Abstract
Context
Dietary fiber intake may relate to bone health.
Objective
To investigate whether dietary fiber intake is associated with bone mineral density (BMD), and the modification effect of genetic variations related to gut microbiota-derived short-chain fatty acids (SCFAs).
Design
The associations of dietary fiber intake with estimated BMD derived from heel ultrasound and fractures were assessed in 224 630 and 384 134 participants from the UK Biobank.
Setting
UK Biobank.
Main Outcome Measures
Estimated BMD derived from heel ultrasound
Results
Higher dietary fiber intake (per standard deviation) was significantly associated with higher heel-BMD (β [standard error] = 0.0047 [0.0003], P = 1.10 × 10–54). Similarly significant associations were observed for all the fiber subtypes including cereal, fruit (dried and raw), and vegetable (cooked and raw) (all P < .05). A positive association was found in both women and men but more marked among men except for dietary fiber in cooked vegetables (all Pinteraction < .05). A protective association was found between dietary fiber intake and hip fracture (hazard ratio, 95% confidence interval: 0.94, 0.89-0.99; P = 3.0 × 10–2). In addition, the association between dietary fiber and heel BMD was modified by genetically determined SCFA propionate production (Pinteraction = 5.1 × 10–3). The protective association between dietary fiber and heel BMD was more pronounced among participants with lower genetically determined propionate production.
Conclusions
Our results indicate that greater intakes of total dietary fiber and subtypes from various food sources are associated with higher heel-BMD. Participants with lower genetically determined propionate production may benefit more from taking more dietary fiber.
Keywords: bone mineral density, dietary fiber, short-chain fatty acids
The world population is aging. Since 2000, the average length of life has increased by 5.5 years to 72.0 years in 2016 worldwide (1). By 2050, the world’s population aged 60 years and older is expected to total 2 billion. Bone loss is among the major conditions adversely affecting life quality and mortality in the aging population (2). While a variety of risk factors have been related to bone loss, contributors affecting bone health are not yet completely understood.
Recently emerging evidence shows that gut microbiota is closely related to bone health (3-6). Changes in the composition of gut microbiota lead to bone loss, and strategies targeting manipulation of gut microbiota were supposed to prevent or reverse bone loss (7). Gut microbiota ferments dietary fiber in the intestines to produce short-chain fatty acids (SCFAs) (7), which may account for the effect of gut microbiota on bone as increasing evidence suggests the beneficial effect of SCFAs, especially butyrate and propionate, on improvement of bone health (8).
In this study, we assessed the association between dietary fiber and estimated bone mineral density (BMD) derived from heel ultrasound and prospectively examined the relation between fiber intake and the risk of hip fracture and all fractures. We particularly examined whether SCFA-related genetic variants (9) modified the association between dietary fiber intake and bone health. Given the health effect of dietary fiber may differ in various sex and age groups (10, 11), we further analyzed these relations in men and women separately.
Materials and Methods
Study and participants
The UK Biobank is a prospective cohort study of 0.5 million men and women aged 40 to 69 years recruited from across the UK between 2006 and 2010 (12). Only self-reported White British participants were included in the study. The following were excluded from the analysis: participants (1) without information on dietary fiber intake; (2) with a mismatch between genetic sex and self-reported sex; (3) without information on covariates; (4) with implausible energy intake at baseline (for men, <800 or >4200 kcal/day; for women, <600 or >3500 kcal/day); (5) with fracture at baseline; (6) who withdrew from the UK Biobank cohort. Our final analysis included 384 134 participants for fracture and 224 630 for BMD measurement (all supplementary material and figures are located in a digital research materials repository (13)).
UK Biobank received ethics approval from the North West Multi-Center Research Ethics Committee (REC reference: 11/NW/03820). This study was approved by the Biomedical Committee of the Tulane University (New Orleans, Louisiana) Institutional Review Board. All participants gave written informed consent before enrolment in the study, which was conducted in accordance with the principles of the Declaration of Helsinki.
Assessment of dietary intakes
The frequency of dietary intake was obtained through the touchscreen questionnaire, which was shown on the UK Biobank website (https://www.ukbiobank.ac.uk/). Briefly, for cooked vegetables, participants were asked: “On average how many heaped tablespoons of cooked vegetables would you eat per day?”; for salad or raw vegetables: “On average how many heaped tablespoons of salad or raw vegetables would you eat per day?”; for fresh fruit: “About how many pieces of fresh fruit would you eat per day?”; for dried fruit: “About how many pieces of dried fruit would you eat per day?”; for bread: “How many slices of bread do you eat each week?” and “What type of bread do you mainly eat?”; for cereal: “How many bowls of cereal do you eat a week?” and “What type of cereal do you mainly eat?” Detailed information about the questionnaire is available elsewhere (http://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=100052). A dietary fiber score was calculated according to the established methods (14, 15). Total energy intake was obtained from the web-based 24-hour assessment. We also included data of dietary fiber based on a 24-hour dietary recall of the previous day.
