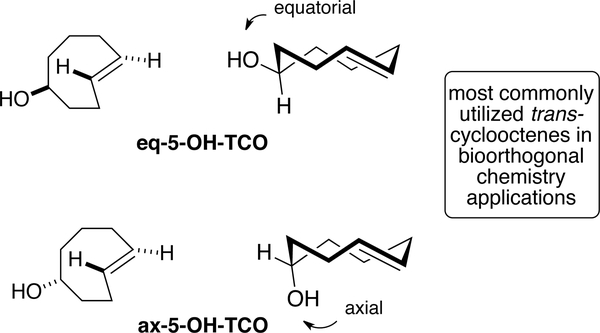
Due to their exceptionally fast reaction rates with tetrazines, trans-cyclooctenes have become broadly utilized for applications in bioorthogonal chemistry. Flow photochemistry has been integral to the discovery and synthesis of a range of functionalized trans-cyclooctenes that have been used throughout this field. Most commonly, the diastereomers of 5-hydroxy-trans-cyclooctene are used for bioorthogonal chemistry applications (Figure 10). The equatorial diastereomer is produced as the major product in photoisomerization reactions, and can be produced on relatively large scale using flow chemistry.[78] With a second order rate constant of 8.0 × 104 M–1s−1 at 25 °C with an amido-substituted dipyridyltetrazine in water, the axial diastereomer is ~4 times faster than the equatorial diastereomer. This rate difference is even more pronounced for carbamate derivatives of 5-hydroxy-trans-cyclooctene, and derivatives of ax-5-OH-TCO have been advanced for applications in pretargeted nuclear medicine.[120] Both diastereomers of 5-hydroxy-trans-cyclooctene display good stability toward isomerization and have been used for applications as bioorthogonal reporters where long-term cellular or in vivo stability is required. The stability properties of trans-cyclooctenes has been summarized in a recent report.[135]
Figure 10.
Equatorial and axial diastereomers are produced as a separable mixture upon photoisomerization of 5-hydroxy-cis-cyclooctene. These dienophiles have been widely used for applications in bioorthogonal chemistry.
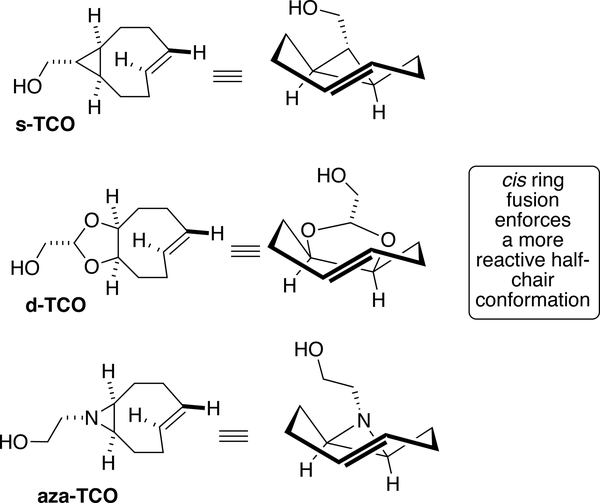
Even more rapid bioorthogonal reactions can be realized with the conformationally strained dienophiles (Figure 11).[23– 24] These bicyclic compounds adopt a half-chair conformation that in the ground state is 5.6–5.9 kcal/mol higher in energy than the crown conformer of unconstrained trans-cyclooctenes. As a result, cycloadditions with tetrazines are more than 2 orders of magnitude faster with these compounds. With an amido-substituted dipyridyltetrazine in water, s-TCO reacts with a second order rate constant of 3.3 × 106 M−1s−1, and has the advantage that the photochemistry precursors can be made simply on large scale.[136] With the same tetrazine, d-TCO reacts with a rate of 3.7 × 105 M–1s−1, and displays better long-term stability toward isomerization.[24] The more recently described aza-TCO also displays rapid reactivity that is intermediate between s-TCO and d-TCO.[93] aza-TCO has been utilized in the formation of fluorescent products in tetrazine ligations that do not require attachment of an extra fluorophore moiety.
Figure 11.
Conformationally strained trans-cyclooctenes have faster rates of reaction than the crown conformer trans-cyclooctenes and can be employed in a number of bioothogonal applications.
The hydrophobicity of TCO and s-TCO can negatively impact the physiochemical properties of bioconjugates for some biological experiments. This has recently been linked to high levels of non-specific background fluorescence during imaging experiments, necessitating lengthy washout protocols (>2 h) to dissociate the excess reagent from the cell.[103, 137–138] While d-TCO displays reduced lipophilicity, the compound is relatively bulky compared to the parent trans-cyclooctene system. Accordingly, the more hydrophilic trans-cyclooctene dienophiles were sought.
