Abstract
Although primarily affecting the respiratory system, COVID-19 causes multiple organ damage. One of its grave consequences is a prothrombotic state that manifests as thrombotic, microthrombotic and thromboembolic events. Therefore, understanding the effect of antiplatelet and anticoagulation therapy in the context of COVID-19 treatment is important. The aim of this rapid review was to highlight the role of thrombosis in COVID-19 and to provide new insights on the use of antithrombotic therapy in its management. A rapid systematic review was performed using preferred reporting items for systematic reviews. Papers published in English on antithrombotic agent use and COVID-19 complications were eligible. Results showed that the use of anticoagulants increased survival and reduced thromboembolic events in patients. However, despite the use of anticoagulants, patients still suffered thrombotic events likely due to heparin resistance. Data on antiplatelet use in combination with anticoagulants in the setting of COVID-19 are quite scarce. Current side effects of anticoagulation therapy emphasise the need to update treatment guidelines. In this rapid review, we address a possible modulatory role of antiplatelet and anticoagulant combination against COVID-19 pathogenesis. This combination may be an effective form of adjuvant therapy against COVID-19 infection. However, further studies are needed to elucidate potential risks and benefits associated with this combination.
Keywords: epidemiology, pharmacology, COVID-19
Introduction
Coronaviruses are a major cause of public health concern due to their highly infectious nature and propensity to cause acute outbreaks as portrayed historically by severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome.1 2 COVID-19 proves to be no exception. As of 3 December 2020, there have been more than 60 million confirmed cases and more than 1.4 million deaths worldwide related to COVID-19 according to the latest report by the WHO.3
Although primarily affecting the respiratory system, COVID-19 causes multiple organ damage. After transmission via droplet inhalation, the virus (SARS-CoV-2) propagates within the respiratory tract and attaches to the lung epithelium via ACE2 expressed on the cell’s surface.4 5 Successful containment of the infection by the immune system is essential for viral eradication and disease resolution. However, some patients develop a maladaptive immune response against the virus marked by hypercytokinaemia (cytokine storm), resulting in constant viral shedding and progression to advanced disease.6 Extrapulmonary manifestations of severe COVID-19 involve complications related to the heart, kidney, bladder, oesophagus and ileum due to elevated ACE2 expression within these organs.7
COVID-19-associated thrombosis and coagulopathy are a major cause of morbidity and mortality in affected patients with the underlying mechanisms incompletely understood. The disease itself causes a hypercoagulable state by altering the natural balance of circulating prothrombotic factors in severe infections.8 9 Moreover, hyperviscosity was demonstrated in a series of 15 critically ill patients wherein plasma viscosity as assessed by capillary viscometry was elevated.10 Endothelial dysfunction may be the common pathophysiological process underlying these complications, as SARS-CoV-2 exhibits tropism towards the endothelial tissue and previous coronaviruses have been implicated in endothelial dysfunction as well.8–10 Endothelial cells play a vital role in maintaining homeostasis by secreting factors that regulate blood flow, vascular tone, coagulation and vessel wall inflammation.11 Disruption of normal endothelial function results in an imbalance which favours a proinflammatory and procoagulant state.12 Infection of endothelial cells by SARS-CoV-2 through ACE2 results in the internalisation and downregulation of this receptor, which prevents its normal functioning. The end result of this downregulation would be an increase in prothrombotic signalling due to the accumulation of angiotensin II.13 SARS-CoV-2 further leads to the activation of the complement system, primarily through the lectin pathway, resulting in endothelial injury and the production of more prothrombotic factors.12–14 It is through these diverse and interconnected systems that COVID-19 favours a prothrombotic environment which clinically manifests as thromboembolic and microthrombotic phenomena.15
Although further studies are required to fully understand COVID-19 aetiology, coagulopathy appears to be linked to its severe manifestations and complications.16 17 A model wherein endothelial dysfunction provides adequate explanation for COVID-19’s diverse clinical manifestations and guides future endeavours in treatment and patient management is needed.18 Consequently, it is vital to address antiplatelet and anticoagulation therapy in this context. The aims of this rapid review were to highlight the role of thrombosis in COVID-19 and to provide new insight regarding the use of antithrombotic therapy in its management.
Methods
This review was performed using a rapid review methodology in which the steps of a systematic review are streamlined or accelerated to produce evidence in a shortened time frame.19 A comprehensive systematic literature search was conducted to examine the possible use of anticoagulation and antiplatelet therapies in reducing thrombotic events associated with COVID-19. Four major databases were searched: Ovid MEDLINE, Web of Science, PubMed and Google Scholar from March 2020 until January 2021. The following combination of key terms were employed: combination 1: “COVID-19” OR “SARS” AND “platelet aggregation inhibitors” AND “anticoagulants”; combination 2: “COVID-19” OR “SARS” AND “anticoagulants” OR “platelet aggregation inhibitors”. Moreover, bibliographies of relevant studies were hand-searched.
Studies included were in English and had clearly defined outcomes such as patient mortality, survival rates and thrombotic status. Studies without a clearly defined untreated control group and that did not clearly define whether antithrombotic treatment was administered before or after COVID-19 infection or if it was administered as a treatment for any other comorbidity were excluded. Also, studies with a primary endpoint other than mortality and/or the development of thrombotic or thromboembolic events were excluded. Review articles, case reports, case series, commentaries and short communications were not included. One author (RF) performed title/abstract screening and data extraction. A stringent examination of study methods was performed before data extraction to ensure the quality of each study and the applicability of all eligibility criteria. Discrepancies regarding the inclusion or exclusion of articles were resolved by discussion between authors (KM, RF and CC) until consensus was reached.
