Highlights
-
•
Language and memory interact as a composite function based on the LMN network.
-
•
LMN configuration is state- and condition-dependent (healthy–pathological).
-
•
LMN reconfiguration is reflected in network segregation and integration changes.
-
•
LMN reconfiguration in HC depends on flexibility of key language and memory regions.
-
•
LMN reconfiguration in TLE shows increased segregation and closer modules.
Keywords: Language, Memory, Connectivity, Temporal lobe epilepsy, Extrinsic, Intrinsic
Abstract
Current theoretical frameworks suggest that human behaviors are based on strong and complex interactions between cognitive processes such as those underlying language and memory functions in normal and neurological populations. We were interested in assessing the dynamic cerebral substrate of such interaction between language and declarative memory, as the composite function, in healthy controls (HC, N = 19) and patients with temporal lobe epilepsy (TLE, N = 16). Our assumption was that the language and declarative memory integration is based on a language-and-memory network (LMN) that is dynamic and reconfigures according to task demands and brain status. Therefore, we explored two types of LMN dynamics, a state reconfiguration (intrinsic resting-state compared to extrinsic state assessed with a sentence recall task) and a reorganization of state reconfiguration (TLE compared to HC). The dynamics was evaluated in terms of segregation (community or module detection) and integration (connector hubs). In HC, the level of segregation was the same in both states and the mechanism of LMN state reconfiguration was shown through module change of key language and declarative memory regions with integrative roles. In TLE patients, the reorganization of LMN state reconfiguration was reflected in segregation increase and extrinsic modules that were based on shorter-distance connections. While lateral and mesial temporal regions enabled state reconfiguration in HC, these regions showed reduced flexibility in TLE. We discuss our results in a connectomic perspective and propose a dynamic model of language and declarative memory functioning. We claim that complex and interactive cognitive functions, such as language and declarative memory, should be investigated dynamically, considering the interaction between cognitive networks.
1. Introduction
Recent neurocognitive frameworks suggest that human behaviors are enabled by complex interactions between cognitive functions, instead of isolated involvement of each of them (Kellermann et al., 2016, Roger et al., 2020b, Van Der Maas et al., 2006). The evidence of integration can be found in everyday life such as recalling an old episode (Larsen et al., 2002, Park et al., 2011) or maintaining a conversation by relying on what is called the common ground (Clark & Marshall, 1981). When formulating an utterance during a conversation, interlocutors are relying on background information that they believe is shared by the participants in the conversation (Stalnaker, 2002). Hence, language must rely on the declarative memory and previous studies described a wide language-and-memory network (LMN) (Banjac et al., 2020, Roger et al., 2020a, Roger et al., 2020b). This perspective is in line with the current transition towards new integrative and dynamic neurocognitive and connectomic models (Dick et al., 2014, Garcia-Ramos et al., 2016, Herbet and Duffau, 2020, Kellermann et al., 2016, Zamora-Lopez et al., 2011, Zhou et al., 2020). The connectomic perspective assumes that complex cognitive systems are shaped by the interactions between processes (Meunier et al., 2010) and functional integration and specialization are supported by the modular architecture (Bertolero et al., 2015, Fornito et al., 2016, Meunier et al., 2010, Park and Friston, 2013, Zamora-Lopez et al., 2011). Modularity enables the adaptation of a global network to environmental changes (Finc et al., 2017, Meunier et al., 2010) shaping it into local modules or specialized communities, composed of densely intra-connected regions (nodes) that share common functions (segregation property). These modules are sparsely connected with other communities via inter-module connections that provide the integration property (Fornito et al., 2016, Guimerà and Amaral, 2005, Rubinov and Sporns, 2010). Nodes that are highly interconnected within their communities, but not so strongly to other communities are called provincial hubs and they support segregation (Bertolero et al., 2015, Meunier et al., 2009, Schedlbauer and Ekstrom, 2019). The integration between modules is based on the connector nodes that are highly connected with other communities and can be divided into satellites and connector hubs depending on their status within their own community (Bertolero et al., 2015, Bullmore and Sporns, 2012, Fornito et al., 2016, Guimerà and Amaral, 2005, Meunier et al., 2010). The difference is that connector hubs are, unlike satellites, also highly interconnected within their communities (Bertolero et al., 2015, Meunier et al., 2009, Schedlbauer and Ekstrom, 2019).
To understand composite functions such as the language and declarative memory and its cerebral substrate, the language-and-declarative memory network (LMN), it is mandatory to focus on the communication or the dynamics between the interactive functions, within a connectomic perspective. Traditionally, the connectomic approach is based on examining resting-state correlations between spontaneous BOLD signals of regions which are functionally or anatomically connected (Bullmore & Sporns, 2012). This approach reveals large hierarchical and distributed brain networks related to various functional domains (Power et al., 2011, Yeo et al., 2011), reflecting “intrinsic” activity intervening in the absence of any stimulation or task (Bolt et al., 2017, Fox and Raichle, 2007). Although these resting-state networks (RSN) are robust (De Luca et al., 2006, Yeo et al., 2011) and were found to be associated with behavior (e.g. Arnemann et al., 2015, van den Heuvel et al., 2009), it is difficult to make comprehensive conclusions on network architecture and connectivity, without considering the brain activity during task (extrinsic brain activity). Studies exploring connectomic features of extrinsic or task-related networks have indeed found that they differ from intrinsic resting-state networks (Bolt et al., 2017, Cohen and D’Esposito, 2016, Keerativittayayut et al., 2018, Mennes et al., 2013, Spadone et al., 2015) although others found significant similarities between them (Cole et al., 2014, Krienen et al., 2014). Therefore, intrinsic brain architecture does not provide a complete repertoire of extrinsic functional properties, such as flexible reconfiguration when facing changing environment and task demands (Mennes et al., 2013). The differences between intrinsic and extrinsic networks were reported for the cognitive control (Mennes et al., 2013, Tomasi et al., 2014), working memory (Rzucidlo et al., 2013, Stanley et al., 2015) and semantic memory (DeSalvo et al., 2014). Modern connectomic approaches allow to assess this state-dependent reconfiguration of brain architecture (Cole et al., 2014, Fornito et al., 2016, Sporns and Betzel, 2016) for specific cognitive functions and tasks (e.g. He et al., 2018, Hearne et al., 2017, Schedlbauer and Ekstrom, 2019). Task-induced changes of network modularity can predict behavioral outcomes (Finc et al., 2017). Indeed, decreased modularity was observed for high cognitive demands (Finc et al., 2017, Hearne et al., 2017) and successful memory retrieval is associated with reconfiguration of modular structure (Schedlbauer and Ekstrom, 2019, Westphal et al., 2017). Overall, results suggest significant flexible reconfiguration of large-scale functional networks along rest and task-activity states for different cognitive functions (Bassett et al., 2011, Hearne et al., 2017, Yue et al., 2017). However, the majority of studies explored cognitive functions separately, without investigating possible interactions between functions, such as a composite language and declarative memory function. A complete description of this composite function and underlying LMN should thus be based on both extrinsic and intrinsic activities to capture flexibility and the dynamic architecture that underlies its complex links.
Moreover, a more comprehensive understanding of functional interaction based on a specific LMN network can be provided by studying conditions showing the reorganization of language and memory functions, such as temporal lobe epilepsy (TLE) (Tracy & Boswell, 2008). This neurological condition is characterized by seizures induced by an epileptogenic network centered on medial temporal structures (Barr, 2015), associated or not with hippocampal atrophy (Thom and Bertram, 2012). The cognitive deficits of TLE patients suggest dynamic relationship between language and declarative memory (Alessio et al., 2006, Bartha-Doering and Trinka, 2014, Zhao et al., 2014, Allone et al., 2017, Bell et al., 2011, Tramoni-Negre et al., 2017). This relationship also supports the necessity of evaluating this composite function within a connectomic perspective instead of language and declarative memory separately (Waites et al., 2006). Significant reorganization of LMN occurs in TLE (Liao et al., 2010, Richardson, 2012) and results from complex interactions between neurophysiological activity (epileptic activity) and neuroplasticity (Dinkelacker et al., 2016). By neurocognitive plasticity we refer to reorganization of neurosynaptic maps that is related to efficient cognitive functioning (Duffau, 2006).
Resting-state studies in TLE patients showed reduced functional connectivity (Bettus et al., 2009) within “high-level” RSN such as default mode network (DMN), dorsal attention network (DAN) and salience network (SAL) (Burianová et al., 2017, Liao et al., 2010, Zhang et al., 2009b), as well as within “low-level” RSN such as auditory and sensorimotor networks (Zhang et al., 2009a), and within language network (Waites et al., 2006). In addition, TLE patients showed reduced synchronization between multimodal “high-level” RSN (Burianová et al., 2017), as well as between “high-level” and “low-level” RSN (sensorimotor, Yang et al., 2018). Increased connectivity within medial temporal lobes together with decreased connectivity between them and distal networks (Englot et al., 2016, Haneef et al., 2014, Liao et al., 2010, Roger et al., 2020a) were described in these patients and identified as dynamic diaschisis, a reorganization pattern based on hyper- and hypo-connected remotely-located regions (Cataldi et al., 2013, Roger et al., 2020a). Using resting-state Liao et al., (2010) found that global topological measures of TLE functional networks are disrupted showing reduced clustering.
Similarly, studies focusing on extrinsic activity found global reduction in connectivity within language network (Pravatà et al., 2011, Vlooswijk et al., 2010) and recruitment of additional networks located more posteriorly, due to anterior seizure activity (Protzner & McAndrews, 2011). Recently, He et al. (2018) reported that left temporal and right frontal regions in TLE patients showed reduced flexibility and ability to dynamically adapt to demands of a verb generation task. These regions also showed reduced communication with a core left frontal subnetwork. Overall, these authors suggested that the effect of pathology on network dynamics is more likely to manifest during language operations than during resting-state (He et al., 2018).
Given that the majority of studies in healthy individuals and patients explored cerebral networks based on either intrinsic or extrinsic activity, it is difficult to understand how are brain networks dynamically reconfigured between resting-state and task-based activity. Additionally, the majority of studies did not address directly the question of interaction between language and declarative memory, as mentioned above. This fMRI study is set to bridge this gap by evaluating both intrinsic and extrinsic LMN functional connectivity (FC) in healthy controls (HC) and TLE patients and describe the network properties by using a graph theory approach (Fornito, 2016, Rubinov and Sporns, 2010). This allows to understand the mechanisms supporting the language and declarative memory interaction on the basis of LMN dynamic reconfiguration according to brain activity state (intrinsic, extrinsic) and physiological (health, epilepsy) condition. The intrinsic connectivity was assessed with a resting-state protocol, while the extrinsic task-based was assessed with a language-and-memory fMRI protocol that recruits LMN (Banjac et al., 2020). We first explored segregation property by testing how LMN separates into modules for each state (LMN configuration) using a data-driven community detection algorithm (Blondel et al., 2008, Rubinov and Sporns, 2010, Schedlbauer and Ekstrom, 2019) and then analyzing the state reconfiguration (i.e. how the configuration changes between intrinsic and extrinsic state) in healthy participants. Then we explored the integration of LMN modules based on connector hubs first within each configuration and then comparing them in order to evaluate state reconfiguration. The reorganization of LMN configurations and state reconfiguration in terms of segregation and integration was tested using the same approach in TLE patients and comparing the results between the groups. We evaluated cognitive efficiency of reorganization in TLE patients, as an indication of neuroplasticity, by associating cognitive scores with several segregation and integration parameters. We finally attempted to describe a neurocognitive integrative model of language and declarative memory interaction that is supported by a flexible LMN. Based on the previous research, we expected that brain modular structure in HC shows less segregation during the task, due to a more complex cerebral activity required by the task. In addition, regions showing more flexibility between states should be the ones that allow for functional integration. Furthermore, TLE patients should show alterations of the modular structure due to disruption of functional connectivity and reduced flexibility of temporal regions. The potential difference between HC and TLE in “connector” regions should reflect compensatory mechanisms used by patients.
