Abstract
Lessons Learned
Androgen receptor as assessed by immunohistochemistry is expressed in a high proportion of patients with hepatocellular carcinoma (HCC).
Enzalutamide at 160 mg orally daily is safe and tolerable in patients with advanced HCC but has no single‐agent antitumor activity.
Enzalutamide, a CYP3A4 inducer, at a standard dose of 160 mg reduces the exposure of sorafenib, a CYP3A4 substrate.
Enzalutamide and sorafenib is safe and tolerable in patients with advanced HCC, but the addition of enzalutamide to sorafenib did not enhance the antitumor activity of sorafenib.
Background
Androgen receptor (AR) interference is deleterious to hepatocellular carcinoma (HCC) in preclinical models.
Methods
This is a multicenter, phase Ib study of enzalutamide ± sorafenib in patients with advanced HCC. In part 1, a 3 + 3 dose de‐escalation design with expansion established the recommended phase II dose (RP2D) of enzalutamide in patients in whom sorafenib treatment had failed. In part 2, a 3 + 3 dose escalation with expansion established the safety of enzalutamide with sorafenib in treatment‐naive patients with HCC. Secondary objectives included objective response rate (ORR), progression‐free survival (PFS), overall survival (OS), pharmacokinetics (PK), and determination of AR expression by immunohistochemistry. A 7‐day run‐in with sorafenib alone in part 2 allowed assessment of the impact of enzalutamide on sorafenib pharmacokinetics.
Results
In part 1, 16 patients received enzalutamide 160 mg daily. No dose‐limiting toxicity (DLT) occurred; 1 patient required dose reduction. Responses were not observed; median PFS and OS were 1.8 (95% confidence interval [CI]: 1.6–3.6) and 7 (95% CI: 3.6 to not reached [NR]) months, respectively. In part 2, patients received sorafenib 400 mg daily (4) or twice a day (8) both with enzalutamide at the recommended phase II dose—no DLTs were observed. ORR was 10% (95% CI: 0.3–44.5), and median PFS and OS were 2.9 (95% CI: 1.6 to NR) and 6.7 (95% CI: 4.6 to NR) months, respectively. Enzalutamide reduced sorafenib exposure by 60%. Tumor AR expression did not associate with outcome.
Conclusion
Enzalutamide is ineffective in HCC; further development is not supported by this study.
Discussion
Ample data indicate that the transcriptional factor AR promotes hepatocarcinogenesis and blocking AR by multiple methods leads to HCC growth suppression [1, 2]. However, preclinical and clinical studies suggest that reduction of circulating androgens and thereby AR inactivation in a ligand‐dependent context is insufficient to produce antitumor effects [1]. Thus, pharmacologic interference with ligand‐independent AR activation and AR nuclear translocation may be required to impair HCC growth [1]. Clinical evaluation of the selective AR antagonist, enzalutamide, in patients with treatment‐refractory advanced HCC is therefore warranted. As AR signaling also drives angiogenic signaling pathways, combination treatment with enzalutamide and the antiangiogenic multitargeted inhibitor, sorafenib, in advanced HCC is also worth evaluating [3, 4, 5, 6]. Thus, this is a multicenter, open label, phase Ib study of enzalutamide with or without sorafenib in patients with advanced HCC with Child‐Pugh class A liver function.
At the time of the study conception and conduct, the only drug available for the treatment of advanced HCC was sorafenib [7]. In part 1, we assessed the safety of enzalutamide in patients with HCC in whom prior sorafenib had failed. Given the tolerability and the dose de‐escalation design, all 16 patients received the standard 160 mg orally daily dose of enzalutamide. There was no single‐agent antitumor activity in either AR‐positive or AR‐negative patients. Emerging preclinical and translational data confirm our important clinical findings. Since the completion of the study, it is now apparent that that innate resistance to enzalutamide in HCC may be mediated by compensatory feedback AKT‐MTOR activation [4] and AR splice variants, known to be insensitive to AR antagonism [8].
In part 2, the combination of sorafenib and enzalutamide in treatment‐naive patients also exhibited limited antitumor activity and certainly was not greater than what has been reported previously for sorafenib monotherapy [7]. Although sorafenib, a CYP3A4 substrate, is predicted to be cleared by enzalutamide, a CYP3A4 inducer, available clinical data supporting a meaningful drug–drug interaction are conflicting [9, 10]. Thus, a 7‐day sorafenib run‐in was embedded into part 2 of the study to measure the steady‐state sorafenib PK and compare this with sorafenib PK on enzalutamide. We documented a clear drug–drug interaction—enzalutamide reduced sorafenib Cmax and Area under the curve, 0 – 8 hours (AUC0–8hr) by 59% and 60%, respectively. Given the totality of these data, opening the combina$tion expansion cohort was not pursued and the study was terminated [11, 12, 13, 14] (Fig. 1). Further development of enzalutamide in HCC as single agent or in combination with sorafenib is not warranted.
Figure 1.
