Abstract
Social spiders are thought to predominantly receive information about their environment through vibrational cues. Thus, group living introduces the challenge of distinguishing useful vibrational information from the background noise of nestmates. Here we investigate whether spatial proximity between colony-mates may allow social spiders (Stegodyphus dumicola) to reduce background noise that might obstruct vibrational information from prey. To do so, we constructed experimental colonies and measured whether the number of spiders in proximity to one another whilst resting could predict the number of spiders that participated in prey capture. Additionally, we exposed spider colonies to five different simulated vibrational cues mimicking prey to determine which cue types spiders were most responsive to. We found that the number of spiders huddled together prior to foraging trials was positively correlated with the number of spiders participating in collective foraging. Furthermore, colonies responded more quickly to pulsed vibrational cues over other types of vibrational patterns. Together these data reveal that both social interactions and prey cues shape how social sit-and-wait predators experience and respond to their environment.
Keywords: collective action, communication, foraging, seismic cues, sociality, spiders
Introduction
A spider’s web serves many important functions. Primarily, the web acts as a net trap to ensnare and retain prey (Chacon and Eberhard 1980; Scharf et al. 2011). However, the web also acts to extend the spider’s senses, interfacing them with the surrounding environment. Through vibrational cues transmitted along their webs, spiders receive important information about their environment, such as the size, type, and distance of prey items in their webs, the presence and/or types of nearby predators, the presence and/or sex of nearby conspecifics, and more (Barth 1982). The majority of spiders that construct a capture web are solitary (Lubin and Bilde 2007), and therefore the vibrations they experience are either from prey, enemies, potential mates, or environmental noise. A small minority of web-building spiders, however, live in cooperative groups (Avilés and Guevara 2017; Pruitt and Avilés 2017) which can add noise from colony mates when interpreting vibrational cues.
Group living in spiders is rare, but has evolved several times to allow successful capture of large and infrequent prey items that would otherwise be impossible for a single spider to subdue unassisted (Whitehouse and Lubin 2005; Lubin and Bilde 2007; Powers and Aviles 2007; Yip et al. 2008). Although group living may solve this particular problem, it also introduces obstacles that are not encountered by solitary living spiders. One major obstacle that needs to be overcome in the transition from solitary to group living is the ability to distinguish prey cues from background vibrations caused by colony mates. Furthermore, there is need to coordinate the collective prey capture dynamics of the group. Some spiders appear to have solved this problem by approaching prey using pulses of movement interspersed with collective quiescence, which allows the group to reorient towards prey without signal jamming (Krafft and Pasquet 1991). Other observations suggestion that some species of social spider use vibratory recruitment signals to attract colony mates to assist with prey capture (Bradoo 1980). However, apart from these studies, very little is known about how these cooperative sit-and-wait predators sense and respond to their vibratory world.
Stegodyphus dumicola is a social spider native to southern Africa which lives in colonies containing up to several hundred individuals (Parthasarathy and Somanathan 2018), and they construct dense three-dimensional silken retreats with one to several two-dimensional capture webs radiating away from it (Seibt and Wickler 1990). While in their retreats, the spiders tend to rest in groups in which they interact and are often in physical contact (Hunt et al. 2018). When a prey item becomes ensnared, vibratory cues from the struggling prey recruit spiders from the retreat to the capture web, where they cooperatively subdue and eventually consume the prey (Whitehouse and Lubin 1999; Amir et al. 2000). Unlike many social insects and some social spiders, all individuals in S. dumicola colonies are the same age and thus do not exhibit any age-related division of labor such as temporal polyethism (Seeley 1982) or repertoire expansion (Seid and Traniello 2006).
Collective actions in many taxa are coordinated through local social interactions. For many animals, spatial proximity is necessary for a social interaction to occur and therefore the spatial organization of individuals can impact the collective behavior of a group (Pinter-Wollman et al. 2017a). In S. dumicola, resting interactions up to four days before a collective prey attack can impact an attack’s efficiency (Hunt et al. 2019). In short, greater connectivity among adult nestmates immediately prior to an attack increased attack speed, possibly due to higher rates of information sharing through vibrations between individuals. It is unknown, however, if colony size influences resting networks although we know that individuals in smaller colonies are more likely to participate in collective prey capture. Furthermore, nest structures that facilitate close social proximity lead to more swift and efficient collective prey capture (Modlmeier et al. 2014a) and when colonies are fragmented between more than one nest structure, the structure with the most individuals capture prey fastest (Najm et al. 2019). Therefore, here we aim to test the hypothesis that participation in prey capture will scale positively with the number of individuals resting together prior to the prey capture event.
