Abstract
Like many seemingly inhospitable environments on our planet, the highly acidic human stomach harbors a diverse bacterial microflora. The best-known member of the human gastric flora, Helicobacter pylori, causes a number of gastric diseases, including peptic ulcer disease and gastric adenocarcinoma. In the absence of Helicobacter pylori infection, the gastric microbiota displays some features similar to the oral cavity with Firmicutes the most common phylum, followed by Proteobacteria and Bacteroidetes. When present, H. pylori dominates the gastric microbiome and reduces diversity and composition of other taxa. The composition of the gastric microbiome also is altered in the setting of proton pump inhibitor therapy and gastric neoplasia. This review summarizes foundational and recent studies that have investigated the composition of the human gastric microbiome in a variety of patient groups, with a focus on potential mechanisms involved in regulation of gastric microbial community structure.
Keywords: Microbiome, Stomach, Helicobacter, gastric cancer
1. Introduction
Although studies from as early as the 1940s and 1950s describe bacteria in human gastric samples [1–4], the stomach has generally been considered an inhospitable environment for microbes due to its high level of acidity [5]. This concept was challenged by Marshall and Warren’s discovery of Helicobacter pylori (H. pylori) as a highly common stomach-specific pathogen in 1983 [6]. The emergence of culture-independent high throughput and next-generation sequencing methods in the mid-2000s opened the door for in depth analyses of microbiota in different environments, including the stomach [7–10]. In a hallmark study from 2006, Bik et al. were the first to use unbiased 16S ribosomal RNA (rRNA) gene sequencing to characterize bacterial diversity in the human gastric mucosa [7]. Since 2010, more than 300 primary research papers focusing on the gastric microbiome have been published. However, the role of the gastric microbiota for human health and H. pylori-associated disease processes is not fully understood. This review will highlight and summarize findings from the past three years (2018–2021) that have provided key insights into the interactions between H. pylori and the gastric microbiome in human health and disease.
2. The gastric microbiota: H. pylori and beyond
While not a sterile environment, the human stomach harbors several orders of magnitude fewer culturable bacteria than the small and large intestine, with only 102-104 colony forming units (CFUs) per mL content, compared with 1010-1012 CFUs per mL in the colon [8]. H. pylori is the most well-known and clinically relevant member of the human gastric microbiota and is associated with the development of peptic ulcer disease, gastric adenocarcinoma and MALT lymphoma [11]. Since only a small proportion of H. pylori-infected humans, mainly adults, develop clinical disease, H. pylori is considered a pathobiont, a natural member of the microbiota that has pathogenic potential under certain conditions. A key feature of H. pylori that enables its survival in the acidic gastric lumen is the ability to convert urea to ammonia using the enzyme urease, which leads to local neutralization of the gastric acid around the bacteria [12]. When H. pylori is present, this species dominates the gastric microbiome, representing up to 72% of culturable gastric bacteria and up to 97% of transcriptionally active taxa [7,13].
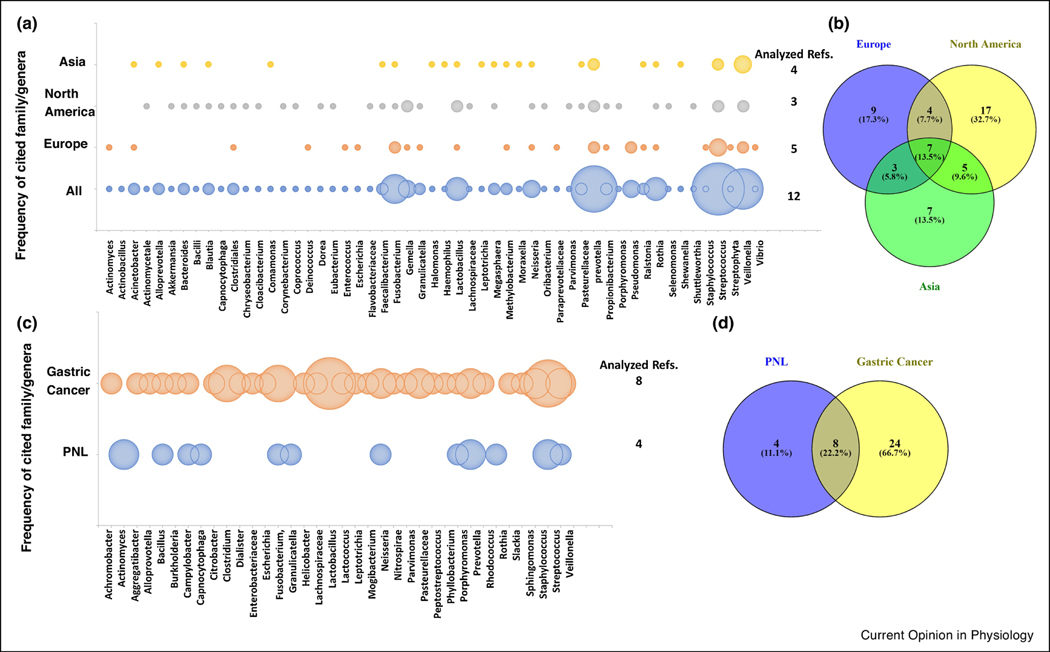
In the absence of H. pylori, the microbiota of the stomach were found to be less diverse, based on the richness (number) and abundance (frequency) of the bacteria, than those in the ileum and colon [14,15]. Whether gastric bacteria represent a truly resident flora has been a matter of debate that has recently been resolved by Spiegelhauer et al. who found no significant difference between tissue-adherent and non-adherent gastric bacteria [16]. Representative taxa from five major bacterial phyla are routinely detected in H. pylori negative gastric samples, with Firmicutes being the most common, followed by Proteobacteria, Bacteroidetes, Actinobacteria and Fusobacteria [7,8,17–19]. How these gastric microbes survive in the acidic environment of the stomach is not well understood, but periplasmic chaperones that protect protein structures and specific transcriptional pathways involved in the acid tolerance response and the amino acid-dependent extreme acid resistance have been implicated as protective mechanisms [20]. Notably, Firmicute classes in the stomach are mostly composed of Bacilli and Negativicutes as opposed to Clostridia and Erysipelotrichia, which dominate in the lower gastrointestinal tract [21]. An overview of commonly detected bacterial taxa in non-H. pylori infected stomach is provided in Fig. 1A,B. At the genus level, Prevotella, Streptococcus, Neisseria, Lactobacillus, Veillonella, Gemella, Fusobacterium, Rothia and Haemophilus have been detected as dominant taxa