Abstract
Overcharge abuse can trigger thermal runaway when a device is left unattended. Redox shuttles, as economic and efficient electrolyte additives, have been proven to provide reliable and reversible protection for state-of-art Li-ion batteries (LIBs) against overcharge. Here, a functional organic salt, trisaminocyclopropenium perchlorate (TAC•ClO4), is developed and employed as a redox shuttle for overcharge protection in a Na-ion battery system. This type of novel redox shuttle molecule is reported for the first time. As a unique ionic compound with the smallest aromatic ring structure, TAC•ClO4 exhibits distinctive attributes of fast diffusion, high solubility, and ultrahigh chemical/electrochemical stability in both redox states. With merely 0.1 M TAC•ClO4 in electrolyte, Na3V2(PO4)3 cathode can carry overcharge current even up to 10C or 400% SOC. Na3V2(PO4)3/hard carbon cells demonstrated strong anti-overcharging ability of 176 cycles at 0.5C rate and 54 cycles at 1C rate with 100% overcharge. Moreover, TAC•ClO4 addition has little impact on the electrochemical performance of Na-ion batteries, especially on the rate performance and the initial Columbic efficiency. Interestingly, a unique and reversible electrochromic behavior of TAC•ClO4 electrolyte can promptly provide the device an overcharge alarm under a designed potential to further enhance the safety level.
Keywords: cyclopropenium salt, sodium ion batteries, redox shuttle, overcharge protection, electrochromic effect
1. Introduction
Sodium-ion batteries (SIBs) have aroused world-wide attention as a competitive technology for electrochemical energy storage (EES) [1,2]. However, a safety accident in a large-scale EES station could have catastrophic consequences and lead to tremendous economic loss [3–7]. Therefore, the development of intrinsically safe SIBs is most critical for the pursuit of extensive application. Among all the safety issues of SIBs, overcharge abuse can induce particularly harsh thermal runaway [8]. During overcharge, Na dendrites can grow at the anode surface while the cathode structure can undergo collapse, leading to a series of side reactions that would generate heat and gas, eventually evolving into a disastrous thermal runaway [9–11]. To construct safer SIBs, one of the most adopted strategies is to prevent electrolyte burning, known as the last stage before entering uncontrollable thermal runaway [12], through e.g. developing non-flammable organic electrolyte [13–18], aqueous electrolyte [19–23] and solid-state electrolyte [24–27]. Those approaches can marginally improve the safety performance by reducing or eliminating the flammability of electrolyte at the expense of the performance of the battery. These issues highlight the importance of exploiting a self-actuated and reversible protection technique which takes effect before permanent damage occurs under abusive conditions of SIBs.
Redox shuttles represent a class of electroactive molecules that can serve as electrolyte additives to achieve reversible overcharge protection [28]. Under overcharge conditions, redox shuttles become oxidized to form corresponding radical cations at a cathode surface. The resulting positively charged radicals subsequently diffuse to the anode surface where they are reduced. Through continuously shuttling between cathode and anode, the cell voltage can be clamped at the reverse redox potential of the shuttle molecules until the end of overcharge. Thus, all the cell components will be exempt from any potential damages. During the past two decades, a lot of redox shuttles have been successfully developed for LIB systems, most of which were based on 1,4-di-tert-butyl-2,5-dimethoxybenzene (DDB) [29–35], phenothiazine (PT) [36–40], triphenylamines (TPA) [41], 2,2,6,6-tetramethylpiperinyl-oxides (TEMPO) [42] as well as their derivatives. Meanwhile, Na anodes as well as other components in the SIB are considerably different and more reactive than their LIB counterparts. Thus, state-of-the-art redox shuttles that have worked well in LIBs may not function as well in SIBs. Therefore, more adequate redox shuttles need to be developed specifically for SIBs. Ideally, a robust redox shuttle should provide an effective protection under high overcharge current (≥ 1.0C) with a low addition amount (≤ 0.1 M), since overcharge is more likely to occur at the end of fast-charging process and low concentration of the additive could maintain the battery energy density and ensure low cell impedance. Molecules that meet these requirements are those with the attributes of (a) high stability throughout the entire redox process (b) high compatibility with SIB materials and (c) high mobility or diffusivity. These stringent requirements may well be the reason why no such kind of redox shuttle has thus far been devised.
It is clear that a new structural paradigm is needed to address these challenges. As the smallest aromatic cation, the cyclopropenium ion is the canonical example of a two π-electron Hückel system, which thus often displays high chemical stability [43–45]. Especially, trisaminocyclopropenium (TAC) ions are exceptionally stable cations that are resistant to hydrolysis and can undergo facile single-electron oxidation to furnish stable dications [45]. Recently, TACs were used by one of our groups (Lambert) as organic electrophotocatalysts [46–48] and functional polymer materials [49, 50]. Sanford also demonstrated that TACs can serve as the catholytes in organic redox flow batteries [51–54]. We envisioned that TACs could also be employed as a redox shuttle and provide a new platform for overcharge protection of SIBs. Notably, the unique aromatic and ionic character of the TACs would represent a significant departure from state-of-the-art redox shuttle additives.
Herein, we report trisaminocyclopropenium perchlorate (TAC•ClO4) as a novel redox shuttle molecule for overcharge protection in SIBs. We demonstrate that upon the occurrence of overcharge, the TAC molecule can immediately lock the potential of Na3V2(PO4)3 cathode at 3.75 V (vs. Na+/Na) by reversibly shuttling between its cationic and dicationic forms via a single-electron redox reaction. Upon overcharging under harsh conditions e.g. 400% SOC or at 10C rate, TAC can still offer effective protection against voltage increase, with a low concentration of 0.1 M. Na3V2(PO4)3/hard carbon full cell survived 167 overcharge cycles at 0.5C and 54 cycles at 1C with 100% overcharge. Heat generated during the overcharge decreased by 20% in the presence of TAC in the electrolyte. There was little impact on the electrochemical performance of the SIB with the addition of TAC. The distinct color switch (colorless to red) of TAC in its different oxidation states also makes it possible to use it as an overcharge indicator.
