Supplemental Digital Content is available in the text.
Keywords: cardiovascular disease, meta-analysis, randomized controlled trial, inhibitor
Abstract
Objectives
To demonstrate a magnitude of the cardiovascular benefits, concomitantly analyzing the safety outcomes of sodium-glucose cotransporter 2 inhibitor (SGLT2-I) comprehensively, as a class effect in a larger sample size combined from recent randomized control trials.
Methods
We searched electronic databases using specific terms and evaluated 6 efficacy and 10 safety outcomes. Odds ratios (ORs) and 95% confidence interval (CI) were used to compare two interventions.
Results
Five studies (n = 41 267) were included, among which 23 539 received SGLT2-I. The SGLT2-I group favored reduction in major adverse cardiovascular events (OR, 0.78; 95% CI, 0.62–0.98; P = 0.03), cardiovascular death (CVD) or heart failure hospitalization (OR, 0.60; 95% CI, 0.46–0.80; P = 0.0004), rate of hospitalization for heart failure (OR, 0.56; 95% CI, 0.44–0.72; P < 0.00001), CVD (OR, 0.68; 95% CI, 0.50–0.93; P = 0.01), all-cause mortality (OR, 0.67; 95% CI, 0.48–0.93; P = 0.02) and myocardial infarction (OR, 0.79; 95% CI, 0.64–0.99; P = 0.04) when compared to the placebo group. Safety analysis showed higher diabetic ketoacidosis (DKA) rate in SGLT2-I group (OR, 2.33; 95% CI, 1.40–3.90; P = 0.001); in contrast, major hypoglycemic events were significantly lower (OR, 0.79; 95% CI, 0.73–0.87; P < 0.00001). AKI was significantly higher in the placebo group (OR, 0.76; 95% CI, 0.65–0.88; P = 0.0004). There were no statistically significant effects on other outcomes.
Conclusion
In selected high-risk patients of cardiovascular disease, the SGLT2-I is a potential effective class of drugs for improving cardiovascular outcomes and all-cause mortality without an increased risk of all other major complications except DKA on this meta-analysis.
Introduction
Sodium-glucose cotransporter 2 inhibitors (SGLT2-Is) are approved for the management of type 2 diabetes mellitus (T2D) and have recently been investigated in several randomized control trials (RCTs) for cardiovascular safety and efficacy in patients with T2D. The first RCT to investigate the effects of SGLT2-I on cardiovascular outcomes in T2D was with empagliflozin [1]. The benefits of SGLT2-I on reducing hospitalization for heart failure and progression of renal disease regardless of existing atherosclerotic cardiovascular disease (ASCVD) or a diagnosis of heart failure have been discussed in the literature [2]. Previous meta-analyses have shown moderate benefits of SGLT2-I on major adverse cardiovascular events (MACEs) that seem confined to patients with established ASCVD [2]. The studies on dapagliflozin and canagliflozin on T2D did not show reduction of cardiovascular death (CVD) [3,4]. Furthermore, the studies that have shown reduction in the composite of CVD or heart failure hospitalization was primarily driven by a reduction in hospitalization, not mortality [3,5]. A recently published study on dapagliflozin showed promising cardiovascular benefits in patients without diabetes in patients with heart failure [6]. Therefore, it is also possible that the differences in pharmacokinetics of SGLT2-I play a role in the differences in the cardiovascular outcomes.
Even though the SGLT2-I has been emerging as new therapeutic option, the safety profile of SGLT2-I has raised concerns regarding their use [7]. The true significance of adverse effects such as volume depletion, infections and rare diabetic ketoacidosis (DKA) are not known [8]. Similarly, Fournier’s gangrene, bladder cancer and bone fractures are worrisome side effects commonly discussed in the literature, and events rates vary in all recent studies [3,6,9]. Some studies in T2D patients reported contradictory results on the association of SGLT2-I treatment with amputation risk [10]. The data from CANVAS program and Udell et al. reported an increase in the occurrence of lower limb amputations with the use of SGLT2-I [5,7,9]. In the EMPA-REG OUTCOME study, rates of amputations were not significantly higher in those randomized to empagliflozin [11].