2. Material and methods
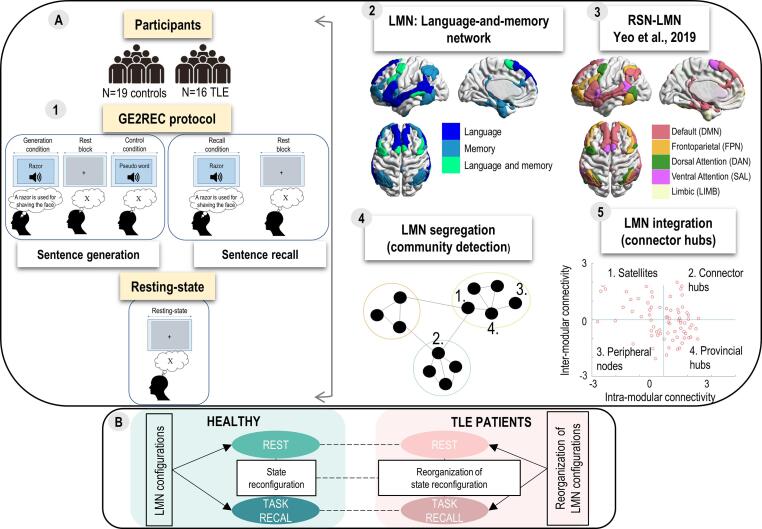
A schematic illustration of the study design is presented in Fig. 1.
Fig. 1.
Schematic representation of study pipeline. Panel A: Schematic representation of methodology. Subpanel 1. Two groups of participants, healthy participants (N = 19) and TLE patients (N = 16), performed resting-state and sentence recall task. Subpanel 2. We focused on the language-and-memory ROIs defined in previous work (Roger et al., 2019). Subpanel 3. ROIs were separated based on the resting-state network (RSN) they belonged to. Subpanel 4. We performed community detection in two groups and two tasks to explore LMN configurations, state reconfiguration and their reorganization. Subpanel 5. We determined the role (connector hub, provincial hub, satellite or peripheral node) of each LMN region based on its connectivity within module (intra-modular connectivity) and with other modules (inter-modular connectivity). In order to explore LMN integration and its state reconfiguration we focused on the connector hubs. The roles are schematically presented on Subpanel 4 with numbers corresponding the role in Subpanel 5. Panel B: Schematic representation of main terms and analyses. Both segregation and integration of LMN were explored for each state, extrinsic and intrinsic (state LMN configuration) as well as its reconfiguration between the sates in healthy participants (green). LMN configurations and state reconfiguration in terms of segregation and integration were tested using the same approach in TLE patients (pink), as well as the differences in LMN configurations and state reconfiguration between healthy controls and TLE patients (dashed lines). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.1. Participants
Nineteen healthy volunteers (age 21.2 ± 2.97; 9 females; all self-reported right-handed) and 16 TLE patients (11 left TLE and 5 right TLE, age 33.8 ± 10.5; 9 females; 14 right-handed) were included in the study. The handedness was determined according to The Edinburgh Handedness Inventory (Oldfield, 1971). The demographic and clinical features are presented in Table 1, Table 2. Participants were native French speakers and had normal or corrected-to-normal vision. Healthy participants received financial compensation for their participation. Patients were diagnosed with drug-resistant temporal epilepsy between 2017 and 2019. The diagnoses were made by neurologists based on the recommendations of the ILAE (International League Against Epilepsy) committee report (Wieser et al., 2001) and on a synthesis of several evaluations (clinical, scalp/depth-EEG, MRI/PETscan). Patients were candidates for a curative surgery and the fMRI evaluations were performed as a part of their presurgical assessment. Patients as well as healthy participants provided written informed consent for the study that was approved by the local ethic committee (CPP: 09-CHUG-14, 04/06/2009 and 2017-A00384-49).
Table 1.
Demographic and clinical data for healthy controls.
| Demographic information | Clinical data | ||||
|---|---|---|---|---|---|
| Gender | Age | Handedness | Vol hippo R | Vol hippo L | |
| 1 | F | 19 | R | 3.38 | 3.19 |
| 2 | M | 19 | R | 3.61 | 3.42 |
| 3 | F | 19 | R | 3.45 | 3.52 |
| 4 | M | 21 | R | 3.78 | 3.53 |
| 5 | F | 18 | R | 3.56 | 3.5 |
| 6 | F | 18 | R | 3.59 | 3.8 |
| 7 | M | 20 | R | 3.8 | 3.66 |
| 8 | M | 23 | R | 3.99 | 3.93 |
| 9 | M | 23 | R | 4.46 | 3.92 |
| 10 | F | 19 | R | 3.67 | 3.47 |
| 11 | F | 18 | R | 3.84 | 3.93 |
| 12 | F | 29 | R | 3.9 | 3.79 |
| 13 | M | 21 | R | 4.82 | 4.64 |
| 14 | F | 25 | R | 3.55 | 3.63 |
| 15 | M | 19 | R | 3.64 | 3.59 |
| 16 | M | 21 | R | 4.44 | 4.44 |
| 17 | M | 23 | R | 3.74 | 3.9 |
| 18 | M | 25 | R | 4.22 | 4.28 |
| 19 | M | 23 | R | 4.54 | 4.14 |
| Mean | 8F/11 M | 21 | 19R | 3.89 | 3.80 |
Note: F – female; M – male; Age – age at the time of examination; Hand. – self-reported handedness; R – right; EZ lat. – laterality of epileptogenic zone; Vol hippo R – volume of the right hippocampus in cm3; Vol hippo L – volume of the left hippocampus in cm3.
Table 2.
Demographic, clinical and neuropsychological data for TLE patients.
| Demographic
information |
Clinical
data |
Language and memory
cognitive scores |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Hand. | EZ lat. | HA | Vol hippo R | Vol hippo L | Age onset | Epilepsy duration | AED | VCI | DO80 | SFL | PFL | AMI | IMI | DMI | |
| P1 | F | 54 | R (+90%) | Left | No | 3.41 | 3.11 | 52 | 2 | 2 | 0.00 | 0.67 | 1.39 | −0.18 | 0.33 | −0.99 | −0.28 |
| P2 | F | 37 | R (+100%) | Left | No | 3.82 | 4.08 | 35 | 2 | 2 | 0.00 | −0.30 | 0.34 | −0.30 | 1.34 | −0.47 | −0.74 |
| P3 | F | 32 | R (+90%) | Left | Yes | 3.01 | 1.81 | 29 | 3 | 2 | −1.08 | −6.43 | −0.62 | 0.24 | −1.08 | −1.56 | −1.41 |
| P4 | M | 45 | R (+20%) | Left | Yes | 3.47 | 1.97 | 40 | 5 | 5 | −0.28 | −1.30 | −0.67 | −1.28 | −0.52 | −0.08 | −0.28 |
| P5 | M | 24 | R (+100%) | Left | Yes | 4.16 | 2.98 | 17 | 7 | 3 | / | −1.30 | −0.34 | −1.65 | / | / | / |
| P6 | M | 24 | L (-80%) | Left | Yes | 2.04 | 2.35 | 16 | 9 | 3 | −0.41 | −2.20 | −2.17 | −0.97 | −2.33 | −1.88 | −2.33 |
| P7 | M | 27 | R (+80%) | Left | No | 3.99 | 4.19 | 21 | 6 | 3 | 0.00 | −0.03 | −0.34 | −0.34 | 1.34 | 0.13 | 0.00 |
| P8 | F | 43 | R (+100%) | Left | No | 3.92 | 4.10 | 12 | 31 | 3 | 0.28 | −1.30 | −1.65 | −1.44 | 0.09 | −0.08 | −1.13 |
| P9 | F | 38 | R (+100%) | Left | Yes | 3.95 | 2.58 | 10 | 29 | 3 | −1.56 | −5.30 | −0.93 | −0.32 | 0.47 | 0.41 | 0.08 |
| P10 | M | 24 | R (+40%) | Left | Yes | 5.10 | 3.56 | 20 | 4 | 4 | −0.13 | −4.30 | 1.66 | −0.17 | 0.61 | 0.47 | −0.08 |
| P11 | M | 28 | R (+100%) | Left | Yes | 3.37 | 2.39 | 1 | 27 | 4 | 0.28 | 0.70 | −0.24 | −1.03 | 0.67 | 1.08 | 1.34 |
| P12 | F | 43 | R (+100%) | Right | Yes | 2.24 | 3.71 | 3 | 40 | 6 | 0.13 | −2.30 | −1.51 | −2.02 | −1.08 | −2.33 | −2.05 |
| P13 | F | 19 | L (-100%) | Right | No | 3.48 | 3.46 | 14 | 5 | 3 | −1.28 | −0.39 | −0.37 | −0.40 | 0.67 | 0.08 | −0.61 |
| P14 | M | 38 | R (+100%) | Right | No | 3.54 | 3.82 | 8 | 30 | 2 | 0.13 | −4.30 | −1.65 | −1.75 | 0.08 | −0.08 | −0.28 |
| P15 | F | 45 | R (+100%) | Right | No | 3.39 | 3.18 | 40 | 5 | 2 | 0.13 | −0.30 | 0.34 | −0.30 | 1.18 | 1.28 | −0.15 |
| P16 | M | 20 | R (+90%) | Right | Yes | 4.72 | 4.80 | 16 | 4 | 3 | 0.00 | −3.30 | −1.73 | −1.82 | 0.33 | 1.08 | 0.33 |
| Mean | 8F/8M | 34 | 14R/2L | 11L/5R | 9 | 3.60 | 3.26 | 20.88 | 13.06 | 3.13 | −0.25 | −1.98 | −0.53 | −0.86 | 0.14 | −0.19 | −0.51 |
| Diff. | 0.29 | 29.5 | 0.37 | / | 0.78 | 31 | 14 | 37 | 21.5 | 30 | 18.5 | 32 | 37 | 42.5 | 25.5 | 19.5 | 25 |
| p | 1 | 0.82 | 1 | / | 0.593 | 0.692 | 0.126 | 0.281 | 0.495 | 0.764 | 0.42 | 0.609 | 0.281 | 0.089 | 0.951 | 0.499 | 1 |
Note: F – female; M – male; Age – age at the time of examination; Hand. – handedness evaluated with Edinburgh quotient (Oldfield, 1971) ; L – left, R – right; EZ lat. – laterality of epileptogenic zone; HA – hippocampal atrophy; Vol hippo R – volume of the right hippocampus in cm3; Vol hippo L – volume of the left hippocampus in cm3; Age onset – age of onset of seizures; AED – number of epileptic drugs taken; VCI – standardized score of verbal comprehension index for verbal semantic memory (Wechsler, 2008); DO80 - standardized score for French version of naming task (Deloche & Hannequin, 1997); SFL – semantic fluency, z core of performance on the task of categorical word generation (Godefroy et al., 2008), PFL – phonological fluency, z score of performance on the task of alphabetical word generation (Godefroy et al., 2008); AMI – standardized score of auditory memory (Wechsler, 2009); IMI - standardized score of immediate memory (Wechsler, 2009); DMI – standardized score for delayed memory (Wechsler, 2009). Diff – difference between LTLE and RTLE patients: for variables sex, handedness and HA values of χ2 are presented and for all the others values of Mann-Whitney U test are provided with corresponding p value.
2.2. Neuropsychological data in patients
All patients underwent complete neuropsychological assessment including language and memory functions carried out by a neuropsychologist and a speech therapist. This general cognitive assessment was used in further analyses of the efficiency of LMN configurations and state reconfiguration in TLE patients. Specifically, the following cognitive scores were used in the analyses: (a) language scores: verbal comprehension index (VCI; WAIS IV, Wechsler, 2008), naming (DO80; Deloche & Hannequin, 1997), semantic fluency (SFL) and phonological fluency (PFL; Godefroy, 2008); and (b) memory scores: auditory memory index (AMI), immediate memory index (IMI) and delayed memory index (DMI; WMS IV, Wechsler, 2009). Test scores were standardized by gender, age and sociocultural level. Detail data on patients’ cognitive performance is presented in Table 2.
2.3. Experimental protocol
Participants first performed the GE2REC protocol for interactive mapping of language and memory previously validated (Banjac et al., 2020) that is composed of three runs, sentence generation (GE) and two retrieval tasks (2REC), a recognition of stimuli presented in the first run and a sentence recall task. In order to access brain networks that reflect language and declarative memory interaction, we focused on the sentence recall task (abbreviated RA). During RA, participants received the auditory words previously presented GE and were asked to recall and covertly repeat the sentences that they have previously generated. The recall run was designed as a block paradigm with 5 task (8 stimuli/condition, 40 words in total) and 5 control (fixation cross displayed for 10 s) conditions. The total duration of the run was 4.17 min (for other details see Banjac et al., 2020). Following the recall run, each participant underwent a resting-state for 13.20 min to measure cerebral intrinsic activity. Participants were required to lay down into the magnet, to rest with eyes open while fixating a cross centered on the screen during the entire duration of the acquisition period.
