Abstract
Cardiovascular disease is one of the leading causes of morbidity and mortality in persons with cancer. The elevated risk is thought to derive from the combination of cardiovascular risk factors and direct cardiotoxicity from cancer therapies. Exercise may be a potential strategy to counteract these toxicities and maintain cardiovascular reserve. In this article, we review the evidence for the potential cardioprotective effects of exercise training in cancer patients before, during, and following treatment. We also propose a patient-tailored approach for the development of targeted prescriptions based on individual exercise capacity and cardiovascular reserve.
Keywords: cancer, cardioprotection, cardiotoxicity, cardiovascular complications, cardiovascular disease prevention, exercise
Introduction
The relationship between cancer therapy and cardiovascular disease (CVD) is well-recognized. This is in part due to some chemotherapeutic agents being inherently toxic to cardiac cells (cardiotoxic), a phenomenon driven by cellular injury and cardiac myocytes’s susceptibility to damage compounded by its very limited regenerative capacity. Unfortunately, this makes the cardiovascular system (CVS) particularly vulnerable to irreparable damage when exposed to certain agents used in the treatment of cancer [1]. While much of the current research on the impact of cancer therapy on the CVS focuses on changes to the heart, cancer therapy (including chemo and radiation therapy) also affect oxygen utilization by the body. Specifically, exposure to various chemotherapeutic regimens produces declines in cardiovascular function and reserve, as can be seen with findings of decreased peak oxygen consumption uptake (peak VO2) in patients post-chemotherapy [2,3]. Additionally, cardiorespiratory fitness (CRF), a global measure of cardiovascular function, declines between 5 and 26% during exposure to cancer therapy, and may remain depressed even after cessation of treatment [2,4].
Notably, there is an emerging paradigm of involvement of the cardiovascular-skeletal muscle axis in CRF in cancer patients [5]. This is corroborated by data suggesting that cancer survivors may experience decrements in skeletal muscle quality in addition to cardiovascular decrements which together contribute to CRF [6,7]. It is clear that rehabilitation focused on improving cardiovascular function has the potential to mitigate damage from cancer therapy and improve patient outcomes. However, the extent to which these programs should focus on muscle quality is yet unclear. While exercise therapy and cardiac rehabilitation are recommended and mainstay for many cardiac and pulmonary conditions, this strategy is not routinely offered to cancer patients despite a growing body of evidence that indicates these patients would likely see cardiovascular benefit from exercise [8]. Here, we review the current evidence exploring the impact of exercise on cardiovascular outcomes in adults with cancer, and propose a framework for targeted exercise prescription in this population.
Utilizing multiple approaches, several studies have been conducted to explore the impact of exercise on cardiac outcomes in subjects with cancer. These predominantly involve examining variables such as the timing of introduction of exercise, whether initiated pre- or post-cancer diagnosis/treatment, and include both animal and human subject studies.
Impact of exercise before cancer treatment on preventing future cardiovascular outcomes
As noted, certain cancer treatments may result in profound damage to the CVS. Until recently, only animal studies had examined whether exercise in the period before cancer diagnosis alters subsequent risk of cardiovascular events (CVEs) extending to the post-treatment period. Most of these studies have focused on anthracyclines, a therapeutic class of chemotherapy that is known to be cardiotoxic. One such study demonstrated the cardioprotective effects of exercise preconditioning after as little as 5 days to 3 weeks of endurance training in rodents [9]. Two other rodent studies similarly showed that doxorubicin-induced cardiovascular toxicity was attenuated by exercise training before treatment [10,11]. Additionally, both studies concluded that exercise training prevented a decline in the expression of sarcoplasmic/endoplasmic reticulum calcium ATPase 2a, which has been implicated in the pathogenesis of other types of cardiomyopathies. Other cardioprotective benefits of exercise before exposure to chemotherapy include the attenuation in acute rises in cardiac troponin and markers of oxidative stress and apoptosis as well as decreases in levels of heat shock protein expression, and the extent of cardiac mitochondrial dysfunction which can be seen in the context of cardiac injury [9,12–14].
Exercise has been shown to improve cardiovascular outcomes in patients with cancer even when initiated before the diagnosis of cancer. This was demonstrated in a recent large prospective observational cohort study (the first human study of its kind) which showed that higher levels of physical activity before cancer diagnosis were associated with lower risks of CVEs in women with breast cancer [15]. Using cardiovascular endpoints such as heart failure, myocardial infarction, angina, coronary revascularization, peripheral and coronary artery disease, transient ischemic attack, stroke, and death; exercise was shown to be beneficial in this cohort. Specifically, the study demonstrated that individuals with high levels of physical activity, defined as those in the top quartile of physical activity levels before the diagnosis of cancer, had significantly lower risk of future composite CVEs [hazard ratio (HR): 0.63, 95% confidence interval (CI) 0.45–0.88, P = 0.02] as well as death secondary to coronary heart disease (HR: 0.41, 95% CI 0.21–0.78, P < 0.01) when compared to their counterparts with lower levels of physical activity before diagnosis of cancer. Not only does this study provide added incentive to encourage exercise in the general population but acts as a basis to better define whether this observed benefit is present when exercise is initiated after diagnosis of cancer in previously inactive individuals.