This meta-analysis aims to demonstrate the magnitude of benefit of SGLT2-I, as a class effect, concomitantly analyzing safety outcomes in a comprehensive way irrespective of diabetic history incorporating large numbers of patients with a high risk of ASCVD from all recent innovative trials.
Methods
Selection of studies
Two authors (M.R.R. and M.B.) independently searched PubMed, Web of Science, Google Scholars and Cochrane from database inception to 30 January 2020. Conference proceedings, clinicaltrials.gov, reference lists of published trials, reviews and meta-analysis were also searched for studies. Keyword and medical subject heading search (MSH) terms used included ‘SGLT2-I and cardiovascular outcomes’, ‘dapagliflozin and cardiovascular outcomes’, ‘canagliflozin and cardiovascular outcomes’, ‘empagliflozin and cardiovascular outcomes’ and ‘ertugliflozin and cardiovascular outcomes’. All authors contributed to sort out the disagreement to select final eligible studies. We followed the PRISMA flow diagram (Fig. 1) and have provided the search strategy to obtain all eligible studies. Reasons for exclusion are also presented in Fig. 1.
Fig. 1.
PRISMA flow diagram demonstrating study search strategy on SGLT2 Inhibitors and cardiovascular outcomes. SGLT2-I, sodium-glucose cotransporter 2 inhibitors.
After removing the duplicates, a total 635 studies were screened. A total of 119 studies were selected for abstracts with or without full study review. The following standard criteria was set with the consensus of all authors to finalize the eligible studies: (1) RCTs with a placebo control group on SGLT2-I with established ASCVD or high risk of ASCVD and (2) RCTs who clearly reports main outcomes of interest including adverse effects. Nine studies were included from a full article review, in which four studies were excluded as they did not meet our inclusion criteria. One RCT did not match our outcomes of the cardiovascular benefit as its outcome was improvement in left ventricular systolic function [12]. Multiple observational studies were excluded [13,14]. The study that compares SGLT-2-I with dipeptidyl peptidase 4 (DDP4) rather than placebo was also not included in the meta-analysis [15]. Several trials have not yet released the final results [16,17]. The five-placebo controlled RCTs met the inclusion criteria. Any discrepancies in data extraction or risk-of-bias assessment were resolved with consensus of all authors.
Quality assessment
The modified Jadad score was used to assess the methodological quality of RCTs. The quality of each trial was quantified by a score of 0–8. High-quality studies (≥3) were included as shown in Supplementary Table S1, Supplemental digital content 1, http://links.lww.com/CAEN/A26.
Outcomes
The primary efficacy outcomes were MACE, cardiovascular death or heart failure hospitalization, hospitalization for heart failure, cardiovascular death, myocardial infarction (MI) and all-cause mortality. Primary safety outcomes were DKA, bone fracture, amputation, Fournier’s gangrene, volume depletion, acute kidney injury (AKI), major hypoglycemia, bladder cancer and urinary tract infection (UTI). MACE was defined as death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. Both safety and efficacy outcomes were analyzed performing meta-analysis. The exclusion and inclusion criteria of each study including primary and secondary outcomes are all presented separately (Supplementary Table S2, Supplemental digital content 1, http://links.lww.com/CAEN/A26).
Data analysis
Pairwise analysis was performed using Cochrane Review Manager (RevMan) 5.3 software (The Nordic Cochrane Center, Copenhagen, Denmark). The data from included trials was used to calculate risk ratio and 95% confidence interval (CI). The Mantel–Haenszel equation with random effects model was used in the analysis of all outcomes. A two-sided P value of <0.05 was considered statistically significant for all analyses. I-square (I2) and Chi-square tests were used to test for heterogeneity.
A Bayesian network meta-analysis was conducted to compare indirectly between multiple interventions and was performed using NetMetaXL 1.6.1 (Canadian Agency for Drugs and Technologies in Health, Ottawa, Canada) and WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). The Bayesian Markov chain Monte-Carlo model* was used for the analysis of the random and fixed effects models. The random effects model was used for final interpretation. Odds ratios (ORs) and 95% CI used to compare the two interventions. A hierarchical Bayesian network was used to rank treatments according to their comparative effectiveness. Rankograms with surface under the cumulative ranking curve (SUCRA) probabilities were reported. A SUCRA of 90% means that the intervention of interest achieves 90% of effectiveness or safety relative to other interventions. All-cause mortality informative priors were chosen based on non-pharmacological interventions with objective outcomes. Heterogeneity (τ2) was evaluated, with τ2 estimate of 0.04 interpreted as a low, 0.14 as a moderate, and 0.40 as a high degree of heterogeneity. We assumed 10 000 baselines values and all outcomes convergence were achieved at 20 000 iterations and lack of autocorrelation was checked and confirm ed.