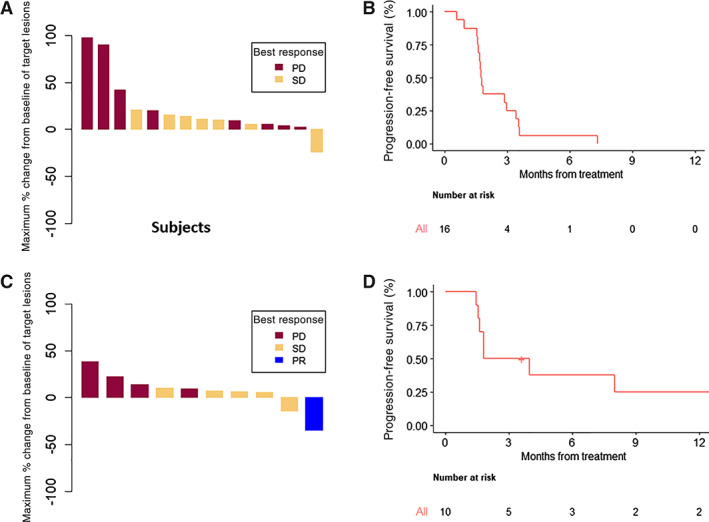
Efficacy and outcomes for advanced HCC patients treated with enzalutamide +/− sorafenib. Waterfall plot (A) and progression‐free survival (B) of enzalutamide. Waterfall plot (C) and progression‐free survival (D) of enzalutamide and sorafenib.
Abbreviations: PD, progressive disease; PR, partial response; SD, stable disease.
Trial Information
| Disease | Hepatocellular carcinoma |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase I, 3 + 3 |
| Primary Endpoints | Safety, tolerability, recommended phase II dose |
| Secondary Endpoints | Efficacy, pharmacokinetics, correlative endpoint |
|
Additional Details of Endpoints or Study Design Dose Escalation Design: For Part 1: Three patients will be enrolled at enzalutamide 160 mg daily. If no DLTs are observed, an additional three patients will be enrolled for confirmation of safety. If zero out of six or one out of six DLTs are observed, then 160 mg will be defined as the dose to move forward as the recommended phase II dose (RP2D). If two out of three or two out of six DLTs are observed, we will de‐escalate to 120 mg and again follow the 3 + 3 design. Upon defining the RP2D, 10 additional patients receive the enzalutamide at the RP2D to further assess safety, obtain additional PK analysis, and explore efficacy in the second‐line setting. In total, 16 patients were enrolled into part 1. Dose Escalation Design: For Part 2: A dose escalation scheme will be used whereby patients will be treated in sequential cohorts of three. If no patients experience a DLT at dose level 1 in an initial group of three patients, cohort 2 will open and re‐enroll three patients. If one of three patients experiences a DLT, the cohort will be expanded to six. If no further DLTs occur, this dose will be considered the RP2D. If two of six patients experience a DLT, the maxium tolerated dose (MTD) has been exceeded. The MTD will be defined as the highest dose for which no more than one of six patients develops a DLT. After the establishment of the RP2D and schedule, an expansion cohort was planned for a total of 39 additional patients who were treatment naive. Using a Simon minimax design with 39 patients, we can show an improvement in 4‐month PFS from 50% to 70% using a type I and type II error rate of 10% each. In the first stage, we would need 23 patients, out of whom we need 12 to be alive and progression free at 4 months, in which case we would accrue an additional 16 patients. If at the end of the study 24 or more are alive and progression free, we would call this promising. This cohort was not explored based on interim analysis of the study showing limited antitumor activity and a drug–drug interaction with the combination. Immunohistochemistry for AR: Deparaffinized tissue sections from HCC tumors were treated with antigen retrieval solution followed by incubation with standard blocking reagents. Primary antibody for AR (DAKO) was then applied with dilution 1:70 (clone AR441, Dako, catalog number M3562) and incubated overnight at 4°C. Appropriate secondary antibodies labeled with polymer −30’ were applied at room temperature (Envision Kit, Dako catalog number K4006) followed by detection using DAB as substrate‐chromogen. Positive and negative controls were performed in parallel (prostate, positive control for AR). The number of cells that are AR positive, the intensity of staining, and the percentage of nuclear staining was assessed. Nuclear staining >5% will be considered positive. AR testing was performed and analyzed without knowledge of the patients' clinical status. PK Determination: Whole blood samples were obtained at Cycle 1: Day −1, before dose and 1 hour, 4 hours, 8 hours, and 24 hours after dose (Cycle 1 Day 1) for sorafenib PKs and Cycle 2 Day 1, before dose and 1 hour, 4 hours, 8 hours, and 24 hours (Cycle 2 Day 2) for both sorafenib and enzalutamide PKs on the first 20 patients in the Part 2 dose escalation. A 7‐day run‐in of sorafenib alone allowed intrapatient comparison of sorafenib steady state PK on and off enzalutamide. Sorafenib and sorafenib N‐oxide in plasma was determined using a validated liquid chromatography tandem mass spectrometry method on an AB Sciex 5500 triple quadrupole. The software program used was Phoenix 64 WinNonlin (Pharsight Corp., St. Louis, MO, version 7.0). | |
| Investigator's Analysis | Lack of efficacy and drug–drug interaction |
Drug Information: Enzalutamide Monotherapy (Part 1)
| Drug 1 | |
| Generic/Working Name | Enzalutamide |
| Drug Type | Small molecule |
| Drug Class | Androgen receptor |
| Dose | 160 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | Daily for 28‐day cycles |