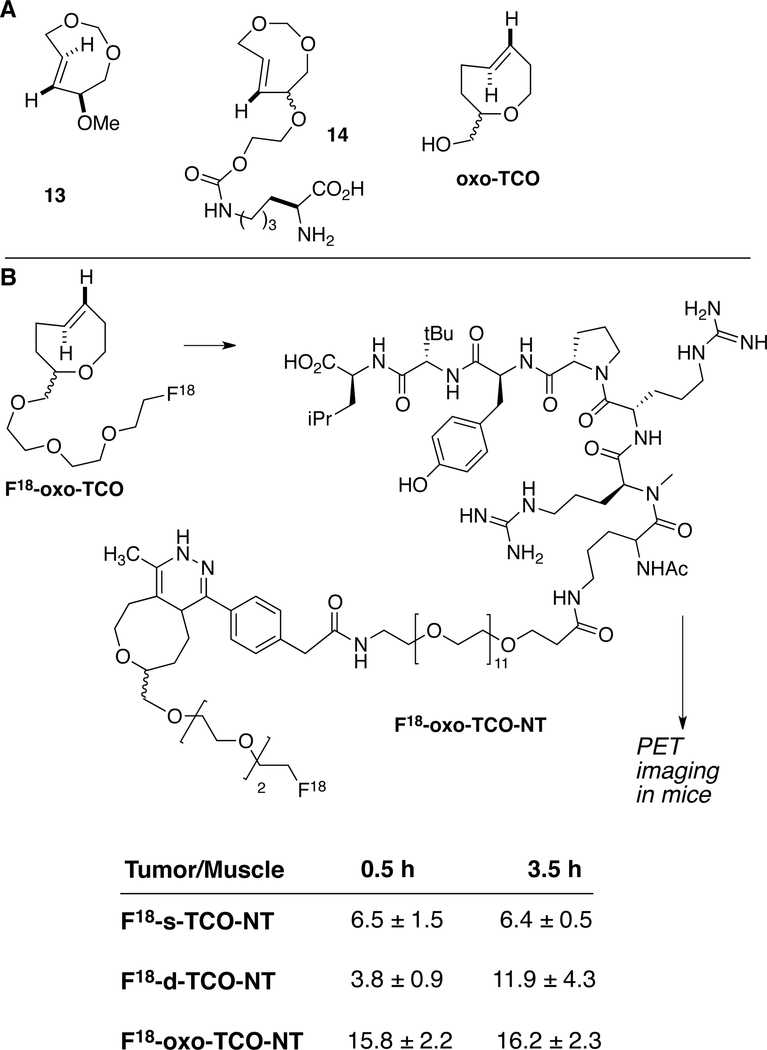
In a seminal contribution, Jendrella synthesized 4,6-dioxo-TCO 13 and showed it to be more reactive than trans-cyclooctene in cycloadditions with cyclopentadiene, 2,3-dimethylbutadiene, mesitonitriloxide and diphenylketene.[139] Woerpel[49–57] and Tomooka[62] have synthesized trans-oxasilacycloalkenes, and have studied their reactivity in Diels-Alder and azide cycloadditions. Kele, Lemke and coworkers reported the genetic incorporation of dioxo-TCO 14 and demonstrated that the lower lipophilicity of this molecule resulted in improved washout times during imaging experiments.[103] In Diels-Alder reactions with tetrazines, the reaction rate with 2 is similar to that with the parent TCO.[103] Separately, trans-5-oxocene (oxo-TCO) was shown to display enhanced reactivity and hydrophilicity compared to trans-cyclooctene (TCO) in the tetrazine ligation reaction.[140] oxo-TCO has an improved logP 0.51 relative to 5-hydroxy-trans-cyclooctene (logP 1.11), d-TCO (logP 0.91). The reaction of oxo-TCO (2.2 : 1 d.r.) with an amido-substituted 3,6-dipyridyltetrazine in water occurs with a second order rate constant of 9.5 × 104 M–1s–1 in PBS at 25 °C, which is faster than either diastereomer of 5-hydroxy-trans-cyclooctene, and approaching the rate of bicyclic d-TCO.
As shown in Figure 12B, an 18F-labeled trans-5-oxocene (18F-oxoTCO) was utilized to develop probes for positron emission tomography imaging in mice.[137] A probe was constructed from a tetrazine conjugate of a peptide that targets the neurotensin (NT) receptor, which is upregulated in prostate, pancreatic, lung, and colorectal cancer. The tracer showed comparable tumor uptake with analogous probes constructed from 18F-labeled s-TCO and d-TCO. However, the increased hydrophilicity from the oxo-TCO enabled a faster clearance rate of the tracer from non-targeting organs, which lead to significantly higher tumor to background ratio compared with s-TCO and d-TCO counterparts.
Figure 12.
(A) Hydrophilic trans-cyclooctene analogs. (B) 18F-labeled probe based on oxo-TCO displays improved hydrophilicity for in vivo PET imaging in mice.
In the bioorthogonal probe-reporter strategy,[141] there are different stability requirements for the bioorthogonal reaction partners. Stability requirements are higher for the reporter molecule, which resides in the biological environment for entire duration of a biological experiment, whereas the probe need only be stable for the labeling portion of the experiment. The stability of more reactive trans-cyclooctenes such as s-TCO, d-TCO and oxo-TCO have been described in detail,[135] and have been used by a number of groups for cellular and in vivo experiments. Unfortunately, the stabilities of these compounds have sometimes been misquoted as too unstable for cellular experiments. For live cell applications, these compounds have high utility as probes, where short incubation times in the cellular enviroment are required due to the very fast kinetics of reactions with tetrazines. The primary mechanism for the deactivation of TCO reagents is isomerization to the cis-isomer, which decreases labeling efficiency but has the merit of not causing non-selective off-target labeling in cellular experiments. For the use of trans-cyclooctenes as chemical reporters, it is advisable to use the more resilient parent trans-cyclooctenes based on 5-hydroxy-trans-cyclooctene.
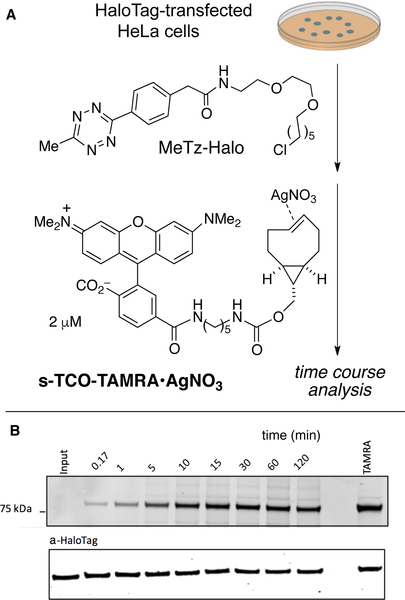
An additional, practical consideration for strained TCO derivatives is that non-crystalline derivatives of s-TCO and d-TCO can isomerize upon prolonged storage. The shelf-life of the most reactive trans-cyclooctenes can be greatly extended by ‘protecting’ them as stable Ag(I) metal complexes. [135, 142] NMR studies showed that Ag-complexation is thermodynamically favorable for trans-cyclooctenes with dissociation kinetics that are very rapid. Additionally, TCO•AgNO3 complexes are immediately dissociated upon addition of NaCl. Thus, the most highly strained trans-cyclooctenes can be stabilized for long term storage through Ag(I) complexation, and then liberated on demand by addition of NaCl which is present in high concentration in cell media. The utility of Ag-TCO complexes was demonstrated in several live cell labeling experiments.[135, 142] For example, the silver nitrate complex of a highly reactive s-TCO-TAMRA conjugate was prepared, and was shown to label a protein-tetrazine conjugate in live cells with faster kinetics and similar labeling yield relative to an ‘ordinary’ TCO-TAMRA conjugate (Figure 13).
Figure 13.
(A) Incorporation of MeTz-Halo into HaloTag-transfected HeLa cells was followed by labeling with s-TCO-TAMRA•AgNO3. (B) Kinetics were studied by timecourse quenching with a non-fluorescent TCO and following analysis by in-gel fluorescence.