Results
A total of five RCTs were included in the analysis involving 41 267 patients in a high-risk cardiac disease study group. All the patients who received SGLT2-I were compared with patients taking placebo (Table 1). A total of 23 539 patients received SGLT2-I and 17 728 received placebo. A total of 65% in the SGLT2-I group and 61.4% in the placebo group had established ASCVD or at least had cardiovascular risk factors. A total of 14 outcomes were evaluated that included six efficacy and nine adverse outcomes in Figs 2a and b, respectively.
Table 1.
Baseline characteristics of participants of included studies
| M | EMPA-REG outcome | CANVAS | DAPA-heart failure | DECLARE-TIMI 58 | CREDENCE |
|---|---|---|---|---|---|
| Zinman et al. | Neal et al. | McMurray et al. | Wiviott et al. | Perkovic et al. | |
| Year | 2015 | 2017 | 2019 | 2019 | 2019 |
| Type of SGLT2-I | Empagliflozin | Canagliflozin | Dapagliflozin | Dapagliflozin | Canagliflozin |
| (dose in mg) | 10 and 25 | 100 and 300 | (10) | (10) | (100) |
| Study type | RCT | RCT | RCT | RCT | RCT |
| (placebo control) | (placebo control) | (placebo control) | (placebo control) | (placebo control) | |
| SGLT2-I (n)/placebo (n) | 2333/4687 | 5795/4347 | 2373/2371 | 8582/8578 | 2202/2199 |
| Follow up (year) | 3.1 | 2.4 | 1.52 | 4.2 | 2.62 |
| Age (mean, SD) | 63.1 (8.7) | 63.3 (8.3) | 66.3 (10.9) | 63.9 (6.8) | 63.0 (9.2) |
| Female (%) | 28.5 | 35.8 | 23.4 | 37.4 | 33.9 |
| Established cardiovascular disease (%) | 7020 (100%) | 6656 (65.6%) | 4744 (100%) | 6974 (40.6%) | 2223 (50.5%) |
| History of CHF | 706 (10.1) | 1461 (14.4) | 4744 (100%) | 1724 (10.0) | 652 (14.8) |
| eGFR <60 mL/min per 1.73 m2 | 1819 | 2039 | 1226 | 1265 | 2592 |
| HgbA1c | 8.1 (0.8) | 8.2 (0.9) | NA | 8.3 (1.2) | 8.3 (1.3) |
| BMI (SD) | 30.6(5.3) | 32(6) | 28.2(6) | 32(6) | 31.3(6.2) |
CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; SGLT2-I, sodium-glucose cotransporter 2 inhibitors.
Fig. 2.
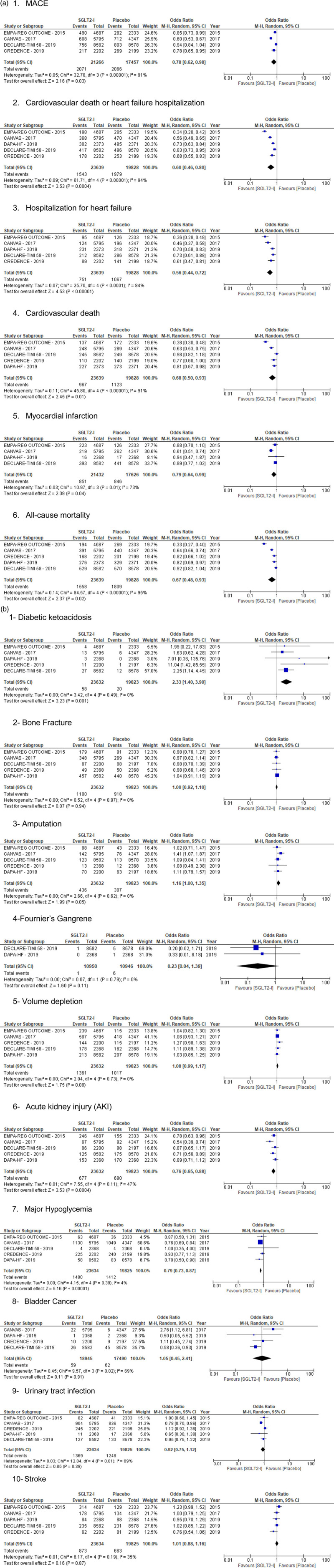
(a) Forest plot showing efficacy outcomes on cardiovascular efficacy. (b) Common safety outcomes of SGLT2-I in patients with high risk of cardiovascular disease. SGLT2-I, sodium-glucose cotransporter 2 inhibitors.