Taken together, these studies provide support for stronger consideration for exercise as primordial and primary prevention to mitigate cardiovascular risks associated with cancer and cancer therapy. It is therefore suggested that typical cardiovascular risk management in cancer patients should be pursued in the same manner or more aggressively as with the general population [16].
Exploring the safety and efficacy of exercise in patients undergoing cancer treatment
Several recent reviews have concluded that exercise in patients with cancer is not only safe, but also improves quality of life, improves aerobic fitness, reduces risk of cancer recurrence, and reduces risk of all-cause mortality [8,17–20]. Specifically, while results have been widely variable, exercise studies during cancer treatment suggest that overall, exercise diminishes cancer treatment-associated decline and improves cardiovascular outcomes.
The randomized controlled trials (RCTs) examining exercise during treatment are summarized in Table 1 and have variable findings, likely due to the heterogeneous nature of these studies as elaborated further.
Table 1.
Summary of randomized controlled trials for effects of exercise on cardiovascular outcomes during cancer treatment
| Study (year) | N | Cohort, settinga | Baseline CVD | Cardiotoxic cancer therapies | Modality, intensity, frequency (day/week), duration (weeks) | Cardiovascular outcomes | Protocol adherence LTF % |
|---|---|---|---|---|---|---|---|
| MacVicar et al. (1989) (25) | 45 | Breast cancer patients: AT, stretching, UC | NR | Adjuvant CT | CE | VO2p | NR |
| 60–85% HRR | AT: 40% increase | LTF: 27% | |||||
| 3 days/week | UC: NC | ||||||
| 10 weeks | P = 0.05 | ||||||
| Segal et al. (2001) (26) | 123 | Breast cancer patients: AT, self-directed AT, UC | NR | Previous XRT: 37% | TM | VO2p (estimated) | NR |
| Most common CT regimens: Fluorouracil, doxorubicin and cyclophosphamide: 35% | 50–60% VO2p | Supervised: 2.4% increase | LTF: 27% | ||||
| 3–5 days/week | Self: 3.5% increase | ||||||
| Adriamycin and cyclophosphamide: 31% | 26 weeks | UC: NC | |||||
| P = NS | |||||||
| Kim et al. (2006) (27) | 41 | Breast cancer patients: AT, UC | None | CT: 40.9% | CE, TM, or walking, jogging, running on track | VO2p | 78.3% |
| XRT: 31.8% | Moderate to High | AT: 8.3% increase | NR | ||||
| Combination: 27.3% | 3 days/week | UC: 2.1% increase | |||||
| 8 weeks | P < 0.001 | ||||||
| Resting SBP | |||||||
| AT: t39 = 2.09 | |||||||
| UC: NC | |||||||
| P < 0.05 | |||||||
| Courneya et al. (2007) (28) | 242 | Breast cancer patients: AT, RT, or UC | NR | Taxane CT: 31% | AT: CE, ET, TM | VO2p | AT: 93% |
| Non-taxane CT: 61% | 60–80% VO2p | AT: 0.2% increase | RT: 96% | ||||
| RT: 8–12 reps | RT: 5% decrease | LTF: 9% | |||||
| 60–70 RM | UC: 6% decrease | ||||||
| 3 days/week | P = 0.006 | ||||||
| 17 weeks | |||||||
| Daley et al. (2007) (29) | 108 | Breast cancer patients: AT, UC | None | Exercise | AT: 1:1 supervised therapy | 8-minute TM walk test | 77% |
| CT: 79.4% | 65–85% max HR | AT versus UC | NR | ||||
| XRT: 79.4% | 3 days/week | P = 0.002 | |||||
| Control | 8 weeks | ||||||
| CT: 73% | |||||||
| XRT: 78.9% | |||||||
| Courneya et al. (2009) (30) | 122 | Lymphoma patients: AT or UC | HTN 29% | CT: % NR | CE | VO2p | 95% |
| DLD 30% | 60–100% VO2p | AT: 17% increase | LTF: 11% | ||||
| 3 days/week | UC: 2% decrease | ||||||
| 12 weeks | P = 0.021 | ||||||
| Segal et al. (2009) (31) | 121 | Prostate cancer: AT, RT, UC | NR | XRT: % NR | AT: CE, ET, TM | VO2p | NR |
| ADT: 61.2% | 50–75% VO2p | AT: 0.1% increase | LTF: 7% | ||||
| RT: 8–12 reps | RT: 0.5% increase | ||||||
| 60–70% RM | UC: 5% decrease | ||||||
| 3 days/week | P = 0.01 | ||||||
| 24 weeks | |||||||
| Courneya et al. (2013) (32) | 301 | Breast cancer patients: AT, high-dose AT, combined AT/RT | Obese 23% | Taxane: 74.1% | AT: CE, ET, TM, row | VO2p | NR |
| Trastuzumab: 16.6% | RT: 8–12 reps | AT: 12% decrease | LTF: 7% | ||||
| Neither: 9.3% | 60–70% RM | High-dose AT: 9% decrease | |||||
| 3 days/week | Combined: 13% decrease | ||||||
| 16 weeks | P = 0.03 | ||||||
| Jones et al. (2013) (33) | 20 | Breast cancer patients: AT, UC | NR | Neoadjuvant CT | CE | VO2p | 66% |
| 55–100% VO2p | AT: 13% increase | LTF: 5% | |||||
| 3 days/week | UC: 9% decrease | ||||||
| 12 weeks | P = NS | ||||||
| Samuel et al. (2013) (34) | 48 | Head and neck cancer patients: AT or RT, UC | NR | CT: % NR | AT: walking | 6-minute walk test | Adherence not measured |