Social interactions, however, are not be the only property that influences how or whether a colony attacks prey. Previous studies in S. dumicola have shown that the mere presence of predator cues (e.g. vibratory, olfactory, etc.) can greatly reduce both the speed and number of spiders during prey attack (Wright et al. 2017). Additionally, vibrational cues communicate information to spiders about the identity of the ensnared item, like whether it is innocuous prey or a dangerous ant predator (Keiser et al. 2015; Pruitt et al. 2016; Wright et al. 2016). Experiments have further shown that S. dumcola colonies can be trained to associate different vibrational cues with prey and predators, and respond appropriately (Pruitt et al. 2016), and that the personality distribution of groups may also determine aspects of collective behavior (Beleyur et al. 2015; Wright et al. 2015; Parthasarathy et al. 2019). Thus, it is clear that both contact-based interactions as well as vibrational cues are important for determining collective hunting behavior (Pruitt et al. 2016; Hunt et al. 2019). Therefore, the second hypothesis that we test here is that different vibrational cues will elicit different collective hunting behavior, and we predict that pulsed vibratory cues will elicit greater attack proficiency than continuous or more random vibratory cues because pulsed cues may give the attackers updated information about the prey’s location, similar to the synchronized pausing behavior mentioned earlier.
Methods
Animal collection
Resting interactions
In the summer of 2015 naturally-occurring S. dumicola colonies were collected from roadside fences and bushes around Upington (28°27′22″S 21°14′7″E), located in the Northern Cape of South Africa. Colonies were then brought back to the laboratory at University of Pittsburgh where the experiments were performed.
Vibration cues
In November of 2015 we collected naturally-occurring S. dumicola colonies (N = 7) from roadside fences and bushes around the cities of Upington (28°27′22″S 21°14′7″E) and Groblershoop (28°53′50″S 21°59′4″E) in the Northern Cape of South Africa. Colonies were then taken to an apartment in Upington where the experiment was performed.
Colony construction
Resting interactions
We set up 23 colonies of six subadult spiders in 1L clear plastic cups containing three twigs of Acacia mellifera in a criss-cross pattern for the spiders to use as a substrate for constructing uniform capture webs. Average prosoma widths of all experimental colonies combined were 2.21 mm, and average prosoma widths for each experimental colony (mean of six spiders) ranged from 1.38 mm to 2.57 mm. Spiders from different source colonies were never mixed to maintain natural levels familiarity and relatedness between individuals (Modlmeier et al. 2014b; Laskowski et al. 2016). Colonies were given 48 hours to construct capture webs prior to prey attack assays. Colonies were fed one cricket each day as part of each foraging trial.
Vibration cues
From the seven original source colonies we created 49 experimental groups each containing 10 subadult spiders of similar size by haphazardly selecting spiders from the same source colony and placing them into new containers (300 ml parfait cups) containing three A. mellifera twigs for the spiders to use as a substrate for constructing capture webs. Capture web area was uniform for each colony because they were constructed on the rim of each cup, which each had a rim diameter of 8 cm. Thus the area for each capture web was approximately 50 cm2. Spiders were not fed during this experiment given its short duration.
Collective prey attack
Resting interactions
In order to standardize hunger levels, each colony was fed three crickets before the start of the experiment and then starved for a week. We tested each colony’s collective foraging 5 times by placing a cricket in the center of the capture web and measuring the latency until the first spider made contact with the prey as well as the number of attackers in the capture web during the attack. Before each foraging assay, we recorded the number of spiders that were resting in physical contact with one another inside of the retreat. The latency to attack, number of attackers, and number of spiders in physical contact with one another were averaged over these 5 trials, and these averages were used as our final colony scores quantifying prey attack behavior and resting interactions. These tests were performed once daily over five days, and ordered haphazardly each day.
