Abstract
Aims
To evaluate the safety and effectiveness of a compliant multi-electrode radiofrequency balloon catheter (RFB) used with a multi-electrode diagnostic catheter for pulmonary vein isolation (PVI).
Methods and results
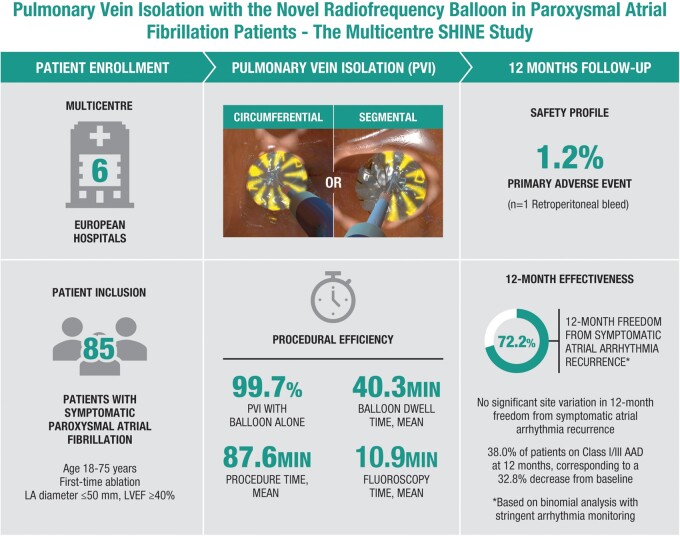
This prospective, multicentre, single-arm study was conducted at six European sites and enrolled patients with symptomatic paroxysmal atrial fibrillation. The primary effectiveness endpoint was entrance block in treated pulmonary veins (PVs) after adenosine/isoproterenol challenge. The primary safety endpoint was the occurrence of primary adverse events (PAEs) within 7 days. Cerebral magnetic resonance imaging and neurological assessments were performed pre- and post-ablation in a subset of patients. Atrial arrhythmia recurrence was assessed over 12 months via transtelephonic and Holter monitoring. Quality of life was assessed by the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire. Of 85 patients undergoing ablation per study protocol, PV entrance block was achieved in all (one PV required touch-up with a focal catheter). Acute reconnection of ≥1 PVs after adenosine/isoproterenol challenge was observed in 9.3% (30/324) of PVs ablated. Post-ablation, silent cerebral lesions were detected in 9.7% (3/31) of patients assessed, all of which was resolved at 1-month follow-up. One patient experienced a PAE (retroperitoneal bleed). Freedom from documented symptomatic and all arrhythmia was 72.2% and 65.8% at 12 months. Four patients (4.7%) underwent repeat ablation. Significant improvements in all AFEQT subscale scores were seen at 6 and 12 months.
Conclusion
PVI with the novel RFB demonstrated favourable safety and effectiveness, with low repeat ablation rate and clinically meaningful improvement in quality of life.
ClinicalTrials.gov Registration Number
Keywords: Atrial fibrillation, Radiofrequency ablation, Pulmonary vein isolation, Electrophysiological mapping, Radiofrequency balloon catheter, Quality of life
Graphical Abstract
What’s new?
The novel multi-electrode radiofrequency balloon catheter (RFB) consists of a compliant balloon with the ability to directionally tailor the dose of ablative energy simultaneously delivered along the balloon circumference.
The prospective, multicentre SHINE study evaluated the safety and effectiveness of the multi-electrode RFB when used with a multi-electrode diagnostic catheter for pulmonary vein isolation and electrophysiological mapping in 85 patients with symptomatic paroxysmal atrial fibrillation.
The SHINE study demonstrated safe and effective use of the multi-electrode circular diagnostic and RFB, with only one primary adverse event reported and sustained pulmonary vein entrance block achieved in all patients. At 12 months after ablation, 72.2% of the patients were free of documented symptomatic arrhythmia recurrence and the patient cohort experienced significant improvements in quality of life.
Introduction
Pulmonary vein isolation (PVI) with radiofrequency (RF) catheter ablation is an effective treatment option for patients with paroxysmal atrial fibrillation (PAF).1 Point-by-point RF ablation is a technically challenging procedure, with a considerable learning curve required to safely and effectively achieve PVI. Thus, balloon ablation catheters have been developed with the aim of achieving PVI using fewer applications of ablation energy. The cryoballoon is the most commonly used balloon ablation catheter, with others including the laser balloon and RF balloon.1,2 However, since it is not possible to directionally titrate energy circumferentially from their single ablative surface, the varying thickness of the tissue at the pulmonary vein (PV) ostia means that this could give rise to under- or over-ablation depending on tissue thickness. Also, these balloon catheters are most often used without electroanatomical mapping, potentially lengthening fluoroscopy and/or procedure times.
The multi-electrode RF balloon catheter (RFB; HELIOSTAR, Biosense Webster, CA, USA) is a 28-mm spherical diameter, compliant balloon that is compatible with the three-dimensional electroanatomical mapping system (CARTO® 3, Biosense Webster, CA, USA).3,4 The RFB can perform circumferential or segmental ablation with 10 irrigated, flexible gold electrodes, each capable of independently delivering varying levels of RF energy to adjust for differences in target tissues (Figure 1). The RADIANCE first-in-human study established the feasibility of the RFB to achieve electrical PVI with a favourable acute safety profile, without using a second focal catheter.3,4 However, the first generation RFB was limited by the inability to monitor PV electrograms ‘online’ during RF energy delivery.