Flow Photochemical Synthesis of trans-Cycloheptenes and Sila-trans-Cycloheptenes
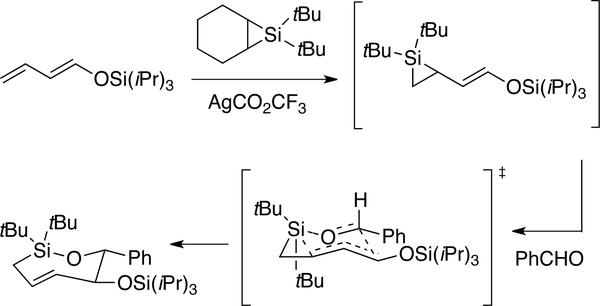
Like trans-cyclooctene, trans-cycloheptene has long captured the imagination of chemists. trans-Cycloheptene was first trapped from trans-1,2-cycloheptenethionocarbonate through treatment with P(OMe)3.[143] In photoprotonation reactions of cyclic alkenes, including cycloheptene, photoisomerization reactions are driven by selective addition reactions of trans-cycloalkenes.[82–83, 144] Cycloheptene was first directly observed by Inoue who studied the singlet sensitized photoisomerization of cis-cycloheptene at –35 °C.[145–146] Unlike trans-cyclooctene, trans-cycloheptene is very labile and undergoes rapid isomerization under ambient conditions.[147] trans-Cycloheptene has also been prepared via ligand exchange from a trans-cycloheptene•CuOTF complex.[148] Because C-Si bonds are long, the inclusion of silicon into the cyclic backbone can alleviate olefinic strain and impart stability to trans-cycloalkenes. [52–57, 62] Woerpel has developed a general method for the preparation of trans-oxasilacycloheptenes—seven membered rings that contain trans-alkenes and siloxy bonds in the backbone (Figure 14), and has described their selective addition, difunctionalization and cycloaddition reactions.[52–57]
Figure 14.
Woerpel’s synthesis of trans-oxasilacycloheptenes
Several studies had shown that metal complexes of trans-cycloheptene can be isolated.[16, 149–151] Jendralla described the preparation of AgOTf and AgClO4 complexes of 3-methoxy-trans-cycloheptene and 6-Methoxy-(Z),4(E)-cycloheptadiene through Ag-mediated ring opening of a nitrosourea derivative of bicyclo[4.1.0]heptane.[16, 150] In unspecified yields, the AgClO4•3-methoxy-trans-cycloheptene complex was combined with a number of dienes to give the products of metal decomplexation and [4+2] cycloaddition.
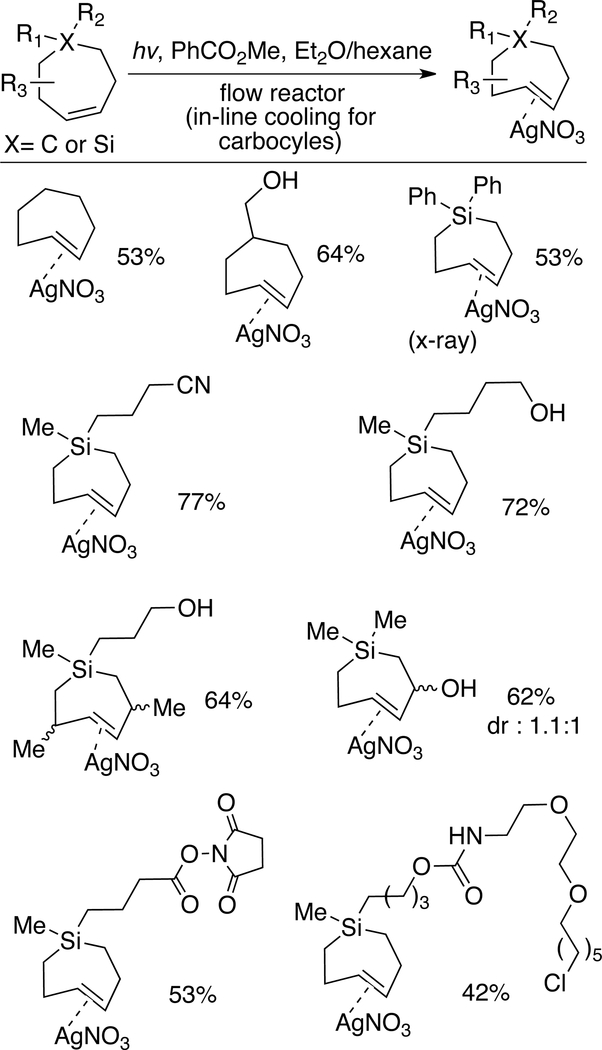
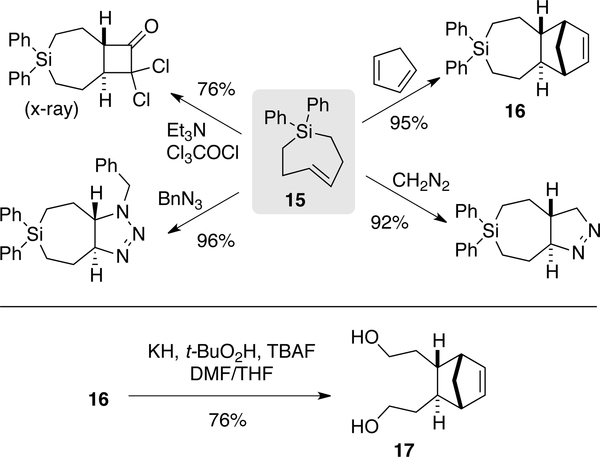
Inital attempts to directly prepare trans-cycloheptenes and sila-analogs by flow photochemical isomerization were unsuccessful, most likely due to the susceptibility of carbocyclic TCHs to isomerization. However, flow photochemistry can be used to prepare trans-cycloheptenes and sila-trans-cycloheptenes as their isolable Ag-complexes.[152] Photoisomerizations to form sila-trans-cycloheptene silver nitrate (Si-TCH•AgNO3) complexes were carried out at r.t. using the standard flow-photoisomerization apparatus (254 nm), with the modification that Si-TCH•AgNO3 complexes were directly isolated from SiO2 without Ag-decomplexation. The Si-TCH•AgNO3 complexes are stable in neat form for >1 month in the freezer. For carbocyclic trans-cycloheptene•silver nitrate (TCH•AgNO3) synthesis, the reactor design was modified to allow for in-line cooling and shortened residence time in the photoreactor (Figure 15). With this modified reactor, TCH•AgNO3 complexes were isolated as semisolids that are moderately stable at r.t. but stable for weeks in the freezer. The scope of TCH and Si-TCH synthesis is shown in Figure 16. Aryl, Cyano, carbamate, and hydroxyl groups were tolerated in the reaction as were NHS ester and chloroalkane groups that were then used to enable conjugation to fluorophores and HaloTag fusion proteins, respectively.