Drug Information: Enzalutamide and Sorafenib Combination (Part 2)
| Drug 1 | |
| Generic/Working Name | Enzalutamide |
| Drug Type | Small molecule |
| Drug Class | Androgen receptor |
| Dose | 160 mg per flat dose |
| Schedule of Administration | Daily for 28‐day cycles |
| Drug 2 | |
| Generic/Working Name | Sorafenib |
| Drug Type | Small molecule |
| Drug Class | Angiogenesis ‐ antivascular |
| Dose | 400 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | Daily or twice a day for 28‐day cycles |
Dose Escalation Table: Enzalutamide Monotherapy (Part 1)
| Dose level | Dose of drug: enzalutamide | No. enrolled | No. evaluable for toxicity |
|---|---|---|---|
| Expansion cohort | 160 mg | 10 | 10 |
| Cohort 1 | 160 mg | 6 | 6 |
| Cohort −1 | 0 | 0 | 0 |
Dose Escalation Table: Enzalutamide and Sorafenib Combination (Part 2)
| Dose level | Dose of drug: enzalutamide | Dose of drug: sorafenib | No. enrolled | No. evaluable for toxicity |
|---|---|---|---|---|
| Expansion | 160 mg daily | 400 mg twice a day | 0 | 0 |
| Cohort 2 | 160 mg daily | 400 mg twice a day | 8 | 8 |
| Cohort 1 | 160 mg daily | 400 mg daily | 4 | 4 |
| Cohort −1 | 120 mg daily | 400 mg daily | 0 | 0 |
Patient Characteristics: Enzalutamide Monotherapy (Part 1)
| Number of Patients, Male | 12 |
| Number of Patients, Female | 4 |
| Stage |
Stage 1: 0 Stage 2: 2 Stage 3: 0 Stage 4: 14 |
| Age | Median (range): 70 (64–78) years |
| Number of Prior Systemic Therapies | Median (range): 3 (2–7) |
| Performance Status: ECOG |
0 — 4 1 — 12 2 — 0 3 — 0 Unknown — 0 |
| Etiologic factor | |
| Hepatitis B virus | |
| Hepatitis C virus | 2 |
| Hepatitis C virus, hepatitis B virus | 0 |
| Nonviral | 11 |
| Androgen receptor expression | |
| Unknown | 2 |
| Negative (<5%) | 4 |
| Positive (≥5%) | 10 |
| Disease burden | |
| Extrahepatic | 14 |
| Intrahepatic | 2 |
| Vascular involvement | 0 |
| Alpha‐fetoprotein, median, range | 214 (9–1,546) |
Patient Characteristics: Enzalutamide and Sorafenib Combination (Part 2)
| Number of Patients, Male | 2 |
| Number of Patients, Female | 10 |
| Stage |
Stage 1: 0 Stage 2: 1 Stage 3: 6 Stage 4: 5 |
| Age | Median (range): 62 (61–67) |
| Number of Prior Systemic Therapies | Median (range): 0 |
| Performance Status: ECOG |
0 — 1 1 — 11 2 — 0 3 — 0 Unknown — 0 |
| Etiologic factor | |
| Hepatitis B virus | 0 |
| Hepatitis C virus | 4 |
| Hepatitis C virus, hepatitis B virus | 1 |
| Nonviral | 7 |
| Androgen receptor expression | |
| Unknown | 4 |
| Negative <5%) | 1 |
| Positive (≥5%) | 7 |
| Disease burden | |
| Extrahepatic | 8 |
| Intrahepatic | 1 |
| Vascular involvement | 3 |
| Alpha‐fetoprotein, median, range | 196 (8–2,095) |
Primary Assessment Method: Enzalutamide Monotherapy (Part 1)
| Title | Enzalutamide efficacy (part 1) |
| Number of Patients Enrolled | 16 |
| Number of Patients Evaluable for Toxicity | 16 |
| Number of Patients Evaluated for Efficacy | 15 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 7 (44%) |
| Response Assessment PD | n = 8 (50%) |
| Response Assessment OTHER | n = 1 (6%) |
| (Median) Duration Assessments PFS | 1.76 months, CI: 1.61–3.55 |
| (Median) Duration Assessments OS | 7.03 months, CI: 3.55–NR |
| Outcome Notes | One patient did not complete 80% of dosing in cycle 1 and was replaced for DLT per protocol. The patient clinically deteriorated on study and was evaluable for toxicity and survival but not for response. |
Primary Assessment Method: Enzalutamide and Sorafenib Combination (Part 2)
| Title | Enzalutamide and sorafenib efficacy (part 2) |
| Number of Patients Enrolled | 12 |
| Number of Patients Evaluable for Toxicity | 12 |
| Number of Patients Evaluated for Efficacy | 10 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 1 (6%) |
| Response Assessment SD | n = 5 (42%) |
| Response Assessment PD | n = 4 (33%) |
| Response Assessment OTHER | n = 2 (17%) |
| (Median) Duration Assessments PFS | 2.89 months, CI: 1.61–NR |
| (Median) Duration Assessments OS | 6.69 months, CI: 4.60–NR |
| Outcome Notes | Two of 12 patients were not included in the efficacy analysis. One could not swallow enzalutamide and hence never received combination treatment, and the other had an adverse event related to sorafenib during the sorafenib run‐in and never received combination treatment. These two were not included in the efficacy end points of ORR, PFS, and OS but were assessed for safety. |
Adverse Events: Enzalutamide Monotherapy (Part 1)
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Abdominal distension | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Abdominal pain | 56% | 38% | 6% | 0% | 0% | 0% | 44% |
| Activated partial thromboplastin time prolonged | 81% | 13% | 0% | 6% | 0% | 0% | 19% |