in the healthy stomach in a majority of studies [8,13,18,21–24]. While the majority of studies have focused on antral and corpus mucosa, a study investigating the cardiac region found Halomonas, Shewanella, and Comamonasas to be the most prevalent genera [25]. In total, 57 bacterial genera from eight major phyla have been reported in >20% of the studies included in a recent systematic review and were therefore considered typical gastric bacteria [22]. Importantly, the gastric microbiota showed significant similarity with the microbiota of the duodenum, oral cavity and esophagus, but differed from the colonic microbiome [15,18,21]. Moreover, microbiota in the stomach were more dissimilar across individuals than in other parts of the gastrointestinal tract [8,18,21,22]. This high degree of inter-individual variability may partly explain the discrepant findings obtained in different studies, especially if only a limited number of subjects were included. Further differences in detected taxa appear to be associated with the geographical origin of the samples analyzed (Fig. 1A,B).
Figure 1.
Commonly reported taxa at the genus/family level according to geographical location or presence of gastric lesions. (A) Bubble plot showing frequency of detection (not abundance) for different taxa in H. pylori-free, non-inflamed stomachs according to geographical origin of the samples [15,18,21,22,25,60]. Size of the bubbles represents the number of papers that reported each taxon as present. (B) Venn diagram of the data in (A) showing the number of taxa that were represented in a specific location or were shared between locations. (C) Bubble plot showing frequency of differentially expressed taxa in stomachs with gastric cancer or with preneoplastic lesions (PNL) including atrophic gastritis and intestinal metaplasia and compared to H. pylori gastritis controls. Data were obtained from available comparison plots between PNL and H. pylori gastritis and gastric cancer and H. pylori gastritis [22,42,54–57,59]. Size of the bubbles represents the number of papers that cited each taxon as differentially expressed between lesions and gastritis. (D) Venn diagram of the data in (C) showing the number of taxa that were represented in PNL and gastric cancer or were shared between both.
3. Crosstalk between H. pylori and other gastric microbes alters microbial colonization of the stomach and other sites
Since chronic H. pylori is known to change gastric physiology, including luminal pH and mucin structure [26,27], it has been proposed that H. pylori infection impacts other gastric microbes. Mathematical modeling has demonstrated that a minority of bacterial species are dominant drivers of the overall bacterial community structure [28]. However, to what extent H. pylori infection alters the composition of the gastric microbiome is still a matter of debate. The majority of studies has shown that H. pylori colonization in the absence of gastric atrophy or cancer was associated with a decreased diversity of other taxa [8,13,21,23,24,29,30]. This observation can be partly explained by the dominant abundance of H. pylori in the H. pylori-infected stomach [13]. Other reports failed to demonstrate significant changes in the gastric community structure associated with H. pylori infection or showed an inverse relationship [7,31,32], and one study found significant changes only in subjects infected with CagA-positive H. pylori [33]. In H. pylori-infected subjects, the flora was dominated by H. pylori, but, non-Helicobacter-Proteobacteria [32], Lactobacillus sp., Acinetobacter ursingii, and Streptococcus agalactiae [25] also were increased. Specific taxa found to be decreased in H. pylori-infected samples include Streptococcaceae [24,29] and other Firmicutes [32,33], Actynomicetaceae [29], and Roseburia [33]. The composition of the gastric microbiota in H. pylori-infected subjects may additionally be influenced by disease stage. Thus, different microbial taxa dominated the gastric microbiota in dyspeptic vs. non-dyspeptic patients [34] and in patients with different degrees of inflammation and pathology [23,25]. Certain bacterial strains in the stomach can also interfere with H. pylori infection. Thus, both certain Lactobacilli and Streptococcus mitis were associated with decreased H. pylori growth [5]. Notably, in a recent systematic review that included 65 papers, no overarching gastric microbiome signatures of health or disease were determined beyond the detrimental effects of H. pylori itself [20]. However, longitudinal studies on gastric microbiota acquisition across the human lifespan are not available at this point. Late adult acquisition of H. pylori, characteristic of the developed countries from which most of the studies originate, may represent more rigid ecosystems in terms of its modification. On the other hand, acquisition of the bacterium in early childhood may lead to a more beneficial interaction with the founder microbiota in terms of later clinical outcomes.