2. Results and discussion
2.1. Physicochemical properties of the TAC•ClO4 organic salt
As illustrated in Scheme S1, TAC•ClO4 can be made through a one-pot synthesis from commercially available materials on a multi-gram scale and with no column purification (Fig. S1) [47]. Molecules with various structures were synthesized to optimize solubility, electrochemical reversibility, redox potential, and other properties.
The electrochemical reversibility of TAC•ClO4 was first verified by cyclic voltammetry (CV) on glassy carbon electrode. Unless specifically described otherwise, NaClO4/EC+DMC electrolyte was used. As illustrated in Fig. 1a, a pair of well-defined, highly reversible redox peaks emerged at 3.8 V (vs. Na+/Na), demonstrating the mean potential of the anodic and cathodic peaks. Even after 1000 cycles, the CV profile showed almost no degradation or reduction of both anodic and cathodic current peaks, indicating high electrochemical stability of TAC•ClO4 in a carbonate electrolyte bulk. As displayed in Fig. 1b, a single-electron redox reaction takes place between the TAC cation and its oxidized state, the TAC dication. Fig. 1c shows the high stability and chemical compatibility of TAC with Na metal. Throughout the six-month long storage, the electrolyte solution with 0.1 M TAC•ClO4 remained transparent with no gas bubbles detected on the Na surface, implying a high chemical inertness of TAC cation with Na metal. As displayed in Fig. 1d, the chemical stability of TAC dication can be explained via a 3D structure achieved from our previous result of X-ray diffraction [47]. The protons alpha to nitrogen radical cation are sterically inaccessible for deprotonation and subsequent reductive degradation due to its geometry. Moreover, the unique steric conformation leaves the α-hydrogen atoms orthogonal to the TAC π-system, also making it difficult from being deprotonated [45, 47]. Therefore, TAC dication owns a high chemical stability despite its strong oxidizing character.
Fig. 1.
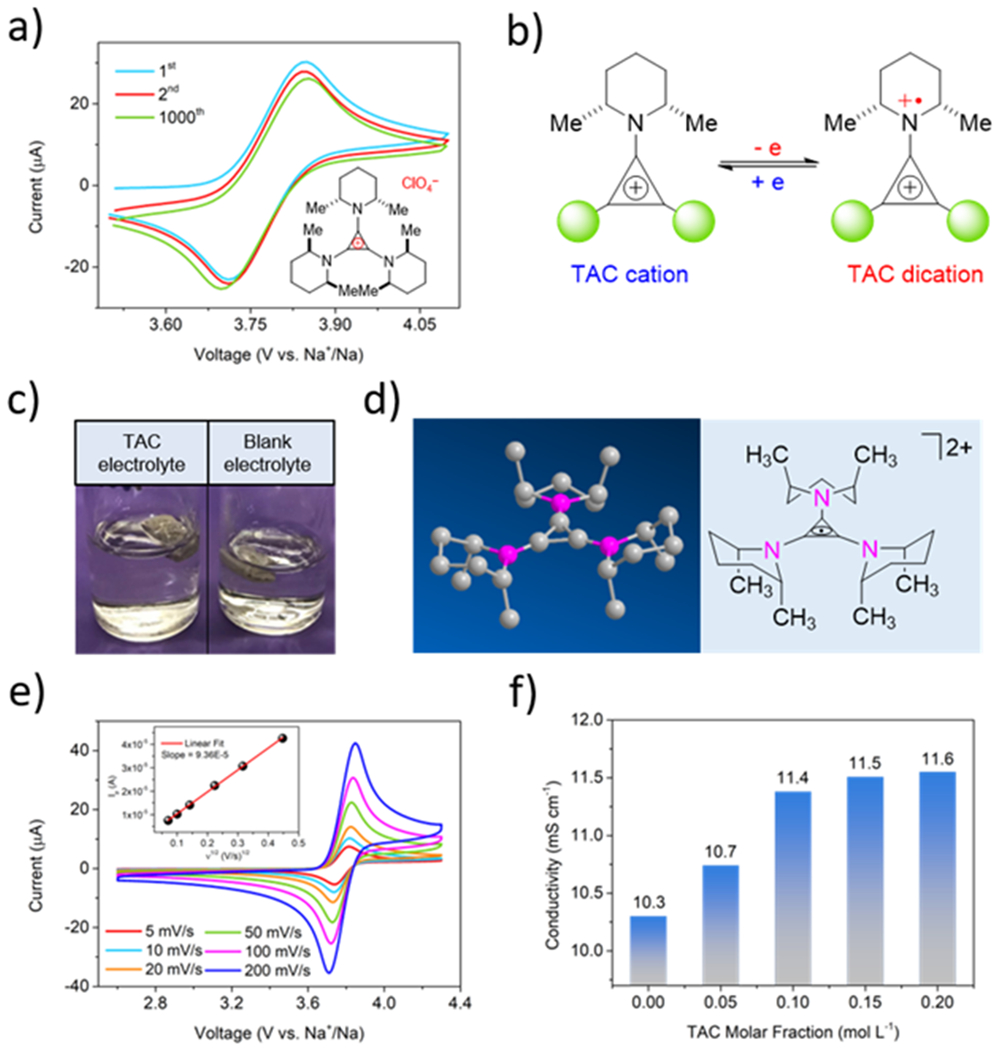
Physicochemical properties of the TAC•ClO4 compound. a) Cyclic voltammograms of 4 mM TAC•ClO4 at 100 mV s−1 for 1000 cycles; b) electrochemical conversion between TAC cation and TAC dication; c) photographs of Na metal stored in electrolyte with or without 0.1 M TAC•ClO4 at ambient temperature after six months; d) 3D structure of TAC dication ; e) cyclic voltammograms of 4 mM TAC•ClO4 at various scan rates; the insert is a plot of the anodic current to Ip vs. ν1/2; f) the dependence of TAC•ClO4 concentration on the ionic conductivity of electrolyte.