Figure 1.
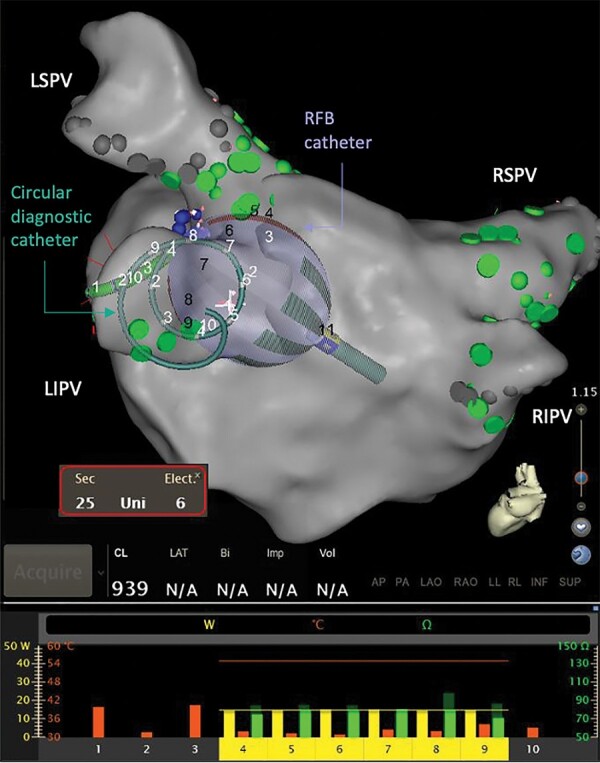
The RFB and circular diagnostic catheter as visualised using the electroanatomical mapping system. The RFB and the circular diagnostic catheter are shown at the ostium of LIPV during ablation, where the bottom panel reflects the RF power being delivered (yellow bar, 0–40 W), temperature measured (orange bar, 0–60 Celsius), starting impedance (dark green, 0–150 Ohms), and current impedance (bright green, 0–150 Ohms) for each of the 10 individual electrodes. The bottom panel also shows the posterior electrodes #1–#3 are turned off. LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RF, radiofrequency; RFB, radiofrequency balloon catheter; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
The multicentre SHINE study was designed to evaluate the safety and effectiveness of the second-generation multi-electrode RFB in combination with a multi-electrode diagnostic catheter for PV-targeted ablation, with a post-procedure follow-up period of 12 months. A subgroup of patients was also screened for silent cerebral lesions (SCLs) using pre- and post-procedural diffusion-weighted magnetic resonance imaging (MRI). The reproducibility of procedural efficiency and clinical outcome were also evaluated under different anaesthesia settings and across different centres.
Methods
Study design and patients
SHINE was a prospective, multicentre, single-arm clinical study evaluating the safety and effectiveness of using the multi-electrode RFB in combination with the multi-electrode circular diagnostic catheter; electrophysiological mapping of the atria (recording and stimulation) was facilitated with the mapping system. The study was conducted at six sites in four European countries in accordance with Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki. It was reviewed and approved by ethics committees at all participating sites and by all national authorities in participating countries.
Eligible patients had symptomatic PAF suitable for PVI and were able to comply with uninterrupted anticoagulation. Exclusion criteria included atrial fibrillation (AF) secondary to reversible or non-cardiac cause, previous ablation for AF, anticipation of receiving ablation other than PVI, and persistent AF.
Atrial fibrillation ablation procedure
Following completion of the RADIANCE study,3,4 several modifications were made to the ablation workflow for the SHINE study to further improve device safety profile, namely eliminating dual transseptal access, bolus dosing with heparin before transseptal puncture, maintaining activated clotting time at 350–400 s, using an integrated 3-Fr diagnostic multi-electrode circular catheter (LASSOSTAR, Biosense Webster, Irvine, CA, USA) to minimise catheter exchange, continuously irrigating all side ports, and using a target temperature setting of 55°C.
Procedures were performed under conscious sedation or general anaesthesia. Computed tomography and/or magnetic resonance angiography images were first reviewed to evaluate the number and size of the PVs and left atrial anatomy. A single transseptal puncture was then performed. Pre-ablation mapping and recording were conducted with either the PENTARAY® or the LASSO® NAV catheters (Biosense Webster, Irvine, CA, USA), and the electroanatomical mapping system, which was then withdrawn from the left atrium. A multi-electrode oesophageal temperature monitoring device was inserted before starting ablation; additional mechanical oesophageal deviation (DV8; Manual Surgical Sciences, Inc.) was performed per physician’s preference. Target activated clotting time was confirmed prior to insertion of the balloon catheter and maintained throughout the procedure. The RFB catheter with the intraluminal circular diagnostic catheter was then introduced into the left atrium and left in situ for the rest of the case. Tissue contact of the RFB was verified through fluoroscopy, CARTO system visualisation of the balloon and balloon size index, or by observing electrode impedance and temperature parameters. During ablation, 15 W was simultaneously delivered to each RFB electrode, and energy delivery to electrodes along the posterior wall was typically stopped after 20 s or earlier in case of oesophageal temperature rise; posterior electrodes were identified by the balloon’s representation on the mapping system. The PV signals were monitored in real time on the circular diagnostic catheter (Figure 2, Supplementary material online, Video S1), and RF duration for the non-posterior wall-facing electrodes was limited to 60 s. Isolation of all targeted PVs was then confirmed with the circular catheter, or in some cases exchanged with the Lasso Nav diagnostic catheter. Isoproterenol/adenosine challenge was administered for all targeted PVs to confirm entrance block. There was no minimum waiting period mandated after PVI was achieved. Antiarrhythmic drug (AAD) therapy was managed per the institution’s standard of care.