Vibration cues
Once capture webs were constructed, colonies were randomly assigned to receive one of 5 vibrational cues as follows: (1) continuous vibration (N = 10); (2) short pulsed vibrations (4 pulses/sec (N = 9)); (3) long pulsed vibrations (2 pulses/sec (N = 10)); (4) vibration on for a random period between 0.5–1 seconds and off for 2 seconds (random on/off) (N=10); and (5) vibration on for a random period between 0.5–1.5 seconds and off for a random period between 0.5–1.5 seconds (N = 10). These vibrations are meant to mimic a variety of prey items from winged insects that buzz more continuously (e.g. bees and flies) (vibrations 1–3) or intermittently (e.g. moths and butterflies) (vibration 4) to insects that may give off more irregular vibratory cues such as beetles or ants (vibration 5). Vibrations 1, 4, and 5 were performed using an Arduino as in (Pinter-Wollman et al. 2017b) (see supplementary document for code), and vibrations 2 and 3 were performed using different settings on a hand-held vibratory device (Magic Purple Bullet). We discuss the potential caveats of using two different devices in the discussion.
Prey capture assays were performed by placing a small piece of white paper (1 cm2) in the center of the capture web and vibrating it with the instruments mentioned above. The devices do not come into contact with the web, just the paper. These vibrations elicited an attack response from 100% of spider colonies. We recorded latency until the contact of the first spider with the paper (latency to attack), as well as the total number of spiders participating in the attack sequence at the moment the first spider made contact (number of attackers). Collective foraging assays were performed 4 times on each group over the course of 4 days, once daily at 11:00 am. We were not able to perform the assays blind, as the vibrations produced by each device are distinct. However, observing latency to attack and the number of attackers responding is straightforward and not overtly susceptible to biased interpretation. The average of these 4 trials were used as our measure of colony responsiveness.
Statistical methods
Resting interactions
To determine the effect of resting interactions on prey attack dynamics, average latency to attack or average number of attackers were used as the dependent variables in general linear mixed models (GLMM) with the fixed effect of average number of spiders resting with one another, and the random effect of experimental colony ID nested in source colony ID.
Vibration cues
To determine the effect of vibratory cue on prey capture behavior, we conducted two GLMM tests with average latency to attack or average number of attackers as dependent variables, and the type of vibratory stimulus as a fixed effect. Experimental colony ID was nested within source colony ID and set as random effects. Differences between treatments were analyzed using post hoc Tukey tests.
We also re-ran the same models on the three treatments using the Arduinos (continuous, random on/off, and random treatments) and separately on the two treatments using the Magic Purple Bullet (long pulse and short) to control for device bias. Post hoc Tukey tests were then performed to see if patterns of significance remained for the vibratory cues in each device.
All statistical analysis was conducted in the software JMP Pro 14.1 (SAS reference).
Results
Resting interactions
Colonies in which more spiders were resting in contact had more individuals that participated in collective foraging: (F1,14.19 = 13.93, p = 0.002; Fig. 1). On day one of observation, 83% of attackers were found to be part of the resting groups, and in 88% of cases the first spider to attack was part of the resting group. The number of spiders resting in contact, however, did not predict colonies’ latency to attack prey: (F1,14.99 = 0.35, p = 0.57).
Figure 1.
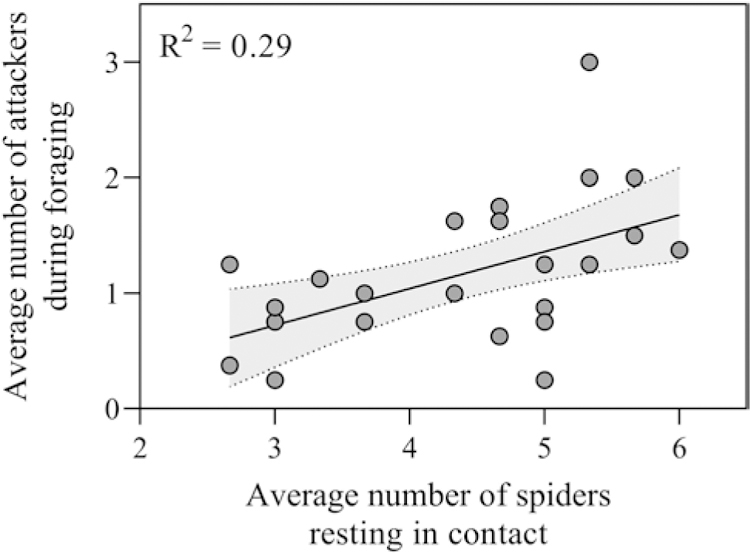
Relationship between the number of spiders resting in contact prior to foraging and the average number of spiders attacking during foraging. The line represents the best fit regression and the shaded area is the 95% confidence intervals.