2.4. MR acquisition
Functional MRI experiments were performed at the MR facility. MR images were acquired with a whole-body 3 T MR Philips imager (Achieva 3.0 T TX Philips, Philips Medical Systems, Best, NL) with a 32-channel head coil for all of the participants. During the recall task, the manufacturer-provided gradient-echo/T2* weighted EPI method was used. Forty-two adjacent axial slices parallel to the bicommissural plane were acquired in sequential mode (3 mm thickness, TR = 2.5 s, TE = 30 ms, flip angle = 82°, in-plane voxel size = 3 × 3 mm; field of view = 240 × 240 × 126 mm; data matrix = 80 × 80 pixels; reconstruction matrix = 80 × 80 pixels). During resting-state, four hundred cerebral rs-fMRI volumes were acquired using a gradient echo planar imaging sequence (FEEPI, 36 axial slices, 3.5 mm thickness, TR = 2.0 s, TE = 30 ms, flip angle = 75°, field of view = 192 × 192 mm, in-plane voxel size = 3 × 3 mm). In addition, a T1-weighted high-resolution three-dimensional anatomical volume (T1TFE, 128 sagittal slices, 1.37 mm thickness, field of view = 224x256 mm, in-plane voxel size = 0.89 × 0.89 mm) was acquired for each participant.
2.5. Prior data analysis and data preprocessing
2.5.1. Statistical analyses of demographic, clinical and neuropsychological characteristics
We included TLE patients with left (LTLE) and right (RTLE) origin of seizures. Since previous studies showed that these patients can differ regarding cognitive functioning and neural organization (Besson et al., 2014, de Campos et al., 2016, Phuong et al., 2021, Roger et al., 2020b), before conducting the main analyses planned in this study, we tested whether the LTLE and RTLE patients in our sample significantly differed regarding their clinical characteristics (age, epilepsy duration, number of AEDs, hippocampal atrophy and gender), hippocampal volume, neuropsychological performance. We did not flip the images of patients in the L-R direction in line with recommendations (Lee et al., 2018) since previous research found significant asymmetries in functional connectivity between two hemispheres mirrored over the longitudinal fissure (Raemaekers et al., 2018). Previous research showed that epilepsy patients show more often atypical language lateralization than healthy participants (Baciu and Perrone-Bertolotti, 2015, Berl et al., 2014). A recent study also showed that language lateralization is related to functional connectivity of language system and whole-brain organization (Wang et al., 2019). Due to this, we controlled language lateralization by only including participants with left lateralization of language activation in frontal lobe and left to bilateral activation in temporal lobe. That way we wanted to exclude the possibility that the potential differences of LMN community structure between healthy participants and TLE patients are a result of differences in language lateralization. The lateralization indices (LI) were calculated on frontal activations during GE task using the bootstrap method of the SPM LI toolbox (Wilke & Lidzba, 2007). The differences between LTLE and RTLE patient groups on mentioned characteristics were tested using the Mann-Whitney U tests and Chi-square tests.
2.5.1.1. Functional MRI preprocessing
The preprocessing was performed using SPM12 (Welcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm/) running under Matlab R2015b (Mathworks Inc., Sherborn, MA, USA) using the standard routines. All images were realigned to correct the head motion, time-corrected with the mean image as the reference slice, spatially normalized to MNI (Montreal Neurological Institute) space and then spatially smoothed with an 8 mm FWHM (Full Width at Half Maximum) Gaussian kernel. The T1-weighted anatomical volume was co-registered to the mean image created by the realignment procedure and was normalized within the MNI (Montreal Neurological Institute) space. The anatomical normalization parameters were subsequently used for the normalization of functional volumes. Motion parameters from the realignment step were then analyzed using ART (Artifact Detection Tool, Gabrieli Lab, Massachusetts Institute of Technology, available at: https://www.nitrc.org/projects/artifact_detect). We considered as outliers those volumes that had more than 3 mm interscan movement of in translation, 0.02 rad in rotation and 3 SD global interscan signal intensity relative to the session mean. Participants who had more than 15% of scans marked as outliers were excluded from the study. Healthy participants and TLE patients did not differ neither during the resting-state or sentence recall regarding the mean movement value nor number of outliers (Supplementary Material, Table S1).
2.5.1.2. Network analysis
-
•
Language-and-memory network: parcellation & node definition
ROIs of LMN explored in this study were previously defined and validated by Roger et al. (2020a) in the space of Atlas of Intrinsic Connectivity of Homotopic Areas (AICHA, Joliot et al., 2015). Despite standard left lateralization of language network, we tested the nodes across both hemispheres since language reorganization in TLE patients can be interhemispheric (Baciu & Perrone-Bertolotti, 2015) and given that language engages nondominant hemisphere in healthy subjects (Hickok and Poeppel, 2007, Vigneau et al., 2011). Additionally, we have separated the hippocampus into anterior and posterior parts since various reorganization patterns were found for the anterior and posterior hippocampal networks (Li et al., 2017). We also used specific ROIs for anterior and posterior hippocampi (left and right) for each participant given that in patients, the hippocampal atrophy may have significant effects (Roger et al., 2020a). However, in order to avoid any artificial differences between two groups of participants subject-specific hippocampal ROIs for healthy participants were also used. Subject-specific hippocampal ROIs were generated from T1w anatomical images with Vol-Brain processing pipeline (http://volbrain.upv.es). This pipeline provided subject-specific MNI registered hippocampal ROIs. The anterior and posterior parts of these subject-specific hippocampal ROIs were defined through their overlap with anterior and posterior hippocampal masks of the AICHA atlas (Joliot et al., 2015). The final LMN network comprised 74 ROIs (37 per hemisphere). Panel A, Subpanel 2 of Fig. 1 shows the LMN on a brain template and a detailed description of ROIs is provided in the Table S2.
ROIs were also classified according to their membership to a specific resting-state network. For this purpose we used the 7 resting-state networks (RSN) atlas defined by Yeo and colleagues (2011). To precisely determine which RSN network each LMN region belonged to, the LMN was overlaid with the RSN map (Yeo et al., 2011). The number of overlapping voxels with each network was calculated for each region. A ROI was determined to belong to the RSN with which it had the largest percentage of overlapping voxels. Panel A, Subpanel 3 of Fig. 1 shows LMN ROIs according to the RSN they belong to.
-
•
Connectivity analyses
The FC within the LMN was calculated based on the resting-state and the recall activity using a Graph theory (GT) analysis (Fornito, 2016, Rubinov and Sporns, 2010). The CONN toolbox (Functional Connectivity Toolbox, Gabrieli Lab, Massachusetts Institute of Technology) was used to obtain FC matrices and the Brain Connectivity Toolbox (BCT, Rubinov & Sporns, 2010, available at https://sites.google.com/site/bctnet/) and GraphVar toolbox (Kruschwitz et al., 2015, available at https://www.nitrc.org/projects/graphvar/) were used for graph theory analyses.
The CONN Toolbox (Functional Connectivity Toolbox, Gabrieli Lab, Massachusetts Institute of Technology) provides ROI-to-ROI correlation analysis according to the temporal fluctuations of BOLD signals. The first step consisted of denoising the pre-processed unsmoothed data by regressing out the BOLD signal from the white matter, the CSF as well as outliers and movements obtained by ART and SPM. For the recall task, we also entered in the regression a separate regressor for the experimental condition, according to other previous studies validating this approach (Cao et al., 2014, Cole et al., 2014, Mohr et al., 2016). The resulting residual time series were temporally filtered to remove low-frequency scanner drifts and/or high-frequency physiological noise using band-pass filtered (0.008–0.09 Hz) for the resting-state and high-pass filter (0.008 Hz) for the recall task. Resting-state data processed in this way reflected intrinsic network, while that resulting from the recall task, reflected extrinsic network. Then the Z score of r-Pearson correlation coefficient was calculated for each participant by CONN toolbox for every possible pair of residual time series (2701 pairs of 74 ROIs of the LMN). In agreement with previous studies (Bolt et al., 2017, Cohen and D’Esposito, 2016; Finc et al., 2020) especially the one using similar community detection algorithm (Schedlbauer & Ekstrom, 2019), the negative correlations were set to zero. However, for validation purposes the algorithm was also run with preserved negative correlations and the main findings remained similar (see Figure S1). The resultant 74x74 matrices were then used in the statistical analyses described hereinafter.
2.6. Data analysis
2.6.1. LMN segregation
-
•
LMN configurations and state reconfiguration
Modularity or community detection enables a data-driven partition of a network into modules showing its segregation properties (Fornito et al., 2012). Community detection algorithm was performed in healthy participants to analyze LMN state configurations. Data-driven community structure was assessed by applying modularity maximization algorithm (Louvain greedy algorithm, Blondel et al., 2008, Rubinov and Sporns, 2010) to individual correlation matrices with positive, weighted edges, for each state separately. Given that the community partition can vary with each run of the algorithm (Rubinov & Sporns, 2010), we applied a consensus approach (Lancichinetti and Fortunato, 2012, Sporns and Betzel, 2016) similarly to prior studies (Dwyer et al., 2014, Hearne et al., 2017, Schedlbauer and Ekstrom, 2019). The applied approach was meant to estimate the most stable network partitions within a group not only across algorithm iterations, but also across the thresholds as proposed by Schedlbauer and Ekstrom (2019). The detailed explanation of the procedure is provided in the Supplementary Material. The community detection on group level provided the modular partition, or LMN configuration, for each state and its corresponding modularity index Q showing the degree to which the matrix was able to be subdivided into nonoverlapping modules (Rubinov and Sporns, 2010, Rubinov and Sporns, 2011). The modules of the final network partitions were named based on the neuroanatomical localization of regions that were forming them. To test state reconfiguration in terms of segregation we calculated the difference in the optimal group modular decomposition measured via the modularity index Q between states and the the variation of information (VIn) to see if the module partitions differ between the states. VIn quantifies the information that is intrinsic to the two partitions corrected by the information that they share (Meilă, 2007). To test the statistical significance of index Q difference and VIn, we implemented repeated measure permutation procedure (Dwyer et al., 2014, Hearne et al., 2017) that is explained in detail in Supplementary Material. Using the group modular partitions, we identified the regions that altered their module alliance between the states, called “movers” (Schedlbauer & Ekstrom, 2019). The modular reconfiguration of modules was quantified by a proportion of reconfiguration (pr) obtained by dividing the number of regions within a resting-state module that change the module during the task with the number of regions within that resting-state module.
-
•
Reorganization of LMN configurations and state reconfiguration
In order to test the reorganization of LMN configurations we performed the above-mentioned data-driven community detection algorithm in TLE patients and we calculated the change of modularity index Q and VIn to explore the reorganization of state reconfiguration. Additionally, the community detection was performed on an individual-level for TLE patients providing for each patient the modular partition for each state and modularity index Q. Based on this, the state reconfiguration on individual level was calculated as the difference between modularity index Q for two states and between the number of modules. The aim of these TLE individual-level analyses was to test the effectiveness of LMN configurations and state reconfiguration by relating them to clinical and neuropsychological characteristics of patients.
We tested the significance of the difference between LMN configurations and state reconfiguration observed in healthy participants and the reorganization observed in TLE patients in three ways. First, we tested the significance of the difference between groups in segregation and modular partition of LMN configurations during each state using mentioned repeated measure permutation procedure for difference of group-level modularity index Q and VIn of group partitions. Second, to test the group differences in the ways that modules were segregated in LMN configurations, we analyzed physical distances within and between observed modules. Euclidian distance was calculated between the regions that composed one module as well between regions forming different modules (Alexander-Bloch et al., 2013). We compared mean within modules and between modules distances among groups for each state using the two-sample t-test. Third, since the epileptogenic zone of our patients is situated in temporal lobe we tested if TLE patients differed from healthy participants in terms of ability of these regions to change modules during state reconfiguration. This was done by comparing the number of “movers” in temporal and frontal lobe between the groups using the Chi-square test.