A data extraction form was developed and piloted and then used to extract study details, antithrombotic therapies used, and complications and outcomes. Extracted data for each source document were verified by a second author (CC), and any discrepancies found in the verification stage were adjudicated by a third author (KM).
Results
Results from databases and bibliographical search yielded 1577 articles (figure 1). After title and abstract screening, deduplication and full-text assessment, 12 articles met the inclusion criteria. Study characteristics of the papers included in this review can be found in online supplemental table 1.
Figure 1.
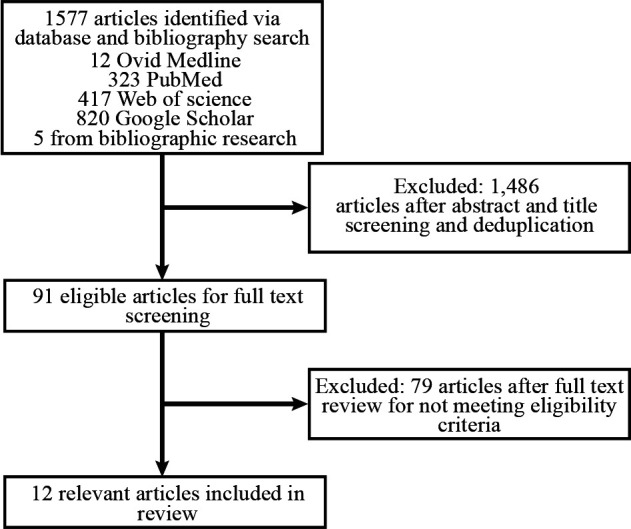
Study flowchart.
openhrt-2021-001628supp001.pdf (248KB, pdf)
Antithrombotic therapy and mortality
Nadkarni et al investigated the impact of prophylactic and therapeutic dose heparin or direct oral anticoagulants on the mortality of 2859 patients with COVID-19 as compared with 1530 non-anticoagulant users in USA.20 Based on the analysis, it was evident that all patients treated with an anticoagulant had a reduced mortality when compared with those not treated. Both prophylactic and therapeutic regimens (HR 0.53, 95% CI 0.45 to 0.62, p<0.001) significantly reduced patient mortality compared with non- anticoagulant-treated patients, with therapeutic dose anticoagulant (HR 0.50, 95% CI 0.45 to 0.57, p<0.001) being associated with a slightly reduced hazard of mortality when compared with prophylactic dose anticoagulant.20 Interestingly, when compared with non-anticoagulant users, the incidence of intubation was reduced in both prophylactic (HR 0.72, 95% CI 0.58 to 0.89, p=0.003) and therapeutic (HR 0.69, 95% CI 0.51 to 0.94, p=0.02) anticoagulants and subgroups.20 These findings were corroborated by Ayerbe et al, who found that heparin administration in 1734 Spanish patients was associated with a statistically significant decreased risk of mortality among patients with COVID- 19 (age and gender adjusted OR 0.55, 95% CI 0.37 to 0.82, p=0.003). This association remained significant even after adjustment for patient fever (>37°C) and oxygen saturation (levels<90%) (OR 0.54, 95% CI 0.36 to 0.82, p=0.003). The protective association between heparin and mortality was also independent of antiviral and adjunct treatment regimens (OR 0.42, 95% CI 0.26 to 0.66, p<0.001).21 However, no data were provided whether heparin was therapeutic or prophylactic.21 To add, by retrospectively comparing the mortality of 611 patients in Spanish hospitals treated with different doses of low-molecular-weight heparin (LMWH) with those not receiving any anticoagulant treatment, Gonzalez-Porras et al reported a major advantage of high-dose prophylactic LMWH in reducing mortality when compared with low-dose prophylactic LMWH treatment and to the complete absence of treatment.22 Absence of anticoagulant treatment led to a sixfold increase in patient mortality when compared with high-dose LMWH (OR 6.2, 95% CI 2.6 to 14.6), and treatment with low-dose LMWH was associated with a twofold increase in patient mortality when compared with high-dose LMWH (OR 2.0, 95% CI 1.1 to 3.6).22 Bousquet et al reported a 4.2-fold increase in mortality in patients not treated with anticoagulant (HR 4.20, 95% CI 1.36 to 12.9), by reference to those on therapeutic doses and prophylactic treatment increases patient mortality 1.2-fold when compared with therapeutic doses (HR 1.20, 95% CI 0.43 to 3.31).23
On the other hand, Tang et al reported that heparin did not reduce mortality in 99 patients receiving therapeutic anticoagulant treatment compared with the non-anticoagulant group (n=350; 30.3% vs 29.7%, respectively; p=0.910) at a Chinese hospital. This observation was seen regardless of underlying comorbidities such as diabetes, hypertension or heart disease.24 Investigations were also done to assess the change in risk of mortality on treatment with heparin among patients suffering from severe sepsis-induced coagulopathy (SIC) and patients at high risk of developing thrombotic events given their elevated D-dimer levels (>3.0 µg/mL). Patients receiving therapeutic dose heparin exhibited a decrease in mortality when compared with their matched controls. Patients on anticoagulants having an SIC score of ≥4 experienced a 40.0% mortality compared with 64.2% mortality in non-anticoagulant controls (p=0.029), while patients suffering from D-dimer elevation experienced a 32.8% mortality compared with 52.4% mortality in non-AC controls (p=0.017).24 Similarly, Russo et al investigated the effect of antiplatelets (acetylsalicylic acid and P2y12 inhibitor) and anticoagulants (non-vitamin K anticoagulant and vitamin K anticoagulant) on mortality of patients and on development of acute respiratory distress syndrome (ARDS) secondary to COVID-19 infection in 192 patients with COVID-19 admitted to Italian hospitals.25 They reported that regardless of antithrombotic therapy type, mortality remain unchanged. Similarly, there was no change in ARDS outcome in treated patients with COVID-19.25 Patients in this study were not put on antiplatelet and anticoagulant combination therapy; therefore, the synergistic effect of these therapies could not be analysed.25