As an ionic compound, TAC•ClO4 is expected to have a high solubility in polar carbonated electrolytes compared to other neutral overcharge molecules. The solubility of TAC•ClO4 was roughly evaluated and compared with DDB, which is considered as a typical standard for the overcharge additives in LIB systems. As displayed in Fig. S2, the electrolyte can dissolve up to 0.3 M TAC•ClO4 in comparison to less than 0.05 M DDB. The diffusion coefficient of TAC was determined by a CV of a 4 mM TAC•ClO4 solution on a glassy carbon electrode at scan rates of 5-200 mV s−1. As depicted in Fig. 1e, TAC still exhibits very symmetric peaks even under a high sweeping rate of 200 mV s−1, demonstrating a fast mass transport and electro-kinetics in the bulk electrolyte. According to the insert of an Ip ~ ν1/2 plot and Randles-Sevick equation listed in Eq. S1, the diffusion coefficient of TAC was calculated to be 7.9×10−6 cm s−1. By contrast, DDB exhibits two orders of magnitude lower diffusion coefficient (9×10−8 cm s−1) compared to TAC as displayed in Fig. S3a despite its high electrochemical stability (Fig. S3b).
The ionic conductivity was also investigated. As shown in Fig. 1f, with the increase of TAC concentration, the conductivity first increases gradually and then reaches a maximum value at around 0.1 M (11.4 mS cm−1 compared to 10.3 mS cm−1 of blank electrolyte). The improvement of the conductivity could be ascribed to the notion that TAC•ClO4 served as an extra amount of supporting electrolyte salt in addition to the NaClO4. It seemed that a maximum value could be ascribed to the increasing viscosity, which was becoming more predominant in affecting the ion mobility. The viscosity of blank electrolyte and 0.1 M TAC electrolyte were 2.88 mPa·s and 2.97 mPa·s at 24 ºC.
2.2. Overcharge protection performance of Na3V2(PO4)3 cathode
To build a reliable SIB system, we selected Na3V2(PO4)3 (NVP) as the cathode material due to its intrinsic safety, high rate capacity retention and long lifespan. Specifically, NVP owns a thermo-stable NASION phosphate host, which has large-sized 3D tunnels and a stable lattice structure, enabling not only fast Na+ ion transport but also zero-strain insertion reaction during cycling [13, 55]. We prepared NVP/carbon composite through a widely adopted sol-gel method (Fig. S4) [56]. The 100% overcharge performance of DDB and TAC were compared in NVP/Na half-cells. As displayed in Fig. 2a, the well-developed LIB redox shuttle DDB cannot provide efficient protection in SIB, which may be due to its poor diffusion and solubility as mentioned in Fig. S3. By contrast, as displayed in Fig. 2b, TAC presents a flat plateau after being fully charged until reaching the cutoff capacity, while uncontrollable voltage increase was observed without TAC. Throughout the whole overcharge process, the voltage was steadily locked at 3.75 V, corresponding to the oxidation-diffusion-reduction process between TAC cation and TAC dication. The CV on NVP and TAC are further compared in Fig. 2c. NVP electrode presented a pair of well-defined redox peaks at around 3.4 V, corresponding to the reversible transformation of V3+/V4+ [57]. Notably, TAC starts to oxidize at 3.7 V, right after the anodic reaction of NVP completes at about 3.5 V. The ~0.2 V potential difference between the initiation of TAC oxidation and the end-of-charge of NVP electrode can perfectly preclude the adverse interference of TAC on the battery’s normal operation. As shown in Fig. 2d, NVP-TAC group can provide a steady 100 cycles protection under 100% overcharge at 0.5C, implying a high reversibility and stability of TAC cation and dication.
Fig. 2.