Assessment of outcome
The primary outcomes for this study were BMD derived from heel ultrasound (heel BMD), which was measured by quantitative ultrasound speed of sound and broadband ultrasound attenuation with the use of a Sahara Clinical Bone Sonometer (Hologic Corporation, Bedford, MA, USA). We performed sensitivity analysis with dual-energy X-ray absorptiometry (DXA) measured total BMD (DXA-BMD) and fracture (all and hip fracture) as outcomes. We used a set of ICD10 codes to identify fracture cases during follow up. We excluded fractures of the skull, face, hands and feet, pathological fractures due to malignancy, atypical femoral fractures, periprosthetic, and healed fracture codes in accordance with a previous report (16). A full list of ICD10 codes used can be found in (13).
Assessment of covariates
Age and sex were obtained from the touchscreen questionnaire. Physical activity was assessed as a weekly frequency of walking, moderate, or vigorous physical activity (17). Metabolic equivalents (MET)-minutes per week were calculated based on walking, or moderate or vigorous physical activity. Smoking status was reported as never, previous, or current; alcohol intake status was reported as never, previous, or current; body mass index (BMI) (kg/m2) was calculated as weight (kg) divided by height (m) squared. The Townsend Deprivation Index was used as an area-based proxy measure for socioeconomic status. Total energy intake, dietary vitamin D, and calcium intake were obtained from the 24-hour dietary recall of the previous day.
Genetic data
Single nucleotide polymorphisms (SNPs) genotyping, imputation, and quality control of the genetic data were performed by the UK biobank team. Detailed information has been described previously (http://www.ukbiobank.ac.uk/scientists-3/genetic-data/). We selected 9 butyrate-related SNPs and 3 propionate-related SNPs reported in a previous genome-wide association study (9, 13). All these SNPs were in Hardy–Weinberg equilibrium (HWE) P > 1 × 10–12 within the white British participants. We calculated the weighted polygenic scores (PGS) base on the SNPs by using the equation: weighted PGS = (β 1 × SNP1 + β 2 × SNP2 +…+ β n × SNPn) × (n/sum of the β coefficients), in which SNP was the number of the risk allele of each SNP and estimates were from a previous genome-wide association study. The PGS for participants with missing genotypes was calculated according to a previous reported method: standardized PGS = PGS with missing genotypes/number of nonmissing SNPs × total number of studied SNPs (18). The weighted PGS for butyrate and propionate ranged from 0 to 13 and 0 to 6, respectively. A higher PGS represents a higher production of butyrate and fecal propionate levels. The distribution of these scores can be found elsewhere (13).
Statistical analysis
Linear regression was used to examine the cross-sectional association of dietary fiber intake with heel-BMD and DXA-BMD. In model 1 we adjusted for age, sex, BMI, and assessment centers. In model 2, we further adjusted for smoking status, alcohol intake status, dietary calcium intake, dietary vitamin D intake, total energy intake, and physical activity. To assess whether the association of dietary fiber intake with heel-BMD can be modified by SCFA PGS, we added an interaction term of SCFA PGS and dietary fiber intake in the model 2. We used Cox regression models to estimate hazard ratios (HRs) for all fractures and hip fractures with adjustment of age, sex, BMI, and assessment centers in model 1 and additional smoking status, alcohol intake status, dietary calcium intake, dietary vitamin D intake, total energy intake, and physical activity in model 2. We evaluated the proportional hazards assumption by testing the interaction term between exposure and time using the Wald test. P for interaction <.05 was regarded as violation of the assumption. Further, we performed strata analyses to examine whether sex, age, BMI, and smoking status modified the association of dietary fiber with heel-BMD. We evaluated a potential modification effect by adding an interaction term of dietary fiber and the stratifying variable. Missing values of covariates from web-based 24-hour assessment and MET-minutes per week was imputed using the mean value. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). All reported P values were nominal and 2-sided. P < .05 was used as the significance level.
Results
Baseline characteristics are shown in Table 1. Of the included 384 134 participants, 206 958 (54.1%) were female. The mean (SD) age was 56 (8) years, and the mean (SD) BMI was 27 (5) kg/m2. The mean (SD) heel-BMD was 0.546 (0.136) g/cm2. Men showed higher heel-BMD than women (0.521 vs 0.576 g/cm2, P < .001). Dietary fiber score was higher in women compared with men (14.5 vs 14.0 g/d, P < .001). During an average follow up of 8.1 years, 11 836 cases were documented for all fractures and 1605 cases for hip fractures.
Table 1.