Four trials reported a 4137 incidence of MACE among 38 723 patients [1,3,4,9]. All studies have reported cardiovascular death or heart failure hospitalization [1,3,4,6,9]. Patients taking SGLT2-I were found to have significant difference in MACE (9.73% vs 11.8%, OR, 0.78; 95% CI, 0.62–0.98; P = 0.03, I2 = 91%) and CVD or heart failure hospitalization (6.5% vs 9.98%; OR, 0.60; 95% CI, 0.46–0.80; P = 0.0004; I2 = 94%) when compared to the placebo group (Figure 2a).
The SGLT2-I decreased the rate of hospitalization for heart failure (3.18% vs 5.38%; OR, 0.56; 95% CI, 0.44–0.72; P < 0.00001; I2 = 84%) and CVD (4.09% vs 5.66%; OR, 0.68; 95% CI, 0.50–0.93; P = 0.01; I2 = 91%) when compared to the placebo group. Four studies evaluated acute MI and demonstrated the outcome in favor of SGLT2-I therapy (3.97% vs 4.80%; OR, 0.79; 95% CI 0.64–0.99; P = 0.04; I2 = 73%). All-cause mortality was less in the SGLT2-I group than in the placebo group (6.59% vs 9.12%; OR, 0.67; 95% CI, 0.48–0.93; P = 0.02; I2 = 95%).
The safety outcomes are presented in Figure 2b. The DKA rate was statistically different between the two groups (OR, 2.33; 95% CI, 1.40–3.90; P = 0.001). The rate of DKA was higher with SGLT2-I when compared to the placebo group (0.24% vs 0.10%). No significant heterogeneity was noted with regards to the DKA rate (I2 = 0%). Comparing bone fracture rate between the two groups, no statistically significant difference was observed (OR, 1.00; 95% CI, 0.92–1.10; P = 0.94). The amputation rate was evaluated in all five trials. The amputation occurrence was not statistically difference between two groups but favored placebo (1.84% vs 1.55%; OR, 1.16; 95% CI; 1.00–1.35) with no significant heterogeneity (I2 = 0%).
Only two large trials included in our study have reported the incidence of fournier’s gangrene (n = 7) in 21 896 patients. DECLARETIMI 58 has reported five cases in placebo group (n = 8578) vs 1 in the SGLT2-I group (n = 8582). DAPA-heart failure reported only one case in the placebo group (n = 2368) with no occurrence reported in the SGLT2-I group (n = 2368). Overall, our results did not show an increased risk of Fournier’s gangrene with SGLT2-I and trended toward less risk.
All included studies had volume depletion and AKI as adverse outcomes (Figure 2b). There was no significant difference in volume depletion between SGLT2-I and placebo; (95% CI, 0.99–1.17; P = 0.08). However, AKI was found to be higher in patients taking placebo (2.86% vs 3.48%; OR 0.76; 95% CI, 0.65–0.88; P = 0.0004). Furthermore, analysis was performed in regard to major hypoglycemia reported by all studies. There was a statistically significantly lower major hypoglycemic events in the SGLT2-I group when compared to placebo (6.26% vs 7.12%, OR, 0.79; 95% CI, 0.73–0.87; P < 0.00001; I2 = 4%). Bladder cancer was observed in four trials. No difference in bladder cancer was observed between the SGLT2-I and placebo groups (0.31% vs 0.35%; OR 1.05, 95% CI, 0.45–2.41, P = 0.91). There was no difference in UTIs (5.79% vs 6.29%, OR 0.92; 95% CI, 0.75–1.12; P = 0.39) between the SGLT2-I and placebo groups.