| Alanine aminotransferase increased | 63% | 25% | 6% | 6% | 0% | 0% | 37% |
| Alkaline phosphatase increased | 56% | 31% | 13% | 0% | 0% | 0% | 44% |
| Anemia | 50% | 31% | 19% | 0% | 0% | 0% | 50% |
| Anorexia | 81% | 13% | 6% | 0% | 0% | 0% | 19% |
| Anxiety | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Ascites | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Aspartate aminotransferase increased | 44% | 31% | 6% | 19% | 0% | 0% | 56% |
| Back pain | 74% | 13% | 13% | 0% | 0% | 0% | 26% |
| Blood bilirubin increased | 69% | 6% | 19% | 0% | 6% | 0% | 31% |
| Bone pain | 88% | 6% | 0% | 6% | 0% | 0% | 12% |
| Constipation | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Cough | 88% | 6% | 6% | 0% | 0% | 0% | 12% |
| Creatinine increased | 81% | 13% | 6% | 0% | 0% | 0% | 19% |
| Death NOS | 94% | 0% | 0% | 0% | 0% | 6% | 6% |
| Depression | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Diarrhea | 75% | 19% | 6% | 0% | 0% | 0% | 25% |
| Dizziness | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Dry mouth | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Dry skin | 81% | 19% | 0% | 0% | 0% | 0% | 19% |
| Dysgeusia | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Dyspnea | 74% | 13% | 13% | 0% | 0% | 0% | 26% |
| Edema face | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Edema limbs | 50% | 25% | 25% | 0% | 0% | 0% | 50% |
| Edema trunk | 94% | 0% | 6% | 0% | 0% | 0% | 6% |
| Encephalopathy | 94% | 0% | 0% | 0% | 0% | 6% | 6% |
| Epistaxis | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Epistaxis | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Fatigue | 11% | 38% | 38% | 13% | 0% | 0% | 89% |
| Gait disturbance | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Generalized muscle weakness | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Gynecomastia | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Headache | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Hematuria | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Hot flashes | 81% | 19% | 0% | 0% | 0% | 0% | 19% |
| Hypercalcemia | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Hyperglycemia | 38% | 50% | 6% | 6% | 0% | 0% | 62% |
| Hyperkalemia | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Hypertension | 75% | 19% | 6% | 0% | 0% | 0% | 25% |
| Hypoalbuminemia | 37% | 38% | 25% | 0% | 0% | 0% | 63% |
| Hypocalcemia | 75% | 25% | 0% | 0% | 0% | 0% | 25% |
| Hypoglycemia | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Hypomagnesemia | 81% | 19% | 0% | 0% | 0% | 0% | 19% |
| Hyponatremia | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Hypophosphatemia | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Hypotension | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| INR increased | 75% | 19% | 6% | 0% | 0% | 0% | 25% |
| Insomnia | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Joint range of motion decreased | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Lipase increased | 68% | 16% | 11% | 0% | 5% | 0% | 32% |
| Lymphocyte count decreased | 87% | 0% | 0% | 13% | 0% | 0% | 13% |
| Muscle weakness upper limb | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Myalgia | 72% | 21% | 7% | 0% | 0% | 0% | 28% |
| Nausea | 62% | 38% | 0% | 0% | 0% | 0% | 38% |
| Neck pain | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Pain | 88% | 6% | 0% | 6% | 0% | 0% | 12% |
| Pain in extremity | 88% | 6% | 6% | 0% | 0% | 0% | 12% |
| Pericardial effusion | 94% | 0% | 0% | 6% | 0% | 0% | 6% |
| Peripheral sensory neuropathy | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Platelet count decreased | 56% | 25% | 13% | 6% | 0% | 0% | 44% |
| Pleural effusion | 88% | 0% | 6% | 6% | 0% | 0% | 12% |
| Pruritus | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Rash acneiform | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Rash maculo‐papular | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Serum amylase increased | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Skin ulceration | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Thromboembolic event | 94% | 0% | 6% | 0% | 0% | 0% | 6% |
| Vomiting | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| Weight loss | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| White blood cell decreased | 81% | 13% | 6% | 0% | 0% | 0% | 19% |
| Adverse Events Legend | |||||||
| Toxicities occurring in at least one patient in all cycles. | |||||||
Abbreviation: INR, international normalized ratio; NC/NA, no change from baseline/no adverse event; NOS, not otherwise specified.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Death NOS | 5 | Unrelated |
| Encephalopathy | 5 | Unrelated |
| Pain | 3 | Unrelated |
Abbreviation: NOS, not otherwise specified.