Baseline characteristics of UK Biobank participants
| N | All (N = 384 134) | Women (N = 207 981) | Men (N = 176 153) | |
|---|---|---|---|---|
| Age, year | 384 134 | 56.8 (8.0) | 56.4 (7.9) | 57.3 (8.0) |
| BMI, kg/m2 | 384 134 | 27.4 (4.8) | 27.0 (5.1) | 27.8 (4.2) |
| Bread intake, times/d | 377 478 | 12.4 (8.5) | 10.0 (6.7) | 15.3 (9.5) |
| Cereal intake, times/d | 383 358 | 4.6 (2.8) | 4.7 (2.7) | 4.6 (2.8) |
| Dried fruit intake, times/d | 380 749 | 0.8 (1.6) | 0.9 (1.6) | 0.7 (1.6) |
| Fresh fruit intake, times/d | 382 961 | 2.2 (1.5) | 2.4 (1.5) | 2.0 (1.5) |
| Cooked vegetable intake, times/d | 380 303 | 2.7 (1.8) | 2.7 (1.7) | 2.7 (1.9) |
| Salad/raw vegetable intake, times/d | 379 761 | 2.1 (2.0) | 2.3 (2.0) | 1.9 (1.9) |
| Englyst_dietary_fibre, g/d | 161 693 | 16.3 (6.3) | 16.1 (6.0) | 16.6 (6.5) |
| Fibre_score, g/d | 369 420 | 14.2 (6.1) | 14.5 (5.8) | 14.0 (6.4) |
| Bread fiber, g/d | 377 478 | 2.5 (1.9) | 2.1 (1.6) | 2.9 (2.2) |
| Cereal fiber, g/d | 383 358 | 2.1 (2.1) | 2.2 (2.1) | 2.1 (2.1) |
| Dried fruit fiber, g/d | 380 749 | 0.4 (0.8) | 0.5 (0.8) | 0.4 (0.8) |
| Fresh fruit fiber, g/d | 382 961 | 4.4 (3.1) | 4.7 (3.0) | 4.0 (3.1) |
| Cooked vegetable fiber, g/d | 380 303 | 2.7 (1.8) | 2.7 (1.7) | 2.7 (1.9) |
| Salad/raw vegetable fiber, g/d | 379 761 | 2.1 (2.0) | 2.3 (2.0) | 1.9 (1.9) |
| Energy intake, kcal/d | 161 693 | 2103.0 (359.7) | 2040.1 (327.2) | 2177.2 (381.5) |
| MET-minutes per wk | 384 134 | 2639.8 (2412.0) | 2535.9 (2156.5) | 2762.6 (2677.3) |
| BMD | 224 630 | 0.5459 (0.1357) | 0.5206 (0.1188) | 0.5756 (0.1478) |
| Whole body BMD | 4192 | 1.2121 (0.1488) | 1.1313 (0.1263) | 1.3009 (0.1178) |
| Smoking status | NA | |||
| Never | NA | 210 425 (54.8) | 123 886 (59.6) | 86 539 (49.1) |
| Previous | NA | 135 586 (35.3) | 66 149 (31.8) | 69 437 (39.4) |
| Current | NA | 38 123 (9.9) | 17 946 (8.6) | 20 177 (11.5) |
| Drinking status | NA | |||
| Never | NA | 12 171 (3.2) | 9094 (4.4) | 3077 (1.8) |
| Previous | NA | 12 744 (3.3) | 7207 (3.5) | 5537 (3.1) |
| Current | NA | 359 219 (93.5) | 191 680 (92.2) | 167 539 (95.1) |
| Hip fracture | NA | |||
| No | NA | 382 529 (99.6) | 206 958 (99.5) | 175 571 (99.7) |
| Yes | NA | 1605 (0.4) | 1023 (0.5) | 582 (0.3) |
| Fracture | NA | |||
| No | NA | 372 298 (96.9) | 200 363 (96.3) | 171 935 (97.6) |
| Yes | NA | 11 836 (3.1) | 7618 (3.7) | 4218 (2.4) |
Abbreviations: BMD, bone mineral density; BMD, estimated bone mineral density derived from heel ultrasound; BMI, body mass index.
In the analysis with adjustment for sex, age, BMI, and assessment centers (model 1), we found significant associations between dietary fiber intake and heel-BMD. Higher intakes of cereal, fruits (fresh and dried), and vegetables (cooked and raw) were associated with higher heel-BMD, whereas a higher bread intake was associated with lower heel-BMD (all P < .005). A higher dietary fiber intake (in SD) was associated with higher heel-BMD (β [SE] 0.0047 [0.0003], P = 1.10 × 10–54). This association was also found for fiber subtypes including bread, cereal, fruits (fresh and dry), and vegetables (cooked and raw) (all P < .005). When further adjusted for socioeconomic status, smoking status, drinking status, dietary vitamin D, dietary calcium, total energy intake, and physical activity in model 2, the associations were slightly attenuated but remained significant for the intake of bread, cereal, and fruit (dried and fresh) (all P < .005). The associations diminished for raw and cooked vegetable intake. For dietary fiber and fiber subtypes, the association with heel-BMD remained significant except for fiber from bread and vegetables (Table 2). We found no significant difference by comparing the BMD of participants’ whole grain bread intake (whole meal or whole grain) with those with refined bread (white and brown bread). The analysis was adjusted for age, sex, assessment center, BMI, deprivation status, physical activity (MET-hours/week), smoking status (never, previous, current), alcohol intake (never, previous, current), dietary calcium intake, dietary vitamin D intake, total energy intake, and times of bread intake.
Table 2.