| 3–5/10 perceived exertion | AT/RT: 42 m increase | ||||||
| RT: 8–12 reps | UC: 96 m decrease | ||||||
| 5 days/week | P < 0.001 | ||||||
| 6 weeks | |||||||
| Study (year) | N | Cohort, settinga | Baseline CVD | Cardiotoxic cancer therapies | Modality, intensity, frequency (day/week), duration (weeks) | Cardiovascular outcomes | Protocol adherence LTF % |
| Hornsby et al. (2014) (35) | 20 | Breast cancer patients: AT, UC | NR | Neoadjuvant doxorubicin + cyclophosphamide | CE | VO2p | 82% |
| Moderate to high intensity | AT: 13.3% increase | NR | |||||
| 3 days/week | UC: 8.6% decrease | ||||||
| 12 weeks | P < 0.05 | ||||||
| Moller et al. (2015) (36) | 45 | Breast and colon cancer patients: hospital or home-based intervention versus UC | NR | Neoadjuvant CT | Home: walking with pedometer | Peak VO2 decreased across study groups 12% | NR |
| Hospital: bikes, resistance and circuit training, dance 3 days/week | P = NS | NR | |||||
| 12 weeks | |||||||
| Van Waart et al. (2015) (37) | 230 | Breast or colon cancer patients: home or supervised AT, CT, UC | NR | Adjuvant CT | Home AT: 12–14 Borg Score | Estimated exercise capacity | NR |
| XRT: 78% | 50–80% maximal workload | Home: 9% decrease | LTF: 11% | ||||
| 5 days/week | Supervised: 14% decrease | ||||||
| NR | UC: 18% decrease | ||||||
| P < 0.001 | |||||||
| Gilbert et al. (2016) (38) | 50 | Prostate cancer: Exercise, UC | Prior MI: 8% | Androgen deprivation therapy | CE, TM, row | FMD of the brachial artery | 93% |
| Angina: 12% | 55–75% max HR | P = 0.04 | NR | ||||
| HTN: 64% | 3 days/week | ||||||
| HTN since ADT: 12% | 12 weeks | ||||||
| Scott et al. (2018) (8) | 65 | Breast cancer patients with metastatic disease: AT, stretching (control) | HTN, DLD, DM, or CAD: 34% | CT: 57% | 55–100% VO2p | VO2p | NR |
| 3days/weeks | AT and control: P = NS | LTF: 3% | |||||
| 12weeks |
Bold value indicates statistically significant of P values.
ADT, androgen deprivation therapy; AT, aerobic training; CE, cycle ergometer; CT, chemotherapy; CVD, cardiovascular disease; DLD, dyslipidemia; ET, elliptical training; FMD, flow-mediated dilatation; HR, hazard ratio; HRR, heart rate reserve; HTN, hypertension; LTF%, lost to follow-up percent; NC, no change; NR, not reported; NS, non-significant; RCT, randomized controlled trial, RM, resistance maximum; RT, resistance training; TM, treadmill; UC, usual care; VO2p, peak oxygen consumption; XRT, radiation therapy.
Supervised unless otherwise stated.
First, there is a lack of standardization in the utilized methodologies and measured outcomes in these studies. For instance, most clinical studies examining this topic during treatment focus on changes in aspects of CRF and peak VO2, rather than clinically relevant outcomes such as reductions in functional status or mortality. Furthermore, the methods used to define CRF are not standardized throughout clinical reviews and often do not consider factors such as age, comorbidities and baseline functional status, all of which are established to have a strong impact on CRF. Similarly, there is inconsistency in exercise specific variables such as the exercise dosing regimen, VO2, and metabolic equivalent targets. To overcome these inconsistencies in methodologies, standardized guidelines by which age-specific CRF categories are defined have been recently proposed [21].
Second, there is paucity of robust clinical data on the effects of exercise during cancer treatment on CVD outcomes such as subclinical reductions in left ventricular ejection fraction (LVEF), ischemic CVD events, and cardiovascular mortality [8]. For instance, a noteworthy study by Haykowsky et al. examined the effect of aerobic training (AT) on 17 women with HER2-positive breast cancer during the first 4 months of adjuvant trastuzumab therapy. They found that despite exercise, initiation of trastuzumab was associated with LV cavity dilation and reduced LVEF (pre: 64 ± 4% versus post: 59 ± 4% P < 0.05). While the findings might suggest that the benefit of exercise might be absent in preventing changes in LVEF post-exposure to trastuzumab, this might be a premature conclusion, given the small population size and absence of a control group [22]. As such, large scale research is on-going to better assess the impact of exercise on improving cardiovascular outcomes in patients during cancer therapy.