Interestingly, H. pylori infection of the stomach may impact microbiota in other segments of the gastrointestinal tract including the oral cavity, esophagus, duodenum and colon [35–40]. These alterations in microbiome structure can be transmitted vertically, since fecal microbiota from vaginally-delivered babies differed based on maternal H. pylori status [39]. α-Diversity, which measures the diversity of bacterial taxa within each sample, was significantly decreased in the oral cavity of H. pylori-positive individuals [37]. In contrast, H. pylori infection correlated with an increase in both α-diversity and β-diversity, which compares between sample diversity, in the duodenum in a study from South America [35]. Likewise, fecal microbiota were generally found to be more diverse in subjects with H. pylori infection compared to uninfected controls [36,41]. Investigators in Japan found that H. pylori infection was associated with an increase in fecal Lactobacillus salivarius abundance, but a decrease in Lactobacillus acidophilus [40]. A large-scale study with >300 participants from China detected 58 microbial species in the feces that correlated with active H. pylori infection [42], whereas other studies found no significant impact of H. pylori in microbial communities in the lower gastrointestinal tract [21,23].
4. Mechanisms of H. pylori-dependent regulation of gut microbiome structure
Microbial communities are shaped by competition for nutrients and host receptors, bacterial metabolites, and host immune mechanisms including antibacterial peptides [19]. Within the stomach, H. pylori occupies specific microniches in the gastric glands, and founder bacteria prevent colonization of these niches by new H. pylori and possibly other microbes [43]. Environmental conditions such as oxygen levels and pH also influence bacterial community structure. Chronic H. pylori infection generally causes hypochlorhydria, i.e., an elevated gastric pH due to H. pylori-induced suppression of the gastric proton pump or gastric atrophy, although hyperchlorhydria is observed in a subset of patients with antral-predominant gastritis and elevated gastrin levels [44]. Atrophic H. pylori gastritis led to increased bacterial diversity and abundance compared to H. pylori gastritis without atrophy, although the degree to which pH sensitive taxa contribute to these alterations is unclear [12].
Several reports indicate that the host immune response also likely impacts the gastric microbiota. β-Defensin 2, a type of innate antimicrobial factor produced by host epithelia, is upregulated by H. pylori and likely affects growth of other gastric microbes [45]. A recent study in mice focusing on gastric innate lymphoid cells (ILCs) revealed that non-H. pylori microbiota from the order Bacteroidales were essential for maintaining ILC2 populations through IL-7 and IL-33 signaling and that the ILC2s promoted gastric IgA secretion, which in turn restricted H. pylori infection [46]. In an in vitro study with human cells, Lactobacillus rhamnosus increased the H. pylori-induced production of IFN-γ and IL-12 in DC-T cell co-cultures indicating that gastric colonizers such as Lactobacilli can modulate the immune response to H. pylori [47]. H. pylori infection in children causes different disease phenotypes and immune responses than in adults, with a more potent gastric regulatory T-cell response, a reduced Th17 response and more diverse microbiota found in children [32,48,49]. Together, these changes promote reduced gastric inflammation in H. pylori-infected children compared with infected adults. Interestingly, H. pylori infection and gastritis in children also were associated with a significant reduction in the fecal Firmicutes-to-Bacteroidetes ratio, a change typically associated with obesity and metabolic syndrome that has not been reported in H. pylori-infected adults [50]. Therefore, health effects of H. pylori-induced gastric dysbiosis also may differ between children and adults.
H. pylori virulence mechanisms also may impact the composition of other microbes in the stomach. Zhao et al. showed that the presence of the H. pylori CagA gene was associated with to an increased proportion of other Gram-negative bacteria in the stomach, possibly contributing to elevated lipopolysaccharide (LPS) biosynthesis and increased inflammation [33]. Others have linked alterations in the gastric microbiota with H. pylori expression of the CagA virulence factors both in humans and mouse models [33,51]. The mechanisms for this relationship are unclear but could relate to the strong inflammatory response induced by CagA signaling in epithelial cells. Additional studies that include analyses of mucus structure, antimicrobial mediators and bacteriocins are needed to better understand why, how and in which circumstances H. pylori infection causes changes to other gastric microbes.