Association of food rich in dietary fiber and estimated bone mineral density
| All | Women | Men | P interaction | ||||
|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | ||
| Model 1 | |||||||
| Bread intake | –0.0002 (0.0000) | 1.10 × 10–6 | –0.0002 (0.0000) | 4.20 × 10–5 | –0.0001 (0.0000) | 1.40 × 10–1 | 2.80 × 10–3 |
| Cereal intake | 0.0018 (0.0001) | 3.70 × 10–70 | 0.0013 (0.0001) | 2.60 × 10–26 | 0.0025 (0.0002) | 2.00 × 10–51 | 1.80 × 10–15 |
| Dried fruit intake | 0.0013 (0.0002) | 4.90 × 10–15 | 0.0013 (0.0002) | 7.50 × 10–10 | 0.0016 (0.0003) | 6.20 × 10–9 | 1.60 × 10–3 |
| Fresh fruit intake | 0.0025 (0.0002) | 5.50 × 10–41 | 0.0018 (0.0002) | 1.20 × 10–16 | 0.0035 (0.0003) | 1.40 × 10–31 | 1.20 × 10–13 |
| Cooked vegetable intake | 0.0005 (0.0002) | 4.50 × 10–3 | 0.0006 (0.0002) | 5.40 × 10–3 | 0.0001 (0.0002) | 6.00 × 10–1 | 2.50 × 10–2 |
| Salad/raw vegetable intake | 0.0005 (0.0001) | 2.10 × 10–4 | 0.0002 (0.0002) | 2.80 × 10–1 | 0.0007 (0.0002) | 1.90 × 10–3 | 3.20 × 10–2 |
| Dietary fiber (1 SD) | |||||||
| Fibre scorea | 0.0047 (0.0003) | 1.10 × 10–54 | 0.0036 (0.0004) | 1.40 × 10–21 | 0.0061 (0.0005) | 1.40 × 10–38 | 2.00 × 10–13 |
| Bread fiber | 0.0008 (0.0003) | 6.60 × 10–3 | 0.0002 (0.0004) | 5.40 × 10–1 | 0.0017 (0.0004) | 1.80 × 10–5 | 1.20 × 10–3 |
| Cereal fiber | 0.0039 (0.0003) | 1.70 × 10–44 | 0.0027 (0.0003) | 1.50 × 10–16 | 0.0054 (0.0005) | 6.60 × 10–33 | 1.20 × 10–8 |
| Dried fruit fiber | 0.0024 (0.0003) | 4.90 × 10–15 | 0.0023 (0.0004) | 7.50 × 10–10 | 0.0028 (0.0005) | 6.20 × 10–9 | 1.60 × 10–3 |
| Fresh fruit fiber | 0.0040 (0.0003) | 5.50 × 10–41 | 0.0029 (0.0004) | 1.20 × 10–16 | 0.0057 (0.0005) | 1.40 × 10–31 | 1.20 × 10–13 |
| Cooked vegetable fiber | 0.0009 (0.0003) | 4.50 × 10–3 | 0.0011 (0.0004) | 5.40 × 10–3 | 0.0003 (0.0005) | 6.00 × 10–1 | 2.50 × 10–2 |
| Salad/raw vegetable fiber | 0.0011 (0.0003) | 2.10 × 10–4 | 0.0004 (0.0004) | 2.80 × 10–1 | 0.0016 (0.0005) | 1.90 × 10–3 | 3.20 × 10–2 |
| Dietary fiberb | 0.0021 (0.0005) | 5.90 × 10–5 | 0.0008 (0.0007) | 2.00 × 10–1 | 0.0038 (0.0008) | 4.20 × 10–6 | 8.10 × 10–5 |
| Model 2 | |||||||
| Bread intake | –0.0001 (0.0000) | 9.10 × 10–5 | –0.0002 (0.0001) | 1.30 × 10–3 | 0.0000 (0.0000) | 3.60 × 10–1 | 7.60 × 10–3 |
| Cereal intake | 0.0013 (0.0001) | 2.00 × 10–35 | 0.0010 (0.0001) | 1.90 × 10–15 | 0.0016 (0.0002) | 2.90 × 10–23 | 4.00 × 10–14 |
| Dried fruit intake | 0.0009 (0.0002) | 1.20 × 10–7 | 0.0009 (0.0002) | 8.90 × 10–6 | 0.0011 (0.0003) | 9.40 × 10–5 | 5.60 × 10–4 |
| Fresh fruit intake | 0.0017 (0.0002) | 1.70 × 10–19 | 0.0012 (0.0002) | 2.10 × 10–8 | 0.0025 (0.0003) | 3.20 × 10–16 | 3.80× 10–13 |
| Cooked vegetable intake | 0.0001 (0.0002) | 3.70 × 10–1 | 0.0003 (0.0002) | 1.90 × 10–1 | –0.0002 (0.0002) | 4.80 × 10–1 | 1.30 × 10–2 |
| Salad/raw vegetable intake | 0.0003 (0.0001) | 7.30 × 10–2 | 0.0000 (0.0002) | 8.90 × 10–1 | 0.0004 (0.0002) | 1.40 × 10–1 | 9.90 × 10–2 |
| Dietary fiber (1 SD) | |||||||
| Fibre scorea | 0.0031 (0.0003) | 1.40 × 10–24 | 0.0024 (0.0004) | 3.40 × 10–10 | 0.0041 (0.0005) | 8.60 × 10–18 | 1.10 × 10–13 |
| Bread fiber | 0.0004 (0.0003) | 1.40 × 10–1 | 0.0001 (0.0004) | 7.70 × 10–1 | 0.0012 (0.0004) | 4.40 × 10–3 | 2.10 × 10–3 |
| Cereal fiber | 0.0028 (0.0003) | 2.70 × 10–23 | 0.0021 (0.0003) | 7.70 × 10–10 | 0.0037 (0.0005) | 4.70 × 10–16 | 7.80 × 10–8 |
| Dried fruit fiber | 0.0016 (0.0003) | 1.20 × 10–7 | 0.0016 (0.0004) | 8.90 × 10–6 | 0.0019 (0.0005) | 9.40 × 10–5 | 5.60 × 10–4 |
| Fresh fruit fiber | 0.0027 (0.0003) | 1.70 × 10–19 | 0.0020 (0.0004) | 2.10 × 10–8 | 0.0040 (0.0005) | 3.20 × 10–16 | 3.80 × 10–13 |
| Cooked vegetable fiber | 0.0003 (0.0003) | 3.70 × 10–1 | 0.0005 (0.0004) | 1.90 × 10–1 | –0.0003 (0.0005) | 4.80 × 10–1 | 1.30 × 10–2 |