Antithrombotic therapy and thrombotic events
Thromboembolic events, namely, deep vein thrombosis (DVT) and pulmonary embolisms (PEs), are involved in COVID-19 pathology. Available data indicate the presence of an association between thromboembolic events and higher patient mortality.26 Li et al investigated the possible therapeutic value of anticoagulants (heparin, novel oral anticoagulant and warfarin) administered orally or parenterally27 among 1125 patients admitted to a Chinese hospital. Results showed that the administration of oral or parenteral anticoagulant successfully reduced the risk of venous thrombosis in 249 treated patients when compared with those not treated (p<0.01). Moreover, only heparin significantly reduced the risk of thromboembolism, bleeding and mortality as a composite outcome (HR 0.70, 95% CI 0.51 to 0.95, p=0.02). However, heparin use was not associated with mortality but was associated with a borderline reduction in thromboembolism and bleeding (HR 0.36, 95% CI 0.13 to 1.01, p=0.053). These observed effects were independent of COVID-19 infection severity, presence of comorbidities, surgery, antiviral drugs, immunomodulators, Chinese herb use and antiplatelet medication (aspirin and clopidogrel).27 Fauvel et al described the benefit of administering prophylactic or therapeutic doses of heparin in reducing the incidence of PE by assessing 1240 patients with COVID-19 admitted to multiple French hospitals. Therapeutic dose heparin was associated with a decreased risk of PE (OR 0.87, 95% CI 0.82 to 0.92, p<0.001), and prophylactic dose was also associated with a decreased risk of PE (OR 0.83, 95% CI 0.79 to 0.85, p<0.001).28 It was evident that the incidence of PE in patients with COVID-19 was also associated with higher levels of C reactive protein (OR 1.03, 95% CI 1.01 to 1.04, p=0.001).28
Helms et al investigated the possible benefit of therapeutic and prophylactic dose heparin in 150 patients with COVID-19 in two French hospitals and suffering from ARDS and presenting with elevated fibrinogen levels and systemic inflammation.29 These patients were matched to patients without COVID-19 ARDS to compare the occurrence of thromboembolic complications and PE in the two groups. Contrary to previous findings, it was evident that despite prophylactic or therapeutic anticoagulation with heparin, patients suffering from COVID-19 ARDS were more likely to develop thromboembolic complications (OR 2.6, 95% CI 1.1 to 6.1, p=0.04) and PEs (OR 6.2, 95% CI 1.6 to 23.4, p=0.01) when compared with controls without nCOVID-19 ARDS.29 Moreover, D-dimer levels were higher in patients with COVID-19 ARDS by a mean 4.5-fold increase than in patients with without COVID-19 (p<0.001).29
Antiplatelet use
The use of antiplatelets has also shown benefit among patients with COVID-19, especially in alleviating respiratory symptoms.30 In a case–control study conducted in Italy, five patients presenting with more than a threefold increase in D-dimer levels were placed on fondaparinux and an antiplatelet therapy regimen (aspirin and/or clopidogrel and a continuous infusion of tirofiban), while controls received standard prophylactic heparin infusions only. Treatment with antiplatelets progressively decreased alveolar–arterial O2 gradient by 138 (49) mm Hg (p=0.005) and progressively increased PAO2/FIO2 by 108 (57) mm Hg (p=0.037) during the 7-day observation period. To add, C reactive protein levels were reduced from 62 mg/dL at baseline to 28 mg/dL in response to treatment after 7 days (p=0.044), thus indicating a possible role of combined antiplatelet therapy in reducing endothelial inflammation.30 However, the small sample size of this study does not allow drawing of valid inferences, and more studies with higher power are warranted. Moreover, a retrospective cohort study conducted by Chow et al 31 revealed that patients who had received aspirin within 24 hours of hospital admission had decreased rates of mechanical ventilation (adjusted HR 0.56, 95% CI 0.37 to 0.85, p=0.007), intensive care unit (ICU) admission (adjusted HR 0.57, 95% CI 0.38 to 0.85, p=0.005) and in-hospital mortality (adjusted HR 0.53, 95% CI 0.31 to 0.90, p=0.02). No significant difference was observed between patients who received aspirin and those who did not, in terms of major bleeding (p=0.69) or overt thrombosis (p=0.82).31 In addition, a retrospective study among 42 patients admitted to the ICU with COVID-19 and who received full dose or prophylactic dose of anticoagulation along with aspirin reported no increase in major bleeds.32
Discussion
