Abstract
Introduction
Desmoid tumors (DT) are rare collagen‐forming tumors that can exhibit locally aggressive patterns of behavior. The aim of this study was to evaluate the efficacy and safety of treatment of DT with single‐agent oral vinorelbine.
Materials and Methods
A retrospective review of patients treated with vinorelbine 90 mg orally on days 1, 8, and 15 of a 28‐day cycle from January 2004 to July 2019 was performed. Response was assessed using RECIST version 1.1. Descriptive statistics were employed.
Results
A total of 29 patients were included. Response rate was 20.7% (6/29), and clinical benefit rate (response by RECIST 1.1 and/or clinical symptom improvement) was 65.5% (19/29). No patient experienced grade 3 or above toxicity. Common toxicities were grade 1–2 nausea (14/26, 48.3%), fatigue (9/26, 31.0%), and diarrhea (4/26, 13.8%).
Conclusion
Single‐agent oral vinorelbine is an effective, safe, and well‐tolerated treatment for DT. It represents a new oral alternative for management of DT.
Short abstract
Methotrexate combined with vinorelbine or vinblastine have been used to treat desmoid tumors combined with either vinorelbine or vinblastine has been effectively used to treat patients with desmoid tumors but requires intravenous treatment. An oral regimen that limits hospital visits would be ideal. This article evaluates the efficacy and safety of single‐agent oral vinorelbine and reports promising results.
Introduction
Desmoid tumors (DTs) are rare collagen‐forming tumors arising from soft tissues that have no metastatic potential but can exhibit locally aggressive patterns of behavior [1]. DTs are seen more frequently in women with peak age of presentation of 30–40 years [2]. They exhibit a variable clinical pattern of behavior, from incidental finding to a discrete mass with pain or restriction in function. An initial period of active surveillance is recommended with treatment only offered after clear clinical or radiological progression [3]. Methotrexate combined with either vinorelbine or vinblastine has been effectively used to treat DTs for over 30 years [4, 5] but requires the need for intravenous (IV) treatment. An oral regimen that limits visits to hospital would be ideal for many patients. The aim of this study was to evaluate the efficacy and safety of single‐agent oral vinorelbine.
Materials and Methods
Institutional approval was obtained prior to commencement. A retrospective review of the prospectively maintained Royal Marsden Hospital (RMH) Sarcoma Unit database was performed to identify patients with DTs treated from January 2004 to July 2019. Patient details were obtained from the database and electronic patient record. In all cases the diagnosis of DT was confirmed by an expert soft tissue pathologist (C.F., K.T.).
A starting dose of vinorelbine 90 mg orally on days 1, 8, and 15 on a 28‐day cycle was used in all patients. Dose interruptions and reductions were implemented as per local institutional guidelines. Repeat imaging was routinely performed every 2–3 cycles. Radiological response was re‐reviewed for this study using the RECIST version 1.1 and toxicity was graded by CTCAE version 4.0. Descriptive statistics were employed.
Results
A total of 29 patients with DTs were treated at RMH between January 2004 and July 2019 with vinorelbine. Patient characteristics are shown in Table 1. The median age at presentation was 31.4 years (interquartile range [IQR], 22.5–47.4). The majority of patients were female (n = 16, 55.2%). Median tumor size was 8.1 cm (IQR, 5.7–10.5 cm). The majority of DTs were in the thorax (n = 9, 31.0%) or upper limb (n = 6, 20.7%). No patients had familial adenomatous polyposis‐associated DT.
TABLE 1.
Baseline patient characteristics
| Characteristic | Total, n = 29 |
|---|---|
| Median age at presentation (IQR), yr | 31.4 (16–47) |
| Gender, n (%) | |
| Female | 16 (55.2) |
| Male | 13 (44.8) |
| Median tumor length (IQR), cm | 8.1 (5.7–10.5) |
| Primary site, n (%) | |
| Thorax | 9 (31.0) |
| Upper limb | 6 (20.7) |
| Intraabdominal | 3 (10.3) |
| Gluteal region | 3 (10.3) |
| Head and neck | 2 (6.9) |
| Lower limb | 2 (6.9) |
| Pelvis | 2 (6.9) |
| Abdominal wall | 1 (3.4) |
| Spine | 1 (3.4) |
| familial adenomatous polyposis‐associated, n (%) | |
| Yes | 0 (0) |
| No | 29 (100) |
| Initial treatment, n (%) | |
| Active surveillance | 16 (55.2) |
| Primary surgery | 10 (34.5) |
| Medical therapy | 3 (10.3) |
| Systemic treatment history, n (%) | |
| No previous treatment | 13 (44.8) |
| 1 previous line | 4 (13.8) |
| 2 previous lines | 5 (17.2) |
| 3 previous lines | 5 (17.2) |
| 4 previous lines | 2 (6.9) |
Abbreviation: IQR, interquartile range.