Figure 15.
Flow photoisomerization apparatus for carbocyclic trans-cycloheptene derivatives uses FEP tubing and inline cooling. For the synthesis of trans-1-sila-4-cycloheptenes, photoisomerizations could be carried out at r.t. using a conventional flow photoisomerization setup.
Figure 16.
Flow-photochemical synthesis of AgNO3 complexes of trans-cycloheptenes and trans-1-sila-4-cycloheptenes.
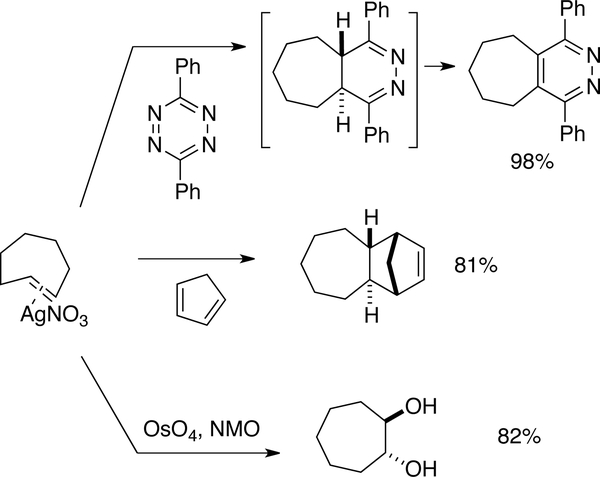
TCH and Si-TCH complexes were shown to engage in a range of reactions as shown in Figures 17 and 18. The AgNO3 complex of trans-cycloheptene underwent several transformations with in situ metal decomplexation. Combination with 3,6-diphenyl-1,2,4,5-tetrazine gave a pyridazine product in 98% yield. Cyclopenta-1,3-diene was also used to trap trans-cycloheptene, delivering the [4+2] cycloaddition adduct in 81% yield as a single diastereomer. Vicinal dihydroxylation with OsO4 and NMO gave the trans-diol in 82% yield as a single diastereomer. [152]
Figure 17.
Reactions of TCH•AgNO3
Figure 18.
Reactions of Si-TCH 15 and cycloadduct 16.
Si-TCH complex 15 was obtained treating the silver complex with NH4OH and directly subjected to reactions cyclopentadiene, diazomethane, dichloroketene, and benzyl azide to give cycloadducts as single diastereomers in 76%–96% yields (Figure 18). With cycloadduct 16, it was shown that the diphenylsila- group could be oxidized to diol product 17 in 76% yield. [152]
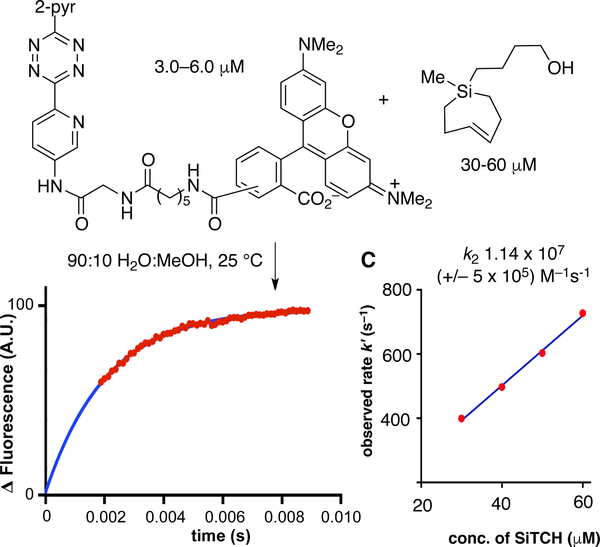
In bioorthogonal reactions, Si-TCH compounds are 1.4–2.8x more reactive than s-TCO depending upon the context of tetrazine reaction partner and reaction conditions. As shown in Figure 19, the reaction of a fluorescent dipyridyltetrazine conjugate with a Si-TCH in 9:1 water:MeOH at 25 °C proceeds with a second order rate constant k2 1.14 × 107 (+/– 5 × 105) M–1s–1. Remarkably, the reaction is complete within 10 milliseconds when the concentration of the excess reagent is only 60 μM. This is the fastest rate constant reported to date for a bioorthogonal reaction. Utility in bioorthogonal protein labeling in live cells was also demonstrated, including labeling of GFP with an unnatrual tetrazine-containing amino acid. An in vitro rate constant of 250,000± 15,000M–1s–1 was measured in PBS at rt. The kinetics of the in vivo tetrazine ligation were monitored in a suspension (PBS) of E. coli overexpressing GFP with the unnatrual tetrazine amino acid. The reaction rate of Si-TCH with GFP-Tet was obtained by measuring the increase in whole-cell fluorescence upon addition of 7c. At room temperature, a second-order rate constant of 155,000 ± 20,000 M–1S–1 was measured. The reactivity and specificity of the Si-TCH reagents with tetrazines in live mammalian cells was also evaluated using the HaloTag platform. The cell labeling experiments show that Si-TCH derivatives are suitably stable to serve as highly reactive probe molecules in the cellular environment.[152]
Figure 19.
Stopped flow kinetics of a Si-TCH with a TAMRA-3,6-dipyridyl-s-tetrazine conjugate were monitored in 9:1 H2O:MeOH with monitoring by fluorescence. Data points are shown in red, and the fit is shown in blue. Second order rate constants (k2) were determined by plotting kobs vs. the concentration of Si-TCH.
Summary
Over the past decade, trans-cyclooctenes and trans-cycloheptenes have emerged from the physical organic literature as building blocks for synthesis and essential tool molecules for chemical biology. Enabling this transition has been the development of flow-chemistry for the photoisomerization of cycloalkenes that is driven by in-line complexation of the trans-isomer by Ag(I). Flow photochemical synthesis of trans-cyclooctenes has been broadly adopted across the chemical biology community, and recent advances have extended the method to isolable silver-complexes of trans-cycloheptenes and sila-trans-cycloheptenes.
Acknowledgements
For financial support we thank NIH GM132460 and Pfizer.