| Salad/raw vegetable fiber | 0.0006 (0.0003) | 7.30 × 10–2 | –0.0001 (0.0004) | 8.90 × 10–1 | 0.0008 (0.0005) | 1.40 × 10–1 | 9.90 × 10–2 |
| Dietary fiberb | 0.0017 (0.0006) | 5.40 × 10–3 | 0.0008 (0.0008) | 2.70 × 10–1 | 0.0025 (0.0009) | 8.50 × 10–3 | 7.20 × 10–5 |
Model 1 was adjusted for age, sex, assessment center, and BMI.
Model 2 was further adjusted for deprivation status, physical activity (MET-h/week), smoking status (never, previous, current), alcohol intake (never, previous, current), dietary calcium intake, dietary vitamin D intake, and total energy intake.
a Fiber score was calculated according the questions on fruit, vegetables, bread, and breakfast cereal. The unit for food intake was times/day. The unit for total and subgroup dietary fiber was g/day.
b Calculated according to a 24-hour dietary recall of the previous day
We tested the association of the PGS with heel-BMD and found no significant results. We next examined whether genetical variations (PGS) related to microbiota-derived SCFA production could modify the association between dietary fiber intake and heel-BMD. We found a significant interaction between propionate PGS and dietary fiber intake on bone health (Pinteraction = 3.9 × 10–3 in model 1 and 5.1 × 10–3 in model 2). The association between dietary fiber and bone health was stronger among participants with lower genetically determined fecal propionate. However, we did not found interaction between butyrate PGS and heel-BMD. The association of dietary fiber intake and bone health was comparable among participants with different levels of genetically determined butyrate production (P > .05 in both models) (Fig. 1).
Figure 1.
Effect of SCFA PGS on the association of dietary fiber (1 SD) with heel-BMD. Values were expressed as adjusted least square means ± SE for heel-BMD. P values were adjusted for age, sex, the Townsend Deprivation Index, physical activity (MET-minutes/week), smoking status (never, previous, current), alcohol intake (never, previous, current), body mass index, dietary calcium intake, dietary vitamin D intake, and total energy intake. T1, T2, and T3 refer to tertiles of SCFA PGS. Low, average and high indicate different levels of dietary fiber intake. We used the raw value of the heel-BMD.
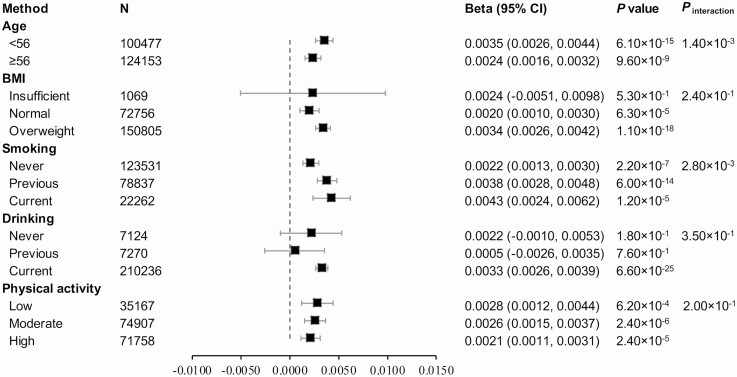
We then tested the association between heel-BMD and dietary fiber intake among women and men separately. In model 1, higher dietary fiber intake was associated with higher heel-BMD and the association was more marked for men (Pinteraction = 2.00 × 10–13). Similar sex-specific associations were also found for fiber subtypes except for dietary fiber from cooked vegetables, which was more marked for women. Further adjustments did not change the direction (Table 2). We also examined the association of dietary fiber intake and bone health among strata of several risk factors including age, BMI, smoking status, drinking status, and physical activity. The associations between dietary fiber intake and bone health were more pronounced in younger participants and smokers (current smokers and former smokers) (Fig. 2), and appeared consistent across the strata of BMI, drinking status, and physical activity.
Figure 2.
Association of dietary fiber intake (1 SD) and bone health stratified by potential confounders. Multivariable model was mutually adjusted for age, sex, the Townsend Deprivation Index, physical activity (MET-minutes/week), smoking status (never, previous, current), drinking status (never, previous, current), BMI, dietary calcium intake, dietary vitamin D intake, and total energy intake.