Dose‐Limiting Toxicities: Enzalutamide Monotherapy (Part 1)
| Dose level | No. enrolled | No. evaluable for toxicity | No. with a dose‐limiting toxicity |
|---|---|---|---|
| Expansion cohort | 10 | 10 | 0 |
| Cohort level 1 | 6 | 6 | 0 |
Adverse Events: Enzalutamide and Sorafenib Combination (Part 2)
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Abdominal pain | 58% | 25% | 17% | 0% | 0% | 0% | 42% |
| Activated partial thromboplastin time prolonged | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Alanine aminotransferase increased | 67% | 25% | 8% | 0% | 0% | 0% | 33% |
| Alkaline phosphatase increased | 58% | 42% | 0% | 0% | 0% | 0% | 42% |
| Anemia | 67% | 33% | 0% | 0% | 0% | 0% | 33% |
| Anorexia | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Ascites | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Aspartate aminotransferase increased | 42% | 33% | 8% | 17% | 0% | 0% | 58% |
| Back pain | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Edema limbs | 67% | 17% | 8% | 8% | 0% | 0% | 33% |
| Blood bilirubin increased | 67% | 0% | 25% | 8% | 0% | 0% | 33% |
| Blurred vision | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Bone pain | 92% | 0% | 8% | 0% | 0% | 0% | 8% |
| Soft tissue infection | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Confusion | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Chills | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Constipation | 75% | 17% | 8% | 0% | 0% | 0% | 25% |
| Cough | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Dehydration | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Depression | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Diarrhea | 75% | 25% | 0% | 0% | 0% | 0% | 25% |
| Dizziness | 75% | 25% | 0% | 0% | 0% | 0% | 25% |
| Dyspnea | 67% | 25% | 8% | 0% | 0% | 0% | 33% |
| Edema face | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Extrapyramidal disorder | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Fatigue | 50% | 33% | 17% | 0% | 0% | 0% | 50% |
| Fever | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Gait disturbance | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Gastritis | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Gastrointestinal disorders—GERD | 83% | 0% | 17% | 0% | 0% | 0% | 17% |
| Headache | 67% | 17% | 8% | 8% | 0% | 0% | 33% |
| Hot flashes | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Hot flashes | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Hyperglycemia | 67% | 17% | 8% | 8% | 0% | 0% | 33% |
| Hypernatremia | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Hypertension | 58% | 25% | 17% | 0% | 0% | 0% | 42% |
| Hypotension | 92% | 0% | 0% | 0% | 0% | 8% | 8% |
| Hypocalcemia | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Hypoglycemia | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Hypokalemia | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Hypomagnesemia | 67% | 25% | 8% | 0% | 0% | 0% | 33% |
| Hyponatremia | 83% | 0% | 0% | 17% | 0% | 0% | 17% |
| Hypophosphatemia | 75% | 0% | 8% | 17% | 0% | 0% | 25% |
| Hypoxia | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| INR increased | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Lipase increased | 67% | 17% | 0% | 8% | 8% | 0% | 33% |
| Lymphocyte count decreased | 75% | 0% | 25% | 0% | 0% | 0% | 25% |
| Myalgia | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Nausea | 75% | 17% | 8% | 0% | 0% | 0% | 25% |
| Neck pain | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Pain | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Pain in extremity | 84% | 8% | 8% | 0% | 0% | 0% | 16% |
| Palmar‐plantar erythrodysesthesia syndrome | 67% | 25% | 8% | 0% | 0% | 0% | 33% |
| Platelet count decreased | 59% | 25% | 8% | 8% | 0% | 0% | 41% |
| Rash maculo‐papular | 75% | 25% | 0% | 0% | 0% | 0% | 25% |
| Serum amylase increased | 75% | 8% | 17% | 0% | 0% | 0% | 25% |
| Skin hyperpigmentation | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Upper respiratory infection | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Urinary incontinence | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Urinary tract infection | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Vomiting | 92% | 0% | 8% | 0% | 0% | 0% | 8% |
| Weight loss | 75% | 25% | 0% | 0% | 0% | 0% | 25% |
| White blood cell decreased | 67% | 33% | 0% | 0% | 0% | 0% | 33% |
| Adverse Events Legend | |||||||
| Adverse events observed in at least one patient among all cycles. | |||||||