5. Altered gastric microbiota are associated with gastric carcinogenesis
Changes to the gastric microbiome associated with H. pylori infection have been proposed to contribute to gastric carcinogenesis, based on early animal experiments that demonstrated that lack of a commensal flora prevents H. pylori-induced carcinogenesis in transgenic insulin-gastrin (INS-GAS) mice [52]. H. pylori virulence factors, particularly CagA [53], directly trigger oncogenic pathways, and additional carcinogens likely accelerate gastric tumorigenesis. In a large prospective study that compared gastric biopsies from subjects before and after development of gastric cancer, significant associations between lesion progression and the abundance of Bacillus, Prevotella and Capnocytophaga were found [54]. Overall, multiple studies suggest that the gastric microbiome of cancer patients differs from that of healthy controls and is influenced by disease stage (Fig. 1C,D). In comparison with the microbiota found in patients with chronic H. pylori gastritis, gastric cancer and preneoplastic lesions are significantly associated with decreased species diversity [23,55–57]. Gastric cancer also is associated with an over-representation of specific taxa, including Streptococcus, Peptostreptococcus, Prevotella, and Fusobacterium [19,22,56,58,59]. While the abundance of H. pylori is significantly decreased in cancer compared to chronic H. pylori gastritis, the abundance of non-Helicobacter Proteobacteria may increase [55,59]. Interestingly, dysbiosis associated with gastric malignancy may persist even after H. pylori has been cleared by antibiotic therapy [60]. An intriguing hypothesis for the involvement of non-Helicobacter bacteria such as Lactobacillus, Escherichia coli and Staphylococcus in gastric carcinogenesis is through production of carcinogenic N-nitroso compounds [16,61]. In support of this hypothesis, Ferreira et al. confirmed that taxa with nitrosating functions were overrepresented in gastric cancer compared to chronic gastritis microbiomes [55]. However, other functional metagenome studies have failed to confirm these associations [56,57,62].
As with non-cancerous H. pylori gastritis, altered microbiota associated with stomach cancer were detected in other sections of the gut and in stool samples [63–65]. While the contributions of gut microbes other than H. pylori to gastric cancer progression remain unclear and warrant further investigations, specific changes in the microbiome associated with stomach cancer may have diagnostic potential as disease biomarkers.
6. Impact of H. pylori therapy on gastric microbiota
Standard H. pylori therapies utilize combination antibiotics and proton pump inhibitors (PPIs). As expected, antibiotic treatment for H. pylori infection will initially lead to reduced microbial colonization of the stomach and other sites including the esophagus and small intestine [5,19]. Several studies have shown near complete normalization of the gastric microbiota two months after antibiotic eradication of H. pylori in both children and adults [30,66,67], although two independent studies reported an increase in gastric Acinetobacter after treatment in a subset of patients [62,68]. Overall, microbes other than H. pylori appear to readily re-colonize the stomach following their elimination [23]. Similarly, the intestinal flora fully recovered after antibiotic treatment following an initial decline in α-diversity [19,67,69,70].
Acid-blocking agents, particularly PPIs, appear to have more lasting effects on the gastric microbiome, possibly because they commonly are used long term. Along with H. pylori-infection, PPI therapy is considered one of the two key microbiome-perturbing mechanism in the stomach [71,72]. In general, PPI treatment promotes bacterial growth, with a higher number of culturable bacterial strains in the stomach [73]. 16S rRNA gene sequencing revealed that PPI induces a higher diversity of bacterial species in the stomach with an increased abundance of Firmicutes and Fusobacteria and a decrease in Bacteroidetes [13,14,22]. At the genus level, Streptococcae were most significantly increased in number following PPI application [13,14,73]. Notably, several of the changes observed in PPI-treated subjects were also found in subjects with atrophic gastritis, implicating luminal pH as a major regulator of gastric microbial community structure.
7. Technical considerations of gastric microbiome studies
The majority of recent studies focusing on gastric microbiota have utilized 16S rRNA gene sequencing. Factors that impact the bacterial community structure of the stomach include the geographical origin, ethnicity, diet and age of the subjects, the sampling method, the type of sample used, and the sequencing and data analysis approach [5,22,24,74,75]. Preventing contamination of the sample with throat oral and esophageal bacteria is a challenge that can be overcome with specialized endoscopes or careful technique [73]. Both biopsy samples and gastric fluids have been used in gastric microbiome studies, with fluids harboring a more diverse flora with increased Actinobacteria, Bacteroidetes and Firmicutes, and tissue samples harboring increased Proteobacteria including H. pylori [17].
One drawback of 16S rRNA gene sequencing is that actively growing bacteria cannot be differentiated from inactive or dead bacteria or gene fragments. To address this issue, several recent studies have used a 16S rRNA transcriptomics approach to identify active taxa and have identified a similar distribution of genera as previously described using 16s rRNA gene [13,21]. Alternatively, culture-based analyses, proteomics, or a combination of the two have been successfully applied to characterize gastric microbes including “culturomics”, a combination of high throughput bacterial culture with nine different growth media, followed by identification of bacterial taxa using MALDI-TOF-MS and the bacterial database Biotyper [8,14,34]. Going forward, multi-omics approaches will provide a more accurate assessment of the gastric microbiome.