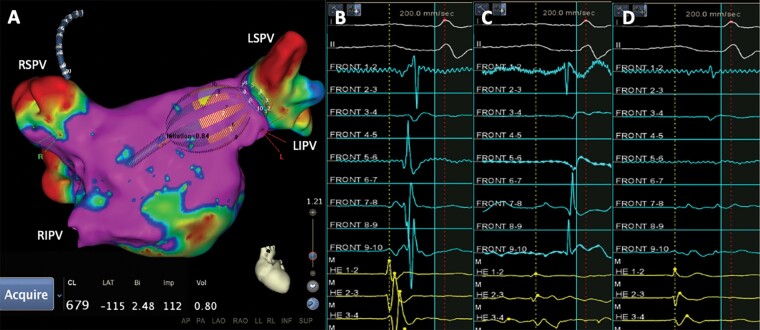
Figure 2.
(A) Voltage map of the left atrium and electrogram recorded by the circular diagnostic catheter (B) before, (C) during, and (D) after LSPV isolation with the RFB. LSPV, left superior pulmonary vein; RFB, radiofrequency balloon; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Rhythm monitoring on follow-up
Stringent arrhythmia monitoring included 24-h Holter monitors, recorded at 6 and 12 months, and transtelephonic monitoring (TTM) at 1, 3, 6, and 12 months after ablation. In addition, patients were asked to record and transmit their electrocardiogram (ECG) weekly though the end of 5-month follow-up, and then monthly until the 12-month follow-up visit. Patients were also asked to transmit any symptom-triggered ECG recording that occurred throughout the study period. All TTM tracings and Holter recordings were independently assessed by a core lab.
Study endpoints
The primary safety endpoint was the occurrence of primary adverse events (PAEs) within 7 days following the initial ablation procedure. Device- or procedure-related death, atrio-oesophageal fistula, and PV stenosis occurring later than 7 days after the ablation procedure were also considered as PAEs. Diaphragmatic paralysis/phrenic nerve palsy were considered PAEs if symptoms had not resolved at the 3-month visit.
The primary effectiveness endpoint was acute procedural success, defined as the confirmation of entrance block in treated PVs after adenosine and/or isoproterenol challenge (with or without the use of a focal catheter).
Secondary endpoints included any serious device adverse events; procedural characteristics; acute reconnection of ≥1 PVs after adenosine/isoproterenol challenge; and symptomatic or asymptomatic recurrence of AF (episodes > 30 s), atrial tachycardia (AT), and/or atrial flutter (AFL), on/off AAD after a 3-month blanking period at 6- and 12 months. Total procedure time was defined as the time of the first femoral puncture until the time of the last catheter removal, meaning that the time needed for transseptal mapping, ablation, and adenosine/isoproterenol was included. Other procedural datapoints related to PVI were also analysed both by subject and by vein.
Silent cerebral lesion assessment
Analyses of MRI data were performed in the neurological assessment evaluable (NAE) analysis population. Incidences of SCLs were evaluated using diffusion-weighted MRI at 72 h prior to the ablation procedure and within 48 h post-procedure. Patients with identifiable lesions or neurological symptoms had a follow-up MRI to determine lesion progress until the lesions were resolved. Neurological and cognitive assessments were also performed using the National Institutes of Health Stroke Scale (NIHSS).
Quality of life assessment
Patients completed the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire at study baseline and at 3, 6, and 12 months after ablation. The AFEQT questionnaire is an AF-specific health-related quality of life questionnaire, which includes 20 questions grouped into four functional subscales, namely symptoms, daily activities, treatment concern, and treatment satisfaction. Overall and subscale scores range from 0 to 100, with an overall score of 0 corresponding to complete disability, while a score of 100 corresponds to no disability. For AFEQT scales, a change of ≥5 points has been identified as a benchmark for minimum clinically important difference in an individual patient.5
Statistical analysis
The safety population comprised all enrolled patients who underwent insertion of the study catheters. The per-protocol population included patients who were enrolled and met all eligibility criteria, underwent RF ablation with study catheters, and were treated for study-related arrhythmia. The NAE population included patients from the per-protocol population who consented to and were eligible for the required neurological assessments and had at least one post-ablation MRI scan.
Patient demographic, cardiovascular medical history, AAD history, baseline CHA2DS2-VASc score, AF history, and procedure data were summarised descriptively. The rate of PAEs and acute procedural success were compared to a performance goal of 15% and 80%, respectively, by using the exact test for a binomial proportion at a one-sided significance level of 5%. If the one-sided 95% lower confidence bound for the primary adverse event and effectiveness rates were greater than the performance goals, the study was considered to have demonstrated safety and effectiveness.
Secondary endpoint analyses were performed descriptively in the proposed analysis populations, excluding the patients with missing outcomes. Secondary effectiveness endpoints, including 6-month and 12-month effectiveness success, were summarised descriptively for patients in the per-protocol population treating the endpoint as a binary variable, based on available follow-up data. The point estimate and the one-sided 95% exact binomial lower bound are presented. Time-to-event analyses were also performed for these secondary effectiveness endpoints. Kaplan–Meier curves and survival estimates were provided using data on all patients in the per-protocol population. Patients who did not have effectiveness failures but were followed up for durations shorter than the endpoint-specified evaluation periods were censored at their last observations.