2.6.2. LMN integration
-
•
LMN configurations and state reconfiguration
In order to analyze the integration in the observed LMN community structures, we calculated the roles of the regions within each LMN configuration in healthy participants. Topological roles were assigned to each node based on its intra- and inter- modular connections. To that end we calculated normalized intra-modular degree (z, Meunier et al., 2009) whose value is higher if a node has a large number of intra-modular connections in comparison to other nodes in the same module. We measured inter-modular connectivity with the participation coefficient (Pc, Rubinov & Sporns, 2010). Due to the narrow distribution (Schedlbauer & Ekstrom, 2019) and dependency on the number of modules (Fornito et al., 2016), the Pc value was standardized within given community partition (Pcs). As in previous studies, the intra- and inter-modular plane was divided into four domains due to the smaller number of nodes within LMN (Meunier et al., 2009, Schedlbauer and Ekstrom, 2019). Nodes were considered as connector hubs if they had both high z (≥0) and Pcs (≥0) and as provincial hubs if they had high intra-modular connectivity (z ≥ 0), but low Pcs (<0). Nodes that had low z (<0) were considered as satellite nodes if they had high Pcs (≥0), or as peripheral nodes if their Pcs was low (<0) (Guimerà and Amaral, 2005, Meunier et al., 2009, Schedlbauer and Ekstrom, 2019). The roles were determined based on final group modular partition for each state and corresponding across-subjects mean FC matrices for group-level roles and corresponding individual FC matrices for individual-level roles. Nodes and their respective roles were grouped based on the RSN network nodes belonged to. State reconfiguration in terms of integration was tested by analyzing the number of connector hubs between two states in healthy participants using the Mann-Whitney for each network using the individual level data (i.e. number of connector hubs in each participant). We also analyzed the distribution of roles in the “mover” regions in each group using Chi-square Goodness of Fit test.
-
•
Reorganization of LMN configurations and state reconfiguration
The same analysis was performed in TLE patients to test the reorganization of LMN configurations and state reconfiguration. Moreover, to test the difference between LMN state reconfiguration and its reorganization in terms of integration, we compared the change in the number of connector hubs within each RSN network between the groups. To this end, the change in the number of connector hubs was calculated as the difference between number of connector hubs during the rest and during the recall task for each network and each participant. Finally, the Mann–Whitney U test was used to test the differences in the connector hub change between the healthy participants and TLE patients for each network separately. The results were FDR corrected for multiple comparisons.
For the purpose of analyzing in more details a possible disorganization of LMN integration in patients, we calculated a specific graph theory parameter, the hub disruption index (HDI, for details on calculation of this index see Achard et al., 2012, Roger et al., 2020a) with Pcs values for LMN configurations. The HDI can indicate whether the integration property of a specific region or node is increased or decreased. The HDI was first calculated on a group level using in order to compare groups and check if there is a general reorganization or disruption of inter-modular integration in TLE patients. The groups were compared with a two-sample t-test. Additionally, we calculated the HDI on regional level in order to identify regions that show the highest increase/decrease of inter-modular connectivity between the groups.
2.6.3. Efficiency of the reorganization of LMN configurations and state reconfiguration
The efficiency of the reorganization of LMN configurations and state reconfiguration observed in TLE patients was tested in two ways. First, the Spearman correlation was calculated between standardized language and memory scores (Table 2) and a) individual modularity index Q and the number of modules for each state and their change between states and b) HDI values. Second, for each neuropsychological test TLE patients were divided into high or low performance group depending on whether their score was above or below the group median. Then the Mann-Whitney U test was used to analyze the differences between high and low performers on FC parameters. For these analyses, both uncorrected and FDR corrected values are reported. The results that do not pass FDR correction are regarded as only exploratory.
3. Results
3.1. Statistical analyses of demographic, clinical and neuropsychological characteristics
We first tested the similarity between left and right TLE patients before combining them in one group. There were no significant differences in demographic and clinical data, and neuropsychological scores between left and right TLE subgroups (Table 2). There was no intra-group difference between left and right hippocampi in neither of the patient groups (LTLE: U = 39, p = .158; RTLE: U = 16, p = .465). When compared to healthy participants, the LTLE patients had smaller left hippocampus (U = 52, p = .024), while the volume of the right hippocampus volume was not different (U = 86.5, p = .438). No significant differences were found between the RTLE patients and healthy participants with respect to the volume of hippocampi (left: U = 41, p = .644; right: U = 23, p = .082). Activations during the language task were found to be consistently left lateralized (LI greater than 0.2) in the frontal lobe for all TLE patients (U = 39.5, p = .173) and they did not differ from healthy participants (U = 196.5, p = .140, Table S3 provides LIs of all participants). In addition, temporal lobe language activations were mostly left lateralized and there was no difference between TLE patients (U = 21.5, p = .496) or between patients and healthy (U = 158.5, p = .829).
Since no significant differences between left and right TLE patients were found, we finally combined them and analyzed them as a single patient group. Nevertheless, the descriptive statistics of the main network parameters for LTLE and RTLE are presented in Table S12.
3.2. LMN segregation
3.2.1. LMN configurations and state reconfiguration
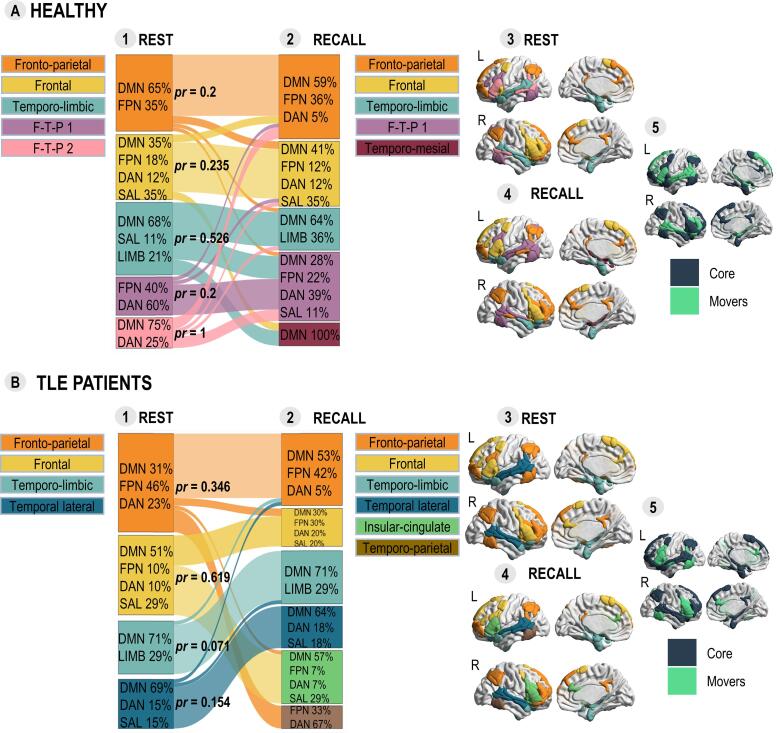
Based on a data-driven community detection algorithm we identified state configurations which show how LMN splits into separate modules (i.e. subset of highly interconnected regions) during each state (Bullmore and Sporns, 2012, Fornito et al., 2016, Meunier et al., 2010). Our results showed that resting-state and task LMN configurations in healthy participants were composed of the same number of modules with no significant difference between the modularity indexes Q (ΔQ = 0.02, p = .391). Although the number of modules was the same, their composition (i.e. partition) was different between the states (VIn = 0.34, p < .001). Module partitions and reconfigurations of resting-state modules in healthy participants are shown in Panel A of Fig. 2 and module affiliation of each region is provided in Table S4.
Fig. 2.
Segregation of LMN in terms of community structures found in healthy participants (Panel A) and TLE patients (Panel B) during the two states. The alluvial diagrams present the dynamics of state reconfiguration of modules from resting-state (Subpanel 1) to sentence recall (Subpanel 2) in healthy participants (Panel A) and its reorganization it TLE patients (Panel B). For each module, the composition is indicated in percentages of networks that form a given module. For each resting-state module the proportion of reconfiguration (pr) is indicated. The architectures of modules are presented in the templates (Subpanels 3 and 4). Subpanels 5 shows the “core” regions of healthy participants and TLE patients that remain in the same module from rest to task (dark blue) and “movers” that change their module (light green). F-T-P = Fronto-temporo-parietal module, REST = resting-state, RECALL = sentence recall task. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The proportion of reconfiguration (pr) used to quantify the extent to which the resting-state modules changed compared to task are presented in the Panel A, Subpanel 1 of Fig. 2 and Table S4 provides details on movement of each region. The most reconfigured modules in healthy participants were the second DMN-DAN fronto-temporo-parietal (pr = 1) and the temporo-limbic module (pr = 0.526).
3.2.2. Reorganization of LMN configurations and state reconfiguration
In TLE patients, the transition between the states was accompanied by a change of the number of modules, as indicated by a significant difference between the modularity indexes Q (ΔQ = 0.05, p < .05) and the module partitions were significantly different between two states (VIn = 0.22, p < .001). The extrinsic configuration of LMN in TLE patients was found to be more modular and to be comprised of a higher number of modules in comparison to healthy participants (ΔQ = 0.07, p < 0.01), while the difference was not significant between intrinsic configurations (ΔQ = 0.004, p = 0.727). In addition, both resting-state (VIn = 0.31, p < .001) and task (VIn = 0.33, p < .001) modular partitions were significantly different between two groups. Module partitions and reconfigurations of resting-state modules in TLE patients are shown in Panel B of Fig. 2 and module affiliation of each region is presented in Table S4.
Additionally, our results indicated that the anatomical (i.e. Euclidean) distance of the regions within modules was significantly smaller in TLE patients than in healthy participants during the task (t = 2.562, p < 0.05). In other words, regions within modules of TLE patients were anatomically closer to each other than in controls.
Furthermore, the temporo-limbic (pr = 0.071) and temporal lateral module (pr = 0.154) in TLE patients showed the smallest change. Consistent with this result, a Chi-square test showed that that mesial and lateral temporal regions were less flexible (i.e. having less “movers”) in TLE patients compared to healthy participants (χ2(1, N = 56) = 7.29, p < .01), while the flexibility of frontal regions was not different between the groups (χ2(1, N = 48) = 0.1, p = .755).
3.3. LMN integration
3.3.1. LMN configurations and state reconfiguration
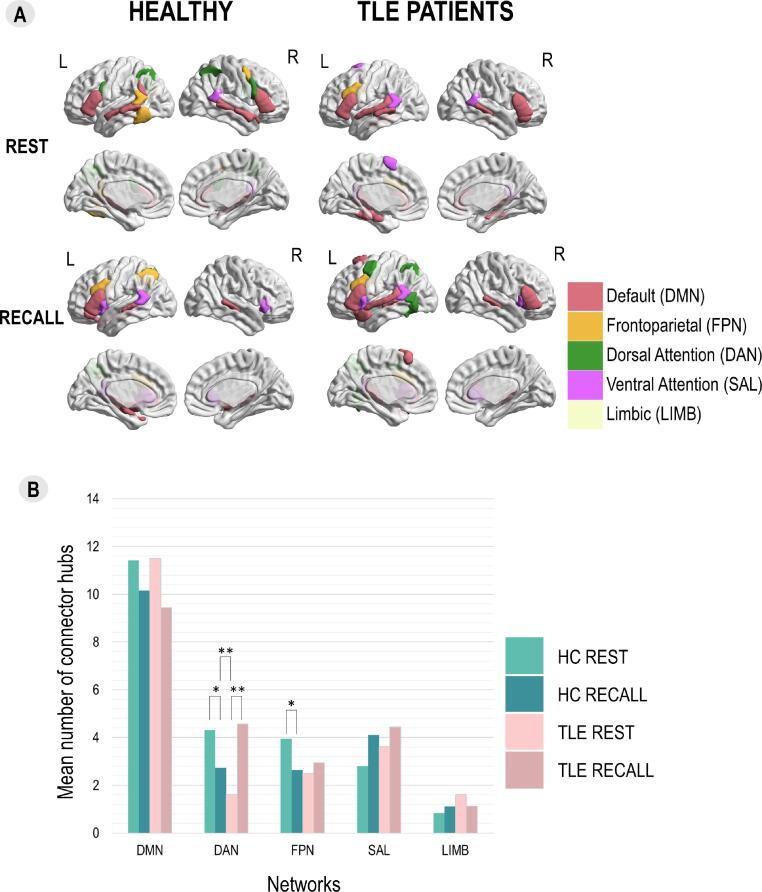
The integration properties were explored via connector hubs, especially their reconfiguration between the tasks. Panel A of Fig. 3 shows the regions that were connector hubs in more than 50% of participants of both groups across the states and Table S5 presents the roles of LMN regions based on their normalized intra-modular degree and standardized participation coefficient obtained with the across-subjects mean FC matrices. Our analyses showed that in healthy participants (Fig. 3, Panel B), less regions belonging to DAN (U = 78.5, pFDR < 0.05) and FPN (U = 99.5, pFDR < 0.05) were connector hubs during recall compared to rest.