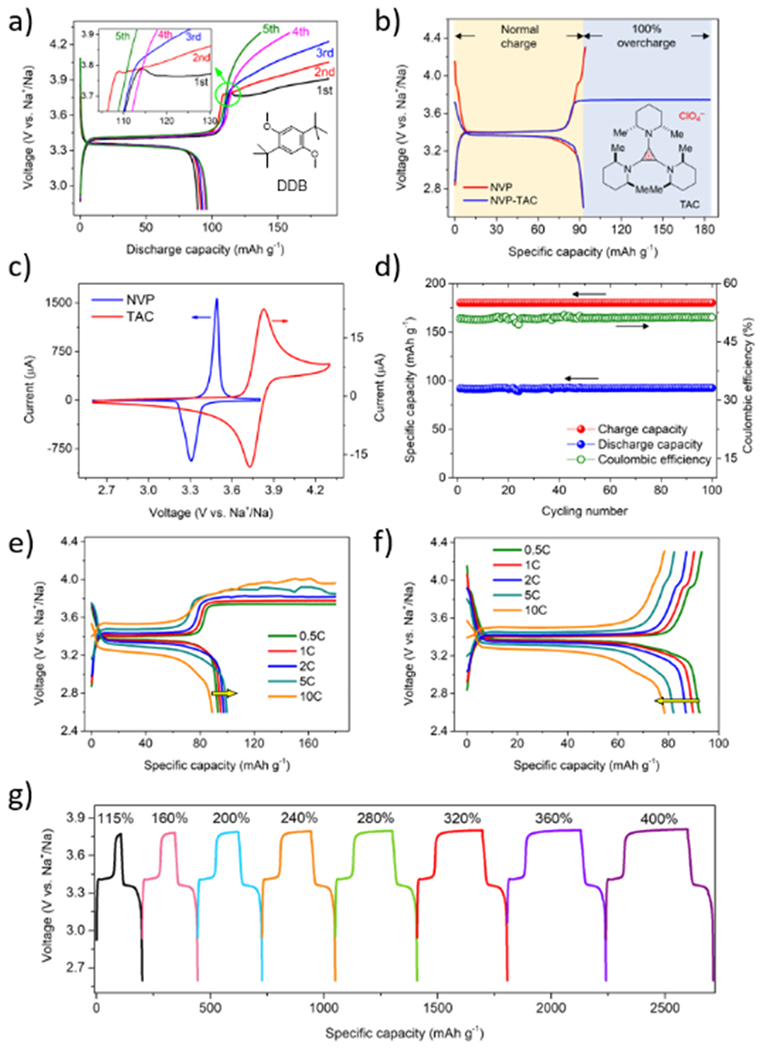
Voltage profile of NVP cathode at 0.5C and with 100% overcharge in electrolyte containing a) saturated DDB and b) 0.1 M TAC and no additive; c) cyclic voltammetry curves of NVP and TAC at a scan rate of 0.1 mV s−1 and 100 mV s−1, respectively; d) capacity retention profile of NVP cathode at 0.5C and with 100% overcharge in 0.1 M TAC electrolyte; rate performance of NVP cathode with 100% overcharge in electrolyte containing e) 0.1 M TAC and f) no additive; g) voltage profiles of NVP cathode at 1C with overcharging to different cut-off capacities.
The effectiveness of the overcharge protection under high charge current densities is very important since overcharge is more likely to take place at the end of fast-charge [58, 59]. Under such conditions, a fast-diffusional electron-transfer process is often required for a redox molecule to prevent a battery from a dangerous situation. However, most reported overcharge currents in LIB systems were unpractically low, e.g. 0.5C or even lower, let alone with relatively large amount of redox shuttle additives (usually 0.2 M -0.4 M), which would pose a negative impact on the battery performance due to the increase of the impedance and the sacrifice of the energy density of the battery. Hence, exploring anti-overcharge effect under high current while maintaining a low concentration of the additive represents a more useful approach. We then investigated the protection capability of NVP/Na cells at various overcharge currents with different TAC concentrations starting from 0.05 M. As shown in Fig. S5, the cell with 0.05 M TAC can provide sound protection at 0.5C but failed at 1C. By contrast, as shown in Fig. 2e, the cell with 0.1 M TAC can successfully provide overcharge protection from 0.5C up to 10C. Interestingly, the discharge capacity of NVP-TAC group gradually increased from 93.2 mAh g−1 to 105 mAh g−1 as the rate increased from 0.5C to 5C. This result could be explained as some TAC dications were trapped within the pores of cathode under the high rate since there was not sufficient time for all of them to diffuse back to the bulk electrolyte. These trapped TAC could contribute to the overall discharge capacity. The maximum capacity TAC can provide when serving as the active material should be less than its theoretical specific capacity, which was calculated to be 71.3 mAh g−1 based on Eq. S2. At 10C rate, the discharge capacity of NVP-TAC group drops to 88.9 mAh g−1, which is evident that the contribution of capacity by TAC molecules cannot offset the capacity decline of NVP material due the sluggish kinetics. The relatively flat plateau around 4 V in Fig. 2e suggests that there were still adequate free-moving TAC molecules to serve as redox shuttle despite the presumed loss of the trapped ones. As rate decreases to 1C, the discharge capacity could fully recover to the original value of 91.5 mAh g−1, implying that the trapped TAC molecules could be liberated back to the electrolyte given adequate relaxation time. On the other hand, as shown in Fig. 2f, the potential of control group (with no additive) quickly rises to the safety cutoff voltage after being fully charged. Besides, the discharge capacity gradually decreases with rate increases owing to the slow kinetics of NVP, 92.6 mAh g−1 at 0.5C to 78.3 mAh g−1 at 10C. Apart from the capacity contribution of TAC, we believe that the superior rate performance of NVP-TAC group could also arise from its higher ionic conductivity compared to blank electrolyte.
The prolonged protection against continuous overcharge is another notable aspect. Usually, the protection duration was considered to be determined by the diffusion coefficient as well as the reversibility of the redox shuttle molecule. As illustrated in Fig. 2g, the NVP-TAC group is overcharged at 1C until reaching various cutoff capacities. With cutoff capacity increase from 115% SOC to 400% SOC, the plateaus at ~3.75 V became longer. The corresponding discharge capacity increased from 93.5 mAh g−1 to 111.7 mAh g−1 and the Coulombic efficiency (CE) decreased from 87.4% to 31.1%. All these phenomena indicate that the high electrochemical reversibility and fast diffusion behavior of TAC molecules has the capacity to offer long-lasting protection up to 400% SOC.