Sensitivity analyses
Sensitivity analyses were conducted by testing the association between dietary fiber intake and DXA-BMD in a subgroup from the UK biobank. The results showed a higher dietary fiber intake (in SD) was associated with higher total body BMD (β [SE] 0.0042 [0.0019], P = 2.5 × 10–2) and less risk of hip fracture (HR, 95% CI: 0.94, 0.89-0.99; P = 3.0 × 10–2) when adjusted for age, sex, center, and BMI. However, these associations were attenuated to be nonsignificant when further adjusted for deprivation status, physical activity, smoking status, alcohol intake, dietary calcium intake, dietary vitamin D intake, and total energy intake. No association was found between dietary fiber intake and all fractures.
We observed similar associations by using data of dietary fiber from 24-hour dietary recall questionnaire. A higher dietary fiber intake (in SD) was associated with higher heel-BMD (β [SE] 0.0021 [0.0005], P = 5.90 × 10–5) when adjusted for age, sex, center, and BMI. Further adjustment did not significantly change the result (Table 2).
Discussion
In this study from the UK biobank, we found that higher intakes of dietary fiber, including total fiber and various subtypes including cereal, bread, and vegetable, were associated with higher heel-BMD, and the association was more pronounced in men than women. In addition, we found that genetically determined gut microbiota-derived SCFA propionate significantly modified the association between dietary fiber and heel-BMD. The association between dietary fiber and bone health was stronger among participants with lower genetically determined propionate.
Growing evidence suggests that gut microbiota is closely related to bone health (3-6, 19). A study in germ-free mice indicated that the composition of gut microbiota directly impacts BMD (20). Several epidemiological studies found altered abundance of gut bacterial taxa with specific functional pathways among participants with low-BMD or osteoporosis (19, 21). Emerging evidence suggests that SCFAs (8), which are produced by gut microbiota via fermentation of complex carbohydrates including dietary fiber, may at least partly account for the link between dietary fiber and bone health (7, 8, 22-25). Two major SCFAs, butyrate and propionate, may directly inhibit osteoclast differentiation (23). In addition, supplementation of sodium propionate was found to have a beneficial effect on bone by decreasing serum parameters for bone resorption in humans (8).
The positive association between dietary fiber intake and bone health observed in our study is in line with previous evidence (26, 27). Data from the Framingham Offspring Study showed that dietary fiber was associated with less bone loss, but the protective association was only reported in men (10). In another study performed in the Korean population, a protective association of dietary fiber with bone was observed and the association was only observed among men aged between 18 and 45 years (11). Furthermore, the consumption of food rich in dietary fiber such as fruits, vegetables, and cereal has been found to be positively related to BMD (28-30). We did not find significant association between dietary fiber intake and risks of fracture, which might be related to the insufficient intake of dietary fiber as well as the relatively moderate effect of dietary fiber on BMD.
Our results suggest that different fiber subtypes were consistently related to BMD in the same direction, while the effect size varied according to different fiber subtypes. The varied association may be attributed to the different fiber types, varied nutrients, and dietary bioactive contents. Beyond providing basic nutrition needs in humans, fruit and vegetables improve bone health by reducing inflammation and other potential preventive effects (31). In addition, the protective associations were stronger for fresh fruit and vegetables than for processed fruit and vegetables, which may result from changes in their structure, chemical composition, nutritional value, and bioavailability of bioactive during processing (32). Of note, bread fiber is mainly from the bran (skin) of whole grain seed, therefore whole grain is supposed to have a more beneficial effect on bone health. However, we did not find a significant difference in the effect for whole grain versus refined bread, which needs further investigation.
Intriguingly, we found that higher levels of genetically determined gut microbiota-derived SCFA propionate could attenuate the association between dietary fiber and BMD. Dietary fiber is the major source of propionate (33), and dietary fiber intervention increased SCFA concentration (34). Propionate and butyrate have been found to promote bone health (8, 22), and intake of sodium propionate was found to encourage good bone health by decreasing serum parameters for bone resorption (8). In addition, it was reported that propionate promoted the expression of vitamin D receptors (35), which might affect bone minerals too (36). Moreover, propionate may contribute to the increase in insulin-like growth factor 1, which promotes bone growth and remodeling (22). We hypothesize that propionate is more likely to reach its maximum beneficial effect on bone health among participants with higher genetically determined propionate. In this case, more dietary fiber intake may have a less beneficial effect on bone health. More investigations are warranted to explore the potential mechanisms underlying the attenuated association between dietary fiber and BMD among people with higher genetically determined propionate levels.
Several other mechanisms may also be involved in the observed associations between dietary fiber and bone health. For example, an intervention trial showed that daily soluble fiber consumption improved the bone calcium balance by increasing bone calcium retention in postmenopausal women (37, 38). In addition, dietary fiber from different sources exhibited varied effects (higher or lower, but in the same direction) in manipulating gut bacteria (39), which might partially explain the varied effect of dietary fiber from different foods on bone health.