Fig. 3.
The integration of LMN assessed via distribution of connector hubs between groups and tasks. Panel A: Topography of the most common connector hubs according to groups and tasks. We present the regions that were connector hubs in more than half of the group (10/19 in healthy and 9/16 in TLE patients). Colors of the regions represent their network affiliation. Panel B: Distribution of connector hubs according to groups and tasks for each network showing LMN state reconfiguration and LMN reorganization in terms of integration. Significant differences at p < 0.01 threshold with FDR correction are marked with ** and those at p < 0.05 are marked with *. HC = healthy controls, REST = resting-state, RECALL = sentence recall task.
Additionally, to explore the regions that changed modules between the states (i.e. “movers”), we analyzed the distribution of their roles across states. Our analyses showed that “movers” were more often connector hubs and satellite nodes during both states in healthy participants (REST: χ2(3, N = 532) = 116.72, pFDR < 0.05; RA: χ2(3, N = 532) = 125.79, pFDR < 0.05) having thus primarily the connecting roles. Table S6 provides the role distribution of “movers” across states and groups.
3.3.2. Reorganization of LMN configurations and state reconfiguration
In TLE patients, higher number of DAN regions were connector hubs during recall than during task (U = 227.5, pFDR < 0.01). Interestingly this variation was different between groups for DAN (U = 16.5, pFDR < 0.01). Detailed results are presented in Tables S7 and S8. Moreover, the “movers” in TLE patients were also more often connector hubs and satellite nodes during both states (REST: χ2(3, N = 400) = 14.12, pFDR < 0.05; RA: χ2(3, N = 400) = 147.02, pFDR = 0.543).
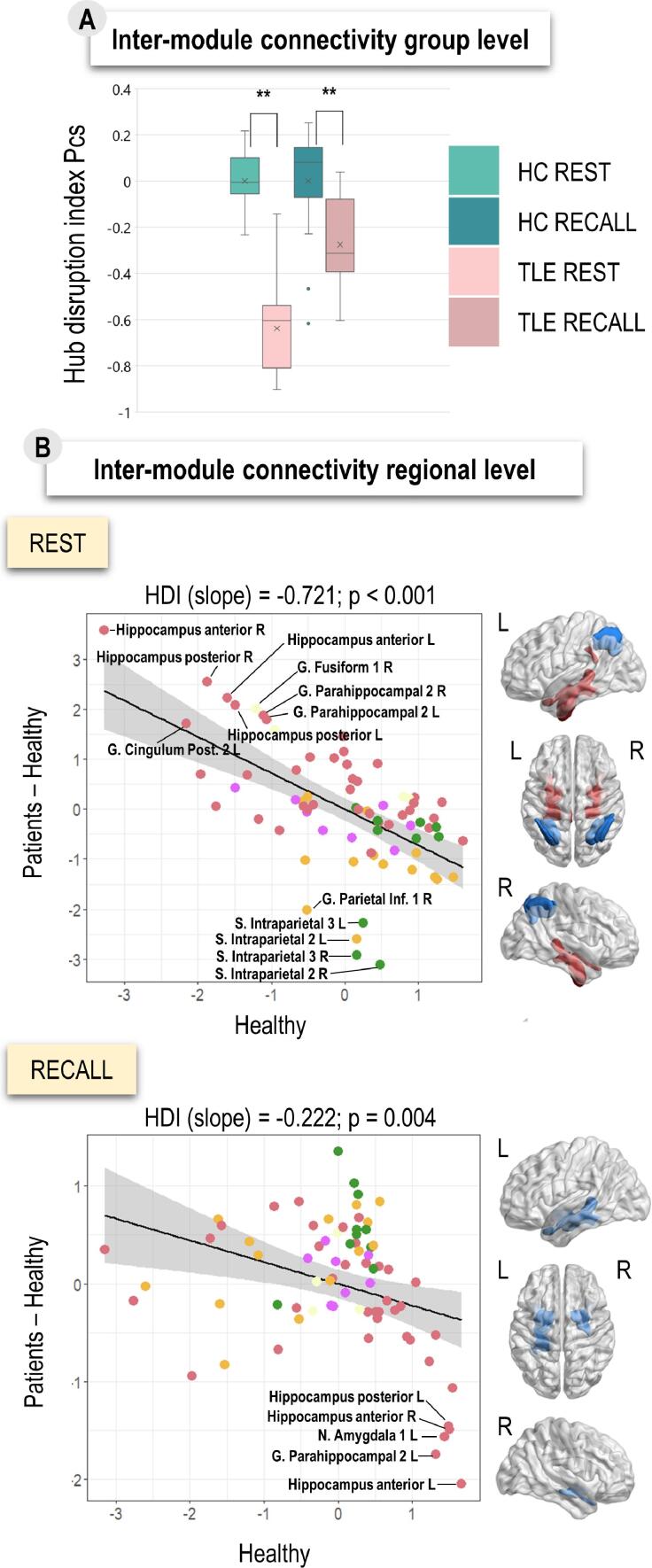
Our HDI results (Fig. 4) indicated that TLE patients showed significant disruption of inter-modular connectivity in both states compared to healthy participants (REST: t = -11.65, pFDR < 0.01; RA: t = -3.86, pFDR < 0.01). We further explored this inter-modular connectivity disruption on a regional level. The biggest disruption of inter-modular connectivity in patients during resting-state was found within FPN and DAN (bilateral intraparietal sulci), while the increase was observed within DMN (bilateral hippocampal gyri, right fusiform, bilateral parahippocampus and left posterior cingulate gyrus). Conversely, the biggest disruption of inter-modular connectivity during the task in patients was identified within DMN (bilateral anterior hippocampus, left posterior hippocampus, bilateral amygdala and left parahippocampus). The HDI results are presented in Fig. 4.
Fig. 4.
Reorganization of LMN state configurations in terms of integration (inter-modular connectivity) expressed via HDI (hub disruption index). Subpanel A shows group differences between healthy participants and TLE patients in terms of HDI for resting-state and sentence recall. Differences that are significant after the FDR correction on the p < 0.01 level are marked with **. Subpanel B shows the differences in inter-modular connectivity on regional level observed when comparing two groups for each state. Colors of the regions represent their network affiliation. Template representations show regions with the greatest differences between the groups. Blue regions show the greatest decrease in inter-modular connectivity in patients compared to control and regions in red show the greatest increase. HC = healthy controls, REST = resting-state, RECALL = sentence recall task, HDI = hub disruption index. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Efficiency of the reorganization of LMN configurations and state reconfiguration
The efficiency of the reorganization of LMN configurations and state reconfiguration found in TLE patients was tested using the Spearman rank correlation and the Mann-Whitney U test. The obtained results were not significant after FDR correction. The results are presented in Tables S9 and S10.
Since there was a significant age difference between healthy participants and TLE patients (U = 269, p < .001), we tested if the main network parameters are age-related and for potential effect of age. No significant effect was obtained. Detailed results are presented in Table S11.
4. Discussion
In the present study, we were interested to describe the LMN (see Roger et al., 2020a) underlying the language and declarative memory composite function. Our hypotheses were that the LMN configuration is dynamic so that it reconfigures according to brain activity (intrinsic, resting-state; extrinsic, task-induced) and condition (normal, HC; neurological, TLE patients). LMN configurations, dynamics of state reconfiguration and their reorganization were assessed by measuring the segregation (community detection) and the integration (connector hubs and inter-modular connectivity) properties. Our objective was to determine how LMN reconfigures across states (resting vs. task-induced) and groups (healthy vs. TLE patients) to support language and declarative memory interaction.
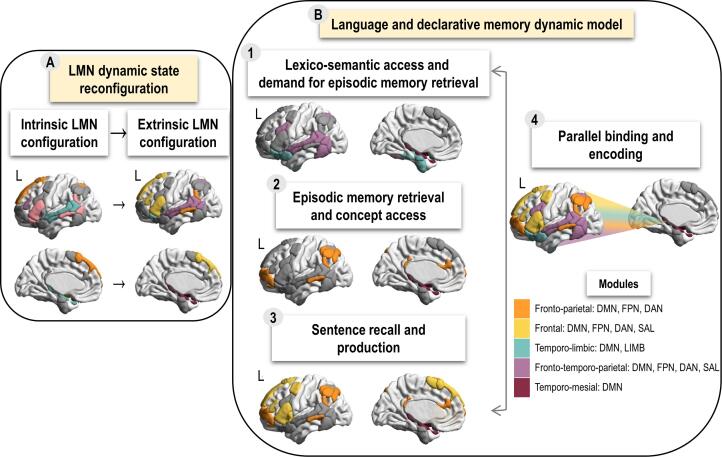
Analyses were first performed for HC to assess LMN configurations and state reconfiguration. Contrary to our expectations, the segregation did not vary across intrinsic and extrinsic states in terms of number of modules. However, rest and task differed in modular compositions of LMN configurations in line with the idea that task-related reconfigurations are necessary to adapt to task-demands (Cohen & D’Esposito, 2016). More specifically, LMN-intrinsic configuration included the following five modules (see Fig. 2, Panel A, Subpanel 1): fronto-parietal, frontal, temporo-limbic and two fronto-temporo-parietal. The second fronto-temporo-parietal module includes language key regions (left triangularis and orbitalis, left superior and middle temporal, supramarginal gyri) which is in agreement with previous results revealing correlated activity of language systems during rest (Alavash et al., 2019, Muller and Meyer, 2014). The segregation of LMN extrinsic configuration, illustrated in in Fig. 2 (Panel A, Subpanel 2), shows five modules: fronto-parietal, frontal, temporo-limbic, fronto-temporo-parietal and temporo-mesial. There were two main segregation changes in task compared to rest in HC: (a) language key–regions from second fronto-temporo-parietal module migrated to other modules; (b) the hippocampus and amygdalae of temporo-limbic module migrated into a separate temporo-mesial module (Fig. 2, Panel A). The temporo-mesial module in sentence recall task could be engaged in the episodic retrieval and simultaneous encoding processes, as well as in the binding of retrieved episodes and lexico-semantic information into a coherent experience (Cooper and Ritchey, 2020, Ranganath and Ritchey, 2012). Therefore, the temporo-mesial (hippocampo-amygdalar) module and its dynamics across states may serve as a specialized interface between language and memory, as suggested by Duff & Brown-Schmidt (2012). This module that emerges during the task, could be supporting language and declarative memory based on its flexible interactions with various cortical networks enabling both episodic retrieval (Geib et al., 2017, Westphal et al., 2017) and language processing (Covington and Duff, 2016, Piai et al., 2016). The composition of the first fronto-temporo-parietal module changed in task compared to rest by including supplementary lateral temporal regions required by lexico-semantic and syntactic processes typically recruited during a sentence recall task (Menenti et al., 2012, Price, 2012). In conclusion, regions showing modular shift between states in HC (see Fig. 2, Panel A, Subpanel 5) were those regions described as key either for language (Price, 2012) or for memory (Duff and Brown-Schmidt, 2012, Ranganath and Ritchey, 2012). The synthetic representation of these findings is presented in Fig. 5 through a model of language and declarative memory dynamic interaction based on LMN. The more detailed description of the model is provided in Supplementary material.
Fig. 5.
Schematic representation of the model of language and declarative memory dynamic interaction based on LMN. Panel A shows state reconfiguration from intrinsic to extrinsic state during sentence recall. Only the regions that changed module (“movers”) are colored and the colors correspond to their modules for each state. Non-mobile regions (that remain in the same module) are presented in gray. Temporo-lateral module in rest divides into temporo-lateral and temporo-mesial modules in task that are engaged in semantic and episodic memory respectively. Fronto-temporo-parietal module found during rest includes language key-regions that separate during the task into modules that are engaged in lexico-semantic access, syntactic processing and sentence generation. Panel B shows cognitive processes recruited by the composite language and declarative memory function and the underlying LMN during the task. Within each subpanel we present language and declarative memory subprocesses and the corresponding modules presented in color. Fronto-temporo-parietal and temporo-limbic modules are engaged in lexico-semantic access while temporo-limbic module is also engaged in episodic retrieval (Subpanel 1). Hippocampo-amygdalar module is engaged in episodic retrieval and fronto-parietal module in concept access (Subpanel 2). Through the connection of hippocampo-amygdalar and fronto-parietal modules, the sentence is recalled and the information is transferred to working memory. Fronto-parietal and frontal modules are engaged in syntactic processing and sentence production (Subpanel 3). Hippocampo-amygdalar module can also access the information from all modules, combine it into a complete experience and perform encoding in parallel (Subpanel 4).