2.3. Electrochemical compatibility of TAC on Na3V2(PO4)3 cathode and hard carbon anode
We selected hard carbon (HC) as the anode material to construct NVP/HC full cell since it has been a commonly reported cell chemistry of SIBs [60]. Before full cell evaluation, the performance of cathode and anode were studied separately in order to understand the influence of TAC on the individual electrode within their normal operating voltage range. The initial CE of a HC anode was critical to its practical application. As shown in Fig. 3a, two distinct voltage regions are displayed, a slope region above 0.2 V and a flat plateau region below 0.2 V, corresponding to the formation of SEI layer and Na+ insertion/extraction in/from the layered carbon materials, respectively [61, 62]. The two HC anodes delivered a similar capacity of ~280 mAh g−1 with a similar initial CE (71.2% for HC vs. 72.4% for HC-TAC). The inset CV curves in Fig. 3a are in consistent with the result of charge/discharge profiles. Rate performance is then compared in Fig. 3b, displaying nearly the same level of reversible capacity of 288 mAh g−1, 279 mAh g−1, 223 mAh g−1, 90 mAh g−1, 80.6 mAh g−1 and 52.1 mAh g−1 under 0.05C, 0.1C, 0.2C, 0.5C, 1C, 2C and 5C, respectively. All of the above results proved that HC-TAC anode was completely capable for the full battery application. The cycling performance of NVP cathodes are presented in Fig. 3c. Both cathodes showed a stable cycling behavior with a capacity retention of 96.2% (NVP-TAC) compared to 97.9% (NVP) and a CE of nearly 100% in 200 cycles. The inset charge/discharge profiles show a capacity of ~92 mAh g−1 with a high initial CE of 97%. Furthermore, EIS spectra shown in Fig. 3d exhibit approximately the same level of impedance after 200 normal cycles, 485 ohms of NVP compared to 518 ohms of NVP-TAC. In addition, SEM images as displayed in Fig. S6 show no obvious change in the electrode surface morphology after normal cycling. Therefore, under normal working condition, TAC can stay inactive within bulk electrolyte and would hardly cause any detrimental impact on the battery, e.g. the increase of self-discharge rate or cell impedance.
Fig. 3.

a) Initial voltage profiles of HC anode at 0.05C in electrolyte containing 0.1 M TAC or no additive, inset is the corresponding CV profiles achieved at a scan rate of 0.1 mV s−1; b) rate performance of HC anodes between 0-2.5 V in electrolyte containing 0.1 M TAC or no additive; c) capacity retention profiles of NVP cathode at 1C in electrolyte containing 0.1 M TAC or no additive, inset are the initial voltage profiles; d) comparison of EIS profiles of NVP cathodes after 200 cycles.
2.4. Overcharge protection performance of Na3V2(PO4)3/hard carbon cells
The application of the TAC redox shuttle additive in a full cell was further validated. As shown in Fig. 4a, the NVP/HC/TAC full cell delivered a capacity of 95 mAh g−1 and an CE of 53% at 0.5C. The discharge plateau was at around 3.24 V and the overcharge potential can be locked at 3.71 V, exhibiting no obvious polarization when compared to half-cell data. The longevity test shows that full cells can withstand 54 overcharge cycles (160 hours) under 1C rate as exhibited in Fig. 4b and 176 overcharge cycles (1020 hours) under 0.5C rate as reflected in Fig. 4c, respectively. In comparison with the previous work in LIB systems as summarized in Table S1, the present study provides significant advancement in the field of overcharge protection by realizing ultralong life of protection with low additive concentration (≤ 0.1 M) and under high rate (≥ 0.5C).
Fig. 4.
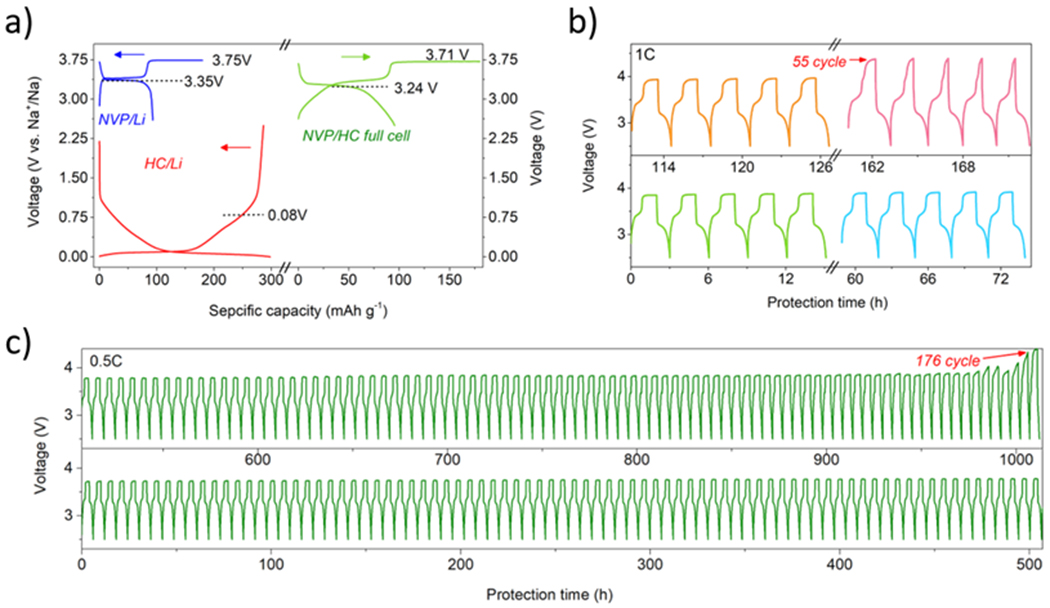
a) Initial voltage profiles of NVP/HC full cell, NVP/Na half-cell and HC/Na half-cell, respectively; voltage-time profile of NVP/HC full cells with 100% overcharge at b) 1C and c) 0.5C.