Initial treatment was active surveillance for over half of the patients (n = 16, 55.2%) and was systemic therapy in the minority of patients (n = 3, 10.3%; nonsteroidal anti‐inflammatory drugs [n = 1], tamoxifen [n = 1], vinorelbine [n = 1]). A relatively high proportion of patients (n = 10, 34.5%) had primary surgery. These patients were originally diagnosed when surgery was the standard first‐line treatment.
Sixteen (55%) patients had received prior systemic therapy; however, the median number of prior therapies was 0 (range, 0–4). Previous systemic therapies included tamoxifen, liposomal doxorubicin, vinblastine plus methotrexate, and vincristine plus actinomycin. Twenty‐eight patients were evaluable by RECIST 1.1 prior to starting vinorelbine, 10 patients had progressive disease (PD), and 18 had stable disease (SD). Of the 18 with stable disease by RECIST 1.1, 15 had worsening symptoms, 2 had non‐RECIST PD deemed clinically significant, and 1 had a desmoid fibromatosis very close to a critical neurovascular structure warranting treatment. The one patient who was not evaluable by RECIST 1.1 at baseline had clinical and symptomatically progressive disease.
The best response as per RECIST 1.1 to vinorelbine was partial response (PR) in 6 patients, (20.7%) and 19 patients (65.5%) had SD. The best response was seen in a patient with a large obturator DT (Figure 1). PD was seen in two patients (6.9%), and two patients (6.9%) were not evaluable. Nineteen patients (65.5%), 13 of which had stable (n = 12) or progressive (n = 1) disease on imaging, reported a clinical symptom improvement, with 18 patients (62.0%) reporting less pain and nine patients (31%) reporting increased function. The clinical benefit rate, defined in our study as response by RECIST 1.1 (n = 6 patients) and/or clinical symptom improvement (n = 13 patients), was 65.5%.
Figure 1.
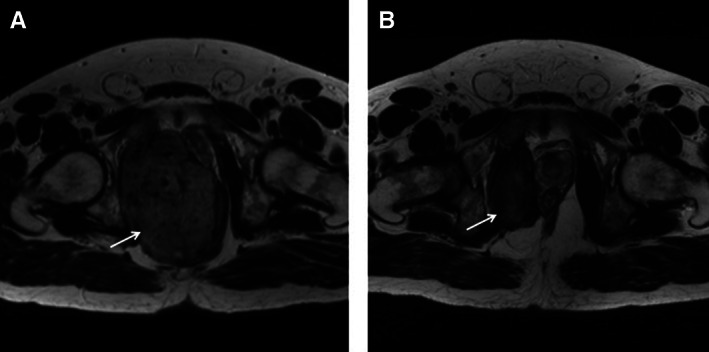
Axial T2 weighted image (T2WI) magnetic resonance imaging (MRI) of the pelvis. MRI at baseline (A) and following 26 months of treatment (B) with vinorelbine. The large intermediate T2 signal tumor (arrow) arises from right obturator internus muscle, compressing and displacing the rectum and prostate. Following treatment (B), the tumor (arrow) has significantly reduced in size achieving partial response by RECIST 1.1. Reduction in T2 signal following treatment also implies response.
Overall median duration of vinorelbine was 7.7 months (IQR, 5.3–13.2 months). Reasons for discontinuation of vinorelbine were stable disease on at least two interval imaging scans (n = 5), clinician choice (n = 6), no further clinical benefit (n = 5), patient choice not due to side effects (n = 5), fertility reasons (n = 1), desmoid fibromatosis now amenable to cryoablation (n = 1), and need for unrelated organ transplantation (n = 1). Four patients had clinical or radiological progressive disease on (n = 2) or following (n = 2) treatment with vinorelbine, with a median time to second treatment of 1.5 months (range, 1–10).
Side effects were mostly gastrointestinal and self‐limiting (Table 2). No patient experienced a grade ≥ 3 side effect or any serious complication due to treatment. One patient (4.2%) had a dose reduction that was to ensure tolerability due to grade 2 nausea and grade 2 abdominal pain. The liver dysfunction, bone marrow suppression, and oral ulcers seen with combination vinca alkaloid and methotrexate [6, 7] were not seen in our cohort.
Table 2.