State reconfiguration of modular composition in HC concerned mainly regions having satellite or connector hub role (see Table 6S), as previously suggested (Schedlbauer & Ekstrom, 2019). Additionally, majority of “movers” that had connecting role (either as connector hub or satellite node) belonged to DMN such as the middle and posterior temporal, superior frontal and prefrontal regions that were proposed to connect DMN to other networks, especially language network, providing thus DMN with linguistic information (Gordon et al., 2020). Globally, the segregation and integration results suggest that LMN state reconfigurations described in HC are mainly based on modular flexibility of connector nodes when facing task requirement.
The further objective of this study was to understand LMN configurations and state reconfigurations in patients with TLE. The state reconfiguration of LMN showed increase in segregation and significant changes in modular composition. LMN was composed of four modules in its intrinsic configuration during rest: fronto-parietal, frontal, temporo-limbic and temporal lateral (Fig. 2, Panel B, Subpanel 1) and six in its extrinsic configuration (Fig. 2, Panel B, Subpanel 2) during task: fronto-parietal, frontal, temporo-limbic, temporal lateral, insulo-cingulate and temporo-parietal. Compared to HC, TLE did not show specific modules composed of language key regions (second fronto-temporo-parietal in HC) during rest. Also, lateral superior and middle temporal regions merged into one specific temporal lateral module during both rest to task which was not the case for HC. Additionally, the temporo-limbic module composed of memory key regions was smaller and limited to antero-lateral temporal regions in patients compared to HC during rest, and temporal mesial module did not separate from temporo-limbic module during the task. Correspondingly, temporo-limbic and temporal lateral modules showed reduced flexibility during state reconfiguration of LMN, as did temporal regions in general compared to HC (Fig. 2, Panel B). The reduced reconfiguration ability of these modules and regions in patients can be explained by the location of the epileptogenic zones in temporal regions (Barr, 2015, Thom and Bertram, 2012) so the epileptic discharges could be preventing normal reconfigurations of these regions. Furthermore, the segregation of insulo-cingulate module in TLE during the task can be related to the task demands for overt speech inhibition (Lœvenbruck et al., 2018, Oh et al., 2014). Similarly, the temporo-parietal module composed exclusively of DAN and FPN regions in TLE patients during task can be explained by a supplementary requirement of control processes during the task (Dixon et al., 2017). Overall, difficulties in episodic retrieval (Rzezak et al., 2017) and naming (Allone et al., 2017) usually observed in TLE patients, might be due to the absence of specialization of the hippocampo-amygdalar module and sharing of the already reduced resources with semantic memory. As a compensatory mechanism, insulo-cingulate and temporo-parietal segregate, suggesting that compared to HC, TLE need more effort to control covert speech and more cognitive control to perform the combined language-and-memory task. Furthermore, regions were grouped into modules more based on their physical proximity in TLE, contrary to what could be expected for complex cognitive tasks such as recall task, which generally rely on long-range integrative connections (Cohen & D’Esposito, 2016). This can be explained by the loss of distant connections as a result of epileptic discharges and their high metabolic cost, as previously suggested (Cohen and D’Esposito, 2016, Englot et al., 2016, Haneef et al., 2014, Lee et al., 2018, Liao et al., 2010, Roger et al., 2020a). The local organization of modules and their greater segregation, may be beneficial for patients since it can prevent from a dysfunction or damage propagation throughout the network (Fornito et al., 2016).
LMN integration and its change between states was assessed via connector hubs in HC and TLE patients and the two groups were compared. Patients showed increased number of DAN connector hubs during task compared to rest (Fig. 3, Panel B), suggesting that patients rely more on DAN regions for enabling the communication and the cooperation between language and memory processes and networks due to dysfunctions within DMN which generally assures this interface (Bettus et al., 2009, Duff and Brown-Schmidt, 2012, Waites et al., 2006). TLE patients indeed have difficulties in language and memory tasks performance (Alessio et al., 2006, Bartha-Doering and Trinka, 2014, Zhao et al., 2014, Allone et al., 2017, Bell et al., 2011, Tramoni-Negre et al., 2017), which is probably the reason they need additional resources that are provided by DAN.
Additional HDI analyses revealed that TLE patients show disruption of inter-modular integration (Fig. 4, Panel A). The regional HDI analyses demonstrated that DMN shows decrease, while DAN and FPN show increase of inter-modular connectivity in task compared to rest in TLE patients relative to HC. Hence, TLE patients may rely less on DMN and more on DAN and FPN to flexibly increase their integration property between states. In addition, we also found the “hippocampal paradox” (Fig. 4, Panel B) previously described in TLE (Roger et al., 2020a). Namely, even though global DMN connectivity is reduced in TLE, there are nodes, such as the hippocampus, that can show hyper-connectivity (Cataldi et al., 2013). We found increased inter-modular connectivity of mesial temporal structures only during rest in line with other studies (Haneef et al., 2014, Roger et al., 2020a). According to Englot et al. (2016), the increase in connectivity of these regions may be explained by the epileptic activity, without any compensatory role. This explanation is supported by the decrease of inter-modular connectivity of these regions during the task (Fig. 4, Panel B). Our results on LMN integration in TLE altogether suggest that classical language-and-memory interactions normally based on DMN regions as an interface (especially mesial temporal structures; see Duff & Brown-Schmidt, 2012) may be enabled in TLE patients, which is compensated by control networks, in particular, the DAN and FPN. The integrative dysfunction of DMN in TLE is due to the loss of long-range connections (Lee et al., 2018, Vaughan et al., 2016).
Overall, our findings validated that intrinsic and extrinsic LMN configurations are neither entirely the same, nor completely different, but rather complementary, providing the justification for their joint exploration. Studying the differences between state-dependent LMN configurations revealed us what are the key language and declarative memory subprocesses the network is trying to support with its adaptation. On the other hand, studying the differences in network state reconfiguration between healthy and pathological condition allowed us to understand what are the additional processes language and declarative memory need when the standard interface is not functional and the corresponding LMN reorganization.
Our study has several limitations. First of all, the low statistical power of the study made difficult to detect correlations with neuropsychological performances. The efficiency of reorganization should be explored in larger samples. Also, larger sample of TLE patients might allow to separate left and right TLE patients and explore the effects of lateralization of epileptogenic zone on modularity. Second, although we aimed to describe the dynamics of reconfiguration, our results are limited by the temporal resolution of the fMRI technique. Future studies could deepen our findings and hypotheses of language and declarative memory model using dynamic functional connectivity or combination of fMRI with electrophysiological data. Furthermore, functional connectivity is based on correlation so we can only discuss association without concluding about causality, therefore dynamic causal modelling could be beneficial for exploring the direction of the interaction between DAN and FPN with DMN. Like previous studies (Bolt et al., 2017, Cohen and D’Esposito, 2016; Finc et al., 2020; Schedlbauer and Ekstrom, 2019, Stanley et al., 2015) we based our analyses only on positive connections and we implemented an algorithm aiming to get the most stable network partitions across algorithm iterations and thresholds. Nevertheless, since there is no consensus on graph theory decisions, the presented data and conclusions may not necessarily generalize to other studies using different network parameters. Finally, the order of task and resting-state could have influenced the results (Grigg et al., 2010, Tailby et al., 2015, Wang et al., 2012). Unfortunately, we were unable to change this aspect of our experimental procedure due to clinical practice. Future studies should examine the stability of modular structure with respect to order of tasks.
5. Conclusion
In this study we were interested in exploring the neural basis of a composite language and declarative memory function based on the dynamical state reconfiguration of LMN and its reorganization in the case of TLE patients. Although resting-state has been found to be predictive of individual differences and various performances, our study joins others showing that it is also necessary to explore the network architecture during task performance in order to understand complex interactive functional networks that are the base of cognitive processing. By using a data driven approach we aimed to understand more fully the changes in main functional networks and regions of LMN that are required when the context explicitly demands their collaboration. Specifically, we have identified flexible community reconfiguration that underlies interaction of these two functions in typical individuals and we have demonstrated how this vital flexibility is reduced in TLE patients, especially in the temporal regions that represent the integrative hubs or interface of language and declarative memory. Finally, a dynamic model of processes involved during language and memory integration has been proposed.
6. Data and code availability statement
In line with the approval of the local ethic committee (CPP: 09-CHUG-14, 04/06/2009 and 2017-A00384-49), all participants were assured raw data would remain confidential and would not be shared. The codes of the main analyses can be found at the following link https://cloud.univ-grenoble-alpes.fr/index.php/s/JBfGGMGaobqQH7P and authors can provide all additional information upon request.
Funding sources
This work has been funded by the French program “AAP GENERIQUE 2017” run by the “Agence Nationale pour la Recherche” grant ‘REORG’ [grant number ANR-17-CE28-0015–01]; and by NeuroCoG IDEX UGA in the framework of the “Investissements d’avenir” program [grant number ANR-15-IDEX-02]. IRMaGe MRI/Neurophysiology facility was partly funded by the French program “Investissement d’Avenir” run by the “Agence Nationale pour la Recherche”; grant “Infrastructure d’avenir en Biologie Santé” [grant number ANR-11-INBS-0006].