The ongoing Multidisciplinary Team IntervenTion in Cardio-Oncology (TITAN) and Exercise to prevent AnthraCycline-base Cardio-Toxicity seek to integrate exercise into treatment plans for cancer patients [23,24]. TITAN specifically will assess cardiac remodeling with imaging and biomarkers at 6–12 months follow-up from supervised to independent exercise training with Cardiology team support in breast cancer or lymphoma patients receiving chemotherapy [24]. The Optimal Training Women with Breast Cancer Trial RCT has a longer follow up of 5 years; and via personalized exercise prescriptions, aims to compare AT and combined resistance training to usual care in breast cancer patients, with CRF as a secondary endpoint. So far, at 2-year follow-up, there has been no statistically significant difference in pre-specified outcomes between exercise and usual care control groups [39]. Nonetheless, a recent protocol has been proposed for a systematic review of the effectiveness of exercise in counteracting cardiotoxicity related to anticancer therapies in women with breast cancer. The proposed primary outcomes for this review include systolic function, diastolic function, and myocardial deformation imaging outcomes [40].
Despite the variations in baseline characteristics across the existing studies on this subject, the overall, results are promising and suggest that exercise during cancer treatment improves cardiovascular health (Table 1), and therefore act as a basis for supporting the call for structured exercise therapy for patients with cancer.
The impact of exercise initiated post-cancer treatment
There is some evidence that exercise improves CRF even when initiated after the completion of cancer therapy. Compared to clinical studies exploring the impact of exercise when started before or during cancer treatment, findings surrounding the post-treatment phase are somewhat mixed. However, while several studies suggest no difference in CRF measured by VO2 in exercise regimens after cancer treatment, the majority of studies point towards efficacy in post-treatment exercise (Table 2) [41,42].
Table 2.
Summary of randomized controlled trials after treatment
| Study (year) | N | Cohort, settinga | Baseline CVD | Timing after treatments | Modality, intensity, frequency, duration | Cardiovascular outcomes | Protocol adherence LTF % |
|---|---|---|---|---|---|---|---|
| Courneya et al. (2003) (51) | 53 | Breast cancer patients: AT, UC | NR | 14 months after CT | CE | VO2p | NR |
| 70–75% VO2 | AT: 15% increase | LTF: 6% | |||||
| 3 days/weeks | UC: NC | ||||||
| 15 weeks | P < 0.001 | ||||||
| Thorsen et al. (2005) (42) | 139 | Breast, gynecological, lymphoma, testicular cancer: unsupervised AT, UC | NR | 30 days after therapy | TM, CE, skiing | VO2p | NR |
| 13–15 Borg Score | AT and UC: | LTF: 20% | |||||
| 3 days/weeks | P = NS | ||||||
| 15 weeks | |||||||
| Daley et al. (2007) (29) | 108 | Breast cancer patients: AT, UC | None | Post therapy | 1:1 specialized AT | Aerobic fitness score AT versus UC | 77% |
| 65–85% max HR | P = 0.002 | NR | |||||
| 3 days/week | |||||||
| 8 weeks | |||||||
| Courneya et al. (2013) (32) | 301 | Breast cancer patients: AT, high dose AT, UC | None | Post-treatment | CE, ET, TM, row | High dose AT superior to AT and UC | NR NR |
| 55–75% VO2 | P = 0.03 | ||||||
| 3 days/week | |||||||
| Ending 3–4 post-CT | |||||||
| Jones et al. (2014) (44) | 90 | HF patients with cancer: 3 months supervised + 4–12 months unsupervised AT, UC | HTN: 94% | Post-HF therapy | CE, TM | VO2p | NR |
| DM: 38% | 60–70% HRR | AT: 4% | LTF: 14% | ||||
| HF: 100% | 4 days/week | Increase | |||||
| 52 weeks | UC: 6% increase | ||||||
| P = NS | |||||||
| Jones et al. (2014) (52) | 50 | Prostate cancer patients: AT, UC | HTN: 54% | 75 days after therapy | TM | VO2p | 79% |
| HPL: 60% | 55–100% speed at VO2p | AT: 9% | LTF: 8% | ||||
| DM: 16% | 5 days/week | UC: 1% | |||||
| CVD: 8% | 24 weeks | P < 0.05 | |||||
| Low CRF: 100% | |||||||
| Rogers et al. (2015) (41) | 222 | Breast cancer patients: supervised or unsupervised AT, UC | HTN: 11% | 54 months after therapy | CE, ET, TM | VO2p | NR |
| 40–59% HRR | AT: 12% increase | LTF: 2% | |||||
| 3–5 days/week | UC: 10% increase | ||||||
| 12 weeks | P = NS | ||||||
| Adams et al. (2017) (43) | 63 | Testicular cancer patients: supervised AT, UC | Obese: 21% | 8 years after therapy | TM | VO2p | 98% |
| Pre-HTN: 19% | 75–95% VO2p | AT: 11% | LTF: 3% | ||||
| Metabolic syndrome: 19% | 3 days/week | Increase | |||||
| 12 weeks | UC: NC | ||||||
| Mild carotid plaque: 57% | Carotid intima-media thickness: | ||||||
| Moderate-severe carotid plaque: 24% | |||||||
| AT: 7% increase in thickness | |||||||
| UC: NC | |||||||
| Carotid distensibility: | |||||||
| AT: 16% increase | |||||||
| UC: NC | |||||||
| Framingham risk score: | |||||||
| AT: 0.5% increase | |||||||
| UC: NC | |||||||
| P < 0.01 | |||||||
| Scott et al. (2020) (46) | 174 | Postmenopausal breast cancer patients: LET, NLET, AC | Impaired VO2p | 2.8 years after therapy | VO2p | Intention-to-treat analysis, regardless of adherence | |
| LET: 0.6 ± 1.7 mLO2/kg·min | |||||||
| NLET: 0.8 ± 1.8mLO2/kg·min → both compared to AC | |||||||
| P = 0.05 |
Bold value indicates statistically significant of P values.