Also of interest, we found a sex difference in associations between dietary fiber and bone health. The associations were stronger in men than in women. Our findings were supported by previous population studies in which protective associations were only found in men (10, 11). Notably, our study extends previous findings by providing evidence that the protective association also existed in women, though not as strong as in men. It was found that decreased estrogen levels promoted bone loss (40) and dietary fiber might reduce estrogen concentrations in women (41, 42), which might partly offset the protective effect of dietary fiber on bone health. In addition, we found that the association between dietary fiber and bone health was more pronounced among younger participants, which is in agreement with a previous study and may be related to accelerated bone loss and changed sex hormone levels during aging (43). Another possible explanation for the sex and age difference of the association is that bone loss occurs earlier in women than men, and there is less variability in BMD for women than men in the study population. Our stratified analyses by menopausal status indicated that the association among premenopausal women was stronger than postmenopausal women, among whom major bone loss had already taken place. This observation partly supported our postulation. In addition, the association between dietary fiber intake and bone health was more pronounced in current smokers and former smokers than never smokers. Smoking adversely affects bone through diverse pathophysiologic mechanisms, including alternating sex hormone production and metabolism and dysregulation of intestinal calcium absorption (44), which might be reverted by higher fiber intake (38).
The strengths of our study include the large sample size and information on risk factors of bone health which enable us to control confounders and perform stratified analysis in risk factor subgroups. There are several potential limitations. First, we used heel ultrasound BMD instead of DXA-derived BMD as our main outcome because the quantitative heel ultrasound method is portable, inexpensive, and without ionizing radiation (45). These 2 BMD measures are highly correlated and have high genetic concordances. More importantly, heel ultrasound BMD could be comparable or even more precise in predicting hip fracture than DXA-derived BMD (16). Second, the fiber score used in the current study was not a complete estimate of fiber intake. Nonetheless, it is not necessary to determine food and nutrient intakes with absolute accuracy when studying the association between dietary factors and outcomes (14), and this score has been verified to separate UK Biobank participants with low and high dietary fiber intake (14, 15). Third, although we have controlled for several lifestyle and dietary factors, unmeasured confounders might still exist. Last, the dietary data were self-reported, which might lead to inaccurate estimates of the associations between dietary fiber and bone. However, the touchscreen dietary questionnaire has been validated to reliably rank participants according to intakes of main food groups (14).
In conclusion, higher dietary fiber consumption was associated with higher BMD, and the association was more marked in men and among participants with lower genetically determined gut microbiota-derived SCFA propionate.
Acknowledgments
Financial Support: This study is supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, and HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, and DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States–Israel Binational Science Foundation Grant 2011036. The funders had no role in study design, data collection and analysis, interpretation of data, writing of the report, and decision to submit the paper for publication. We thank the participants and UK Biobank team for their outstanding commitment and cooperation. This research has been conducted with approved project number 29256.
Glossary
Abbreviations
- BMD
bone mineral density
- BMI
body mass index;
- DXA,
dual-energy X-ray absorptiometry;
- HWE
Hardy–Weinberg equilibrium;
- SCFA
short-chain fatty acid
- SNP
single nucleotide polymorphism;
- PGS
polygenic score;
- WPS
weighted polygenic scores.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The data of UK Biobank is publicly available upon request.
References
- 1. WHO. Global Health Observatory (GHO) data. 2019. ProMED-mail website. https://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends_text/en/#:~:targetText=Global-average-life-expectancy-increased-fastest-increase-since-the-1960s. Accessed April 16, 2020.
- 2. Cerani A, Zhou S, Forgetta V, et al. . Genetic predisposition to increased serum calcium, bone mineral density, and fracture risk in individuals with normal calcium levels: mendelian randomisation study. BMJ. 2019;366:l4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sjögren K, Engdahl C, Henning P, et al. . The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015;26(2):69-74. [DOI] [PubMed] [Google Scholar]
- 5. Rizzoli R. Nutritional influence on bone: role of gut microbiota. Aging Clin Exp Res. 2019;31(6):743-751. [DOI] [PubMed] [Google Scholar]
- 6. Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13(2):125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaiss MM, Jones RM, Schett G, Pacifici R. The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest. 2019;129(8):3018-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lucas S, Omata Y, Hofmann J, et al. . Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanna S, van Zuydam NR, Mahajan A, et al. . Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51(4):600-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dai Z, Zhang Y, Lu N, Felson DT, Kiel DP, Sahni S. Association between dietary fiber intake and bone loss in the Framingham offspring study. J Bone Miner Res. 2018;33(2):241-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee T, Suh HS. Associations between dietary fiber intake and bone mineral density in adult Korean population: analysis of National Health and Nutrition Examination Survey in 2011. J Bone Metab. 2019;26(3):151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bycroft C, Freeman C, Petkova D, et al. . The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou T, Wang M, Ma H, Li X, Heianza Y, Qi L. Supplementary information for Dietary fiber intake, genetic variations of gut microbiota-derived short-chain fatty acids, and bone health in UK biobank.2020. doi:10.6084/m9.figshare.12501860.v2. Deposited September 21, 2020.