Abbreviations: GERD, gastroesophageal reflux disease; INR, international normalized ratio; NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Bone pain | 3 | Unrelated |
| Cellulitis | 3 | Unrelated |
| Hypoxia | 3 | Unlikely |
| Edema, limb | 3 | Unlikely |
| Hypotension | 5 | Unlikely |
| Nausea | 2 | Possible |
| Vomiting | 2 | Possible |
Dose‐Limiting Toxicities: Enzalutamide and Sorafenib Combination (Part 2)
| Dose level | No. enrolled | No. evaluable for toxicity | No. with a dose‐limiting toxicity |
|---|---|---|---|
| Dose level 2 | 8 | 8 | 0 |
| Dose level 1 | 4 | 4 | 0 |
Pharmacokinetics/Pharmacodynamics
| Dose level | No. enrolled | Cmax, mean ± SD, μg/L | Tmax, mean ± SD, hours | AUC0–8, mean ± SD, hours * μg/L | AUCall, hours * μg/mL |
|---|---|---|---|---|---|
| Sorafenib run‐in | 9 | 1.36 ± 0.937 | 9.67 ± 10.8 | 7.78 ± 4.93 | 22.1 ± 12.6 |
| Sorafenib on enzalutamide | 9 | 0.597 ± 0.434 | 10 ± 9.98 | 3.40 ± 2.09 | 9.29 ± 6.91 |
PK/PD Legend: These results indicate that the combination of sorafenib and enzalutamide alters the pharmacokinetics of sorafenib. Seven of the nine subjects have samples collected for both cycles of the study. Two subjects had no C2 samples, which did not allow comparisons to be made between C1 and C2 in these cases. Of the seven remaining subjects with C1/C2 samples, six exhibited a significant decrease in Cmax and AUC0–8hrs for sorafenib when coadministered with enzalutamide. The percentage change from C1 to C2 in Cmax and AUC0–8hrs for sorafenib is −58.7% (−35.9% to −79.9%) and − 59.7% (−18.4% to −82.8%), respectively. This suggests that enzalutamide enhances the metabolism of sorafenib through possible induction of CYP3A4.
Abbreviations: Cmax, concentration maximum; Tmax, time of maximum concentration; AUC, area under the curve; PK/PD, pharmacokinetics/pharmacodynamics.
Assessment, Analysis, and Discussion
| Completion | Study terminated before completion |
| Terminated Reason | Lack of interest |
| Investigator's Assessment | Lack of efficacy and drug–drug interaction |
Hepatocellular carcinoma (HCC), a primary liver tumor, is a leading worldwide cause of cancer‐related morbidity and mortality [15]. Developing in the context of underlying hepatic diseases and fibroinflammatory disorders of the liver, HCC is frequently seen in the background of viral hepatitis, alcohol use, and/or nonalcoholic fatty liver disease [16]. Epidemiologically, HCC is also sexually dimorphic, occurring 8–10 times more frequently in men than in women [17]. These trends do not necessarily correlate with a disproportionate risk due to gender alone for HCC etiologic factors. For example, higher rates of HCC have been observed in men with hepatitis B virus (HBV) who have higher baseline testosterone levels and CAG repeats in androgen receptor (AR) gene when compared with case controls without such findings [17]. Such observations have prompted deep investigation into the role of sex hormones and AR in HCC oncogenesis as well as potential therapeutic targets. Indeed, cell‐based and murine HCC models indicate that AR activation drives liver cancer growth whereas AR signaling blockade leads to tumor regression [1, 2]. In clinical samples, AR overexpression is detected in 40%–80% of cases by various methods and is associated with advanced disease stage and poor overall survival in retrospective series.
Despite these signals, prospective studies designed to demonstrate the therapeutic efficacy of first‐generation antiandrogens in patients with advanced HCC did not improve patient outcomes [18]. The clinical failure of first‐generation antiandrogens may have been due in part to poor specificity and limited potency for AR, leading to an inability to fully abrogate both ligand‐dependent and ligand‐independent AR signaling [1, 2, 3]. Enzalutamide is distinct from first‐generation antiandrogen agents in that it inhibits nuclear translocation of the androgen receptor, DNA binding, and coactivator recruitment [19]. Enzalutamide also has greater affinity for AR than first‐generation compounds and induces tumor stabilization and shrinkage in xenograft models [20]. Thus, there is good rationale for the assessment of enzalutamide monotherapy in HCC in the clinic. It is well established that AR cooperates with vascular endothelial growth factor/hypoxia‐inducible factor α signaling pathways requisite for HCC growth [5, 6]. To address the possibility that AR inhibition with antiandrogens may benefit synergistically from the presence of an antiangiogenic agent, we sought to explore the combination enzalutamide plus sorafenib.