AC, attention control: Control group; AT, aerobic training; CE, cycle ergometer; CRF, cardiorespiratory fitness; CT, chemotherapy; CVD, cardiovascular disease; DM, diabetes; ET, elliptical training; HPL, hyperlipidemia; HR, hazard ratio; HRR, Heart rate reserve; HTN, hypertension; LET, linear, fixed-dose regimen; LTF, lost to follow up; NC, no change; NLET, nonlinear, variable dose regimen; NR, not reported; RCT, randomized controlled trial; RM, resistance maximum; RT, resistance training; TM, treadmill; UC, usual care; VO2p, peak oxygen consumption; XRT, radiation therapy.
Supervised unless otherwise stated.
Similar to clinical studies focusing on exercise during treatment, there are a few notable studies that have assessed the effects of exercise when initiated after completion of cancer therapy using parameters beyond CRF/VO2. Notably, a recent RCT by Adams et al. [43] was the first to provide evidence that high-intensity AT on testicular cancer patients post-treatment improved not only CRF, but other variables such as also arterial thickness, Framingham risk score, arterial stiffness, and low-density lipoprotein (P < 0.01).
On the other hand, two recent RCTs demonstrated mixed benefit of exercise post-cancer treatment in this population. Jones et al. [33] showed that exercise produced increases in VO2 peak, which was associated with improved vascular endothelial function, with no changes in LVEF. Similarly, a recent retrospective intention-to-treat analysis of the Efficacy and Safety of Exercise Training in Patients with Chronic Heart Failure (HF-ACTION) RCT showed that in patients with cancer who have heart failure and randomized to AT had a cardiovascular mortality reduction compared to the usual care group (HR 1.94; CI 1.12–3.16; P = 0.02) [44]. It is notable that although mortality reduction was observed in this study, VO2 improvement was not, which contrasts with what is seen in the general population, in which VO2 has the strongest predictive ability for future mortality [45]. This raises the possibility that VO2 may not be a sufficiently sensitive outcome in the assessment of exercise effect on cardiovascular health in cancer patients despite being the most frequently utilized outcome measure in clinical trials as seen in Tables 1 and 2. It also speaks to the potential presence of alternate mechanisms by which exercise might confer cardiovascular benefit in this population.
Regarding the type of exercise, recently, a RCT by Scott et al. [46] examined patients with primary breast cancer patients who had completed cancer treatment. In this study, patients were randomized to linear AT, non-linear AT, or usual care. Here, linear prescription of exercise defined as having fixed-dosing (per week) and fixed intensity for all patients revealed only modest improvement in CRF independent of dosing of chemotherapy. Of note, this study reported substantial heterogeneity in the response to both linear and non-linear AT in this population of breast cancer survivors with impaired CRF. The authors concluded that exercise programs of greater amount or length, as prescribed in their non-linear programs, may be needed to produce meaningful improvements among this population of post-treatment breast cancer patients [46].
In summary, there is yet insufficient but increasing evidence to conclude that non-linear AT and better-tailored exercise post-cancer treatment improves cardiovascular health. Additional studies are needed to examine the impact of specific approaches to individually-tailored exercise interventions on a variety of outcomes in cancer survivors.
Clinical guidelines and recommendations
Based on current evidence, clinical guidelines recommend moderate-intensity exercise–both aerobic and resistance training–for patients with cancer both during and after treatment. This recommendation also applies to intense cancer treatments such as stem cell transplantation [47,48]. However, such linear exercise recommendations in cancer patients and survivors are fraught with specific problems. For example, the determination of moderate-intensity exercise recommendation may be confounded by the presence of sequela of cancer therapies, such as autonomic dysfunction due to cisplatin or external radiation therapy; or via medications used to treat cardiac complications of chemotherapy such as beta-blockers [49]. Additionally, it is important to consider that there will be significant differences in exercise tolerability due to considerable variability in baseline clinical status such as differences in treatments received, cardiovascular risk factors, and physiologic status (below or comparable to age- and sex-matched VO2peak) [50].
Additional evidence suggests that generalized exercise recommendations might not be appropriate for all patients with cancer. For example, a study by Jones et al. [44], showed that cardiovascular mortality and hospitalization was significantly higher in patients with heart failure and a history of cancer when randomized to aerobic exercise compared to the patients with heart failure and history of cancer assigned to guideline-based usual care. As such, standardized exercise prescription may not be appropriate in this population given the significant potential for patient variability. Based on this, we recommend an individualized approach to exercise recommendations in patients with cancer (Fig. 1).
Fig. 1.