2.5. Electrochromic effect and thermal behavior of TAC added system
Interestingly, the electrochromic behavior of TAC can provide a visual overcharge indication. As shown in Fig. 5a, the TAC electrolyte experiences a reversible and rapid electrochromic transition at 3.5-4.1 V. Specifically, the red color indicated the unsafe status of a device and the lack of color implied that the device was recovered to its normal operating condition again. A UV-vis absorption spectrum is further displayed under a constant voltage of 3.8 V as shown in Fig. 5b. After 30 s, three new peaks emerged at ~457 nm, ~497 nm and ~547 nm, respectively and became stronger as the time at this constant voltage extended. Meanwhile, the electrolyte changed from colorless at 0 s to pink color at 30 s and further to red color at 40 s, indicating an increase in concentration of TAC dication. Technically, this electrochromic effect offered the direct visualization of overcharge, enabling the possible engagement of human-device interaction in the future.
Fig. 5.

The electrochromic effect and thermal stability of TAC added system. a) Photographs of a transparent electrochemical device contained 4 mM TAC electrolyte and scanned at 20 mV s−1 between 3.5 and 4.1 V (vs. Na+/Na); b) UV-vis spectra and the corresponding photographs of the device holding at a constant-voltage of 3.8 V; c) DSC curves of the overcharged NVP cathodes in electrolyte with 0.1 M TAC or no additive.
It is well known that the batteries are more likely to suffer from a harsher thermal runaway under an overcharge condition relative to other abuse conditions because of the excessive electrochemical energy pre-stored in the battery [8]. Therefore, investigating the heat generation from an over de-sodiated cathode during a simulated thermal runaway process would be meaningful. Therefore, we investigated the thermal behavior of the overcharged NVP cathodes via differential scanning calorimetry (DSC) technique. As shown in Fig. 5c, the exothermic peaks of a NVP-TAC cathode and a NVP cathode starts to emerge at 180 ºC and 171 ºC, respectively. Besides, the total heat generation of the NVP-TAC cathode also decreased by 20% by comparing the size of the colored area. Therefore, the TAC additive can dramatically improve the battery safety during overcharge by postponing the initiation temperature of thermal runaway as well as reducing the heat generation of the cathode.
2.6. Mechanistic study on the cause of protection failure
Common wisdom regarding the failure mechanism of overcharge protection was the lack of chemical stability of the oxidized radicals in the solvent bulk [34]. A post-mortem analysis on the electrolyte recovered from the failed battery after overcharge test was carried out by using UPLC-MS. As displayed in Fig. 6a, the mass spectrum exhibits a peak at a molar mass of 372.2 g mol−1, indicating the existence of TAC cation species in the electrolyte of failed battery. Furthermore, as shown in Fig. 6b, the recovered electrolyte exhibits a retention time of chromatographic peak which corresponds well with pure TAC compound. Therefore, TAC processed an ultrahigh stability throughout the overcharge test. It appears that the mechanism of the loss of overcharge protection needs to be further studied. We hypothesized that the electrolyte consumption caused by undesired side reactions during cycling could result in TAC, mostly in its oxidized state, gradually precipitating out of electrolyte solution and hence losing its ability of overcharge protection. It was reported that the discharged state of cyclopropenium-based catholytes in a flow battery had a ten-times higher solubility then its charged state [53]. As illustrated in Fig. 6c, an electrolyte saturated with TAC was charged at constant potential of 3.9 V (vs. Na+/Na). Any visual changes in the cell during the operation can be observed real-time through a quartz window. As shown in Fig. 6d–f, the electrolyte color gradually changed from colorless to dark red, indicating the increase of TAC dication concentration. At the end of charging process, almost all the TAC cation near Pt disk should be oxidized to the dication. Red precipitation can be seen in Fig. 6g, which became more obvious after electrolyte was removed from the cell as shown in Fig. 6h. Therefore, the protection failure could be ascribed to insufficient supply of soluble TAC dication. Our future work will be more focused on the factors that could impact the solubility of cyclopropenium ion derivatives such as the substituents on nitrogen and the electrolyte salts.
Fig. 6.

Mechanistic study for the possible cause of protection failure. a) MS (APCI) and b) UPLC of electrolyte sample recovered from the failed battery after overcharge test; c) schematic illustration of a two-electrode spilt cell using Pt as working electrode and Na ring as both working and reference electrode, the electrolyte was saturated with TAC additive; cell photographs of 3.9 V charging d) at 0 s, e) at 10 s, f) at 2 h, and g-h) completed (with and without the electrolyte, respectively).
3. Conclusion
In summary, this article described the development of a functional organic salt, trisaminocyclopropenium perchlorate (TAC•ClO4) as a new redox shuttle for overcharge protection in SIBs. With the unique aromaticity and ionicity, TAC•ClO4 possesses a combination of merits including fast diffusion, high solubility, and ultrahigh stability in both redox states. With merely 0.1 M TAC, Na3V2(PO4)3 cathode can carry overcharge current up to 10C or 400% SOC. Na3V2(PO4)3/hard carbon full cell displays strong anti-overcharging ability of 176 cycles at 0.5C and 54 cycles at 1C with 100% overcharge. The overcharged cathode also shows a 20% decrease of heat generation with TAC addition. Furthermore, a distinctive electrochromic behavior of TAC electrolyte can provide the device an overcharge alarm to further enhance the safety level.