Amid the COVID-19 pandemic, treatment guidelines for physicians remain inconsistent. An important aspect of COVID-19 progression is the development of coagulopathy, which necessitates the adoption of proper antithrombosis treatment plans that could be administered to patients suffering from mild and moderate to severe diseases. With current guidelines emphasising the importance of thromboprophylaxis in both high-risk and low-risk patients with COVID-19, it has become imperative to assess the efficacy and proper prescription of such treatments.33–35 Overall, the use of anticoagulants has shown an increased survival and reduction in thromboembolic events in patients undergoing treatment when compared with untreated controls. Heparin, whether unfractionated heparin (UFH) or LMWH, was the most common choice of anticoagulant for the management of COVID-19-associated thrombotic events. On the whole, combined antiplatelet and anticoagulant therapy increased patient survival and alleviated respiratory symptoms secondary to PE, which indicates an advantage for combination therapy over antiplatelet or anticoagulant therapy alone.25
Prophylactic versus therapeutic anticoagulants
Clinically, when prescribing anticoagulant regimens, physicians attempt to achieve a balance between healthy blood coagulation and bleeding risk. This balance is contested in COVID-19 as multiple patients develop a hypercoagulable state despite anticoagulation use. The available data indicate that anticoagulation presents patients with a clear advantage over absence of anticoagulation, regardless of minor bleeding risk that accompanies the treatment.20–24 27 28 Thus far, early results of randomised controlled clinical trials on ideal anticoagulant regimen across disease severity in COVID-19 19 have been generated. According to press releases and preprint publications by the multiplatform collaborative clinical trial (ACTIV-4a (NCT04505774), REMAP-CAP (NCT02735707), ATTACC (NCT04372589)), the preliminary results seem to be conflicting across diseases severity.36 37 In moderately ill patients, therapeutic anticoagulation seems to be the preferred regimen.36. Although there was no difference in hospital survival, standard prophylactic dosing is preferred due to more organ support-free days defined as a composite of death, the number of days free of respiratory organ support and cardiovascular organ support, as well as less major bleeding.37 Similar findings in critically ill patients have also been published in the INSPIRATION trial that showed no benefit of intermediate doses of anticoagulation compared with prophylactic doses in terms of the composite endpoint of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation or mortality within 30 days. Less major bleeding was also noted in the prophylactic dose arm.38 Moreover, therapeutic dose anticoagulation seems to reduce endothelial cell lesion (p=0.02), which could also reduce the thromboembolic risk of COVID-19, suggesting another therapeutic target for anticoagulants.39
Bleeding events
Current evidence on the risk of increased bleeding events in patients with COVID-19 receiving therapeutic or prophylactic anticoagulant treatment remains inconclusive. In this context, some data suggest that bleeding events increase in patients placed on antithrombotic therapy especially when comparing high-dose therapies to lower doses (p<0.003).20 22 40 However, other studies indicated lower bleeding incidents in patients treated with therapeutic or prophylactic dose anticoagulant when compared with non-users.24 27 This necessitates further assessment to ascertain whether bleeding risk due to anticoagulant use outweighs the benefits.
C reactive protein
Knowing that C reactive proteins play an important role in activating the blood complement system and that the latter in turn can mediate coagulation increased C reactive protein levels reported in patients with COVID-19 might reflect increased levels of systemic coagulopathy, along with increased inflammatory responses that could be attributed to endothelial dysfunction.20 28 41 42 Complement activation has been well reported in patients with COVID-19, but its root causes remain to be properly delineated.43–45
Heparin resistance
A possible explanation for the failure of heparin to properly inhibit or reduce coagulation in patients with COVID-19 could be the development of heparin resistance in a select group of patients suffering from aggravated disease status. Patients prone to heparin resistance commonly present with a deficiency in antithrombin III, increased fibrinogen and D-dimer level.46 47 Both fibrinogen levels and D-dimer levels have been shown to be elevated in patients with COVID-19, especially in severe cases that develop thromboembolic complications and only respond to higher doses of antithrombotic treatment.9 22–24 29 30 48 49 Additionally, heparin resistance has been reported in patients with COVID-19.50 51 and could therefore explain the failure of antithrombotic therapies in some patients in reducing coagulopathy. The administration of antithrombin III proved useful in abolishing heparin resistance in patients who underwent cardiac surgery and could therefore possibly benefit patients with COVID-19.47 The impact of heparin resistance should be assessed in patients with COVID-19 to identify whether it only affects a few isolated cases or it is actually a key player in the COVID-19-induced coagulopathy.