Vibration Cues
We found significant effects of vibratory cue on both latency to attack (F4,49 = 9.79, p = 0.0006) and the number of attackers (F4,49 = 10.03, p = 0.0005). For latency to attack, descriptively, the random on/off treatment resulted in the slowest attacks (longest latencies) followed by random, continuous, short pulse, and finally long pulse, though not all of these differences were significant. Please see Fig. 2A for the significant differences. The random on/off treatment is characterized by brief vibrations interspersed with longer pauses. For the number of attackers, the random cue resulted in over 60% more attackers than all cues except for the continuous cue (Fig. 2B).
Figure 2.
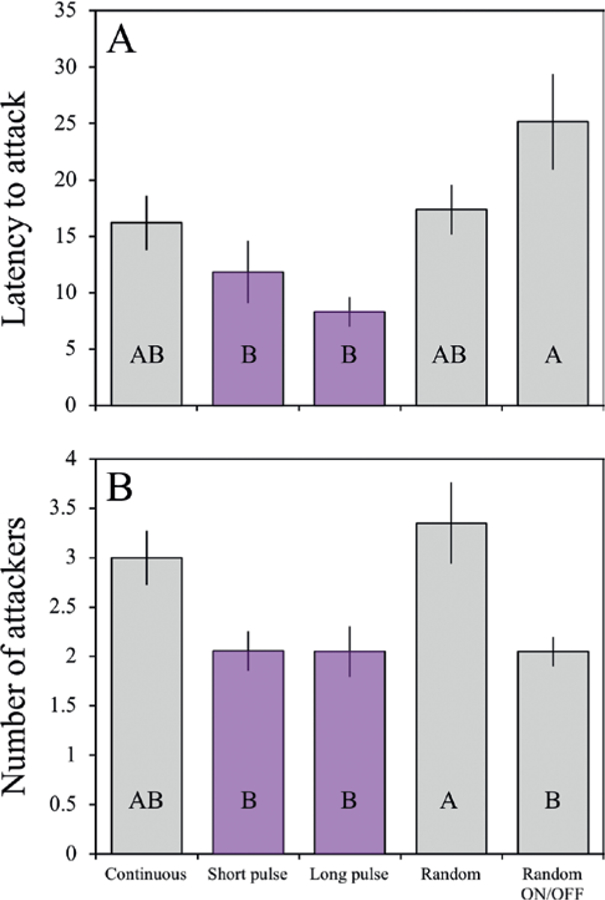
The effect of vibratory cue on collective foraging measured as latency to attack in seconds (A) and total number of attackers (B). Error bars depict standard error. Purple bars refer to cues delivered by the Magic Purple Bullet vibratory device, and grey bars refer to cues given by Arduino devices. Bars that do not share a letter reflect statistically significant differences using a post hoc Tukey test.
For the treatments using either Arduinos or the Magic Purple Bullet in isolation, intra-device post hoc Tukey tests fully recapitulated the patterns of significance in the first analysis that included all vibratory cues regardless of the device used.
Discussion
The speed and voracity with which social predators attack prey can be jointly influenced by social dynamics in the predator and cues from the prey. Our findings support our first hypothesis that greater number of resting interactions, in the moments prior to an attack enhances attack intensity in social spider colonies (Fig. 1). We also found support for our second hypothesis: Different vibrational cues elicited different collective prey attack responses. In particular, pulsed vibratory prey cues resulted in faster attack speeds, whereas random and continuous cues elicited more attackers (Fig. 2).