Stroke (ischemic and hemorrhagic stroke, fatal and nonfatal) was reported in all trials included in this meta-analysis. No significant difference in incidence of stroke was noted between SGLT2-I and placebo groups (3.69% vs 3.34%, 95% CI, 0.88–1.16; P = 0.87).
Discussion
This meta-analysis analyzed comprehensive data from five large randomized placebo-controlled studies: EMPA-REG OUTCOME, CANVAS, CREDENCE, DAPA-heart failure and DECLARE-TIMI 59. This study effectively evaluated a wide range of adverse effects of SGLT2-I while assessing benefits of cardiovascular outcomes. Cardiovascular benefits and mortality reduction by SGLT2-I have been newly studied topic. Our meta-analysis showed that SGLT2-I is superior than placebo in reducing all-cause mortality, MACE, cardiovascular death or heart failure hospitalization, hospitalization for heart failure, cardiovascular death and MI. This is aligned with the findings of the individual trials studied. These trials have provided compelling evidence of cardiovascular, renal and mortality benefits with SGLT2-I agents.
Some unique findings in each trial that are worth reporting is that the CANVAS PROGRAM found evidence of superiority compared to placebo at preventing cardiovascular events and reported similar benefits in both preserved and reduced ejection fractions. The greatest reduction of hospitalization due to heart failure was found among those with reduced ejection fraction [9]. DAPA-heart failure found SGLT2 inhibitor to be superior to placebo in preventing CVD and heart failure hospitalizations across all groups, including those with and without diabetes mellitus and chronic kidney disease [6]. The DECLARE-TIMI 58 found similar benefits in cardiovascular events and heart failure hospitalization in patients with established ASCVD and those at high risk for it, which could suggest a preventive role of SGLT2 inhibitors in heart failure [4].
Individually, all five studies showed that SGLT2-I were associated with lower all-cause mortality compared to placebo. The cardiac benefits have been hypothesized to be due to several mechanisms. SGLT2-I have been associated with the inhibition of cardiac fibrosis, which is considered an important pathway of heart failure [18]. It is also believed that the cardioprotective effects of SGLT2-I are secondary to an improvement in ventricular load through a reduction in preload by eliciting natriuresis and osmotic dieresis, decreasing afterload by lowering blood pressure and improving vascular function. In addition, SGLT2-I has been compared in other studies to loop diuretics, both were associated with a similar natriuretic effect and reduction of interstitial fluid [5,12]. These natriuretic effects may explain the findings in reducing heart failure hospitalizations. SGLT2-I is also believed to improve cardiac metabolism by optimizing utilization of ketones [19].
We also analyzed adverse outcomes of concern which have been reported in the studies [7]. Side effects of SGLT2-I are noted to be an increased risk of volume depletion, DKA, bone fracture, amputation, UTI, Fournier’s gangrene and major hypoglycemia. Due to the SGLT2-Is aforementioned diuretic effect, such population are prone to develop volume depletion. These side effects were studied in all studies. Our meta-analysis did not show any significant association which was similar to the individual trial. Therefore, unless the patient is already volume-depleted or high risk of dehydration, SGLT2 inhibitors may be safely combined with both thiazide and loop diuretics [20].
Our studied showed that AKI was higher in the placebo group. Renal protective effects are seen across all five clinical trials. Furthermore, the CREDENCE trial found patients on canagliflozin had a lower risk for developing end-stage renal disease, worsening creatinine or death by renal or cardiovascular events [3]. A mechanism postulated behind their cardiorenal benefits has been the inhibition of sodium-hydrogen exchanger (NHE)1 and NHE3, hence controlling the reabsorption of sodium [21]. It is also worth noting that these effects are not waived by the glomerular filtration rate (GFR) as demonstrated in the CANVAS program. These mechanisms suggest that benefits can be seen regardless of heart failure and DM2 status.