Analysis of AFEQT overall and subscale scores were conducted in the per-protocol population. Fisher’s exact test or the Kruskal–Wallis test was used to compare differences in procedural data, primary adverse event rate, and treatment success between patients treated with conscious sedation compared with general anaesthesia. Reproducibility of the 6-month and 12-month freedoms from AF/AT/AFL across study sites was analysed using Fisher’s exact test. All statistical analyses were performed in SAS Studio 3.4 or SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study population
The disposition of patients is shown in Supplementary material online, Figure S1. Of the 98 (8 roll-in, 90 evaluable) patients enrolled in the main study, 87 patients met the inclusion criteria, underwent the ablation procedure, and were included in the safety population; among these, 85 were included in the per-protocol populations after the exclusion of two patients post-procedure who did not meet the inclusion criteria. Baseline characteristics and medical history of the patients in the safety population are shown in Table 1. Mean age was 60.2 years, mean CHA2DS2-VASc score was 1.4 ± 1.2, and mean left atrial diameter was 38.5 ± 5.3 mm.
Table 1.
Baseline characteristics and medical history (safety population, N = 87)
| Safety population (N = 87) | |
|---|---|
| Age (years), mean (SD) | 60.2 (10.05) |
| Men, n (%) | 56 (64.4) |
| Time since AF diagnosis (months), mean (SD) | 48.9 (51.26) |
| CHA2DS2-VASc score, mean (SD) | 1.4 (1.23) |
| Left atrial diameter (mm), mean (SD) | 38.5 (5.32) |
| Left ventricle ejection fraction (%), mean (SD) | 61.1 (4.84) |
| Pharmacological cardioversion in the past 12 months, n (%) | 8 (9.2) |
| Direct current cardioversion in the past 12 months, n (%) | 4 (4.6) |
| Number of AADs failed, mean (SD) | 1.3 (0.55) |
| Class I/III AADs, n (%) | 50 (57.5) |
| Comorbidities, n (%) | |
| Coronary artery disease | 3 (3.4) |
| Congestive heart failure, NYHA Class I | 1 (1.1) |
| Hypertension | 42 (48.3) |
| Type II diabetes mellitus | 8 (9.2) |
| Thromboembolic events | 4 (4.6) |
| Atrial flutter | 7 (8.0) |
| Obstructive sleep apnoea | 1 (1.1) |
AAD, antiarrhythmic drug; AF, atrial fibrillation; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischaemic attack/thromboembolism, vascular disease, age 65–74 years, sex category; NYHA, New York Heart Association; SD, standard deviation.
Procedural characteristics
The mean total procedure time was 87.6 min in the per-protocol population, with a mean balloon dwell time of 40.3 min and a mean total procedural fluoroscopy duration of 10.9 min (Table 2). Procedures were performed under general anaesthesia in 54.0% (46/85) of the cases. Procedure and fluoroscopy times were significantly shorter for patients who received general anaesthesia compared with conscious sedation (Table 3). Identification of isolation was performed with the diagnostic catheter during RF application or immediately after the full RF application. Real-time electrogram analysis during ablation was possible in 79.0% (357/452) of PVs. Nearly all (98.8%) patients and 99.7% of PVs were ablated with only the RFB catheter. One patient required ablation with a focal RF catheter to isolate the right inferior PV. Oesophageal temperature rise occurred in 31.8% (27/85) of patients.
Table 2.
General ablation procedural characteristics (per-protocol population, N = 85)
| Per-protocol population (N = 85) | |
|---|---|
| Total procedure time (min), mean (SD) | 87.6 (22.3) |
| Balloon dwell time (min), mean (SD) | 40.3 (16.7) |
| Total duration of RF applications (min), mean (SD) | 6.1 (2.4) |
| Total fluoroscopy duration (min), mean (SD) | 10.9 (9.1) |
| Fluoroscopy time during balloon phase (min), mean (SD) | 7.4 (7.2) |
| Fluid delivered via study catheter (mL), mean (SD) | 993.4 (409.9) |
| Total mapping time (min), mean (SD) | 6.8 (4.7) |
| General anaesthesia, n/N (%) | 46/85 (54.0) |
| Interpretable EGM (%) | 79.0 |
| LIPV (%) | 48.1 |
| LSPV (%) | 86.1 |
| RIPV (%) | 90.3 |
| RSPV (%) | 70.0 |
| RMPV (%) | 100 |
| LPV (common, %) | 76.4 |
| Single-shot isolation (%) | 73.9 |
EGM, electrogram; LIPV, left inferior pulmonary vein; LPV, left pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RF, radiofrequency; RIPV, right inferior pulmonary vein; RMPV, right middle pulmonary vein; RSPV, right superior pulmonary vein; SD, standard deviation.
Table 3.
Procedural efficiency data, safety, and treatment outcome in patients receiving RF ablation under general anaesthesia vs. conscious sedation (per-protocol population, N = 85)
| General anaesthesia (N = 46) | Conscious sedation (N = 39) | P-value | |
|---|---|---|---|
| Mapping time (min), mean (SD) | 7.9 (5.6) | 5.5 (3.0) | 0.06 |
| Balloon dwell time (min), mean (SD) | 36.8 (12.9) | 44.5 (19.7) | 0.06 |
| Fluoroscopy time (min), mean (SD) | 5.9 (4.8) | 16.7 (9.6) | <0.001 |
| Procedure time (min), mean (SD) | 81.8 (19.4) | 94.4 (23.7) | 0.008 |
| Acute effectiveness, n/N (%) | 45/45 (100) | 37/37 (100) | NA |
| Primary adverse event, n/N (%) | 0/39 | 1/45 (2.2) | 1.00 |
| 6-month freedom from symptomatic AF/AT/AFL recurrences, n/N (%) | 38/45 (84.4) | 30/39 (76.9) | 0.42 |
| 12-month freedom from symptomatic AF/AT/AFL recurrences, n/N (%) | 31/41 (75.6) | 26/38 (68.4) | 0.62 |
AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; RF, radiofrequency; SD, standard deviation.