D-dimers
When a blood clot is dissolved, D-dimers are disseminated in the blood as a biproduct of coagulated platelet breakdown.52 Given that coagulopathy is not uncommon in patients with COVID-19, it is expected that D-dimer levels might play an important role in diagnosis or monitoring of patients. Current studies observed elevated D-dimer levels in patients with COVID-19, ranging from a 2.5-fold to a 6.0-fold increase, with these increased levels predicting mortality and the development of thromboembolic events in patients.22–24 29 30 48 Yet, there is no consensus on a specific cut-off level which can accurately predict disease course, mortality or response to antithrombotic therapy. Mouhat et al suggested the use of 2590 ng/mL as threshold level which corresponds to a ≈5-fold increase in normal D-dimer level.53 This 5-fold increase was associated with a 17-fold increase in the incidence of PE among patients (OR 16.9, 95% CI 6.3 to 45.0, p<0.001).53 Similarly, Ventura-Diaz et al reported that a 5.5-fold increase in D-dimer level could predict the occurrence of PE with 81% sensitivity and 59% specificity (p<0.001),54 while Ooi et al examined a larger sample of 974 patients and reported that a 4.5-fold increase in D-dimer level would predict the occurrence of PE with a 72% sensitivity and a 74% specificity.48 Moreover, patients presenting with increased D-dimer levels equivalent to more than fourfolds the normal level exhibit a higher mortality risk when compared with their counterparts, who present with lower levels (OR 10.17, 95% CI 1.10 to 94.38, p=0.041).55 These findings were corroborated by Zhang et al, who had similar findings and showed that D-dimer levels ≥4 times the normal level could predict mortality with 92.3% sensitivity and 83.3%. specificity (HR 51.5, 95% CI 12.9 to 206.7, p<0.001).56 Further research on possible explanations for anticoagulant treatment failure in some patient subgroups is warranted.24 Collectively, these preliminary findings indicate the possible use of D-dimers to guide treatment regimens and to establish different patient subgroups that should receive personalised treatment. However, the use of D-dimer in clinical settings as a stand-alone marker for COVID-19 thrombosis does not seem practical, as a clear cut-off value cannot be easily established since sensitivity of the test is compromised with age.57 58
Arterial thrombotic events
Apart from the venous thrombotic and thromboembolic events, there are also reports of arterial thrombosis including stroke, myocardial infarction, acute limb ischemia, aortic thrombosis and splenic infarcts.59–62 Microvascular thrombosis also is present in COVID-19 disease. Autopsies done on patients who died from COVID-19 infection demonstrated microvascular thrombosis in the lungs. The mechanism of development of this entity is unclear and is thought to be multifactorial.63–66
Guideline recommendations
Guidance on the treatment of COVID-19-associated hypercoagulability has been provided by several international organisations.34 67–69 For outpatients with COVID-19 infection, it is not recommended to initiate anticoagulant and antiplatelet therapy for prevention of venous thromboembolism or arterial thrombosis unless other indications are present.70 However, there is general agreement that thromboprophylaxis should be administered to all patients hospitalised with COVID-19 infection with the prophylactic dose anticoagulation being the backbone of the antithrombotic therapy.34 67–70 Nevertheless, there exist some variations concerning the recommended dosages, duration of treatment and optimal agent. Some societies recommend intermediate dose anticoagulation (eg, enoxaparin 0.5 mg/kg two times per day or 40 mg two times per day or intravenous UFH to achieve antifactor Xa level of 0.3–0.7 units/mL) in a specific subgroup of patients considered at high risk of thrombotic events, whereas others recommend against it.34 67 71 In addition, extended thromboprophylaxis even after patient discharge can be considered in patients at high thrombotic and low bleeding risk.34 67 70 The optimal anticoagulant agent is yet to be determined. Currently, most societies recommend the use of LMWH and UFH as first-line agents for inpatient management of these patients, whereas others recommend the use fondaparinux in addition to the previously mentioned agents.34 71 However, the use of antiplatelet alone for thromboprophylaxis is not recommended.34 When pharmacological anticoagulation is contraindicated, it is recommended to use mechanical thromboprophylaxis.34
Commentary
Coagulation is a highly well-organised procedure that involves the interaction of endothelial cells, platelets and coagulation factors.72 Under physiological conditions, platelets circulate without adhering to intact and inactive endothelia. COVID-19 infection was shown to be highly associated with endothelial dysfunction.73–75 Thus, when endothelial activation and dysfunction occur, disruption of vascular integrity and endothelial cell apoptosis results in exposure of the thrombogenic basement membrane and activation of the clotting cascade by displaying von Willebrand factor, P-selectin and fibrinogen, onto which activated platelets bind and play their primary role in thrombosis.22 76 77 Those activated platelets also produce VEGF, which induces endothelial cells to express the tissue factor, the main activator of the coagulation cascade. The coagulation pathway is also activated when the blood vessels are injured. The transfer of microthrombi into the systemic circulation increases the risk of development of DVT.78 Even though the underlying mechanisms of thrombosis in COVID-19 are incompletely understood, the major contributors of damage are caused by endothelial injury and hypercoagulability.79 80 So far, anticoagulation was associated with better outcomes in patients with COVID-19 with many societies recommending its use as part of the treatment in most patients with COVID-19.20–22 However, these results were not consistent among all studies, with some reporting no added value24 25 and others reporting the occurrence of thrombotic events on anticoagulation.50 51 Recently, increased interest in the use antiplatelets has surfaced for treating and preventing thrombotic events wherein there has been some benefit associated with aspirin use.31 The dual effect of antiplatelet and anticoagulant therapy on COVID-19-induced platelet thrombosis and hypercoagulability, respectively, may result in a synergistic and superior outcome than the use of either medication alone; especially considering that thrombotic manifestations of COVID-19 are heterogenous since they arise from several pathological mechanisms occurring simultaneously. Using a drug combination is not unheard of as it has already been studied in patients with acute coronary syndrome.81
High-quality data to guide the optimal strategy for antithrombotic therapy in patients with COVID-19 are unavailable; enrolment in randomised controlled trials (RCTs) is encouraged and needed to refine guidelines and optimise patient care.82 83 Studies are needed to define the best anticoagulation agent for patients with COVID-19 as so far there is no direct high-certainty evidence which compares different anticoagulants. In addition, examining the role of antiplatelets alone or the combination of anticoagulant and antiplatelet therapies in these patients is highly needed, especially since COVID-19 hypercoagulability is a result of endothelial dysfunction in addition to the activation of the coagulation cascade.22 76–80 To add, the optimal duration for anticoagulation should be determined, especially since COVID-19 has been associated with thrombotic events even after recovery.84 85 Furthermore, the ideal anticoagulation dosage is a matter of huge debate and is yet to be determined by well-conducted studies. Also, studies focusing on antithrombotic strategies in special populations like elderly, pregnant women, children and different ethnicities are also needed.