Authors contribution
Author contributions included conceptualization and investigation methodology (SB, ER, EC, CP, MB), data curation (ER, EC, CP, LL, CM, AK, PK), formal analysis (SB, ER, EC, CP, MB), writing the manuscript or revising it critically for important intellectual content (SB, ER, EC, CP, CM, AK, LL, PK, MB) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (all authors).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102702.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Achard S., Delon-Martin C., Vertes P.E., Renard F., Schenck M., Schneider F., Heinrich C., Kremer S., Bullmore E.T. Hubs of brain functional networks are radically reorganized in comatose patients. Proc. Natl. Acad. Sci. 2012;109(50):20608–20613. doi: 10.1073/pnas.1208933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavash M., Tune S., Obleser J. Modular reconfiguration of an auditory control brain network supports adaptive listening behavior. Proc. Natl. Acad. Sci. 2019;116(2):660–669. doi: 10.1073/pnas.1815321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessio A., Bonilha L., Rorden C., Kobayashi E., Min L.L., Damasceno B.P., Cendes F. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav. 2006;8(3):593–600. doi: 10.1016/j.yebeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch, A. F., Bullmore, E. T., & Gogtay, N. (2013). The Anatomical Distance of Functional Connections Predicts Brain Network Topology in Health and Schizophrenia. Cerebral Cortex, 127– 138. https://doi.org/10.1093/cercor/bhr388. [DOI] [PMC free article] [PubMed]
- Allone C., Lo Buono V., Corallo F., Pisani L.R., Pollicino P., Bramanti P., Marino S. Neuroimaging and cognitive functions in temporal lobe epilepsy: A review of the literature. J. Neurol. Sci. 2017;381:7–15. doi: 10.1016/j.jns.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Arnemann K.L., Chen A.-J.-W., Novakovic-Agopian T., Gratton C., Nomura E.M., D’Esposito M. Functional brain network modularity predicts response to cognitive training after brain injury. Neurology. 2015;84(15):1568–1574. doi: 10.1212/WNL.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu M., Perrone-Bertolotti M. What do patients with epilepsy tell us about language dynamics? A review of fMRI studies. Rev. Neurosci. 2015;26(3) doi: 10.1515/revneuro-2014-0074. [DOI] [PubMed] [Google Scholar]
- Banjac S., Roger E., Cousin E., Perrone-Bertolotti M., Haldin C., Pichat C., Lamalle L., Minotti L., Kahane P., Baciu M. Interactive mapping of language and memory with the GE2REC protocol. Brain Imaging and Behavior. 2020 doi: 10.1007/s11682-020-00355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr W.B. Neuropsychological assessment of patients with epilepsy. In: Barr W.B., Morrison C., editors. Handbook on the neuropsychology of epilepsy. Springer; 2015. pp. 1–36. [Google Scholar]
- Bartha-Doering L., Trinka E. The interictal language profile in adult epilepsy. Epilepsia. 2014;55(10):1512–1525. doi: 10.1111/epi.2014.55.issue-1010.1111/epi.12743. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Wymbs N.F., Porter M.A., Mucha P.J., Carlson J.M., Grafton S.T. Dynamic reconfiguration of human brain networks during learning. Proc. Natl. Acad. Sci. 2011;108(18):7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell B., Lin J.J., Seidenberg M., Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nature Reviews Neurology. 2011;7(3):154–164. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl M.M., Zimmaro L.A., Khan O.I., Dustin I., Ritzl E., Duke E.S., Sepeta L.N., Sato S., Theodore W.H., Gaillard W.D. Characterization of atypical language activation patterns in focal epilepsy: Language Activation Patterns. Ann. Neurol. 2014;75(1):33–42. doi: 10.1002/ana.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero M.A., Yeo B.T.T., D’Esposito M. The modular and integrative functional architecture of the human brain. Proc. Natl. Acad. Sci. 2015;112(49):E6798–E6807. doi: 10.1073/pnas.1510619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson P., Dinkelacker V., Valabregue R., Thivard L., Leclerc X., Baulac M., Sammler D., Colliot O., Lehéricy S., Samson S., Dupont S. Structural connectivity differences in left and right temporal lobe epilepsy. NeuroImage. 2014;100:135–144. doi: 10.1016/j.neuroimage.2014.04.071. [DOI] [PubMed] [Google Scholar]
- Bettus G., Guedj E., Joyeux F., Confort-Gouny S., Soulier E., Laguitton V., Cozzone P.J., Chauvel P., Ranjeva J.-P., Bartolomei F., Guye M. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum. Brain Mapp. 2009;30(5):1580–1591. doi: 10.1002/hbm.v30:510.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel V.D., Guillaume J.-L., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech: Theory Exp. 2008;2008(10):P10008. doi: 10.1088/1742-5468/2008/10/P10008. [DOI] [Google Scholar]
- Bolt T., Nomi J.S., Rubinov M., Uddin L.Q. Correspondence between evoked and intrinsic functional brain network configurations: Functional Brain Network Configurations. Hum. Brain Mapp. 2017;38(4):1992–2007. doi: 10.1002/hbm.23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Burianová H., Faizo N.L., Gray M., Hocking J., Galloway G., Reutens D. Altered functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2017;137:45–52. doi: 10.1016/j.eplepsyres.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Cao H., Plichta M.M., Schäfer A., Haddad L., Grimm O., Schneider M., Esslinger C., Kirsch P., Meyer-Lindenberg A., Tost H. Test–retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. NeuroImage. 2014;84:888–900. doi: 10.1016/j.neuroimage.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Cataldi M., Avoli M., de Villers-Sidani E. Resting state networks in temporal lobe epilepsy. Epilepsia. 2013;54(12):2048–2059. doi: 10.1111/epi.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H.H., Marshall C.R. Definite Reference and Mutual Knowledge. In: Joshi A.K., Webber B.L., Sag I.A., editors. Elements of discourse understanding. Cambridge University Press; 1981. pp. 10–63. [Google Scholar]
- Cohen J.R., D’Esposito M. The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. J. Neurosci. 2016;36(48):12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Bassett D.S., Power J.D., Braver T.S., Petersen S.E. Intrinsic and Task-Evoked Network Architectures of the Human Brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R.A., Ritchey M. Progression from Feature-Specific Brain Activity to Hippocampal Binding during Episodic Encoding. The Journal of Neuroscience. 2020;40(8):1701–1709. doi: 10.1523/JNEUROSCI.1971-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington N.V., Duff M.C. Expanding the Language Network: Direct Contributions from the Hippocampus. Trends in Cognitive Sciences. 2016;20(12):869–870. doi: 10.1016/j.tics.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos B.M., Coan A.C., Lin Yasuda C., Casseb R.F., Cendes F. Large-scale brain networks are distinctly affected in right and left mesial temporal lobe epilepsy. Hum. Brain Mapp. 2016;37(9):3137–3152. doi: 10.1002/hbm.v37.910.1002/hbm.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M., Beckmann C.F., De Stefano N., Matthews P.M., Smith S.M. FMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29(4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Deloche, G., & Hannequin, D. (1997). Test de dénomination orale d’images: DO 80. Éditions du centre de psychologie appliquée.
- DeSalvo M.N., Douw L., Takaya S., Liu H., Stufflebeam S.M. Task-dependent reorganization of functional connectivity networks during visual semantic decision making. Brain and Behavior. 2014;4(6):877–885. doi: 10.1002/brb3.2014.4.issue-610.1002/brb3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Bernal B., Tremblay P. The Language Connectome: New Pathways. New Concepts. The Neuroscientist. 2014;20(5):453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- Dinkelacker V., Dupont S., Samson S. The new approach to classification of focal epilepsies: Epileptic discharge and disconnectivity in relation to cognition. Epilepsy Behav. 2016;64:322–328. doi: 10.1016/j.yebeh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- Dixon M.L., Andrews-Hanna J.R., Spreng R.N., Irving Z.C., Mills C., Girn M., Christoff K. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. NeuroImage. 2017;147:632–649. doi: 10.1016/j.neuroimage.2016.12.073. [DOI] [PubMed] [Google Scholar]
- Duff M.C., Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H. Brain plasticity: From pathophysiological mechanisms to therapeutic applications. Journal of Clinical Neuroscience. 2006;13(9):885–897. doi: 10.1016/j.jocn.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Dwyer D.B., Harrison B.J., Yucel M., Whittle S., Zalesky A., Pantelis C., Allen N.B., Fornito A. Large-Scale Brain Network Dynamics Supporting Adolescent Cognitive Control. J. Neurosci. 2014;34(42):14096–14107. doi: 10.1523/JNEUROSCI.1634-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot D.J., Konrad P.E., Morgan V.L. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. 2016;57(10):1546–1557. doi: 10.1111/epi.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finc K., Bonna K., He X., Lydon-Staley D.M., Kühn S., Duch W., Bassett D.S. Dynamic reconfiguration of functional brain networks during working memory training. Nat. Commun. 2020;11(1):2435. doi: 10.1038/s41467-020-15631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finc K., Bonna K., Lewandowska M., Wolak T., Nikadon J., Dreszer J., Duch W., Kühn S. Transition of the functional brain network related to increasing cognitive demands: Transition of the Functional Brain Network. Hum. Brain Mapp. 2017 doi: 10.1002/hbm.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Harrison B.J., Zalesky A., Simons J.S. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc. Natl. Acad. Sci. 2012;109(31):12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A. Graph Theoretic Analysis of Human Brain Networks. In: Filippi M., editor. Vol. 119. Springer; New York: 2016. pp. 283–314. (FMRI Techniques and Protocols). [DOI] [Google Scholar]
- Fornito A., Zalesky A., Bullmore E.T. Elsevier/Academic Press; 2016. Fundamentals of brain network analysis. [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramos C., Lin J.J., Kellermann T.S., Bonilha L., Prabhakaran V., Hermann B.P. Graph theory and cognition: A complementary avenue for examining neuropsychological status in epilepsy. Epilepsy Behav. 2016;64:329–335. doi: 10.1016/j.yebeh.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geib B.R., Stanley M.L., Dennis N.A., Woldorff M.G., Cabeza R. From hippocampus to whole-brain: The role of integrative processing in episodic memory retrieval: Brain Networks and Memory Retrieval. Hum. Brain Mapp. 2017;38(4):2242–2259. doi: 10.1002/hbm.23518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy O. Fonctions exécutives et pathologies neurologiques et psychiatriques; Evaluation en pratique clinique. Groupe de Boeck: 2008. & GREFEX. [Google Scholar]
- Gordon E.M., Laumann T.O., Marek S., Raut R.V., Gratton C., Newbold D.J., Greene D.J., Coalson R.S., Snyder A.Z., Schlaggar B.L., Petersen S.E., Dosenbach N.U.F., Nelson S.M. Default-mode network streams for coupling to language and control systems. Proc. Natl. Acad. Sci. 2020;117(29):17308–17319. doi: 10.1073/pnas.2005238117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O., Grady C.L., Sporns O. Task-Related Effects on the Temporal and Spatial Dynamics of Resting-State Functional Connectivity in the Default Network. PLoS ONE. 2010;5(10):e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimerà R., Amaral L.A.N. Cartography of complex networks: Modules and universal roles. J. Stat. Mech: Theory Exp. 2005;2005(02):P02001. doi: 10.1088/1742-5468/2005/02/P02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneef Z., Lenartowicz A., Yeh H.J., Levin H.S., Engel J., Stern J.M. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia. 2014;55(1):137–145. doi: 10.1111/epi.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Bassett D.S., Chaitanya G., Sperling M.R., Kozlowski L., Tracy J.I. Disrupted dynamic network reconfiguration of the language system in temporal lobe epilepsy. Brain. 2018;141(5):1375–1389. doi: 10.1093/brain/awy042. [DOI] [PubMed] [Google Scholar]
- Hearne L.J., Cocchi L., Zalesky A., Mattingley J.B. Reconfiguration of Brain Network Architectures between Resting-State and Complexity-Dependent Cognitive Reasoning. The Journal of Neuroscience. 2017;37(35):8399–8411. doi: 10.1523/JNEUROSCI.0485-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G., Duffau H. Revisiting the functional anatomy of the human brain: Toward a meta-networking theory of cerebral functions. Physiol. Rev. 2020;100(3):1181–1228. doi: 10.1152/physrev.00033.2019. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Joliot M., Jobard G., Naveau M., Delcroix N., Petit L., Zago L., Crivello F., Mellet E., Mazoyer B., Tzourio-Mazoyer N. AICHA: An atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods. 2015;254:46–59. doi: 10.1016/j.jneumeth.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Keerativittayayut R., Aoki R., Sarabi M.T., Jimura K., Nakahara K. Large-scale network integration in the human brain tracks temporal fluctuations in memory encoding performance. ELife. 2018;7 doi: 10.7554/eLife.32696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann T.S., Bonilha L., Eskandari R., Garcia-Ramos C., Lin J.J., Hermann B.P. Mapping the neuropsychological profile of temporal lobe epilepsy using cognitive network topology and graph theory. Epilepsy Behav. 2016;63:9–16. doi: 10.1016/j.yebeh.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen F.M., Yeo B.T.T., Buckner R.L. Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1653):20130526. doi: 10.1098/rstb.2013.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschwitz J.D., List D., Waller L., Rubinov M., Walter H. GraphVar: A user-friendly toolbox for comprehensive graph analyses of functional brain connectivity. J. Neurosci. Methods. 2015;245:107–115. doi: 10.1016/j.jneumeth.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Lancichinetti A., Fortunato S. Consensus clustering in complex networks. Sci. Rep. 2012;2(1):336. doi: 10.1038/srep00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S.F., Schrauf R.W., Fromholt P., Rubin D.C. Inner speech and bilingual autobiographical memory: A Polish-Danish cross-cultural study. Memory. 2002;10(1):45–54. doi: 10.1080/09658210143000218. [DOI] [PubMed] [Google Scholar]