Primary effectiveness and safety endpoints
One (1.2%) patient experienced a PAE, with a one-sided 95% upper confidence bound of 5.5%; therefore, the performance goal of 15% was met. This PAE was a vascular access complication (retroperitoneal bleed) during introduction of transseptal needle through the sheath, which was treated conservatively, and the procedure was completed. No deaths, atrio-oesophageal fistula, myocardial infarction, stroke/cerebrovascular accident, transient ischaemic attack, thromboembolism, PV stenosis, permanent phrenic nerve paralysis, or cardiac tamponade/perforation were encountered (Supplementary material online, Table S1). There were no serious device adverse events. One mild transient phrenic nerve palsy was observed and resolved prior to discharge.
The primary effectiveness endpoint of sustained PV entrance block was achieved for all (100%) patients; the one-sided 95% lower confidence bound was 96.4%, above the performance goal of 80%.
Subject- and vein-level PVI procedural data secondary effectiveness endpoints are summarised in Table 4. Acute reconnection of ≥ PVs after adenosine/isoproterenol challenge was observed in 22 out of 82 (26.8%) patients, accounting for 30 out of 324 (9.3%) PVs ablated. After the blanking period, four out of 85 (4.7%) patients underwent repeat ablation. One patient was ablated for typical AFL (so the PVs were not remapped), while three were treated due to AF secondary to reconnection affecting ≥1 PV: left superior PV (n = 1), right superior PV (n = 1), right inferior PV (n = 1), left inferior PV (n = 1), and common left PV (n = 1).
Table 4.
Subject- and vein-level PVI procedural data
| Acute reconnection | n/N (%) | ||
|---|---|---|---|
| Patients | 22/82 (26.8) | ||
| PVs, overall | 30/324 (9.3) | ||
| LIPV | 11/75 (14.7) | ||
| LSPV | 6/75 (8.0) | ||
| RIPV | 6/83 (7.2) | ||
| RSPV | 5/82 (6.1) | ||
| RMPV | 0/1 (0) | ||
| LPV (common) | 2/8 (25.0) | ||
|
| |||
| Focal catheter ablation for PV isolation | Ablated by balloon catheter only, n/N (%) | Ablated by balloon catheter and focal catheter, n/N (%) | |
|
| |||
| Patients | 84/85 (98.8) | 1/85 (1.2) | |
| PVs, overall | 326/327 (99.7) | 1/327 (0.3) | |
| LIPV | 75/75 (100) | 0/75 | |
| LSPV | 76/76 (100) | 0/76 | |
| RIPV | 82/83 (98.8) | 1/83 (1.2) | |
| RSPV | 83/83 (100) | 0/83 | |
| RMPV | 1/1 (100) | 0/1 | |
| LPV (common) | 9/9 (100) | 0/9 | |
|
| |||
| Time to PV isolation by real-time EGMa | Mean (SD) (s) | ||
|
| |||
| PVs, overall | 10.9 (9.1) | ||
| LIPV | 10.9 (9.8) | ||
| LSPV | 12.6 (11.8) | ||
| RIPV | 9.1 (4.8) | ||
| RSPV | 10.4 (8.4) | ||
| RMPV | 6.0 (NA) | ||
| LPV (common) | 18.0 (17.3) | ||
|
| |||
| Number of RF applicationsb | Mean (SD) | ||
|
| |||
| LIPV | 2.0 (1.5) | ||
| LSPV | 2.1 (1.8) | ||
| RIPV | 1.7 (1.0) | ||
| RSPV | 1.8 (1.5) | ||
| RMPV | 1.0 (NA) | ||
| LPV (common) | 3.4 (1.3) | ||
LIPV, left inferior pulmonary vein; LPV, left pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RF, radiofrequency; RIPV, right inferior pulmonary vein; RMPV, right middle pulmonary vein; RSPV, right superior pulmonary vein; SD, standard deviation; NA, not applicable.
LIPV, left inferior pulmonary vein; LPV, left pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RIPV, right inferior pulmonary vein; RMPV, right middle pulmonary vein; RSPV, right superior pulmonary vein.
LIPV, n = 74; LSPV, n = 75; RIPV, n = 80; RSPV, n = 80; RMPV, n = 1; LPV (common), n = 7.
Subclinical lesion on MRI and neurological evaluation
Post-procedure, three of the 31 (9.7%) patients who were assessed had a new SCL detected. One of these occurred in a patient who did not meet the study inclusion criteria (age >75 years). All SCLs were resolved subsequently (i.e. two patients at 1 month and one patient at 10 months—the first repeat MRI obtained).
NIHSS scores are summarised in Supplementary material online, Table S2. There were no cases of clinical stroke; one patient developed limb ataxia, adjudicated as related to stress and fatigue by both the on-site and clinical event committee neurologists.