Our current data show that SGLT2-I does not increase the incidence of major hypoglycemia. These events were variably reported in each trial. This may be due to the result of the continued renal glucose reabsorption capacity of SGLT1, and via metabolic counter-regulatory mechanisms which include decreased insulin release and increased glucagon release leading to increased hepatic gluconeogenesis that are unaffected by SGLT2 inhibition [8].
Our study strengthened the correlation of SGLT2-I with an increased risk of DKA similar to the individual trials that we studied. SGLT2-I is associated with euglycemic DKA. The mechanism behind the cause of DKA is increased glucagon-to-insulin ratio, which stimulates lipolysis, fatty acid oxidation and increased ketogenesis in the liver [22]. While there was a significant increased risk of DKA in the SGLT2-I group, the absolute incidence of this side effect was still quite low, 0.24% in SGLTI vs 0.10% in placebo. In this meta-analysis, there was no significant heterogeneity was noted (P = 0.49, I2 = 0%), and all five trials have reported the events.
Similarly, other reported adverse effects of SGLT2-I are increased risk of bone fractures that were investigated in all five of the clinical trials. In the individual trials, canagliflozin was shown to increase all fracture risks in the CANVAS program but not in the CREDENCE trial [3,9]. Similarly, dapagliflozin was shown to increase the fracture risk in the DAPA-heart failure trial but did not show any increased association in DECLARE-TIMI [4,6]. Also, empagliflozin was not associated with increased risk for fractures in the EMPA-REG outcome [1]. Our meta-analysis did not show an overall increased risk of bone fractures with SGLT2-I compared to placebo. One of the proposed mechanisms for bone fracture with SGLT2-I is decreased sodium reabsorption and increased phosphate reabsorption which increases parathyroid hormone and fibroblast growth factor 23, which then eventually decreases vitamin D and increases bone resorption [23]. Furthermore, patients who have T2D have an increased risk of bone fracture compared to those who are not.
SGLT2 inhibitors have been discussed commonly in association with increased risk of amputations. Our study did not show an increase of risk of amputation with SGLT2-I when compared to placebo. It is likely that uncontrolled diabetes is one of the risk factors for lower limb amputations. The incidence of amputations in diabetes is 1.5–5.0 per 1000 patient-years [10]. Incidence of amputations has been declining with good diabetes control.
The urogenital mycotic infection is an important, albeit rarely serious, adverse effect of SGLT2 inhibitors. Unfortunately, such infections are reported variably in the studies included in the meta-analysis. Moreover, the definition varies widely among the trials. But UTI has been consistently reported. UTI is known to be increased in diabetic patients due to increase in glucose concentration [24]. The SGLT2 inhibitors increase glycosuria by inhibiting sodium glucose co-transporter in the kidney and this could potentially increase the incidence of UTI. A previous study had shown that SGLT2-I is associated with the increased risk of UTI [25]. Our study does not show an overall increased risk of UTI in association with SGLT2-I as a class effect.
The risk of stroke has been assessed in several SGLT2-I trails in patients with high risk of cardiac disease. All trials in our meta-analysis have reported the stroke outcome. Our study conclusively demonstrated that SGLT2 inhibitor therapy was not associated with increase in stroke incidence.
Fournier’s gangrene is a rare but serious side effect discussed in association with SGLT2-I. Two large trials included in our study have reported the incidence of Fournier’s gangrene [4,6]. Our study further support that it is unlikely the treatment with an SGLT2-I is responsible for the development of Fournier’s gangrene because the result was trended toward less risk.
There are several limitations to our study. We did not have access to patient-level data to perform propensity analysis or stratified analysis which could better define differences between treatment groups with respect to patient characteristics, clinical presentation and procedural characteristics. The definition of primary outcomes was variable across studies which may affect the outcome assessment. By excluding nonrandomized and observational studies, we have limited the selection bias. We analyzed all the evidence available and independently analyzed randomized data to reduce the bias to a large extent. The rare side effects of SGLT-I such as Fournier’s gangrene, amputation, DKA from the included studies may not represent definitive incidence because occurrence pattern may vary in a larger population than that the initial trials.