8. Summary and outlook
A large number of studies from the past decade have provided detailed insights into the gastric microbiome beyond H. pylori. Overall, the stomach appears to harbor a low density, but complex resident flora and that is disrupted in the presence of H. pylori infection, H. pylori-induced gastric pathology and cancer. However, impacts of altered gastric microbiota other than H. pylori on human health are unclear, and the majority of studies have been correlative and descriptive. Future studies of the functional interactions between different microbial taxa and their complex relationships with the host should provide more mechanistic insights into the role of gastric microbiota in gastric homeostasis and disease.
Acknowledgements
Funding: This work was supported by the National Institutes of Health [R01GM131408 and U01EB029242; to D.B. and HD088954 to P.D.S.], the Montana Agricultural Experiment Station USDA/NIFA Hatch project [#1026146 to D.B.], a UAB School of Medicine Award, UAB Microbiome Research Center Award and a DiGregorio Family Foundation Award to P.D.S., a Montana INBRE pilot award [P20GM103474; to D.B.] and CONICYT-PIA ANILLO [ACT172097 to P.R.H.].
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barber M, Franklin RH: Bacteriology of stomach and duodenum in cases of peptic ulcer and gastric carcinoma. Br Med J 1946, 1:951–953.*Early paper that demonstrated culture of bacteria, including Streptococci and Lactobacilli, from human gastric contents as well as alterations of the flora composition in achlorhydria.
- 2.Cregan J, Dunlop EE, Hayward NJ: The bacterial content of human small intestine in disease of the stomach. Br Med J 1953, 2:1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howie JW, Duncan IB, Mackie LM: Growth of Clostridium welchii in the stomach after partial gastrectomy. Lancet 1953, 265:1018–1021. [DOI] [PubMed] [Google Scholar]
- 4.Banyai AL: Clinical value of examination of the gastric contents for tubercle bacilli. Am J Med 1948, 4:836–845. [DOI] [PubMed] [Google Scholar]
- 5.Ianiro G, Molina-Infante J, Gasbarrini A: Gastric Microbiota. Helicobacter 2015, 20 Suppl 1:68–71. [DOI] [PubMed] [Google Scholar]
- 6.Marshall BJ, Warren JR: Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1:1311–1315.** Initial description of H. pylori’s association with gastric disease in human subjects.
- 7.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA: Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A 2006, 103:732–737.** First large-scale analysis of gastric microbiota in H. pylori-infected and non-infected human subjects using a 16s rDNA clone library sequencing approach.
- 8.Delgado S, Cabrera-Rubio R, Mira A, Suarez A, Mayo B: Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb Ecol 2013, 65:763–772. [DOI] [PubMed] [Google Scholar]
- 9.Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR, Arrieta JM, Herndl GJ: Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc Natl Acad Sci U S A 2006, 103:12115–12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster SC: Next-generation sequencing transforms today’s biology. Nat Methods 2008, 5:16–18. [DOI] [PubMed] [Google Scholar]
- 11.Reshetnyak VI, Burmistrov AI, Maev IV: Helicobacter pylori: Commensal, symbiont or pathogen? World J Gastroenterol 2021, 27:545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall BJ, Barrett LJ, Prakash C, McCallum RW, Guerrant RL: Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology 1990, 99:697–702. [DOI] [PubMed] [Google Scholar]
- 13.Parsons BN, Ijaz UZ, D’Amore R, Burkitt MD, Eccles R, Lenzi L, Duckworth CA, Moore AR, Tiszlavicz L, Varro A, et al. : Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog 2017, 13:e1006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mailhe M, Ricaboni D, Vitton V, Gonzalez JM, Bachar D, Dubourg G, Cadoret F, Robert C, Delerce J, Levasseur A, et al. : Repertoire of the gut microbiota from stomach to colon using culturomics and next-generation sequencing. BMC Microbiol 2018, 18:157.* This study combined culture techniques and unbiased 16s rRNA sequencing to assess the microbiome in different sections of the human gastrointestinal tract.
- 15.Stearns JC, Lynch MD, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG, Croitoru K, Moreno-Hagelsieb G, Neufeld JD: Bacterial biogeography of the human digestive tract. Sci Rep 2011, 1:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegelhauer MR, Kupcinskas J, Johannesen TB, Urba M, Skieceviciene J, Jonaitis L, Frandsen TH, Kupcinskas L, Fuursted K, Andersen LP: Transient and Persistent Gastric Microbiome: Adherence of Bacteria in Gastric Cancer and Dyspeptic Patient Biopsies after Washing. J Clin Med 2020, 9.* Using a washing protocol for gastric biopsy samples, Spiegelhauer et al. demonstrated that the majority of gastric taxa are derived from mucosa-adherent bacteria and therefore can be considered resident microbiota.