Six- and 12-month effectiveness outcomes and reproducibility
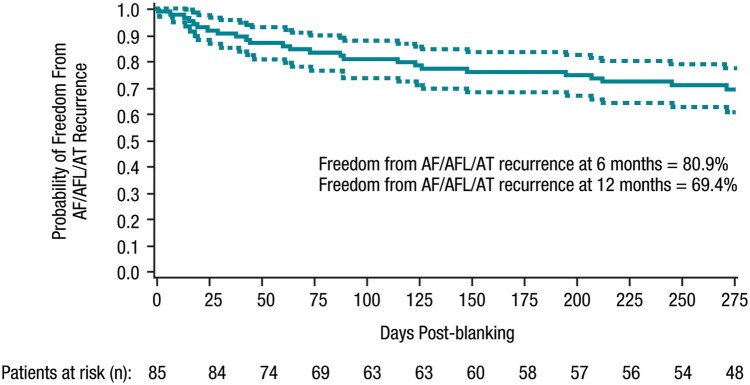
The success rate, defined as freedom from documented symptomatic AF/AT/AFL recurrences, was 81.0% (68/84) at 6 months and 72.2% (57/79) at 12 months, and 65.8% (52/79) for all (symptomatic or asymptomatic) documented atrial arrhythmia recurrence at 12 months (binomial analyses). The outcomes were similar in patients who received ablation under general anaesthesia or conscious sedation (Table 3). The Kaplan–Meier estimate of probability of freedom from documented symptomatic atrial arrhythmia recurrence was 80.9% at 6 months (one-sided 95% lower confidence bound of 73.8%) and 69.4% at 12 months (one-sided 95% lower confidence bound of 60.9%; Figure 3). At 12 months, 38.0% (30/79) of patients were in use of Class I/III AADs, corresponding to a 32.8% decrease in AAD use compared to baseline.
Figure 3.
Kaplan–Meier analysis of 6- and 12-month effectiveness endpoint, time to first documented symptomatic effectiveness failure (per-protocol population, N = 85). AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia.
Recurrence rates were reproducible at 6 and 12 months across study centres (Supplementary material online, Figure S2A and B). No statistical difference was detected between sites for 6- or 12-month recurrence (P = 0.210 and 0.779, respectively).
Quality of life
Patients in the per-protocol population showed substantial improvement in quality of life scores in all subscale categories at 12 months after ablation (Figure 4). Specifically, patients scored an average of 27.1 points higher on the overall AFEQT score at 12 months post-ablation than they did pre-ablation. Gains ranging from 18.5 to 33.0 points, on average, were realised among individual subscores. Mean AFEQT improvements exceeded the universally accepted clinically important differences (+5 points) from 6 to 12 months.
Figure 4.
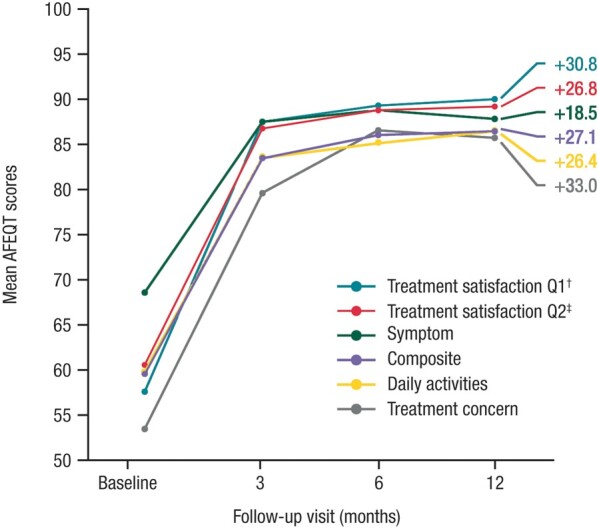
AFEQT scores at study baseline and at 3, 6, and 12 months post-ablation (per-protocol population, N = 85). AFEQT, Atrial Fibrillation Effect on Quality-of-life questionnaire. †Satisfaction level—‘How well current treatment controls your atrial fibrillation’. ‡Satisfaction level—‘The extent to which treatment has relieved your symptoms of atrial fibrillation’.
Discussion
The SHINE study demonstrated the safe and effective use of the multi-electrode circular diagnostic and RFB catheter when used for electrophysiological mapping and RF ablation during treatment of PAF through PVI. The findings from the RADIANCE study were expanded in SHINE to a wider patient cohort treated by more early users of the RFB catheter, with clinical outcome at 1-year follow-up.