Our study, which concomitantly evaluated a wide spectrum of safety outcomes from several well designed large clinical trials, confirms that SGLT2-I has emerged as a potential effective class of drugs for improving cardiovascular morbidity and mortality, including the prevention of heart failure hospitalization and reducing all-cause mortality in selected patients. We did not see an increased risk of major side effects except a mild increase in DKA. The adverse effects due to SGLT2-I are minimal in large numbers of patients from well designed RCTs. Due to the short-term trial durations, future long-term prospective studies and post-marketing surveillance studies are warranted to explore the rate of cardiovascular outcomes and rare side effects.
Acknowledgements
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.cardiovascularendocrinology.com.
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 2.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39. [DOI] [PubMed] [Google Scholar]
- 3.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. ; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357. [DOI] [PubMed] [Google Scholar]
- 5.Lam CSP, Chandramouli C, Ahooja V, Verma S. SGLT-2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. 2019; 8:e013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 7.Ueda P, Svanström H, Melbye M, Eliasson B, Svensson AM, Franzén S, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018; 363:k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Med. 2019; 132:S49–S57.e5. [DOI] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657. [DOI] [PubMed] [Google Scholar]
- 10.Yuan Z, DeFalco FJ, Ryan PB, Schuemie MJ, Stang PE, Berlin JA, et al. Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: a retrospective cohort study. Diabetes Obes Metab. 2018; 20:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018; 41:e4–e5. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019; 140:1693–1702. [DOI] [PubMed] [Google Scholar]
- 13.Cavender MA, Norhammar A, Birkeland KI, Jørgensen ME, Wilding JP, Khunti K, et al. ; CVD-REAL Investigators and Study Group. SGLT-2 inhibitors and cardiovascular risk: an analysis of CVD-REAL. J Am Coll Cardiol. 2018; 71:2497–2506. [DOI] [PubMed] [Google Scholar]
- 14.Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular outcomes and risks after initiation of a sodium glucose cotransporter 2 inhibitor: results from the EASEL population-based cohort study (evidence for cardiovascular outcomes with sodium glucose cotransporter 2 inhibitors in the real world). Circulation. 2018; 137:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patorno E, Pawar A, Franklin JM, Najafzadeh M, Déruaz-Luyet A, Brodovicz KG, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019; 139:2822–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardiovascular outcomes following ertugliflozin treatment in type 2 diabetes mellitus participants with vascular disease, the VERTIS CV study (MK-8835-004) – Full Text View – ClinicalTrials.gov [Internet]. 2020. https://clinicaltrials.gov/ct2/show/NCT01986881. [Accessed 17 April 2020].
- 17.EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR-Reduced) – Full Text View – ClinicalTrials.gov [Internet]. 2020. https://clinicaltrials.gov/ct2/show/NCT03057977. [Accessed 17 April 2020].
- 18.García-Ropero Á, Vargas-Delgado AP, Santos-Gallego CG, Badimon JJ. Inhibition of sodium glucose cotransporters improves cardiac performance. Int J Mol Sci. 2019; 20:3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maejima Y. SGLT2 inhibitors play a salutary role in heart failure via modulation of the mitochondrial function. Front Cardiovasc Med. 2020; 6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitchett D. A safety update on sodium glucose co-transporter 2 inhibitors. Diabetes Obes Metab. 2019; 21:34–42. [DOI] [PubMed] [Google Scholar]
- 21.Packer M. Activation and inhibition of sodium-hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation. 2017; 136:1548–1559. [DOI] [PubMed] [Google Scholar]
- 22.Thiruvenkatarajan V, Meyer EJ, Nanjappa N, Van Wijk RM, Jesudason D. Perioperative diabetic ketoacidosis associated with sodium-glucose co-transporter-2 inhibitors: a systematic review. Br J Anaesth. 2019; 123:27–36. [DOI] [PubMed] [Google Scholar]
- 23.Erythropoulou-Kaltsidou A, Polychronopoulos G, Tziomalos K. Sodium-glucose co-transporter 2 inhibitors and fracture risk. Diabetes Ther. 2020; 11:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. 2015; 8:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figueiredo IR, Rose SCP, Freire NB, Patrocínio MS, Pierdoná N, Bittencourt RJ. Use of sodium-glucose cotransporter-2 inhibitors and urinary tract infections in type 2 diabetes patients: a systematic review. Rev Assoc Med Bras (1992). 2019; 65:246–252. [DOI] [PubMed] [Google Scholar]