Limitations
Due to paucity of research on the use of antiplatelet and antithrombotic therapeutics in the treatment of patients with COVID-19, the recommendations made in this review are not conclusive. Moreover, the lack of comparable studies in terms of study population, clinical intervention and disease severity further restricts drawing generalisable conclusions regarding therapeutic efficacy and safety. RCTs remain the gold standard in clinical research in establishing causation; however, the current lack of such studies reduces this review’s ability in drawing conclusions regarding safety, tolerability and efficacy of the proposed treatments in patients with COVID-19. Evidence synthesised from retrospective and prospective cohorts should therefore be analysed with caution as validity and interpretation of such studies are less clear, lack temporality and are prone to some degree of bias. In addition to observational studies being subject to information bias and reverse causation, one major bias encountered was selection bias, since patients who were treated with antithrombotic agents most likely had a higher mortality risk, due to critical disease status, as compared with those who were left untreated. Additionally, another major issue could be confounding bias, since available studies lacked multivariate techniques such as restriction, matching and stratification, which could have potentially exposed the presence of confounders such as the effect of other administered treatments or COVID-19 variants. Furthermore, this rapid review could be subject to study selection bias as search terms were not tailored to target specific types of antiplatelet or antithrombotic therapies. It is worthwhile to mention that rapid reviews are perishable quickly as new evidence will emerge continuously and its synthesis will require updating.
Conclusion
Current guidelines recommend administration of anticoagulants with varying doses based on case severity in patients with COVID-19.67 71 However, evidence in support of these guidelines is limited due to the paucity of high-quality clinical trials performed to date. Despite the fact that patients with COVID-19 treated with anticoagulants had an overall lower risk of thrombotic events and mortality, the use of anticoagulants or antiplatelets alone was still associated with the occurrence of thrombotic and microthrombotic events in a substantial number of patients. Given the aforementioned shortcomings of anticoagulation when used alone and results from this rapid review showing that combined anticoagulant and antiplatelet therapy in the treatment of COVID-19, despite being poorly studied, implied a better clinical outcome when compared with the use of anticoagulation alone without the occurrence of major bleeding. So, we recommend further clinical trials to evaluate the safety and efficacy of combining antiplatelet and anticoagulants agents in the management of patients with COVID-19.
Footnotes
Correction notice: Since the publication of this article, author Nibal Chamoun’s affiliation has been corrected to ‘School of Pharmacy, Lebanese American University, Byblos, Lebanon’.
Contributors: All coauthors have contributed equally to this work. KM contributed to hypothesis conception and wrote the final draft of manuscript. RF contributed to searching and data extraction, wrote the manuscript text and managed the references. MM wrote the manuscript text and managed the references. CC supervised the searching, data extraction, and manuscript writing and editing and critically reviewed the manuscript. GG and NC contributed to critical revisions of the manuscript. All authors read and approved the final version of the manuscript before submission for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19:141–54. 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lessons of past coronavirus pandemics. Popul Dev Rev 2020;46:633–7. 10.1111/padr.12360 [DOI] [Google Scholar]
- 3. Who coronavirus disease (COVID-19) Dashboard. Available: https://covid19.who.int [Accessed 3 Dec 2020].
- 4. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clinical Immunology 2020;215:108427. 10.1016/j.clim.2020.108427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 2020;55. 10.1183/13993003.00607-2020. [Epub ahead of print: 16 04 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perico L, Benigni A, Casiraghi F, et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol 2021;17:46–64. 10.1038/s41581-020-00357-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zou X, Chen K, Zou J, et al. Single-Cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020;14:185–92. 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020;18:1738–42. 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haemost 2020;18:1747–51. 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maier CL, Truong AD, Auld SC, et al. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 2020;395:1758–9. 10.1016/S0140-6736(20)31209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pearson JD. Normal endothelial cell function. Lupus 2000;9:183–8. 10.1191/096120300678828299 [DOI] [PubMed] [Google Scholar]
- 12. Biswas I, Khan GA. Endothelial dysfunction in cardiovascular diseases. Basic Clin Underst Microcirc 2020. 10.5772/intechopen.89365 [DOI] [Google Scholar]
- 13. Miesbach W. Pathological role of angiotensin II in severe COVID-19. TH Open 2020;4:e138–44. 10.1055/s-0040-1713678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Java A, Apicelli AJ, Liszewski MK, et al. The complement system in COVID-19: Friend and foe? JCI Insight 2020;5. 10.1172/jci.insight.140711. [Epub ahead of print: 06 08 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coccheri S. COVID-19: the crucial role of blood coagulation and fibrinolysis. Intern Emerg Med 2020;15:1369–73. 10.1007/s11739-020-02443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mondal S, Quintili AL, Karamchandani K, et al. Thromboembolic disease in COVID-19 patients: a brief narrative review. J Intensive Care 2020;8:70. 10.1186/s40560-020-00483-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ra C, Wh F. Thrombotic complications of COVID-19 infection: a review. Cardiol Rev 2021;29. 10.1097/CRD.0000000000000347 [DOI] [PubMed] [Google Scholar]