Importantly, 99.7% of the ablated veins (all except for one vein) could be isolated with a single RFB catheter, without the need for touch-up with a focal catheter. With the circular diagnostic catheter, real-time electrogram analysis was possible in nearly 80% of cases. The most common reason for failure of real-time monitoring was noise on the circular diagnostic catheter because of its proximity to one or more active RF electrodes on the RF balloon. The acute reconnection rate after adenosine challenge was 9% of PVs in the current study. All reconnections were targeted by further ablation and persistent isolation after pharmacological challenge was demonstrated in all patients. With a rather stringent arrhythmia monitoring method, 72.2% of patients in the SHINE study were free of documented symptomatic left atrial arrhythmia at 12 months after ablation, and 65.8% including asymptomatic recurrence. These results are comparable with those obtained in early experience of point-by-point RF (contact force or non-contact force) ablation or cryoablation studies.6–8 The long-term success is likely to improve over time with more experience and wider adoption, as has been observed with focal RF and cryoablations.9–11 More recently, pulsed field ablation technology has become available. Early results of PAF ablation with 81 patients showed a 12-month freedom from atrial arrhythmia recurrence of over 80%; however, the majority of the patients received a protocol-mandated remap and reablation (if reconnection occurred) at 75 days which may have helped optimised long-term effectiveness.12
There was no significant variation on freedom from arrhythmia recurrence at 6 and 12 months among the study sites, suggesting reproducibility across sites, most likely due to the simplistic and standardised nature of the ablation workflow with the RFB catheter. This is supported by the short procedure times, balloon dwell times, and fluoroscopy times observed in our study. In general, balloon ablation systems appear to have shorter learning curves and greater reproducibility compared with point-by-point RF ablation.13 PVI with the RFB catheter yielded comparable safety and 1-year treatment outcomes under general anaesthesia and conscious sedation, with shorter procedure and fluoroscopy times under general anaesthesia. The ability to consistently achieve PVI in a short, predictable time with a single catheter may have significant economic impact on the healthcare system in terms of cost saving. This will need to be examined in future studies.
Only one patient in the SHINE study experienced a PAE (a retroperitoneal bleed that was treated conservatively). There were no incidences of sustained phrenic nerve palsy, as the ability to pace from the individual electrodes on the RFB offers another point of confirmation of proximity to the phrenic nerve to avoid phrenic nerve injury, although this was not routinely done during the study. Also, the customisable energy delivery across different electrodes allows minimisation of ablation on the posterior wall; with the reduction in maximum RF duration from 30 to 20 s compared to the RADIANCE study,4 SHINE showed a reduction in the incidence of oesophageal temperature rise as well. SCL is a known complication after invasive cardiac procedures, although its relevance to clinical stroke and long-term neurological impact are unclear at this time.14 SCL rates of up to 40% have been reported following AF ablation, although detection rates vary depending on ablation method, procedural workflow, and lesion detection method.15–17 Diffusion-weighted MRI, as used in the SHINE study, provides a method for the detection of small, clinically silent embolic lesions with high sensitivity.18 A low incidence of SCLs was observed in the current study, with 3 (9.7%) patients showing asymptomatic lesions following ablation, which had all resolved at a follow-up MRI. In the previously published RADIANCE study, 23% of patients showed new SCLs on their pre-discharge MRI, all of which resolved at follow-up.4,19 These results suggest that modifications to the ablation workflow between the RADIANCE and SHINE studies may have contributed to a lower incidence of SCL.
AF has a detrimental effect on many aspects of quality of life,20 and the primary goal of AF ablation is to improve quality of life through the elimination of arrhythmia-related symptoms, such as palpitations, fatigue, or effort intolerance.1 Traditional clinical study outcomes such as freedom from atrial arrhythmia recurrence may not necessarily correlate with patients’ perception of quality of life recovery. For example, in the SMART-AF study, patients reported improvement in physical and mental components of quality of life following RF ablation, irrespective of effectiveness outcomes.7 Thus, formal assessment of quality of life has an increasingly important role in the evaluation of novel ablation procedures.1 In the current study, quality of life as evaluated through the AFEQT questionnaire showed significant benefits in patients’ health-related quality of life and satisfaction with AF control and symptoms as a consequence of the ablation treatment. More patients in this study had AF recurrence documented than those who required repeat AF ablation, probably because their AF recurrences were infrequent and/or did not represent a significant enough symptomatic burden to justify further treatment. An improvement of approximately 20 points in total AFEQT score, as observed in this study, has previously been shown to correlate with improvement on the Patient Global Change assessment.19 This improvement in AFEQT score was also accompanied with a 33% reduction in the Class I/III AAD usage at 12 months compared to baseline.
Limitations
This was a single-arm, non-randomised clinical study, and all cases were performed by operators well experienced with other single-shot PVI devices. Larger studies are needed to evaluate the generalisability of these study results. All cases were performed with oesophageal temperature monitoring and a subset with mechanical oesophageal deviation, and our results may not be applicable to cases performed without. As the definition of success rates and failure modes vary among registration studies, a randomised controlled study is required to draw direct comparisons of the clinical efficacy with other AF ablation technologies.
Conclusion
In this multicentre study, PVI with the novel RFB catheter demonstrated favourable safety and clinical effectiveness at a 12-month follow-up, with a low repeat ablation rate and clinically meaningful improvement in quality of life.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors wish to thank the following individuals for their contribution to study conduct, statistical analysis, and editorial assistance: Nathalie Macours, Liesbeth, Nader Ghaly, Xiangming Zhang, Kendra McInnis, Bharat Kumar, Christina Kaneko, Lee Ming Boo, and MedErgy HealthGroup.
Funding
This work was supported by the Biosense Webster, Inc.
Conflict of interest: R.S. has received personal fees and grants from Biosense Webster, Inc., Abbott, Medtronic, Boehringer Ingelheim, and Daiichi Sankyo; has received non-financial support from Apple; and has served as a consultant for Biosense Webster, Inc. C.T. serves on the advisory boards of Medtronic and Boston Scientific and has received lecture fees from Abbott Medical, Boston Scientific, and Acutus Medical. M.G. has an unrelated patent agreement with Johnson & Johnson. P.N. has received grant support from Biosense Webster, Inc. G.-B.C. has received speaker fees from Biosense Webster, Inc; and has received speaker and teaching fees from Medtronic. A.A., T.T., and L.V. are salaried employees of Biosense Webster, Inc. D.G. has received speaker fees and grants from Biosense Webster, Inc. V.Y.R. is an unpaid consultant to Biosense Webster, Inc; his other disclosures, unrelated to this manuscript, are noted in the Supplement; G.S.D., S.R., F.Q, C.d.A., and W.Y.D. have declared no conflicts of interest.