Supplementary Material
Acknowledgements
This paper is in memory of Professor Quanxin Zha from Wuhan University. We thank Dr. Xingkang Huang for helpful suggestion that greatly assisted the research. Research reported in this publication was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies, under the Vehicle Technology Program, under Contract Number DE-SC0012704 (D.Y.Q) and the National Institutes of Health under R35 GM127135 (T.H.L.).
References
- [1].Hwang J, Myung S, Sun Y, Chem. Soc. Rev 46 (2017) 3529–3614. [DOI] [PubMed] [Google Scholar]
- [2].Pan H, Hu Y-S, Chen L, Energy Environ. Sci 6 (2013) 2338–2360. [Google Scholar]
- [3].Wang Q, Ping P, Zhao X, Chu G, Sun J, Chen C, Power Sources J 208 (2012) 210–224. [Google Scholar]
- [4].Rodrigues M-TF, Babu G, Gullapalli H, Kalaga K, Sayed FN, Kato K, Joyner J, Ajayan PM, Nature Energy 2 (2017) 1–14. [Google Scholar]
- [5].Robinson JB, Finegan DP, Heenan TMM, Smith K, Kendrick E, Brett DJL, Shearing PR, J. Electrochem. En. Conv. Stor 15 (2017) 011010. [Google Scholar]
- [6].Finegan DP, Scheel M, Robinson JB, Tjaden B, Hunt I, Mason TJ, Millichamp J, Di Michiel M, Offer GJ, Hinds G, Brett DJL, Shearing PR, Nature Comm. 6 (2015) 6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bandhauer TM, Garimella S, Fuller TF, J. Electrochem. Soc 158 (2011) R1–R25. [Google Scholar]
- [8].Feng X, Ouyang M, Liu X, Lu L, Xia Y, He X, Energy Storage Mater. 10 (2018) 246–267. [Google Scholar]
- [9].Belov D, & Yang MHJ. Solid State Electrochem. 12 (2008) 885–894. [Google Scholar]
- [10].Kong D, Wen R, Ping P, Peng R, Zhang J, G. Chen. Int. J. Energy Res 43 (2019) 552–567. [Google Scholar]
- [11].Guo Z, Zhou J, Feng J, Du S RSC Adv. 5 (2015) 69514–69521. [Google Scholar]
- [12].Sun Y, Shi P, Xiang H, Liang X, Yu Y, Small 15 (2019) 1805479. [DOI] [PubMed] [Google Scholar]
- [13].Jiang X, Liu X, Zeng Z, Xiao L, Ai X, Yang H, Cao Y, iScience 10 (2018) 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zeng Z, Jiang X, Li R, Yuan D, Ai X, Yang H, Cao Y, Adv. Sci 3 (2016) 1600066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Feng J, An Y, Ci L, Xiong S, J. Mater. Chem. A, 3 (2015) 14539–14544. [Google Scholar]
- [16].Wang J, Yamada Y, Sodeyama K, Watanabe E, Takada K, Tateyama Y, Yamada A, Nature Energy 3 (2018) 2229. [Google Scholar]
- [17].Jiang X, Liu X, Zeng Z, Xiao L, Ai X, Yang H, Cao Y, Adv. Energy Mater 8 (2018) 1802176. [Google Scholar]
- [18].Feng J, Zhang Z, Li L, Yang J, Xiong S, Qian Y, J. Power Sources 284 (2015) 222–226. [Google Scholar]
- [19].Kim H, Hong J, Park K-Y, Kim H, Kim S-W, Kang K, Chem. Rev 114 (2014) 11788–11827. [DOI] [PubMed] [Google Scholar]
- [20].Wu X, Luo Y, Sun M, Qian J, Cao Y, Ai X, Yang H, Nano Energy 13 (2015) 117–123. [Google Scholar]
- [21].Guo Z, Zhao Y, Ding Y, Dong X, Chen L, Cao J, Wang C, Xia Y, Peng H, Wang Y, Chem 3 (2017) 348–362. [Google Scholar]
- [22].Suo L, Borodin O, Wang Y, Rong X, Sun W, Fan X, Xu S, Schroeder MA, Cresce AV, Wang F, Yang C, Hu Y-S, Xu K, Wang C, Adv. Energy Mater 7 (2017) 1701189. [Google Scholar]
- [23].Suo L, Borodin O, Gao T, Olguin M, Ho J, Fan X, Luo C, Wang C, Xu K, Science 350 (2015) 938–943. [DOI] [PubMed] [Google Scholar]
- [24].Janek J, Zeier WG, Nature Energy 1 (2016) 1–4. [Google Scholar]
- [25].Zhou D, Liu R, Zhang J, Qi X, He Y-B, Li B, Yang Q-H, Hu Y-S, Kang F, Nano Energy 33 (2017) 45–54. [Google Scholar]
- [26].Hou H, Xu Q, Pang Y, Li L, Wang J, Zhang C, Sun C, Adv. Sci 4 (2017) 1700072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Banerjee A, Park KH, Heo JW, Nam YJ, Moon CK, Oh SM, Hong S-T, Jung YS, Angew. Chem. Int. Ed 55 (2016) 9634–9638. [DOI] [PubMed] [Google Scholar]
- [28].Chen Z, Qin Y, Amine K, Electrochim. Acta 54 (2009) 5605–5613. [Google Scholar]
- [29].Dahn JR, Jiang J, Moshurchak LM, Fleischauer MD, Buhrmester C, Krause LJ, J. Electrochem. Soc 152 (2005) A1283–A1289. [Google Scholar]
- [30].Feng JK, Ai XP, Cao YL, Yang HX, Electrochem. Comm 9 (2007) 25–30. [Google Scholar]