- 18. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038–44. 10.1093/eurheartj/ehaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garritty C, Gartlehner G, Nussbaumer-Streit B, et al. Cochrane rapid reviews methods group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol 2021;130:13–22. 10.1016/j.jclinepi.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol 2020;76:1815–26. 10.1016/j.jacc.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis 2020;50:298–301. 10.1007/s11239-020-02162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez-Porras JR, Belhassen-Garcia M, Lopez-Bernus A. Low molecular weight heparin in adults inpatient COVID-19 2020. 10.2139/ssrn.3586665 [DOI]
- 23. Bousquet G, Falgarone G, Deutsch D, et al. ADL-dependency, D-dimers, LDH and absence of anticoagulation are independently associated with one-month mortality in older inpatients with Covid-19. Aging 2020;12:11306–13. 10.18632/aging.103583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–9. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russo V, Di Maio M, Attena E, et al. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacol Res 2020;159:104965. 10.1016/j.phrs.2020.104965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malas MB, Naazie IN, Elsayed N, et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine 2020;29:100639. 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W, Xu Z, Xiang H, et al. Risk factors for systemic and venous thromboembolism, mortality and bleeding risks in 1125 patients with COVID-19: relationship with anticoagulation status. Aging 2021;13:9225–42. 10.18632/aging.202769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020;41:3058–68. 10.1093/eurheartj/ehaa500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1–10. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Viecca M, Radovanovic D, Forleo GB, et al. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res 2020;158:104950. 10.1016/j.phrs.2020.104950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg 2021;132:930–41. 10.1213/ANE.0000000000005292 [DOI] [PubMed] [Google Scholar]
- 32. Pavoni V, Gianesello L, Pazzi M, et al. Venous thromboembolism and bleeding in critically ill COVID-19 patients treated with higher than standard low molecular weight heparin doses and aspirin: a call to action. Thromb Res 2020;196:313–7. 10.1016/j.thromres.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020;18:1023–6. 10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTe in patients with coronavirus disease 2019: chest guideline and expert panel report. Chest 2020;158:1143–63. 10.1016/j.chest.2020.05.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Casini A, Alberio L, Angelillo-Scherrer A. Suggestions for thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19. Swiss Med Wkly 2020;150:w20247. [DOI] [PubMed] [Google Scholar]
- 36. NIH . Full-Dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients 2021. https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients
- 37. Zarychanski R. Therapeutic anticoagulation in critically ill patients with Covid-19 – preliminary report. medRxiv 2021. 10.1101/2021.03.10.21252749 [DOI] [Google Scholar]
- 38. INSPIRATION Investigators, Sadeghipour P, Talasaz AH, et al. Effect of Intermediate-Dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the inspiration randomized clinical trial. JAMA 2021;325:1620-1630. 10.1001/jama.2021.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khider L, Gendron N, Goudot G, et al. Curative anticoagulation prevents endothelial lesion in COVID-19 patients. J Thromb Haemost 2020;18:2391–9. 10.1111/jth.14968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Musoke N, Lo KB, Albano J, et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res 2020;196:227–30. 10.1016/j.thromres.2020.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kenawy HI, Boral I, Bevington A. Complement-Coagulation cross-talk: a potential mediator of the physiological activation of complement by low pH. Front Immunol 2015;6:215. 10.3389/fimmu.2015.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu P-cheng, Lin S, Yang X-wei, et al. C-Reactive protein enhances activation of coagulation system and inflammatory response through dissociating into monomeric form in antineutrophil cytoplasmic antibody-associated vasculitis. BMC Immunol 2015;16:10 10.1186/s12865-015-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Showers CR, Nuovo GJ, Lakhanpal A, et al. A Covid-19 patient with complement-mediated coagulopathy and severe thrombosis. Pathobiology 2021;88:28-36. 10.1159/000512503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holter JC, Pischke SE, de Boer E, et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A 2020;117:25018–25. 10.1073/pnas.2010540117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mastellos DC, Pires da Silva BGP, Fonseca BAL, et al. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin Immunol 2020;220:108598. 10.1016/j.clim.2020.108598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Durrani J, Malik F, Ali N, et al. To be or not to be a case of heparin resistance. J Community Hosp Intern Med Perspect 2018;8:145–8. 10.1080/20009666.2018.1466599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kawatsu S, Sasaki K, Sakatsume K, et al. Predictors of heparin resistance before cardiovascular operations in adults. Ann Thorac Surg 2018;105:1316–21. 10.1016/j.athoracsur.2018.01.068 [DOI] [PubMed] [Google Scholar]
- 48. Ooi MWX, Rajai A, Patel R, et al. Pulmonary thromboembolic disease in COVID-19 patients on CT pulmonary angiography - Prevalence, pattern of disease and relationship to D-dimer. Eur J Radiol 2020;132:109336. 10.1016/j.ejrad.2020.109336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iba T, Levy JH, Connors JM, et al. The unique characteristics of COVID-19 coagulopathy. Crit Care 2020;24:360. 10.1186/s13054-020-03077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baccellieri D, Bilman V, Apruzzi L, et al. A case of Covid-19 patient with acute limb ischemia and heparin resistance. Ann Vasc Surg 2020;68:88–92. 10.1016/j.avsg.2020.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. White D, MacDonald S, Bull T, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis 2020;50:287–91. 10.1007/s11239-020-02145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bounds EJ, Kok SJ, Dimer D. StatPearls. StatPearls Publishing, 2019. [Google Scholar]