At the time of the study development, execution, and completion, sorafenib was the only available first‐line systemic treatment for patients with advanced HCC and no effective therapies were approved in the second‐line setting [7]. Given the paucity of active therapies for HCC at that time, we proposed a National Comprehensive Cancer Network–funded, multicenter, open‐label, phase Ib study of enzalutamide with or without sorafenib in patients with advanced HCC with Child‐Pugh class A liver function. In part 1, a 3 + 3 dose de‐escalation design (starting dose 160 mg orally daily) with a 10‐patient expansion cohort was used to define the safety, tolerability, and recommended phase II dose (RP2D) of enzalutamide monotherapy in patients who had experienced disease progression or were intolerant to sorafenib. In part 2, a 3 + 3 dose escalation was used for the combination of sorafenib and enzalutamide with the primary objective of defining safety, tolerability, and RP2D of combination therapy in treatment‐naive patients with advanced HCC. With establishment of the RP2D, a single‐arm phase II expansion was initially planned with the objective of estimating the 4‐month progression‐free survival (PFS) of the combination regimen. Secondary and exploratory objectives for the study included pharmacokinetics, objective response rate (ORR) by RECIST version 1.1, PFS, overall survival (OS), and determination of tumor AR expression by immunohistochemistry. Acknowledging a potential drug–drug interaction between enzalutamide, a CYP3A4 inducer, and sorafenib, a CYP3A4 substrate, sorafenib was administered with a 7‐day run‐in prior to the start of enzalutamide. This dosing schedule allowed for comparison of sorafenib steady‐state exposure (known to occur after ~7 days of dosing) with the sorafenib exposure at enzalutamide steady state (known to occur after 30 days of dosing) [20, 21].
The study established that enzalutamide in patients with HCC is safe and tolerable at 160 mg daily (Part 1). Although formal efficacy testing was not embedded in the protocol, single‐agent enzalutamide showed no meaningful antitumor activity in a patient population with advanced HCC (0% ORR, a median PFS of 1.8 months, and OS of 7 months).
We consider that the failure to observe efficacy was multifactorial and likely includes the study design with a small sample size in a heterogeneous patient population as well as specific factors related to AR biology in HCC. Although AR plays a role in hepatocarcinogenesis irrespective of etiology, for which reason patients of all HCC risk factors were included in this study, translational studies indicate that HBV integration into the TERT promoter renders TERT transcription responsive to AR signaling [22]. These data suggest enzalutamide might have specific therapeutic relevance in HBV‐associated HCC, which made up only 19% of our cohort. Beyond issues potentially related to patient selection, recently reported biologic factors appear to underscore critical reasons for HCC antiandrogen insensitivity. AR‐splice variants, known to mediate enzalutamide resistance, have now been observed in 78% of HCC samples (290/372) in The Cancer Genome Atlas [8]. Furthermore, AR inhibition in vivo activates AKT‐TOR signaling pathways that serve to sustain HCC growth and bypass AR inhibition [4].
The combination of sorafenib and enzalutamide was safe and tolerable, but a clear drug–drug interaction was observed on Part 2 of the study. Enzalutamide reduced sorafenib Cmax and AUC0–8hr by 59 and 60%, respectively. Definitive conclusions related to the clinical significance of the drug–drug interaction were limited by the sample size and the dose escalation design. Although a higher dose of sorafenib in combination with enzalutamide may have been explored, the study was terminated given the totality of the results, which included limited activity of monotherapy; the modest activity of the combination that appeared similar to the historic operating characteristics of sorafenib; the drug–drug interaction; and the shift in landscape to newer tyrosine kinase inhibitors and immunotherapy. Further development of enzalutamide in HCC as a single agent or in combination with sorafenib is not warranted based on these findings.