- 17.Sung J, Kim N, Kim J, Jo HJ, Park JH, Nam RH, Seok YJ, Kim YR, Lee DH, Jung HC: Comparison of Gastric Microbiota Between Gastric Juice and Mucosa by Next Generation Sequencing Method. J Cancer Prev 2016, 21:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seekatz AM, Schnizlein MK, Koenigsknecht MJ, Baker JR, Hasler WL, Bleske BE, Young VB, Sun D: Spatial and Temporal Analysis of the Stomach and Small-Intestinal Microbiota in Fasted Healthy Humans. mSphere 2019, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao ZH, Han JX, Fang JY: Helicobacter pylori infection and eradication: Exploring their impacts on the gastrointestinal microbiota. Helicobacter 2020, 25:e12754. [DOI] [PubMed] [Google Scholar]
- 20.Lund P, Tramonti A, De Biase D: Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev 2014, 38:1091–1125. [DOI] [PubMed] [Google Scholar]
- 21.Vasapolli R, Schutte K, Schulz C, Vital M, Schomburg D, Pieper DH, Vilchez-Vargas R, Malfertheiner P: Analysis of Transcriptionally Active Bacteria Throughout the Gastrointestinal Tract of Healthy Individuals. Gastroenterology 2019, 157:1081–1092 e1083.** This study used 16s rRNA gene expression analysis to describe active bacteria in the human gastrointestinal tract. Interestingly, major bacterial taxa identified with this approach were similar to taxa identified using the more common 16s rRNA gene sequencing
- 22.Rajilic-Stojanovic M, Figueiredo C, Smet A, Hansen R, Kupcinskas J, Rokkas T, Andersen L, Machado JC, Ianiro G, Gasbarrini A, et al. : Systematic review: gastric microbiota in health and disease. Aliment Pharmacol Ther 2020, 51:582–602.** Excellent compilation of all relevant, high quality studies published on human gastric microbiota with detailed overview tables.
- 23.Guo Y, Zhang Y, Gerhard M, Gao JJ, Mejias-Luque R, Zhang L, Vieth M, Ma JL, Bajbouj M, Suchanek S, et al. : Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020, 69:1598–1607.* This report demonstrates that successful eradication of H. pylori infection leads to a restoration of the gastric microbiome similar to that of H. pylori-negative subjects.
- 24.Miftahussurur M, Waskito LA, El-Serag HB, Ajami NJ, Nusi IA, Syam AF, Matsumoto T, Rezkitha YAA, Doohan D, Fauzia KA, et al. : Gastric microbiota and Helicobacter pylori in Indonesian population. Helicobacter 2020, 25:e12695. [DOI] [PubMed] [Google Scholar]
- 25.Yan R, Guo Y, Gong Q, Chen M, Guo Y, Yang P, Huang H, Huang H, Huang W, Ma Z, et al. : Microbiological evidences for gastric cardiac microflora dysbiosis inducing the progression of inflammation. J Gastroenterol Hepatol 2020, 35:1032–1041. [DOI] [PubMed] [Google Scholar]
- 26.van Herwaarden MA, Samsom M, Smout AJ: 24-h recording of intragastric pH: technical aspects and clinical relevance. Scand J Gastroenterol Suppl 1999, 230:9–16. [PubMed] [Google Scholar]
- 27.Newton JL, Jordan N, Oliver L, Strugala V, Pearson J, James OF, Allen A: Helicobacter pylori in vivo causes structural changes in the adherent gastric mucus layer but barrier thickness is not compromised. Gut 1998, 43:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang X, Li X, Yang L, Liu C, Wang Q, Chi W, Zhu H: How Microbes Shape Their Communities? A Microbial Community Model Based on Functional Genes. Genomics Proteomics Bioinformatics 2019, 17:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troncoso C, Pavez M, Cerda A, Oporto M, Villarroel D, Hofmann E, Rios E, Sierralta A, Copelli L, Barrientos L: MALDI-TOF MS and 16S RNA Identification of Culturable Gastric Microbiota: Variability Associated with the Presence of Helicobacter pylori. Microorganisms 2020, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serrano CA, Pierre R, Van Der Pol WJ, Morrow CD, Smith PD, Harris PR: Eradication of Helicobacter pylori in Children Restores the Structure of the Gastric Bacterial Community to That of Noninfected Children. Gastroenterology 2019, 157:1673–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan MP, Kaparakis M, Galic M, Pedersen J, Pearse M, Wijburg OL, Janssen PH, Strugnell RA: Chronic Helicobacter pylori infection does not significantly alter the microbiota of the murine stomach. Appl Environ Microbiol 2007, 73:1010–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brawner KM, Kumar R, Serrano CA, Ptacek T, Lefkowitz E, Morrow CD, Zhi D, Kyanam-Kabir-Baig KR, Smythies LE, Harris PR, et al. : Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol 2017, 10:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Gao X, Guo J, Yu D, Xiao Y, Wang H, Li Y: Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter 2019, 24:e12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira V, Abraham P, Nallapeta S, Shetty A: Gastric bacterial Flora in patients Harbouring Helicobacter pylori with or without chronic dyspepsia: analysis with matrix-assisted laser desorption ionization time-of-flight mass spectroscopy. BMC Gastroenterol 2018, 18:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez-Jaramillo A, Baldeon ME, Prado B, Fornasini M, Cohen H, Flores N, Salvador I, Cargua O, Realpe J, Cardenas PA: Duodenal microbiome in patients with or without Helicobacter pylori infection. Helicobacter 2020, 25:e12753. [DOI] [PubMed] [Google Scholar]