- 53. Mouhat B, Besutti M, Bouiller K, et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respir J 2020;56. 10.1183/13993003.01811-2020. [Epub ahead of print: 22 10 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ventura-Díaz S, Quintana-Pérez JV, Gil-Boronat A, et al. A higher D-dimer threshold for predicting pulmonary embolism in patients with COVID-19: a retrospective study. Emerg Radiol 2020;27:679–89. 10.1007/s10140-020-01859-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yao Y, Cao J, Wang Q, et al. D-Dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care 2020;8:1–11. 10.1186/s40560-020-00466-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang L, Yan X, Fan Q, et al. D-Dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020;18:1324–9. 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Righini M, Van Es J, Den Exter PL, et al. Age-Adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014;311:1117–24. 10.1001/jama.2014.2135 [DOI] [PubMed] [Google Scholar]
- 58. Verma N, Willeke P, Bicsán P. [Age-adjusted D-dimer cut-offs to diagnose thromboembolic events: validation in an emergency department]. Med Klin Intensivmed Notfallmedizin 2014;109:121–8. 10.1007/s00063-013-0265-8 [DOI] [PubMed] [Google Scholar]
- 59. Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a new York City health system. JAMA 2020;324:799–801. 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qureshi AI, Abd-Allah F, Al-Senani F, et al. Management of acute ischemic stroke in patients with COVID-19 infection: insights from an international panel. Am J Emerg Med 2020;38:1548.e5–1548.e7. 10.1016/j.ajem.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg 2020;72:72. 10.1016/j.jvs.2020.04.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Roquetaillade C, Chousterman BG, Tomasoni D, et al. Unusual arterial thrombotic events in Covid-19 patients. Int J Cardiol 2021;323:281–4. 10.1016/j.ijcard.2020.08.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020;220:1–13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost 2020;18:1559–61. 10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020;77:198–209. 10.1111/his.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1859–65. 10.1111/jth.14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv 2021;5:872–88. 10.1182/bloodadvances.2020003763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. National Institute for Health and Care Excellence . COVID-19 rapid guideline: reducing the risk of venous thromboembolism in over 16S with COVID-19. London: National Institute for Health and Care Excellence (UK), 2020. http://www.ncbi.nlm.nih.gov/books/NBK566720/ [PubMed] [Google Scholar]
- 70. Information on COVID-19 treatment, prevention and research. COVID-19 treat. Guidel. Available: https://www.covid19treatmentguidelines.nih.gov/ [Accessed 20 Apr 2021].
- 71. Marietta M, Ageno W, Artoni A, et al. COVID-19 and haemostasis: a position paper from Italian Society on thrombosis and haemostasis (SISET). Blood Transfus 2020;18:167–9. 10.2450/2020.0083-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Transfusion Medicine and Hemostasis - 3rd Edition. Available: https://www.elsevier.com/books/transfusion-medicine-and-hemostasis/shaz/978-0-12-813726-0 [Accessed 20 Jan 2021].
- 73. Gavriilaki E, Anyfanti P, Gavriilaki M, et al. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr Hypertens Rep 2020;22:63. 10.1007/s11906-020-01078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nägele MP, Haubner B, Tanner FC, et al. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis 2020;314:58–62. 10.1016/j.atherosclerosis.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huertas A, Montani D, Savale L, et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J 2020;56. 10.1183/13993003.01634-2020. [Epub ahead of print: 30 07 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sturtzel C. Endothelial cells. Adv Exp Med Biol 2017;1003:71–91. 10.1007/978-3-319-57613-8_4 [DOI] [PubMed] [Google Scholar]
- 77. Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med 2008;359:1261–70. 10.1056/NEJMra0800887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jin Y, Ji W, Yang H, et al. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther 2020;5:293. 10.1038/s41392-020-00454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Esmon CT. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev 2009;23:225–9. 10.1016/j.blre.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wolberg AS, Rosendaal FR, Weitz JI, et al. Venous thrombosis. Nat Rev Dis Primers 2015;1:15006. 10.1038/nrdp.2015.6 [DOI] [PubMed] [Google Scholar]
- 81. Eikelboom JW, Anand SS, Malmberg K, et al. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without ST elevation: a meta-analysis. Lancet 2000;355:1936–42. 10.1016/S0140-6736(00)02324-2 [DOI] [PubMed] [Google Scholar]
- 82. Maldonado E, Tao D, Mackey K. Antithrombotic therapies in COVID-19 disease: a systematic review. J Gen Intern Med 2020;35:2698–706. 10.1007/s11606-020-05906-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ciavarella A, Peyvandi F, Martinelli I. Where do we stand with antithrombotic prophylaxis in patients with COVID-19? Thromb Res 2020;191:29. 10.1016/j.thromres.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vadukul P, Sharma DS, Vincent P. Massive pulmonary embolism following recovery from COVID-19 infection: inflammation, thrombosis and the role of extended thromboprophylaxis. BMJ Case Rep 2020;13:e238168. 10.1136/bcr-2020-238168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zuin M, Rigatelli G, Zuliani G, et al. The risk of thrombosis after acute-COVID-19 infection. QJM 2021:hcab054. 10.1093/qjmed/hcab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2021-001628supp001.pdf (248KB, pdf)
Data Availability Statement
No data are available.