Biography
Jessica Pigga is a native of Scranton PA. She received the B.S. degree from Elizabethtown College where she carried out undergraduate research with Jeffrey Rood. She is currently a 3rd year Ph.D. student in the research group of Joseph Fox at the University of Delaware.

Joseph Fox is Professor of Chemistry and Biochemistry at the University of Delaware, where he also directs an NIH-funded Center of Biomedical Research Excellence. Prior to his appointment at UD in 2001, Fox received the A.B. degree from Princeton University with Maitland Jones Jr., carried out Ph.D. research at Columbia University with Thomas Katz, and was an NIH postdoctoral fellow with Stephen Buchwald at MIT.

References
- [1].Ziegler K, Wilms H, Liebigs Ann. Chem. 1950, 567, 1. [Google Scholar]
- [2].Cope AC, Pike RA, Spencer CF, J. Am. Chem. Soc. 1953, 75, 3212. [Google Scholar]
- [3].Cope AC, Ganellin CR, Johnson HW, Van Auken TV, Winkler HJS, J. Am. Chem. Soc. 1963, 85, 3276–3279. [Google Scholar]
- [4].Cope AC, Pawson BA, J. Am. Chem. Soc. 1965, 87, 3649–3651. [Google Scholar]
- [5].Allinger NL, Sprague JT, J. Am. Chem. Soc. 1972, 94, 5734–5747. [Google Scholar]
- [6].Bach RD, Mazur U, Hamama I, Lauderback SK, Tetrahedron 1972, 28, 1955–1963. [Google Scholar]
- [7].Barrows SE, Eberlein TH, J. Chem. Educ. 2005, 82, 1334–1339. [Google Scholar]
- [8].Shea KJ, Kim JS, J. Am. Chem. Soc. 1992, 114, 4846–4855. [Google Scholar]
- [9].Palacios F, de Heredia IP, Rubiales G, J. Org. Chem. 1995, 60, 2384–2390. [Google Scholar]
- [10].Weyler WJ, Byrd LR, Caserio MC, Moore HW, J. Am. Chem. Soc. 1972, 94, 1027–1029. [Google Scholar]
- [11].Ganis P, Lepore U, Martusce E, J. Phys. Chem. 1970, 74, 2439. [Google Scholar]
- [12].Kinoshita I, Terai Y, Kashiwabara K, Kido H, Saito K, J. Organomet. Chem. 1977, 127, 237–243. [Google Scholar]
- [13].Komiya S, Kochi JK, J. Organomet. Chem. 1977, 135, 65–72. [Google Scholar]
- [14].Nicolaides A, Smith JM, Kumar A, Barnhart DM, Borden WT, Organometallics 1995, 14, 3475–3485. [Google Scholar]
- [15].Cope AC, Banholzer K, Keller H, Pawson BA, Whang JJ, Winkler HJS, J. Am. Chem. Soc. 1965, 87, 3644–3649. [Google Scholar]
- [16].Jendralla H, Angew. Chem. Int. Ed. 1980, 19, 1032–1033. [Google Scholar]
- [17].Thalhammer F, Wallfahrer U, Sauer J, Tetrahedron Lett. 1990, 31, 6851–6851. [Google Scholar]
- [18].Carboni RA, Lindsey RV, J. Am. Chem. Soc. 1959, 81, 4342–4346. [Google Scholar]
- [19].Hamasaki A, Zimpleman JM, Hwang I, Boger DL, J. Am. Chem. Soc. 2005, 127, 10767–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Blackman ML, Royzen M, Fox JM, J. Am. Chem. Soc. 2008, 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Devaraj NK, Weissleder R, Hilderbrand SA, Bioconjugate Chem. 2008, 19, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pipkorn R, Waldeck W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K, J. Pep. Sci. 2009, 15, 235–241. [DOI] [PubMed] [Google Scholar]
- [23].Taylor MT, Blackman ML, Dmitrenko O, Fox JM, J. Am. Chem. Soc. 2011, 133, 9646–9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Darko A, Wallace S, Dmitrenko O, Machovina MM, Mehl RA, Chin JW, Fox JM, Chem. Sci. 2014, 5, 3770–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R, Angew. Chem. Int. Ed. 2010, 49, 2869–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R, Angew. Chem. Int. Ed. 2009, 48, 7013–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rossin R, Verkerk PR, van den Bosch SM, Vulders RCM, Verel I, Lub J, Robillard MS, Angew. Chem. Int. Ed. 2010, 49, 3375–3378. [DOI] [PubMed] [Google Scholar]
- [28].Selvaraj R, Fox JM, Curr. Opin. Chem. Biol. 2013, 17, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].(a) Lang K, Chin JW, ACS Chem. Biol. 2014, 9, 16–20. [DOI] [PubMed] [Google Scholar]; (b) Row RD, Prescher JD, Acc. Chem. Res. 2018, 51, 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rossin R, Robillard MS, Curr. Opin. Chem. Biol. 2014, 21, 161–169. [DOI] [PubMed] [Google Scholar]
- [31].Kang K, Park J, Kim E, Proteome Sci. 2016, 15, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wu H, Devaraj NK, in Top. Curr. Chem, Vol. 374 (Eds.: Carell T, Vrabel M), 2016, p. 3. [DOI] [PubMed] [Google Scholar]
- [33].Oliveira BL, Guo Z, Bernardes GJL, Chem. Soc. Rev. 2017, 46, 4895–4950. [DOI] [PubMed] [Google Scholar]
- [34].Wu H, Devaraj NK, Acc. Chem. Res. 2018, 51, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Knall AC, Slugovc C, Chem. Soc. Rev. 2013, 42, 5131–5142. [DOI] [PubMed] [Google Scholar]
- [36].Devaraj NK, Weissleder R, Acc. Chem. Res. 2011, 44, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Devaraj NK, ACS Cent. Sci. 2018, 4, 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Reese CB, Shaw A, J. Am. Chem. Soc. 1970, 92, 2566–2568. [Google Scholar]