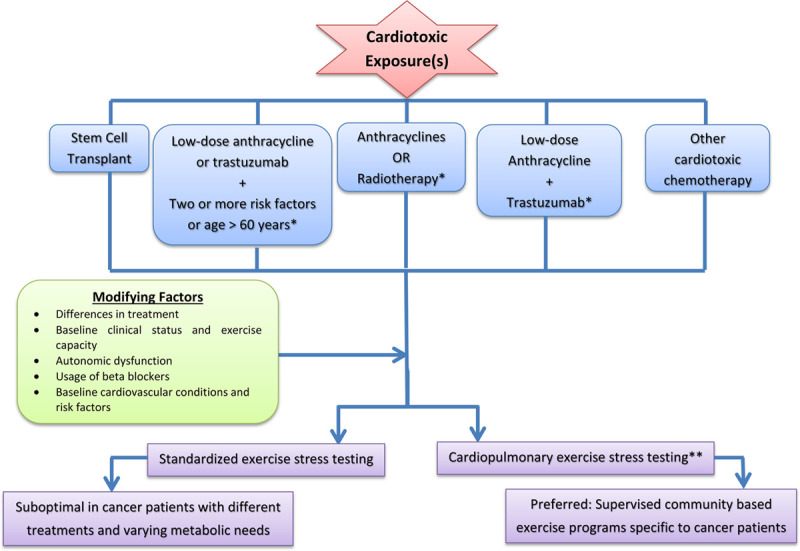
Suggested algorithm for exercise prescription in adult cancer patients peri- and post-cancer therapy. *Considered to be at increased cardiovascular risk by ASCO guidelines (52): Treatment with high-dose anthracycline therapy, high dose radiation therapy (when the heart is included in the treatment field), or low dose anthracycline therapy in combination with low dose radiotherapy; Treatment with low-dose anthracycline or trastuzumab alone plus the presence of two or more risk factors (smoking, hypertension, diabetes mellitus, obesity, dyslipidemia, age greater than 60 years at time of treatment, or the presence of compromised cardiac function); Treatment of low-dose anthracycline followed by trastuzumab. **In settings where CPET unavailable investigators can use maximal incremental exercise tolerance testing (ETT) to determine workload and peak exercise heart rate. CPET, cardiopulmonary exercise testing.
To determine which patients with cancer might safely tolerate exercise before, during or post-chemotherapy, exercise stress testing may be a worthwhile risk stratification tool. Specifically, exercise stress testing and can identify individuals unlikely to tolerate the recommendations for moderate-intensity exercise based on submaximal and maximal exercise reached [53,54]. For example, among deconditioned patients, standardized functional and submaximal exercise testing heart rate and blood pressure responses can be used to prescribe exercises of varying intensities independent of disease severity or baseline fitness status [55].
On the other hand, exercise prescriptions that are determined by baseline physiologic endpoints in the absence of up-to-date objective determination of exercise capacity, are at increased susceptibility of underdosing or overdosing exercise therapy. For example, in primary breast cancer patients with autonomic dysfunction and decreased heart rate reserve, the use of standardized age-predicted maximum heart rate may result in exercise overdosing [49].
To date, no organization has reached a consensus on the impact of cancer therapies on overall CVD risk. However, the American Society of Clinical Oncology has provided evidence-based guidelines regarding selected therapies that predispose cancer patients to CVD. The guideline recommends that those who should be considered at increased CVD risk include: (1) treatment with high-dose anthracycline therapy, high dose radiation therapy (when the heart is included in the treatment field), or low-dose anthracycline therapy in combination with low-dose radiation therapy; (2) treatment with low-dose anthracycline or trastuzumab alone plus the presence of two or more risk factors (smoking, hypertension, diabetes mellitus, obesity, dyslipidemia, age greater than 60 years at time of treatment, or the presence of compromised cardiac function); (3) treatment with low-dose anthracycline followed by trastuzumab [56].
Another strategy to determine exercise capacity before prescribing exercise therapy in patients with cancer is cardiopulmonary exercise testing (CPET). CPET-based metabolic and ventilatory responses allow for the generation of 3 to 5 different exercise intensity zones and provides information on the patient’s VO2peak, thus providing data for individualized tailoring of exercise training [57]. Beyond prediction of exercise tolerability, CPET can be repeated after training to objectively measure and document improvement in cardiac fitness and refine training levels. This is supported by a systematic review which found that CPET is a safe, noninvasive method to measure cardiopulmonary fitness in cancer patients both during and after treatment [58]. As discussed, VO2 may not be the most appropriate method for assessing exercise effect on cardiovascular health in cancer patients; nonetheless, it is the most constructive method currently available for assessing exercise needs. We therefore recommend formal CPET in addition to clinical risk stratification to guide moderate-intensity recommendations (Fig. 1). In settings where CPET is unavailable, investigators may use maximal incremental exercise tolerance testing as the next (although suboptimal) alternative to determine workload and peak exercise heart rate [59].
Finally, we recommend exercising in a group or supervised setting. Data suggest greater and more consistent benefit with exercise interventions that occurred in group settings compared with individual settings [44,60–62]. A supervised setting can provide more motivation for patients and lend initial educational components to ensure that professionals have the opportunity to review how to perform exercises safely with the patient. Exercise prescriptions should be delivered by the American College of Sports Medicine/American Cancer Society-certified exercise trainers. Fitness trainers should be encouraged as much as possible to learn about specific cancer diagnoses and treatments rendered. They should be included as part of the medical team, as a diagnosis of cancer can affect multiple parts of the body, and treatments are becoming increasingly customized.