Disclosures
James J. Harding: Bristol‐Meyers Squibb, Eli Lilly, Exelexis, Eisai (C/A); Robin K. Kelley: Genentech/Roche, Gilead (C/A), Adaptimmune, Agios, AstraZeneca, Bayer, Bristol‐Myers Squibb, Eli Lilly, Exelixis, EMD Serono, Merck, Novartis, QED, Taiho, Partner Therapeutics (RF), Ipsen (Other: travel); Hooman Yarmohammadi: Genentech (C/A); Danny N. Khalil: property rights/interests related to CD40 and in situ vaccination (IP); Ghassan K. Abou‐Alfa: Agios, Astra Zeneca, Autem, Bayer, Beigene, Berry Genomics, Celgene, CytomX, Debio, Eisai, Eli Lilly, Flatiron, Genentech, Gilead, Incyte, Ipsen, LAM, Loxo, Merck, MINA, Polaris, QED, Redhill, Roche, Silenseed, Sillajen, Sobi, Therabionics, Twoxar, Vector, Yiviva (C/A), ActaBiologica, Agios, Astra Zeneca, Bayer, Beigene, Berry Genomics, Bristol‐Myers Squibb, Casi, Celgene, Exelixis, Genentech, Halozyme, Incyte, Mabvax, Puma, QED, Roche, Sillajen, Yiviva (RF), Articles and Methods for Preventing and Treating Dermatologic Adverse Events, identified by International Patent Application No. PCT/US2014/031545 filed on March 24, 2014, and priority application Serial No.: 61/804,907; Filed: March 25, 2013 (IP). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was approved and funded in part by the National Comprehensive Cancer Network Oncology Research Program from general research support provided by Astellas. This research was funded in part through the NIH/National Cancer Institute (NCI) Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center. This work was partially supported by NCI grant P30 CA016056 involving the use of Roswell Park's Bioanalytics, Metabolomics and Pharmacokinetics Shared Resource.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02642913
- Sponsor: Memorial Sloan Kettering Cancer Center
- Principal Investigator: James J. Harding
- IRB Approved: Yes
References
- 1. Ma WL, Hsu CL, Wu MH et al. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology 2008;135:947–955, 955 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ren H, Ren B, Zhang J et al. Androgen enhances the activity of ETS‐1 and promotes the proliferation of HCC cells. Oncotarget 2017;8:109271–109288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun H, Yang W, Tian Y et al. An inflammatory‐CCRK circuitry drives mTORC1‐dependent metabolic and immunosuppressive reprogramming in obesity‐associated hepatocellular carcinoma. Nat Commun 2018;9:5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang H, Li XX, Yang Y et al. Significance and mechanism of androgen receptor overexpression and androgen receptor/mechanistic target of rapamycin cross‐talk in hepatocellular carcinoma. Hepatology 2018;67:2271–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nicholson B, Gulding K, Conaway M et al. Combination antiangiogenic and androgen deprivation therapy for prostate cancer: A promising therapeutic approach. Clin Cancer Res 2004;10:8728–8734. [DOI] [PubMed] [Google Scholar]
- 6. Wang SH, Yeh SH, Shiau CW et al. Sorafenib action in hepatitis B virus X‐activated oncogenic androgen pathway in liver through SHP‐1. J Natl Cancer Inst 2015;107:djv190. [DOI] [PubMed] [Google Scholar]
- 7. Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 8. Dauki AM, Blachly JS, Kautto EA et al. Transcriptionally active androgen receptor splice variants promote hepatocellular carcinoma progression. Cancer Res 2020;80:561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleming MT, Rathkopf DE, Gibbons J et al. Enzalutamide in combination with docetaxel in men with prostate cancer (PC): Preliminary results from a phase I study. J Clin Oncol 2013;31(suppl 6):63a. [Google Scholar]
- 10. Efstathiou E, Titus MA, Wen S et al. Enzalutamide (ENZA) in combination with abiraterone acetate (AA) in bone metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol 2014;32(suppl 15):5000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abou‐Alfa GK, Meyer T, Cheng AL et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruix J, Qin S, Merle P et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- 13. Finn RS, Ryoo BY, Merle P et al. Pembrolizumab as second‐line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE‐240: A randomized, double‐blind, phase III trial. J Clin Oncol 2020;38:193–202. [DOI] [PubMed] [Google Scholar]
- 14. Zhu AX, Kang YK, Yen CJ et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha‐fetoprotein concentrations (REACH‐2): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2019;20:282–296. [DOI] [PubMed] [Google Scholar]
- 15. Mittal S, El‐Serag HB. Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol 2013;47(suppl 0):S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fattovich G, Stroffolini T, Zagni I et al. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004;127(5 suppl 1):S35–S50. [DOI] [PubMed] [Google Scholar]
- 17. Yu MW, Cheng SW, Lin MW et al. Androgen‐receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B‐related hepatocellular carcinoma. J Natl Cancer Inst 2000;92:2023–2028. [DOI] [PubMed] [Google Scholar]
- 18. Groupe d'Etude et de Traitement du Carcinome H . Randomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifen. Hepatology 2004;40:136–139. [DOI] [PubMed] [Google Scholar]
- 19. Scher HI, Fizazi K, Saad F et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–1197. [DOI] [PubMed] [Google Scholar]
- 20. Scher HI, Beer TM, Higano CS et al. Antitumour activity of MDV3100 in castration‐resistant prostate cancer: A phase 1‐2 study. Lancet 2010;375:1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strumberg D, Richly H, Hilger RA et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43‐9006 in patients with advanced refractory solid tumors. J Clin Oncol 2005;23:965–972. [DOI] [PubMed] [Google Scholar]
- 22. Li CL, Li CY, Lin YY et al. Androgen receptor enhances hepatic telomerase reverse transcriptase gene transcription after hepatitis B virus integration or point mutation in promoter region. Hepatology 2019;69:498–512. [DOI] [PubMed] [Google Scholar]