- [39].Braddock DC, Cansell G, Hermitage SA, White AJP, Tetrahedron: Asymmetry 2004, 15, 3123. [Google Scholar]
- [40].Whitham GH, Wright M, J. Chem. Soc. (C) 1971, 886. [Google Scholar]
- [41].Whitham GH, Wright M, J. Chem. Soc. (C) 1971, 891. [Google Scholar]
- [42].Hines JN, Peagram MJ, Thomas EJ, Whitham GH, J. Chem. Soc., Perkin Trans. 1 1973, 2332. [Google Scholar]
- [43].Corey EJ, Shulman JI, Tetrahedron Lett. 1968, 33, 3655–3658. [Google Scholar]
- [44].Vedejs E, Snoble KAJ, Fuchs PL, J. Org. Chem. 1973, 38, 1178. [Google Scholar]
- [45].Schmidt JAR, Mahadevan V, Getzler YDYL, Coates GW, Org. Lett. 2004, 6, 373–376. [DOI] [PubMed] [Google Scholar]
- [46].Wang XN, Krenske EH, Johnston RC, Houk KN, Hsung RP, J. Am. Chem. Soc. 2014, 136, 9802–9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang XN, Krenske EH, Johnston RC, Houk KN, Hsung RP, J. Am. Chem. Soc. 2015, 137, 5596–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ito T, Tsutsumi M, Yamada K, Takikawa H, Yamaoka Y, Takasu K, Angew. Chem. Int. Ed. 2019, 58, 11836–11840. [DOI] [PubMed] [Google Scholar]
- [49].Prévost M, Ziller JW, Woerpel KA, Dalton Trans. 2010, 39, 9275–9281. [DOI] [PubMed] [Google Scholar]
- [50].Ventocilla CC, Woerpel KA, J. Am. Chem. Soc. 2010, 133, 406–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ventocilla CC, Woerpel KA, J. Am. Chem. Soc. 2011, 133, 406–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Greene MA, Prevost M, Tolopilo J, Woerpel KA, J. Am. Chem. Soc. 2012, 134, 12482–12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hurlocker B, Hu CH, Woerpel KA, Angew. Chem. Int. Ed. 2015, 54, 4295–4298. [DOI] [PubMed] [Google Scholar]
- [54].Santucci J, Sanzone JR, Woerpel KA, Eur. J. Org. Chem. 2016, 2933–2943. [Google Scholar]
- [55].Sanzone JR, Woerpel KA, Angew. Chem. Int. Ed. 2016, 55, 790–793. [DOI] [PubMed] [Google Scholar]
- [56].Sanzone JR, Hu CT, Woerpel KA, J. Am. Chem. Soc. 2017, 139, 8404–8407. [DOI] [PubMed] [Google Scholar]
- [57].Sanzone JR, Woerpel KA, Synlett 2017, 28, 2478–2482. [Google Scholar]
- [58].Tomooka K, Komine N, Fujiki D, Nakai T, Yanagitsuru S, J. Am. Chem. Soc. 2005, 127, 12182–12183. [DOI] [PubMed] [Google Scholar]
- [59].Tomooka K, Suzuki M, Shimada M, Yanagitsuru S, Uehara K, Org. Lett. 2006, 8, 963–965. [DOI] [PubMed] [Google Scholar]
- [60].Uehara K, Tomooka K, Chem. Lett. 2009, 38, 1028–1029. [Google Scholar]
- [61].Tomooka K, Suzuki M, Shimada M, Ni R, Uehara K, Org. Lett. 2011, 13, 4926–4929. [DOI] [PubMed] [Google Scholar]
- [62].Tomooka K, Miyasaka S, Motomura S, Igawa K, Chem. Eur. J. 2014, 20, 7598–7602. [DOI] [PubMed] [Google Scholar]
- [63].Machida K, Yoshida Y, Igawa K, Tomooka K, Chem. Lett. 2018, 47, 186–188. [Google Scholar]
- [64].Maeda R, Wada T, Mori T, Kono S, Kanomata N, Inoue Y, J. Am. Chem. Soc. 2011, 133, 10379–10381. [DOI] [PubMed] [Google Scholar]
- [65].Kaneda M, Asaoka S, Ikeda H, Mori T, Wada T, Inoue Y, Chem. Commun. 2002, 1272–1273. [DOI] [PubMed] [Google Scholar]
- [66].Nakamura M, Inoue T, Nakamura E, J. Organomet. Chem. 2001, 624, 300–306. [Google Scholar]
- [67].Nakamura M, Inoue T, Sato A, Nakamura E, Org. Lett. 2000, 2, 2193–2196. [DOI] [PubMed] [Google Scholar]
- [68].Inoue T, Matsuyama K, Inoue Y, J. Am. Chem. Soc. 1999, 121, 9877. [Google Scholar]
- [69].Shi M, Inoue Y, J. Chem. Soc., Perkin Trans. 2 1998, 2421. [Google Scholar]
- [70].Inoue Y, Matsushima E, Wada T, J. Am. Chem. Soc. 1998, 120, 10687. [Google Scholar]
- [71].Tsuneishi H, Inoue Y, Hakushi T, Tai A, J. Chem. Soc., Perkin Trans. 2 1993, 457. [Google Scholar]
- [72].Inoue Y, Yamasaki N, Yokoyama T, Tai A, J. Org. Chem. 1993, 58, 1011. [Google Scholar]
- [73].Yamasaki N, Inoue Y, Yokoyama T, Tai A, J. Photochem. Photobiol., A 1989, 48, 465. [Google Scholar]
- [74].Inoue Y, Yokoyama T, Yamasaki N, Tai A, J. Am. Chem. Soc. 1989, 111, 6480–6482. [Google Scholar]
- [75].Inoue Y, Ueoka T, Kuroda T, Hakushi T, J. Chem. Soc., Perkin Trans. 2 1983, 983–988. [Google Scholar]
- [76].Inoue Y, Kunitomi Y, Takamuku S, Sakurai H, J. Chem. Soc., Chem. Commun. 1978, 1024–1025. [Google Scholar]
- [77].Blanco-Ania D, Maartense L, Rutjes FPJT, ChemPhotoChem 2018, 2, 898–905. [Google Scholar]
- [78].Royzen M, Yap GPA, Fox JM, J. Am. Chem. Soc. 2008, 130, 3760–3761. [DOI] [PubMed] [Google Scholar]
- [79].Cope AC, Bach RD, Org. Synth. Coll. Vol. 5 1973, 315. [Google Scholar]
- [80].Cedeno DL, Sniatynsky R, Organometallics 2005, 24, 3882–3890. [Google Scholar]
- [81].Marshall JA, Science 1970, 170, 137. [DOI] [PubMed] [Google Scholar]
- [82].Kropp PJ, Mol. Photochem. 1978, 9, 39–65. [Google Scholar]
- [83].Moran J, Cebrowski PH, Beauchemin AM, J. Org. Chem. 2008, 73, 1004–1007. [DOI] [PubMed] [Google Scholar]
- [84].Mander LN, Williams CM, Tetrahedron 2016, 72, 1133–1150. [Google Scholar]