Future directions
In this review of exercise in the care of cancer patients, we proposed an algorithm to risk stratify cancer patients and develop a personalized approach to exercise implementation. Transition from the research setting to widespread clinical availability presents significant challenges including lack of staff education on the complexities involved in the overall health and management of cancer patients. The effects of cancer stage, treatment type, and patient-specific risk factors should be clarified when identifying periods of greatest physical decline and recovery. Exercise programs will require infrastructures that enable them to provide services uniquely aligned with exposures and needs of cancer patients. The responsibility of identifying and referring patients with cancer at risk for cardiac dysfunction to exercise programs remains in the hands of both cardiologists and oncologists. Cardiologists have traditionally worked with oncologists to care for cancer patients after cardiac toxicity has occurred, and a proactive stance between specialties is pivotal to developing cardio-oncologic exercise-based rehabilitation.
Further work is needed to demonstrate the benefits of exercise in producing a reduction in cardiac dysfunction in the cancer population and to generate guidelines to help shape referrals and reimbursements. Reimbursement for cardiac rehabilitation was first established in 1982 for patients that experienced myocardial infarctions, coronary artery bypass graft surgery, and stable angina [48]. Unfortunately, there is currently no reimbursement strategy available in the USA that can provide patients with access to exercise-based cardiac rehabilitation programs on the bases of their malignancy and associated potentially cardiotoxic treatment. With further research and the development of formal guidelines, exercise-based cardiac rehabilitation has the potential to provide a widely accessible comprehensive program that has the potential to be significantly beneficial to cancer patients across the United States, regardless of treatment phase.
Conclusion
Persons with cancer, particularly those receiving certain chemotherapeutic agents are at heightened risk of developing cardiovascular sequela which can be measured using a number of outcomes. There is convincing evidence that exercise whether performed before, during, or following cancer treatment can mitigate these risks. As such a patient-tailored, supervised, exercise program using predictions of exercise capacity from stress testing or CPET, is likely to maximize the benefit of this approach and assist in the prevention of poor cardiovascular outcomes in patients with cancer.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lenneman CG, Sawyer DB. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res. 2016; 118:1008–1020. [DOI] [PubMed] [Google Scholar]
- 2.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012; 30:2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squires RW, Shultz AM, Herrmann J. Exercise training and cardiovascular health in cancer patients. Curr Oncol Rep. 2018; 20:27. [DOI] [PubMed] [Google Scholar]
- 4.Hurria A, Jones L, Muss HB. Cancer treatment as an accelerated aging process: assessment, biomarkers, and interventions. Am Soc Clin Oncol Educ Book. 2016; 35:e516–e522. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrist SC, Barac A, Ades PA, et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019; 139:e997–e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaudry RI, Kirkham AA, Thompson RB, Grenier JG, Mackey JR, Haykowsky MJ. Exercise intolerance in anthracycline-treated breast cancer survivors: the role of skeletal muscle bioenergetics, oxygenation, and composition. Oncologist. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reding KW, Brubaker P, D’Agostino R, Jr, et al. Increased skeletal intermuscular fat is associated with reduced exercise capacity in cancer survivors: a cross-sectional study. Cardiooncology. 2019; 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018; 137:1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashraf J, Roshan VD. Is short-term exercise a therapeutic tool for improvement of cardioprotection against DOX-induced cardiotoxicity? An experimental controlled protocol in rats. Asian Pac J Cancer Prev. 2012; 13:4025–4030. [PubMed] [Google Scholar]
- 10.Dolinsky VW, Rogan KJ, Sung MM, et al. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am J Physiol Endocrinol Metab. 2013; 305:E243—E253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lien CY, Jensen BT, Hydock DS, Hayward R. Short-term exercise training attenuates acute doxorubicin cardiotoxicity. J Physiol Biochem. 2015; 71:669–768. [DOI] [PubMed] [Google Scholar]
- 12.Ascensao A, Lumini-Oliveira J, Machado NG, et al. Acute exercise protects against calcium-induced cardiac mitochondrial permeability transition pore opening in doxorubicin-treated rats. Clin Sci (Lond). 2011; 120:37–49. [DOI] [PubMed] [Google Scholar]
- 13.Ascensao A, Magalhaes J, Soares J, et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005; 100:451–460. [DOI] [PubMed] [Google Scholar]
- 14.Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008; 52:470–482. [DOI] [PubMed] [Google Scholar]
- 15.Okwuosa TM, Ray RM, Palomo A, et al. Pre-diagnosis exercise and cardiovascular events in primary breast cancer: women’s health initiative. j Am Coll Cardiol CardioOnc. 2019; 1:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown SA. Preventive cardio-oncology: the time has come. Front Cardiovasc Med. 2019; 6:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: a systematic review. Curr Oncol. 2017; 24:e290–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. 2017; 39:71–92. [DOI] [PubMed] [Google Scholar]
- 19.Wang HL, Cousin L, Fradley MG, et al. Exercise interventions in cardio-oncology populations: a scoping review of the literature. J Cardiovasc Nurs. 2020. [DOI] [PubMed] [Google Scholar]
- 20.Lakoski SG, Willis BL, Barlow CE, et al. Midlife cardiorespiratory fitness, incident cancer, and survival after cancer in men: the Cooper Center Longitudinal Study. JAMA Oncol. 2015; 1:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokkinos P, Myers J, Franklin B, Narayan P, Lavie CJ, Faselis C. Cardiorespiratory fitness and health outcomes: a call to standardize fitness categories. Mayo Clin Proc. 2018; 93:333–336. [DOI] [PubMed] [Google Scholar]