- 36.Dash NR, Khoder G, Nada AM, Al Bataineh MT: Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS One 2019, 14:e0218274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chua EG, Chong JY, Lamichhane B, Webberley KM, Marshall BJ, Wise MJ, Tay CY: Gastric Helicobacter pylori infection perturbs human oral microbiota. PeerJ 2019, 7:e6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji Y, Liang X, Lu H: Analysis of by high-throughput sequencing: Helicobacter pylori infection and salivary microbiome. BMC Oral Health 2020, 20:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez CD, Shin H, Troncoso PA, Vera MH, Villagran AA, Rodriguez-Rivera SM, Ortiz MA, Serrano CA, Borzutzky A, Dominguez-Bello MG, et al. : Maternal H. pylori is associated with differential fecal microbiota in infants born by vaginal delivery. Sci Rep 2020, 10:7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iino C, Shimoyama T, Chinda D, Arai T, Chiba D, Nakaji S, Fukuda S: Infection of Helicobacter pylori and Atrophic Gastritis Influence Lactobacillus in Gut Microbiota in a Japanese Population. Front Immunol 2018, 9:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frost F, Kacprowski T, Ruhlemann M, Bang C, Franke A, Zimmermann K, Nauck M, Volker U, Volzke H, Biffar R, et al. : Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci Rep 2019, 9:20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Li Y, Zhong H, Ding Q, Lin Y, Tang S, Zong Y, Wang Q, Zhang X, Yang H, et al. : Alterations in the human gut microbiome associated with Helicobacter pylori infection. FEBS Open Bio 2019, 9:1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fung C, Tan S, Nakajima M, Skoog EC, Camarillo-Guerrero LF, Klein JA, Lawley TD, Solnick JV, Fukami T, Amieva MR: High-resolution mapping reveals that microniches in the gastric glands control Helicobacter pylori colonization of the stomach. PLoS Biol 2019, 17:e3000231.** Fundamental publication demonstrating local colonization resistance conferred by H. pylori colonization of individual gastric glands.
- 44.Smolka AJ, Schubert ML: Helicobacter pylori-Induced Changes in Gastric Acid Secretion and Upper Gastrointestinal Disease. In Molecular Pathogenesis and Signal Transduction by Helicobacter pylori. Edited by Tegtmeyer N, Backert S: Springer International Publishing; 2017:227–252. [DOI] [PubMed] [Google Scholar]
- 45.Pero R, Brancaccio M, Laneri S, Biasi MG, Lombardo B, Scudiero O: A Novel View of Human Helicobacter pylori Infections: Interplay between Microbiota and Beta-Defensins. Biomolecules 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satoh-Takayama N, Kato T, Motomura Y, Kageyama T, Taguchi-Atarashi N, Kinoshita-Daitoku R, Kuroda E, Di Santo JP, Mimuro H, Moro K, et al. : Bacteria-Induced Group 2 Innate Lymphoid Cells in the Stomach Provide Immune Protection through Induction of IgA. Immunity 2020, 52:635–649 e634.** This study demonstrated a functional pathway involved in the regulation of gastric microbiota through innate lymphoid cells and IgA.
- 47.Wiese-Szadkowska M, Helmin-Basa A, Eljaszewicz A, Gackowska L, Januszewska M, Motyl I, Andryszczyk M, Wieczynska J, Michalkiewicz J: Selected commensal bacteria change profiles of Helicobacter pylori-induced T cells via dendritic cell modulation. Helicobacter 2019, 24:e12614. [DOI] [PubMed] [Google Scholar]
- 48.Serrano C, Wright SW, Bimczok D, Shaffer CL, Cover TL, Venegas A, Salazar MG,Smythies LE, Harris PR, Smith PD: Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children. Mucosal Immunol 2013, 6:950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Pena A, Rollan A, Viviani P, Guiraldes E, et al. : Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 2008, 134:491–499. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Zhang J, Xu J, Wei X, Yang J, Liu Y, Li H, Zhao C, Wang Y, Zhang L, et al. : Helicobacter pylori Infection Aggravates Dysbiosis of Gut Microbiome in Children With Gastritis. Front Cell Infect Microbiol 2019, 9:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noto JM, Zackular JP, Varga MG, Delgado A, Romero-Gallo J, Scholz MB, Piazuelo MB, Skaar EP, Peek RM Jr.: Modification of the Gastric Mucosal Microbiota by a Strain-Specific Helicobacter pylori Oncoprotein and Carcinogenic Histologic Phenotype. mBio 2019, 10.* Findings from this study indicate that H. pylori CagA is involved in the modulation of gastric microbial diversity and community structure associated with H. pylori infection.
- 52.Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, et al. : Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 2011, 140:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amieva M, Peek RM Jr.: Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 2016, 150:64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadeerhan G, Gerhard M, Gao JJ, Mejias-Luque R, Zhang L, Vieth M, Ma JL, Bajbouj M, Suchanek S, Liu WD, et al. : Microbiota alteration at different stages in gastric lesion progression: a population-based study in Linqu, China. Am J Cancer Res 2021, 11:561–575. [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C: Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67:226–236.** This recent study found that pathways involved in the generation of carcinogenic nitrite and nitric oxide were upregulated in microbiota from subjects with gastric carcinoma, supporting a hypothesis on the role of non-H. pylori bacteria in gastric carcinogenesis that was originally proposed by Mowat et al. in 2000.