- [85].Darko A, Boyd SJ, Fox JM, Synthesis-Stuttgart 2018, 50, 4875–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yokoi T, Ueda T, Tanimoto H, Morimoto T, Kakiuchi K, Chem. Commun. 2019, 55, 1891–1894. [DOI] [PubMed] [Google Scholar]
- [87].Siegl SJ, Galeta J, Dzijak R, Vázquez A, Del Río-Villanueva M, Dračínský M, Vrabel M, ChemBioChem 2019, 20, 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ravasco JMJM, Coelho JAS, Trindade AF, Afonso CAM, Pure. Appl. Chem. 2019. [Google Scholar]
- [89].Kara SS, Ateş MY, Deveci G, Cetinkaya A, Kahveci MU, J. Polym. Sci. A 2019, 57, 673–680. [Google Scholar]
- [90].Dadhwal S, Fairhall JM, Goswami SK, Hook S, Gamble AB, Chem. Asian J. 2019, 14, 1143–1150. [DOI] [PubMed] [Google Scholar]
- [91].Versteegen RM, ten Hoeve W, Rossin R, de Geus MAR, Janssen HM, Robillard MS, Angew. Chem. Int. Ed. 2018, 57, 10494–10499. [DOI] [PubMed] [Google Scholar]
- [92].Van Der Gracht AMF, De Geus MAR, Camps MGM, Ruckwardt TJ, Sarris AJC, Bremmers J, Maurits E, Pawlak JB, Posthoorn MM, Bonger KM, Filippov DV, Overkleeft HS, Robillard MS, Ossendorp F, Van Kasteren SI, ACS Chem. Biol. 2018, 13, 1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Siegl SJ, Vázquez A, Dzijak R, Dračínský M, Galeta J, Rampmaier R, Klepetářová B, Vrabel M, Chem. Eur. J. 2018, 24, 2426–2432. [DOI] [PubMed] [Google Scholar]
- [94].Demeester KE, Liang H, Jensen MR, Jones ZS, D’Ambrosio EA, Scinto SL, Zhou J, Grimes CL, J. Am. Chem. Soc. 2018, 140, 9458–9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bruins JJ, Blanco-Ania D, Van Der Doef V, Van Delft FL, Albada B, Chem. Commun. 2018, 54, 7338–7341. [DOI] [PubMed] [Google Scholar]
- [96].Bernard S, Kumar RA, Porte K, Thuéry P, Taran F, Audisio D, Eur. J. Org. Chem. 2018, 2000–2008. [Google Scholar]
- [97].Vázquez A, Dzijak R, Dračínský M, Rampmaier R, Siegl SJ, Vrabel M, Angew. Chem. Int. Ed. 2017, 56, 1334–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Siegl SJ, Dzijak R, Vázquez A, Pohl R, Vrabel M, Chem. Sci. 2017, 8, 3593–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Marjanovic J, Baranczak A, Marin V, Stockmann H, Richardson PL, Vasudevan A, MedChemComm 2017, 8, 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rossin R, Van Duijnhoven SMJ, Ten Hoeve W, Janssen HM, Kleijn LHJ, Hoeben FJM, Versteegen RM, Robillard MS, Bioconjugate Chem. 2016, 27. [DOI] [PubMed] [Google Scholar]
- [101].Maggi A, Ruivo E, Fissers J, Vangestel C, Chatterjee S, Joossens J, Sobott F, Staelens S, Stroobants S, Van Der Veken P, Wyffels L, Augustyns K, Org. Biomol. Chem. 2016, 14, 7544–7551. [DOI] [PubMed] [Google Scholar]
- [102].Lorenzo MM, Decker CG, Kahveci MU, Paluck SJ, Maynard HD, Macromol. 2016, 49, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kozma E, Nikić I, Varga BR, Aramburu IV, Kang JH, Fackler OT, Lemke EA, Kele P, ChemBioChem 2016, 17, 1518–1524. [DOI] [PubMed] [Google Scholar]
- [104].Denk C, Svatunek D, Mairinger S, Stanek J, Filip T, Matscheko D, Kuntner C, Wanek T, Mikula H, Bioconjugate Chem. 2016, 27, 1707–1712. [DOI] [PubMed] [Google Scholar]
- [105].Altai M, Perols A, Tsourma M, Mitran B, Honarvar H, Robillard M, Rossin R, ten Hoeve W, Lubberink M, Orlova A, Karlstrom AE, Tolmachev V, J. Nucl. Med. 2016, 57, 431–436. [DOI] [PubMed] [Google Scholar]
- [106].Wang K, Wang D, Ji K, Chen W, Zheng Y, Dai C, Wang B, Org. Biomol. Chem. 2015, 13, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hoffmann JE, Plass T, Nikic I, Aramburu IV, Koehler C, Gillandt H, Lemke EA, Schultz C, Chem. Eur. J. 2015, 21, 12266–12270. [DOI] [PubMed] [Google Scholar]
- [108].Cserép GB, Demeter O, Bätzner E, Kállay M, Wagenknecht HA, Kele P, Synthesis 2015, 47, 2738–2744. [Google Scholar]
- [109].Blizzard RJ, Backus DR, Brown W, Bazewicz CG, Li Y, Mehl RA, J. Am. Chem. Soc. 2015, 137, 10044–10047. [DOI] [PubMed] [Google Scholar]
- [110].Wollack JW, Monson BJ, Dozier JK, Dalluge JJ, Poss K, Hilderbrand SA, Distefano MD, Chem. Biol. Drug. Des. 2014, 84, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mejía Oneto JM, Gupta M, Leach JK, Lee M, Sutcliffe JL, Acta Biomaterialia 2014, 10, 5099–5105. [DOI] [PubMed] [Google Scholar]
- [112].Kurra Y, Odoi KA, Lee YJ, Yang Y, Lu T, Wheeler SE, Torres-Kolbus J, Deiters A, Liu WR, Bioconjugate Chem. 2014, 25, 1730–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Erdmann RS, Takakura H, Thompson AD, Rivera-Molina F, Allgeyer ES, Bewersdorf J, Toomre D, Schepartz A, Angew. Chem. Int. Ed. 2014, 53, 10242–10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Denk C, Svatunek D, Filip T, Wanek T, Lumpi D, Fröhlich J, Kuntner C, Mikula H, Angew. Chem. Int. Ed. 2014, 53, 9655–9659. [DOI] [PubMed] [Google Scholar]
- [115].Versteegen RM, Rossin R, Ten Hoeve W, Janssen HM, Robillard MS, Angew. Chem. Int. Ed. 2013, 52, 14112–14116. [DOI] [PubMed] [Google Scholar]