- 14. Bradbury KE, Young HJ, Guo W, Key TJ. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7(e6):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. 2020;49(1):246-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris JA, Kemp JP, Youlten SE, et al. ; 23andMe Research Team . An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019;51(2):258-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yates T, Zaccardi F, Dhalwani NN, et al. . Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur Heart J. 2017;38(43):3232-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Television watching, leisure time physical activity, and the genetic predisposition in relation to body mass index in women and men. ProMED-mail website. https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.112.098061. Accessed September 9, 2020. [DOI] [PMC free article] [PubMed]
- 19. Li C, Huang Q, Yang R, et al. . Gut microbiota composition and bone mineral loss—epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int. 2019;30(5):1003-1013. [DOI] [PubMed] [Google Scholar]
- 20. Villa CR, Ward WE, Comelli EM. Gut microbiota-bone axis. Crit Rev Food Sci Nutr. 2017;57(8):1664-1672. [DOI] [PubMed] [Google Scholar]
- 21. Das M, Cronin O, Keohane DM, et al. . Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxford). 2019;58(12):2295-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan J, Herzog JW, Tsang K, et al. . Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113(47):E7554-E7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan J, Takakura A, Zandi-Nejad K, Charles JF. Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes. 2018;9(1):84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tyagi AM, Yu M, Darby TM, et al. . The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity. 2018;49(6):1116-1131.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahman MM, Kukita A, Kukita T, Shobuike T, Nakamura T, Kohashi O. Two histone deacetylase inhibitors, trichostatin A and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood. 2003;101(9):3451-3459. [DOI] [PubMed] [Google Scholar]
- 26. Farrell VA, Harris M, Lohman TG, et al. . Comparison between dietary assessment methods for determining associations between nutrient intakes and bone mineral density in postmenopausal women. J Am Diet Assoc. 2009;109(5):899-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karamati M, Yousefian-Sanni M, Shariati-Bafghi SE, Rashidkhani B. Major nutrient patterns and bone mineral density among postmenopausal Iranian women. Calcif Tissue Int. 2014;94(6):648-658. [DOI] [PubMed] [Google Scholar]
- 28. Tucker KL, Chen H, Hannan MT, et al. . Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr. 2002;76(1):245-252. [DOI] [PubMed] [Google Scholar]
- 29. Sahni S, Mangano KM, McLean RR, Hannan MT, Kiel DP. Dietary approaches for bone health: lessons from the Framingham Osteoporosis Study. Curr Osteoporos Rep. 2015;13(4):245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dreher ML. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients. 2018;10(12):1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gunn CA, Weber JL, Kruger MC. Midlife women, bone health, vegetables, herbs and fruit study. The Scarborough Fair study protocol. BMC Public Health. 2013;13(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oude Griep LM, Verschuren WM, Kromhout D, Ocké MC, Geleijnse JM. Raw and processed fruit and vegetable consumption and 10-year stroke incidence in a population-based cohort study in the Netherlands. Eur J Clin Nutr. 2011;65(7):791-799. [DOI] [PubMed] [Google Scholar]
- 33. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. So D, Whelan K, Rossi M, et al. . Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6): 965-983. [DOI] [PubMed] [Google Scholar]
- 35. Lin H, Huang Y, Tian T, Wang P, Li Y. Propionate promotes vitamin D receptor expression via yes-associated protein in rats with short bowel syndrome. Biochem Biophys Res Commun. 2020;523(3):645-650. [DOI] [PubMed] [Google Scholar]
- 36. Jurutka PW, Bartik L, Whitfield GK, et al. . Vitamin D receptor: Key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J Bone Miner Res. 2007;22(Suppl. 2):2-10. [DOI] [PubMed] [Google Scholar]
- 37. Whisner CM, Martin BR, Nakatsu CH, et al. . Soluble corn fiber increases calcium absorption associated with shifts in the gut microbiome: a randomized dose-response trial in free-living pubertal females. J Nutr. 2016;146(7):1298-1306. [DOI] [PubMed] [Google Scholar]
- 38. Jakeman SA, Henry CN, Martin BR, et al. . Soluble corn fiber increases bone calcium retention in postmenopausal women in a dose-dependent manner: a randomized crossover trial. Am J Clin Nutr. 2016;104(3):837-843. [DOI] [PubMed] [Google Scholar]
- 39. Patnode ML, Beller ZW, Han ND, et al. . Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell. 2019;179(1):59-73.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791-801. [DOI] [PubMed] [Google Scholar]
- 41. Goldin BR, Woods MN, Spiegelman DL, et al. . The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer. 1994;74(3 Suppl):1125-1131. [DOI] [PubMed] [Google Scholar]
- 42. Goldin BR, Adlercreutz H, Gorbach SL, et al. . Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med. 1982;307(25):1542-1547. [DOI] [PubMed] [Google Scholar]
- 43. Ego S. Pathogenesis of bone fragility in women and men. Lancet. 2002;359(9320):1841-1850. [DOI] [PubMed] [Google Scholar]
- 44. Yoon V, Maalouf NM, Sakhaee K. The effects of smoking on bone metabolism. Osteoporos Int. 2012;23(8):2081-2092. [DOI] [PubMed] [Google Scholar]
- 45. Moayyeri A, Adams JE, Adler RA, et al. . Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int. 2012;23(1):143-153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of UK Biobank is publicly available upon request.