Incidence and grade of adverse events on single‐agent vinorelbine
| Adverse event on vinorelbine | Grade 1 or 2, number of patients (%) | Grade 3 or 4, number of patients (%) |
|---|---|---|
| Nausea | 14 (58.8) | 0 (0) |
| Fatigue | 10 (34.4) | 0 (0) |
| Diarrhea | 5 (17.2) | 0 (0) |
| Constipation | 3 (12.5) | 0 (0) |
| Vomiting | 2 (8.3) | 0 (0) |
| Gastritis | 2 (8.3) | 0 (0) |
| Abdominal pain | 2 (8.3) | 0 (0) |
| Excessive sweating | 1 (4.2) | 0 (0) |
| Dizziness | 1 (4.2) | 0 (0) |
| Anemia | 1 (4.2) | 0 (0) |
Discussion
Methotrexate combined with either vinorelbine or vinblastine is an established treatment for DT based on multiple studies [5, 8, 9]. In two recent single‐center retrospective studies, objective response rate (ORR) for vinorelbine with low dose methotrexate was 35.2%–85.4% with a clinical benefit rate (CBR) of 87.3%–98% [6, 7]. Notably, in these studies, the clinical benefit rate was defined as complete response plus PR plus SD; however, there are limitations to the inclusion of SD to the CBR. Patients with desmoid fibromatosis have a varied natural history, with many patients having long‐term stable disease on active surveillance; thus, a CBR that includes SD may overestimate the true effect of a treatment. Our study of single‐agent vinorelbine in DT showed an ORR of 20.6% and CBR (complete response plus PR and/or symptomatic improvement) of 65.5%. These results are similar to a retrospective study of 50 patients treated with vinorelbine in patients with and without hormonal blockade [10]. Patients in our cohort did not have similar hematological, hepatotoxicity, and oral mucositis that are commonly seen with combination vinca alkaloids and methotrexate [6, 7]. Importantly, only one patient (3.4%) required a dose reduction. No patients stopped treatment because of intolerance. This is in contrast to data from a recent retrospective cohort of patients treated with vinorelbine with low dose methotrexate in which the rate of treatment discontinuation was 79% (38/48 patients), mainly due to treatment intolerance and patient preference [6]. Thus, single‐agent vinorelbine has similar CBR to vinca alkaloids and methotrexate but has a more favorable tolerability profile. In our study, despite the overall response rate of 20.7%, 19 patients (65.5%) reported a clinical improvement in symptoms, 18 patients (62.0%) reported less pain, and 9 patients (31.0%) reported increased function as a result of vinorelbine therapy. Notably, quality of life outcomes were not reported in either of the recent retrospective studies of low‐dose vinca alkaloid and methotrexate [6, 7].
The oral administration of vinorelbine allows outpatient administration with clinic reviews up to 12‐weekly when appropriate. Reduced visits to hospital coupled with favorable side effect profile are likely to contribute to a better quality of life.
Conclusion
Single‐agent vinorelbine is a safe, effective, and well tolerated first‐line treatment for patients with DT who have clinical, radiological, or symptomatic progressive disease following a period of active surveillance. Patients should be referred to specialist centers with experience in diagnosis and management of these rare tumors.
Disclosures
Winette van der Graaf: Novartis (RF), Bayer (C/A); Robin L. Jones: Merck Sharpe & Dohme, GlaxoSmithKline (RF), Adaptimmune, Athenex, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Eli Lilly & Co, Merck, Pharmamar, Upto Date (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The authors acknowledge funding to the Royal Marsden/ Institute of Cancer Research National Institute for Health Research Biomedical Research Centre.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Fisher C, Thway K. Aggressive fibromatosis. Pathology 2014;46:135–140. [DOI] [PubMed] [Google Scholar]
- 2. Demoid Tumor Working Group . The management of desmoid tumours: A joint global consensus‐based guideline approach for adult and paediatric patients. Eur J Cancer 2020;127:96–107. [DOI] [PubMed] [Google Scholar]
- 3. Gronchi A, Jones RL. Treatment of desmoid tumors in 2019. JAMA Oncol 2019;5:567–568. [DOI] [PubMed] [Google Scholar]
- 4. Palassini E, Frezza AM, Mariani L et al. Long‐term efficacy of methotrexate plus vinblastine/vinorelbine in a large series of patients affected by desmoid‐type fibromatosis. Cancer J 2017;23:86–91. [DOI] [PubMed] [Google Scholar]
- 5. Weiss AJ, Lackman RD. Low‐dose chemotherapy of desmoid tumors. Cancer 1989;64:1192–1194. [DOI] [PubMed] [Google Scholar]
- 6. Ingley KM, Burtenshaw SM, Theobalds NC et al. Clinical benefit of methotrexate plus vinorelbine chemotherapy for desmoid fibromatosis (DF) and correlation of treatment response with MRI. Cancer Med 2019;8:5047–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li S, Fan Z, Fang Z et al. Efficacy of vinorelbine combined with low‐dose methotrexate for treatment of inoperable desmoid tumor and prognostic factor analysis. Chin J Cancer Res 2017;29:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toulmonde M, Pulido M, Ray‐Coquard I et al. Pazopanib or methotrexate–vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): A non‐comparative, randomised, open‐label, multicentre, phase 2 study. Lancet Oncol 2019;20:1263–1272. [DOI] [PubMed] [Google Scholar]
- 9. Constantinidou A, Jones RL, Scurr M et al. Advanced aggressive fibromatosis: Effective palliation with chemotherapy. Acta Oncol 2011;50:455–461. [DOI] [PubMed] [Google Scholar]
- 10. Mir O, Rahal C, Rimareix F et al. Efficacy of oral vinorelbine in advanced/progressive desmoid tumours: An updated retrospective study in 50 patients. J Clin Oncol 2016;34(suppl):11050a. [Google Scholar]