- [116].Emmetiere F, Irwin C, Viola-Villegas NT, Longo V, Cheal SM, Zanzonico P, Pillarsetty N, Weber WA, Lewis JS, Reiner T, Bioconjugate Chem. 2013, 24, 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yang J, Šečkute J, Cole CM, Devaraj NK, Angew. Chem. Int. Ed. 2012, 51, 7476–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Plass T, Milles S, Koehler C, Szymański J, Mueller R, Wießler M, Schultz C, Lemke EA, Angew. Chem. Int. Ed. 2012, 51, 4166–4170. [DOI] [PubMed] [Google Scholar]
- [119].Keliher EJ, Reiner T, Turetsky A, Hilderbrand SA, Weissleder R, ChemMedChem 2011, 6, 424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rossin R, Van Den Bosch SM, Ten Hoeve W, Carvelli M, Versteegen RM, Lub J, Robillard MS, Bioconjugate Chem. 2013, 24, 1210–1217. [DOI] [PubMed] [Google Scholar]
- [121].Schoch J, Staudt M, Samanta A, Wiessler M, Jäschke A, Bioconjugate Chem. 2012, 23, 1382–1386. [DOI] [PubMed] [Google Scholar]
- [122].Svatunek D, Denk C, Rosecker V, Sohr B, Hametner C, Allmaier G, Frohlich J, Mikula H, Monatsh. Chem. 2016, 147, 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Billaud EMF, Shahbazali E, Ahamed M, Cleeren F, Noel T, Koole M, Verbruggen A, Hessel V, Bormans G, Chem. Sci. 2017, 8, 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Igawa K, Machida K, Noguchi K, Uehara K, Tomooka K, J. Org. Chem. 2016, 81, 11587–11593. [DOI] [PubMed] [Google Scholar]
- [125].Nubbemeyer U, Eur. J. Org. Chem. 2001, 1801–1816. [Google Scholar]
- [126].Edstrom ED, J. Am. Chem. Soc. 1991, 113, 6690–6692. [Google Scholar]
- [127].Sudau A, Munch W, Nubbemeyer U, Bats JW, J. Org. Chem. 2000, 65, 1710–1720. [DOI] [PubMed] [Google Scholar]
- [128].Surprenant S, Lubell WD, Org. Lett. 2006, 8, 2851–2854. [DOI] [PubMed] [Google Scholar]
- [129].Cerè V, Peri F, Pollicino S, Antonio A, J. Chem. Soc., Perkin Trans. 2 1998, 977–980. [Google Scholar]
- [130].Cere V, Paolucci C, Pollicino S, Sandri E, Fava A, J. Org. Chem. 1991, 56, 4513–4520. [Google Scholar]
- [131].Ceré V, Paolucci C, Pollicino S, Sandri E, Fava A, J. Org. Chem. 1992, 57, 1457–1461. [Google Scholar]
- [132].Royzen M, Taylor MT, DeAngelis A, Fox JM, Chem. Sci. 2011, 2, 2162–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Lauritsen A, Madsen R, Org. Biomol. Chem. 2006, 4, 2898–2905. [DOI] [PubMed] [Google Scholar]
- [134].Scinto SL, Ekanayake O, Seneviratne UI, Pigga JE, Brannick SJ, Taylor MT, Liu J, am Ende CW, Rozovsky S, Fox JM, J. Am. Chem. Soc. 2019, 141, 10932–10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Fang Y, Judkins JC, Boyd SJ, am Ende CW, Rohlfing K, Huang Z, Xie Y, Johnson DS, Fox JM, Tetrahedron 2019, 75, 4307–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].O’Brien JGK, Chintala SR, Fox JM, J. Org. Chem. 2018, 83, 7500–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang M, Vannam R, Lambert WD, Xie Y, Wang H, Giglio B, Ma X, Wu Z, Fox J, Li Z, Chem. Commun. 2019, 55, 2485–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Uttamapinant C, Howe JD, Lang K, Beranek V, Davis L, Mahesh M, Barry NP, Chin JW, J. Am. Chem. Soc. 2015, 137, 4602–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Jendralla H, Tetrahedron 1983, 39, 1359–1363. [Google Scholar]
- [140].Lambert WD, Scinto SL, Dmitrenko O, Boyd SJ, Magboo R, Mehl RA, Chin JW, Fox JM, Wallace S, Org. Biomol. Chem. 2017, 15, 7476–7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Prescher JA, Bertozzi CR, Nat. Chem. Biol. 2005, 1, 13–21. [DOI] [PubMed] [Google Scholar]
- [142].Murrey HE, Judkins JC, am Ende CW, Ballard TE, Fang Y, Riccardi K, Di L, Guilmette ER, Schwartz JW, Fox JM, Johnson DS, J. Am. Chem. Soc. 2015, 137, 11461–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Corey EJ, Carey FA, Winter RAE, J. Am. Chem. Soc. 1965, 87, 934–934. [Google Scholar]
- [144].Marshall JA, Hammond CS, Turro NJ, Leermakers PA, Acc. Chem. Res. 1969, 2, 33–40. [Google Scholar]
- [145].Hoffmann R, Inoue Y, J. Am. Chem. Soc. 1999, 121, 10702–10710. [Google Scholar]
- [146].Inoue Y, Ueoka T, Kuroda T, Hakushi T, Chem. Commun. 1981, 1031–1031. [Google Scholar]
- [147].Squillacote ME, DeFellipis J, Shu Q, J. Am. Chem. Soc. 2005, 127, 15983–15988. [DOI] [PubMed] [Google Scholar]
- [148].Wallraff GM, Michl J, J. Org. Chem. 1986, 51, 1794–1800. [Google Scholar]
- [149].Evers JTM, Mackor A, Recl. Trav. Chim. Pays-Bas 1979, 98, 423–423. [Google Scholar]
- [150].Jendralla H, Chem. Ber. 1980, 113, 3557–3569. [Google Scholar]
- [151].Nishiyama H, Naitoh T, Motoyama Y, Aoki K, Chem. Eur. J. 1999, 5, 3509–3513. [Google Scholar]
- [152].Fang Y, Zhang H, Huang Z, Scinto SL, Yang JC, am Ende CW, Dmitrenko O, Johnson DS, Fox JM, Chem. Sci. 2018, 9, 1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]