Data availability
Johnson & Johnson Medical Devices Companies have an agreement with the Yale Open Data Access (YODA) Project to serve as the independent review panel for evaluation of requests for clinical study reports and patient-level data from investigators and physicians for scientific research that will advance medical knowledge and public health. Requests for access to the study data can be submitted through the YODA Project site at http://yoda.yale.edu.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. et al. ; Document Reviewers. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Metzner A, Wissner E, Lin T, Ouyang F, Kuck KH.. Balloon devices for atrial fibrillation therapy. Arrhythm Electrophysiol Rev 2015;4:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dhillon GS, Honarbakhsh S, Di Monaco A, Coling AE, Lenka K, Pizzamiglio F. et al. Use of a multi-electrode radiofrequency balloon catheter to achieve pulmonary vein isolation in patients with paroxysmal atrial fibrillation: 12-month outcomes of the RADIANCE study. J Cardiovasc Electrophysiol 2020;31:1259–69. [DOI] [PubMed] [Google Scholar]
- 4. R Reddy VY, Schilling R, Grimaldi M, Horton R, Natale A, Riva S. et al. Pulmonary vein isolation with a novel multielectrode radiofrequency balloon catheter that allows directionally tailored energy delivery: short-term outcomes from a multicenter first-in-human study (RADIANCE). Circ Arrhythm Electrophysiol 2019;12:e007541. [DOI] [PubMed] [Google Scholar]
- 5. Holmes DN, Piccini JP, Allen LA, Fonarow GC, Gersh BJ, Kowey PR. et al. Defining clinically important difference in the atrial fibrillation effect on quality-of-life score. Circ Cardiovasc Qual Outcomes 2019;12:e005358. [DOI] [PubMed] [Google Scholar]
- 6. Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG. et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713–23. [DOI] [PubMed] [Google Scholar]
- 7. Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT. et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014;64:647–56. [DOI] [PubMed] [Google Scholar]
- 8. Reddy VY, Dukkipati SR, Neuzil P, Natale A, Albenque JP, Kautzner J. et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) study. Circulation 2015;132:907–15. [DOI] [PubMed] [Google Scholar]
- 9. Duytschaever M, De Pooter J, Demolder A, El Haddad M, Phlips T, Strisciuglio T. et al. Long-term impact of catheter ablation on arrhythmia burden in low-risk patients with paroxysmal atrial fibrillation: the CLOSE to CURE study. Heart Rhythm 2020;17:535–43. [DOI] [PubMed] [Google Scholar]
- 10. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L. et al. ; For the CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 11. Duytschaever M, Vijgen J, De Potter T, Scherr D, Van Herendael H, Knecht S. et al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: the multicentre VISTAX trial. Europace 2020;22:1645–52. [DOI] [PubMed] [Google Scholar]
- 12. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H. et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 13. Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, Halimi F. et al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace 2017;19:48–57. [DOI] [PubMed] [Google Scholar]
- 14. Büsing KA, Schulte-Sasse C, Flüchter S, Suselbeck T, Haase KK, Neff W. et al. Cerebral infarction: incidence and risk factors after diagnostic and interventional cardiac catheterization—prospective evaluation at diffusion-weighted MR imaging. Radiology 2005;235:177–83. [DOI] [PubMed] [Google Scholar]
- 15. Deneke T, Nentwich K, Krug J, Müller P, Grewe PH, Mügge A. et al. Silent cerebral events after atrial fibrillation ablation—overview and current data. J Atr Fibrillation 2014;6:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyazaki S, Kajiyama T, Yamao K, Hada M, Yamaguchi M, Nakamura H. et al. Silent cerebral events/lesions after second-generation cryoballoon ablation: how can we reduce the risk of silent strokes? Heart Rhythm 2019;16:41–8. [DOI] [PubMed] [Google Scholar]
- 17. Nakamura K, Sasaki T, Take Y, Okazaki Y, Inoue M, Motoda H. et al. Postablation cerebral embolisms in balloon-based atrial fibrillation ablation with periprocedural direct oral anticoagulants: a comparison between cryoballoon and HotBalloon ablation. J Cardiovasc Electrophysiol 2019;30:39–46. [DOI] [PubMed] [Google Scholar]
- 18. Lickfett L, Hackenbroch M, Lewalter T, Selbach S, Schwab JO, Yang A. et al. Cerebral diffusion-weighted magnetic resonance imaging: a tool to monitor the thrombogenicity of left atrial catheter ablation. J Cardiovasc Electrophysiol 2006;17:1–7. [DOI] [PubMed] [Google Scholar]
- 19. Dorian P, Burk C, Mullin CM, Bubien R, Godejohn D, Reynolds MR. et al. Interpreting changes in quality of life in atrial fibrillation: how much change is meaningful? Am Heart J 2013;166:381–7.e8. [DOI] [PubMed] [Google Scholar]
- 20. Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM. et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Johnson & Johnson Medical Devices Companies have an agreement with the Yale Open Data Access (YODA) Project to serve as the independent review panel for evaluation of requests for clinical study reports and patient-level data from investigators and physicians for scientific research that will advance medical knowledge and public health. Requests for access to the study data can be submitted through the YODA Project site at http://yoda.yale.edu.