- [31].Zhang L, Zhang Z, Wu H, Amine K, Energy Environ. Sci 4 (2011) 2858–2862. [Google Scholar]
- [32].Zhang L, Zhang Z, Redfern PC, Curtiss LA, Amine K, Energy Environ. Sci 5 (2012) 8204–8207. [Google Scholar]
- [33].Huang J, Azimi N, Cheng L, Shkrob IA, Xue Z, Zhang J, Dietz Rago NL, Curtiss LA, Amine K, Zhang Z, Zhang L, J. Mater. Chem. A 3 (2015) 10710–10714. [Google Scholar]
- [34].Weng W, Huang J, Shkrob IA, Zhang L, Zhang Z, Adv. Energy Mater 6 (2016) 1600795. [Google Scholar]
- [35].Leonet O, Colmenares LC, Kvasha A, Oyarbide M, Mainar AR, Glossmann T, Blázquez JA, Zhang Z, ACS Appl. Mater. Interfaces 10 (2018) 9216–9219. [DOI] [PubMed] [Google Scholar]
- [36].Buhrmester C, Moshurchak L, Wang RL, Dahn JR, J. Electrochem. Soc 153 (2006) A288–A294. [Google Scholar]
- [37].Ergun S, Elliott CF, Kaur AP, Parkin SR, Odom SA, J. Phys. Chem. C 118 (2014) 14824–14832. [DOI] [PubMed] [Google Scholar]
- [38].Kaur AP, Ergun S, Elliott CF, Odom SA, J. Mater. Chem. A 2 (2014) 18190–18193. [Google Scholar]
- [39].Casselman MD, Kaur AP, Narayana KA, Elliott CF, Risko C, Odom SA, Phys. Chem. Chem. Phys 17 (2015) 6905–6912. [DOI] [PubMed] [Google Scholar]
- [40].Kaur AP, Elliott CF, Ergun S, Odom SA, J. Electrochem. Soc 163 (2016) A1–A7. [Google Scholar]
- [41].Moshurchak LM, Buhrmester C, Dahn JR, J. Electrochem. Soc 155 (2008) A129–A131. [Google Scholar]
- [42].Buhrmester C, Moshurchak LM, Wang RL, Dahn JR, J. Electrochem. Soc 153 (2006) A1800–A1804. [Google Scholar]
- [43].Weiss R, Schloter K, Tetrahedron Lett. 16 (1975) 3491–3494. [Google Scholar]
- [44].Johnson RW, Tetrahedron Lett. 17 (1976) 589–592. [Google Scholar]
- [45].Bandar JS, Lambert TH, Synthesis 45 (2013) 2485–2498. [Google Scholar]
- [46].Huang H, Strater ZM, Lambert TH, J. Am. Chem. Soc (2020) DOI: 10.1021/jacs.9b11472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Huang H, Strater ZM, Rauch M, Shee J, Sisto TJ, Nuckolls C, Lambert TH, Angew. Chem. Int. Ed 58 (2019) 13318–13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang H, Lambert TH, Angew. Chem. Int. Ed 59 (2020) 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jiang Y, Freyer JL, Cotanda P, Brucks SD, Killops KL, Bandar JS, Torsitano C, Balsara NP, Lambert TH, Campos LM, Nature Comm. 6 (2015) 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Griffin PJ, Freyer JL, Han N, Geller N, Yin X, Gheewala CD, Lambert TH, Campos LM, Winey KI, Macromolecules 51 (2018) 1681–1687. [Google Scholar]
- [51].Sevov CS, Samaroo SK, Sanford MS, Adv. Energy Mater 7 (2017) 1602027. [Google Scholar]
- [52].Hendriks KH, Robinson SG, Braten MN, Sevov CS, Helms BA, Sigman MS, Minteer SD, Sanford MS, ACS Cent. Sci 4 (2018) 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Robinson SG, Yan Y, Hendriks KH, Sanford MS, Sigman MS, J. Am. Chem. Soc 141 (2019) 10171–10176. [DOI] [PubMed] [Google Scholar]
- [54].Yan Y, Robinson SG, Sigman MS, Sanford MS, J. Am. Chem. Soc 141 (2019) 15301–15306. [DOI] [PubMed] [Google Scholar]
- [55].Fang Y, Xiao L, Chen Z, Ai X, Cao Y, Yang HJEER, Electrochem. Energy Rev 1 (2018) 294–323. [Google Scholar]
- [56].Liu D, Huang X, Qu D, Zheng D, Wang G, Harris J, Si J, Ding T, Chen J, Qu D, Nano Energy 52 (2018) 1–10. [Google Scholar]
- [57].Jian Z, Han W, Lu X, Yang H, Hu Y-S, Zhou J, Zhou Z, Li J, Chen W, Chen D, Chen L, Adv. Energy Mater 3 (2013) 156. [Google Scholar]
- [58].Lopez J, Gonzalez M, Viera JC, Blanco C, IEEE (2004) 19–24 [Google Scholar]
- [59].Liu Y, Zhu Y, Cui Y, Nature Energy 4 (2019) 540–550. [Google Scholar]
- [60].Komaba S, Murata W, Ishikawa T, Yabuuchi N, Ozeki T, Nakayama T, Ogata A, Gotoh K, Fujiwara K, Adv. Funct. Mater 21 (2011) 3859–3867. [Google Scholar]
- [61].Hwang J, Myung S, Sun Y-K, Chem. Soc. Rev 46 (2017) 3529–3614 [DOI] [PubMed] [Google Scholar]
- [62].Pan H, Hu Y-S, Chen L, Energy Environ. Sci 6 (2013) 2338–2360. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