- Lee K., Khoo H.M., Lin J.-M., Dubeau F., Gotman J., Grova C. Disruption, emergence and lateralization of brain network hubs in mesial temporal lobe epilepsy. NeuroImage: Clinical. 2018;20:71–84. doi: 10.1016/j.nicl.2018.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Ji C., Zhu L., Huang P., Jiang B., Xu X., Sun J., Chen Z., Ding M., Zhang M., Wang S. Reorganization of anterior and posterior hippocampal networks associated with memory performance in mesial temporal lobe epilepsy. Clin. Neurophysiol. 2017;128(5):830–838. doi: 10.1016/j.clinph.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Liao W., Zhang Z., Pan Z., Mantini D., Ding J., Duan X., Luo C., Lu G., Chen H., Valdes-Sosa P.A. Altered Functional Connectivity and Small-World in Mesial Temporal Lobe Epilepsy. PLoS ONE. 2010;5(1):e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lœvenbruck H., Grandchamp R., Rapin L., Nalborczyk L., Dohen M., Perrier P., Baciu M., Perrone-Bertolotti M. A cognitive neuroscience view of inner language: To predict and to hear, see, feel. In: Langland-Hassan P., Vicente A., editors. Inner Speech: New Voices. Oxford University Press; 2018. pp. 131–167. [Google Scholar]
- Meilă M. Comparing clusterings—An information based distance. Journal of Multivariate Analysis. 2007;98(5):873–895. doi: 10.1016/j.jmva.2006.11.013. [DOI] [Google Scholar]
- Menenti L., Segaert K., Hagoort P. The neuronal infrastructure of speaking. Brain Lang. 2012;122(2):71–80. doi: 10.1016/j.bandl.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Mennes M., Kelly C., Colcombe S., Castellanos F.X., Milham M.P. The Extrinsic and Intrinsic Functional Architectures of the Human Brain Are Not Equivalent. Cereb. Cortex. 2013;23(1):223–229. doi: 10.1093/cercor/bhs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D., Achard S., Morcom A., Bullmore E. Age-related changes in modular organization of human brain functional networks. NeuroImage. 2009;44(3):715–723. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Meunier D., Lambiotte R., Bullmore E.T. Modular and Hierarchically Modular Organization of Brain Networks. Front. Neurosci. 2010;4 doi: 10.3389/fnins.2010.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr H., Wolfensteller U., Betzel R.F., Mišić B., Sporns O., Richiardi J., Ruge H. Integration and segregation of large-scale brain networks during short-term task automatization. Nat. Commun. 2016;7(1):13217. doi: 10.1038/ncomms13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A.M., Meyer M. Language in the brain at rest: New insights from resting state data and graph theoretical analysis. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh A., Duerden E.G., Pang E.W. The role of the insula in speech and language processing. Brain Lang. 2014;135:96–103. doi: 10.1016/j.bandl.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park H.-J., Friston K. Structural and Functional Brain Networks: From Connections to Cognition. Science. 2013;342(6158):1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Park L., St-Laurent M., McAndrews M.P., Moscovitch M. The immediacy of recollection: The use of the historical present in narratives of autobiographical episodes by patients with unilateral temporal lobe epilepsy. Neuropsychologia. 2011;49(5):1171–1176. doi: 10.1016/j.neuropsychologia.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Phuong T.H., Houot M., Méré M., Denos M., Samson S., Dupont S. Cognitive impairment in temporal lobe epilepsy: Contributions of lesion, localization and lateralization. J. Neurol. 2021;268(4):1443–1452. doi: 10.1007/s00415-020-10307-6. [DOI] [PubMed] [Google Scholar]
- Piai V., Anderson K.L., Lin J.J., Dewar C., Parvizi J., Dronkers N.F., Knight R.T. Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proc. Natl. Acad. Sci. 2016;113(40):11366–11371. doi: 10.1073/pnas.1603312113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional Network Organization of the Human Brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravatà E., Sestieri C., Mantini D., Briganti C., Colicchio G., Marra C., Colosimo C., Tartaro A., Romani G.L., Caulo M. Functional Connectivity MR Imaging of the Language Network in Patients with Drug-Resistant Epilepsy. American Journal of Neuroradiology. 2011;32(3):532–540. doi: 10.3174/ajnr.A2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzner A.B., McAndrews M.P. Network Alterations Supporting Word Retrieval in Patients with Medial Temporal Lobe Epilepsy. J. Cognit. Neurosci. 2011;23(9):2605–2619. doi: 10.1162/jocn.2010.21599. [DOI] [PubMed] [Google Scholar]
- Raemaekers M., Schellekens W., Petridou N., Ramsey N.F. Knowing left from right: Asymmetric functional connectivity during resting state. Brain Struct. Funct. 2018;223:1909–1922. doi: 10.1007/s00429-017-1604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2012;13(10):713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Richardson M.P. Large scale brain models of epilepsy: Dynamics meets connectomics. J. Neurol. Neurosurg. Psychiatry. 2012;83(12):1238–1248. doi: 10.1136/jnnp-2011-301944. [DOI] [PubMed] [Google Scholar]
- Roger E., Pichat C., Torlay L., David O., Renard F., Banjac S., Attyé A., Minotti L., Lamalle L., Kahane P., Baciu M. Hubs disruption in mesial temporal lobe epilepsy. A resting-state fMRI study on a language-and-memory network. Hum. Brain Mapp. 2020;41(3):779–796. doi: 10.1002/hbm.v41.310.1002/hbm.24839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger E., Torlay L., Gardette J., Mosca C., Banjac S., Minotti L., Kahane P., Baciu M. A machine learning approach to explore cognitive signatures in patients with temporo-mesial epilepsy. Neuropsychologia. 2020;142:107455. doi: 10.1016/j.neuropsychologia.2020.107455. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage. 2011;56(4):2068–2079. doi: 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Rzezak P., Lima E.M., Gargaro A.C., Coimbra E., de Vincentiis S., Velasco T.R., Leite J.P., Busatto G.F., Valente K.D. Everyday memory impairment in patients with temporal lobe epilepsy caused by hippocampal sclerosis. Epilepsy Behav. 2017;69:31–36. doi: 10.1016/j.yebeh.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Rzucidlo J.K., Roseman P.L., Laurienti P.J., Dagenbach D., Zhan W. Stability of Whole Brain and Regional Network Topology within and between Resting and Cognitive States. PLoS ONE. 2013;8(8):e70275. doi: 10.1371/journal.pone.0070275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlbauer A.M., Ekstrom A.D. Flexible network community organization during the encoding and retrieval of spatiotemporal episodic memories. Network Neurosci. 2019;3(4):1070–1093. doi: 10.1162/netn_a_00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadone S., Della Penna S., Sestieri C., Betti V., Tosoni A., Perrucci M.G., Romani G.L., Corbetta M. Dynamic reorganization of human resting-state networks during visuospatial attention. Proc. Natl. Acad. Sci. 2015;112(26):8112–8117. doi: 10.1073/pnas.1415439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Betzel R.F. Modular Brain Networks. Annu. Rev. Psychol. 2016;67(1):613–640. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker R. Common ground. Linguist. Philos. 2002;25(5/6):701–721. [Google Scholar]
- Stanley M.L., Simpson S.L., Dagenbach D., Lyday R.G., Burdette J.H., Laurienti P.J., He Y. Changes in Brain Network Efficiency and Working Memory Performance in Aging. PLoS ONE. 2015;10(4):e0123950. doi: 10.1371/journal.pone.012395010.1371/journal.pone.0123950.g00110.1371/journal.pone.0123950.t00110.1371/journal.pone.0123950.t002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby C., Masterton R., Huang J., Jackson G., Abbott D. Resting state functional connectivity changes induced by prior brain state are not network specific. NeuroImage. 2015;106:428–440. doi: 10.1016/j.neuroimage.2014.11.037. [DOI] [PubMed] [Google Scholar]
- Thom, M., & Bertram, E. H. (2012). Temporal lobe epilepsy. In Handbook of Clinical Neurology (Vol. 107, pp. 225–240). Elsevier. https://doi.org/10.1016/B978-0-444-52898-8.00014-8. [DOI] [PubMed]
- Tomasi D., Wang R., Wang G.-J., Volkow N.D. Functional Connectivity and Brain Activation: A Synergistic Approach. Cereb. Cortex. 2014;24(10):2619–2629. doi: 10.1093/cercor/bht119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy J.I., Boswell S.B. Mesial temporal lobe epilepsy: A model for understanding the relationship between language and memory. In: Stemmer B., Whitaker H.A., editors. Handbook of the Neuroscience of Language. Elsevier; 2008. pp. 319–328. [Google Scholar]
- Tramoni-Negre E., Lambert I., Bartolomei F., Felician O. Long-term memory deficits in temporal lobe epilepsy. Revue Neurologique. 2017;173(7–8):490–497. doi: 10.1016/j.neurol.2017.06.011. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Stam C.J., Kahn R.S., Hulshoff Pol H.E. Efficiency of Functional Brain Networks and Intellectual Performance. J. Neurosci. 2009;29(23):7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Maas H.L.J., Dolan C.V., Grasman R.P.P.P., Wicherts J.M., Huizenga H.M., Raijmakers M.E.J. A dynamical model of general intelligence: The positive manifold of intelligence by mutualism. Psychol. Rev. 2006;113(4):842–861. doi: 10.1037/0033-295X.113.4.842. [DOI] [PubMed] [Google Scholar]
- Vaughan D.N., Rayner G., Tailby C., Jackson G.D. MRI-negative temporal lobe epilepsy: A network disorder of neocortical connectivity. Neurology. 2016;87(18):1934–1942. doi: 10.1212/WNL.0000000000003289. [DOI] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Hervé P.-Y., Jobard G., Petit L., Crivello F., Mellet E., Zago L., Mazoyer B., Tzourio-Mazoyer N. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? NeuroImage. 2011;54(1):577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Vlooswijk M.C.G., Jansen J.F.A., Majoie H.J.M., Hofman P.A.M., de Krom M.C.T.F.M., Aldenkamp A.P., Backes W.H. Functional connectivity and language impairment in cryptogenic localization-related epilepsy. Neurology. 2010;75(5):395–402. doi: 10.1212/WNL.0b013e3181ebdd3e. [DOI] [PubMed] [Google Scholar]
- Waites A.B., Briellmann R.S., Saling M.M., Abbott D.F., Jackson G.D. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann. Neurol. 2006;59(2):335–343. doi: 10.1002/ana.v59:210.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Wang S., Van der Haegen L., Tao L., Cai Q. Brain Functional Organization Associated With Language Lateralization. Cereb. Cortex. 2019;29(10):4312–4320. doi: 10.1093/cercor/bhy313. [DOI] [PubMed] [Google Scholar]
- Wang Z., Liu J., Zhong N., Qin Y., Zhou H., Li K. Changes in the brain intrinsic organization in both on-task state and post-task resting state. NeuroImage. 2012;62(1):394–407. doi: 10.1016/j.neuroimage.2012.04.051. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Pearson; 2008. Wechsler Adult Intelligence Scale—Fourth Edition. [Google Scholar]
- Wechsler, D. (2009). Wechsler Memory Scale—Fourth Edition. Pearson.
- Westphal A.J., Wang S., Rissman J. Episodic Memory Retrieval Benefits from a Less Modular Brain Network Organization. The Journal of Neuroscience. 2017;37(13):3523–3531. doi: 10.1523/JNEUROSCI.2509-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser H.G., Blume W.T., Fish D., Goldensohn E., Hufnagel A., King D., Sperling M.R., Luders H. Proposal for a New Classification of Outcome with Respect to Epileptic Seizures Following Epilepsy Surgery. Epilepsia. 2001;42(2):282–286. doi: 10.1046/j.1528-1157.2001.4220282.x. [DOI] [PubMed] [Google Scholar]
- Wilke M., Lidzba K. LI-tool: A new toolbox to assess lateralization in functional MR-data. J. Neurosci. Methods. 2007;163(1):128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Yang H., Zhang C., Liu C., Yu T., Zhang G., Chen N., Li K. Brain network alteration in patients with temporal lobe epilepsy with cognitive impairment. Epilepsy Behav. 2018;81:41–48. doi: 10.1016/j.yebeh.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q., Martin R., Fischer-Baum S., Ramos-Nuñez A.I., Ye F., Deem M.W. Brain Modularity Mediates the Relation between Task Complexity and Performance. J. Cognit. Neurosci. 2017;29(9):1532–1546. doi: 10.1162/jocn_a_01142. [DOI] [PubMed] [Google Scholar]
- Zamora-Lopez G., Russo E., Gleiser P.M., Zhou C., Kurths J. Characterizing the complexity of brain and mind networks. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2011;369(1952):3730–3747. doi: 10.1098/rsta.2011.0121. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lu G., Zhong Y., Tan Q., Liao W., Chen Z., Shi J., Liu Y. Impaired perceptual networks in temporal lobe epilepsy revealed by resting fMRI. J. Neurol. 2009;256(10):1705–1713. doi: 10.1007/s00415-009-5187-2. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lu G., Zhong Y., Tan Q., Yang Z., Liao W., Chen Z., Shi J., Liu Y. Impaired attention network in temporal lobe epilepsy: A resting FMRI study. Neurosci. Lett. 2009;458(3):97–101. doi: 10.1016/j.neulet.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Zhao F., Kang H., Llbo Y., Rastogi P., Venkatesh D., Chandra M. Neuropsychological deficits in temporal lobe epilepsy: A comprehensive review. Annals of Indian Academy of Neurology. 2014;17(4):374–382. doi: 10.4103/0972-2327.144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, D., Lynn, C. W., Cui, Z., Ciric, R., Baum, G. L., Moore, T. M., Roalf, D. R., Detre, J. A., Gur, R. C., Gur, R. E., Satterthwaite, T. D., & Bassett, D. S. (2020). Efficient Coding in the Economics of Human Brain Connectomics. ArXiv:2001.05078 [q-Bio]. http://arxiv.org/abs/2001.05078. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In line with the approval of the local ethic committee (CPP: 09-CHUG-14, 04/06/2009 and 2017-A00384-49), all participants were assured raw data would remain confidential and would not be shared. The codes of the main analyses can be found at the following link https://cloud.univ-grenoble-alpes.fr/index.php/s/JBfGGMGaobqQH7P and authors can provide all additional information upon request.