- 56.Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, Wu WK, Wong SH, Chen Z, Sung JJY, et al. : Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu YL, Pang W, Huang Y, Zhang Y, Zhang CJ: The Gastric Microbiome Is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Front Cell Infect Microbiol 2018, 8:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sjostedt S, Kager L, Heimdahl A, Nord CE: Microbial colonization of tumors in relation to the upper gastrointestinal tract in patients with gastric carcinoma. Ann Surg 1988, 207:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu G, Torres J, Hu N, Medrano-Guzman R, Herrera-Goepfert R, Humphrys MS, Wang L, Wang C, Ding T, Ravel J, et al. : Molecular Characterization of the Human Stomach Microbiota in Gastric Cancer Patients. Front Cell Infect Microbiol 2017, 7:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe T, Nadatani Y, Suda W, Higashimori A, Otani K, Fukunaga S, Hosomi S, Tanaka F, Nagami Y, Taira K, et al. : Long-term persistence of gastric dysbiosis after eradication of Helicobacter pylori in patients who underwent endoscopic submucosal dissection for early gastric cancer. Gastric Cancer 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mowat C, Williams C, Gillen D, Hossack M, Gilmour D, Carswell A, Wirz A, Preston T, McColl KE: Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation. Gastroenterology 2000, 119:339–347. [DOI] [PubMed] [Google Scholar]
- 62.Sung JJY, Coker OO, Chu E, Szeto CH, Luk STY, Lau HCH, Yu J: Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020, 69:1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youssef O, Lahti L, Kokkola A, Karla T, Tikkanen M, Ehsan H, Carpelan-Holmstrom M, Koskensalo S, Bohling T, Rautelin H, et al. : Stool Microbiota Composition Differs in Patients with Stomach, Colon, and Rectal Neoplasms. Dig Dis Sci 2018, 63:2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou G, Yang J: Correlations of gastrointestinal hormones with inflammation and intestinal flora in patients with gastric cancer. J BUON 2019, 24:1595–1600. [PubMed] [Google Scholar]
- 65.Qi YF, Sun JN, Ren LF, Cao XL, Dong JH, Tao K, Guan XM, Cui YN, Su W: Intestinal Microbiota Is Altered in Patients with Gastric Cancer from Shanxi Province, China. Dig Dis Sci 2019, 64:1193–1203. [DOI] [PubMed] [Google Scholar]
- 66.Gudra D, Pupola D, Skenders G, Leja M, Radovica-Spalvina I, Gorskis H, Vangravs R, Fridmanis D: Lack of significant differences between gastrointestinal tract microbial population structure of Helicobacter pylori-infected subjects before and 2 years after a single eradication event. Helicobacter 2020, 25:e12748. [DOI] [PubMed] [Google Scholar]
- 67.He C, Peng C, Wang H, Ouyang Y, Zhu Z, Shu X, Zhu Y, Lu N: The eradication of Helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults. Helicobacter 2019, 24:e12590. [DOI] [PubMed] [Google Scholar]
- 68.Shin CM, Kim N, Park JH, Lee DH: Changes in Gastric Corpus Microbiota With Age and After Helicobacter pylori Eradication: A Long-Term Follow-Up Study. Front Microbiol 2020, 11:621879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kakiuchi T, Yamamoto K, Imamura I, Hashiguchi K, Kawakubo H, Yamaguchi D, Fujioka Y, Okuda M: Gut microbiota changes related to Helicobacter pylori eradication with vonoprazan containing triple therapy among adolescents: a prospective multicenter study. Sci Rep 2021, 11:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liou JM, Chen CC, Chang CM, Fang YJ, Bair MJ, Chen PY, Chang CY, Hsu YC, Chen MJ, Chen CC, et al. : Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis 2019, 19:1109–1120. [DOI] [PubMed] [Google Scholar]
- 71.Ohno H, Satoh-Takayama N: Stomach microbiota, Helicobacter pylori, and group 2 innate lymphoid cells. Exp Mol Med 2020, 52:1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang I, Nell S, Suerbaum S: Survival in hostile territory: the microbiota of the stomach. FEMS Microbiol Rev 2013, 37:736–761. [DOI] [PubMed] [Google Scholar]
- 73.Arroyo Vazquez JA, Henning C, Park PO, Bergstrom M: Bacterial colonization of the stomach and duodenum in a Swedish population with and without proton pump inhibitor treatment. JGH Open 2020, 4:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He C, Cheng D, Peng C, Li Y, Zhu Y, Lu N: High-Fat Diet Induces Dysbiosis of Gastric Microbiota Prior to Gut Microbiota in Association With Metabolic Disorders in Mice. Front Microbiol 2018, 9:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, Li W, Wang X, Ding C, Liu J, Li Y, Li W, Sun Y: High-Salt Diet-Induced Gastritis in C57BL/6 Mice is Associated with Microbial Dysbiosis and Alleviated by a Buckwheat Diet. Mol Nutr Food Res 2020, 64:e1900965. [DOI] [PubMed] [Google Scholar]