- 22.Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009; 15:4963–4967. [DOI] [PubMed] [Google Scholar]
- 23.Keats MR, Grandy SA, Giacomantonio N, MacDonald D, Rajda M, Younis T. EXercise to prevent AnthrCycline-based Cardio-Toxicity (EXACT) in individuals with breast or hematological cancers: a feasibility study protocol. Pilot Feasibility Stud. 2016; 2:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pituskin E, Haykowsky M, McNeely M, Mackey J, Chua N, Paterson I. Rationale and design of the multidisciplinary team IntervenTion in cArdio-oNcology study (TITAN). BMC Cancer. 2016; 16:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989; 38:348–351. [PubMed] [Google Scholar]
- 26.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001; 19:657–665. [DOI] [PubMed] [Google Scholar]
- 27.Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006; 29:156–165. [DOI] [PubMed] [Google Scholar]
- 28.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007; 25:4396–4404. [DOI] [PubMed] [Google Scholar]
- 29.Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007; 25:1713–1721. [DOI] [PubMed] [Google Scholar]
- 30.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009; 27:4605–4612. [DOI] [PubMed] [Google Scholar]
- 31.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009; 27:344–351. [DOI] [PubMed] [Google Scholar]
- 32.Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013; 105:1821–1832. [DOI] [PubMed] [Google Scholar]
- 33.Jones LW, Fels DR, West M, et al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res (Phila). 2013; 6:925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuel SR, Maiya GA, Babu AS, Vidyasagar MS. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res. 2013; 137:515–520. [PMC free article] [PubMed] [Google Scholar]
- 35.Hornsby WE, Douglas PS, West MJ, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014; 53:65–74. [DOI] [PubMed] [Google Scholar]
- 36.Moller T, Lillelund C, Andersen C, et al. The challenge of preserving cardiorespiratory fitness in physically inactive patients with colon or breast cancer during adjuvant chemotherapy: a randomised feasibility study. BMJ Open Sport Exerc Med. 2015; 1:e000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Waart H, Stuiver MM, van Harten WH, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015; 33:1918–1927. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert SE, Tew GA, Fairhurst C, et al. Effects of a lifestyle intervention on endothelial function in men on long-term androgen deprivation therapy for prostate cancer. Br J Cancer. 2016; 114:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolam KA, Mijwel S, Rundqvist H, Wengstrom Y. Two-year follow-up of the OptiTrain randomised controlled exercise trial. Breast Cancer Res Treat. 2019; 175:637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antunes P, Esteves D, Nunes C, et al. Effects of physical exercise on outcomes of cardiac (dys)function in women with breast cancer undergoing anthracycline or trastuzumab treatment: study protocol for a systematic review. Syst Rev. 2019; 8:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers LQ, Courneya KS, Anton PM, et al. Effects of the BEAT cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2015; 149:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005; 23:2378–2388. [DOI] [PubMed] [Google Scholar]
- 43.Adams SC, DeLorey DS, Davenport MH, et al. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer. 2017; 123:4057–4065. [DOI] [PubMed] [Google Scholar]
- 44.Jones LW, Douglas PS, Khouri MG, et al. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF-ACTION randomized trial. J Clin Oncol. 2014; 32:2496–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016; 134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 46.Scott JM, Thomas SM, Peppercorn JM, et al. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer: a randomized controlled trial. Circulation. 2020; 141:560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: a clinical practice guideline. Curr Oncol. 2017; 24:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010; 42:1409–1426. [DOI] [PubMed] [Google Scholar]
- 49.Scott JM, Jones LW, Hornsby WE, et al. Cancer therapy-induced autonomic dysfunction in early breast cancer: implications for aerobic exercise training. Int J Cardiol. 2014; 171:e50–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002; 347:1483–1492. [DOI] [PubMed] [Google Scholar]
- 51.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003; 21:1660–1668. [DOI] [PubMed] [Google Scholar]
- 52.Jones LW, Hornsby WE, Freedland SJ, et al. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014; 65:852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013; 128:873–934. [DOI] [PubMed] [Google Scholar]
- 54.American Thoracic S, American College of Chest P. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003; 167:211–277. [DOI] [PubMed] [Google Scholar]
- 55.Seiler KS, Kjerland GO. Quantifying training intensity distribution in elite endurance athletes: is there evidence for an ‘optimal’ distribution? Scand J Med Sci Sports. 2006; 16:49–56. [DOI] [PubMed] [Google Scholar]
- 56.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017; 35:893–911. [DOI] [PubMed] [Google Scholar]
- 57.Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. 2015; 6:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steins Bisschop CN, Velthuis MJ, Wittink H, et al. Cardiopulmonary exercise testing in cancer rehabilitation: a systematic review. Sports Med. 2012; 42:367–379. [DOI] [PubMed] [Google Scholar]
- 59.Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008; 9:757–765. [DOI] [PubMed] [Google Scholar]
- 60.Baumann FT, Zopf EM, Bloch W. Clinical exercise interventions in prostate cancer patients – a systematic review of randomized controlled trials. Support Care Cancer. 2012; 20:221–233. [DOI] [PubMed] [Google Scholar]
- 61.Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011; 41:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keogh JW, MacLeod RD. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manage. 2012; 43:96–110. [DOI] [PubMed] [Google Scholar]