There are several reasons why group cohesion may influence the voracity in which S. dumicola responds to prey cues. The first has to do with information transfer. Because S. dumicola lives in colonies often containing hundreds of other individuals, the ability of nestmates to both send and receive information about potential prey is central to survival, as failing to respond quickly and forcefully to large and infrequent prey could be extremely costly. Given that most information for social spiders is vibrational in nature, adaptations that increases the reliability of vibrational information should be favored. Interacting with nestmates may be one such method of ensuring information reliability, as the movement of nearby conspecifics responding to perceived prey cues and catalyzing a prey response in their neighbors may result in a faster and larger prey response than would occur if every spider ignored social cues and simply acted individually in response to perceived prey cues (Treherne and Foster 1981). The degree of group cohesion, then, may undergird the dynamics of social influence and susceptibility observed in this species (Pinter-Wollman et al. 2016; Pruitt et al. 2018). Additionally, responding to conspecific social cues may help dilute risk (Foster and Treherne 1981), especially for potentially dangerous prey items like wasps, ants, and other well-defended venomous species commonly found in S. dumicola capture webs that can occasionally kill attacking spiders (Wright et al. pers. obs). It is notable that we did not find a relationship between resting interactions and latency to attack. This may be due to the size of the containers used, which may not have allowed for substantial variation between colonies to emerge, or resting interactions may just cause others to join in an attack and thus may not influence attack speed per se.
We further found that colonies attacked more swiftly in response to steady, pulsed vibratory cues than random and nearly continuous (Fig. 2). In Anelosimus eximius social spiders, individuals participating in collective attacks exhibit a synchronized stop-and-go behavior called synchronized pausing (Krafft and Pasquet 1991). Iterative pauses during attack have been observed in solitary species as well, and the behavior is thought to give the spider a brief moment to update their information about the prey’s location and reorient (Mielle 1978). In social spiders, however, this is not straightforward because the presence of often several hundred nestmates can contribute to vibrational noise. Therefore, at least one species of social spider (A. eximius) has evolved the ability to collectively synchronize their pauses to give attacking spiders a “silent” moment to reorient to prey cues (Krafft and Pasquet 1991). Whether this behavior is present in other social spiders is currently unknown, but differences in the web structure and individual size between A. eximius and S dumicola may result in different ways to achieve the same noise-cancelling effect. Our finding that prey cues composed of regular long and/or short pulsed vibrations elicit the swiftest attack responses suggests that short pauses in prey cues may allow the spiders to have those ‘silent’ moment. In addition, it is possible that these pulsed vibrations are more similar to vibrations of struggling prey, rather than the consistent cues of something like wind or the constant movement of co-attacking conspecifics. Given that synchronized pausing has not been observed in S. dumicola, this species may rely more heavily on pauses in their prey’s movement, rather than their nestmates, to reorient and to differentiate prey cues from other vibratory noise. A reliance on prey cues for predators have been observed across a broad range of animal taxa (Peake 2005; Creel and Christianson 2008).
For the number of attackers, the random treatment elicited the greatest number of attackers, while all other vibratory cues except for the continuous treatment exhibited only two-thirds the number of attackers. For the random cue, we observed that spiders were constantly being recruited and then abandoning the chase when the vibrations stopped, resulting in an ever-growing number of seemingly interested and alert spiders being recruited to the capture web, but then taking considerable time to discover the source. Another qualitative observation was that the random vibration cue resulted in a slower attack speed too, because attackers so often abandon their pursuit in these cases. Conversely, treatments where spiders responded more quickly had less time for other spiders to be recruited, which explains the fewer number of attackers in response to pulsed vibrational cues. Thus, the number of attackers per se may not always be a reliable indicator of attack proficiency. A potentially important caveat to our findings stems from the use of two different types of vibratory devices to deliver vibratory cues to spider colonies. Because of this, we cannot rule out the possibility that our results may be driven by the spiders favoring vibratory properties, such as vibration intensity, which was stronger in the device delivering the pulsed cues (Magic Purple Bullet) compared with the device delivering all other cues (Arduino).
To conclude, our findings show that both interactions and vibratory cues are important for response to prey by social sit and wait predators. More investigations about the role of resting interactions in social spiders would be particularly useful for understanding how social groups optimize collective hunting speed and magnitude, and the underlying mechanisms that cause groups with greater contact to behave more aggressively. Moreover, studies investigating the way various social spider taxa respond to an even wider spectrum of vibrational types and intensities will help to shape our understanding of the unique adaptations to group living social spiders have made to efficiently hunt in a social group. Studies that can detect and recreate (via playbacks) the vibratory cues of prey and conspecifics will be particularly useful in understanding these animals’ vibratory world.
Supplementary Material
References
- Amir N, Whitehouse MEA, Lubin Y (2000) Food consumption rates and competition in a communally feeding social spider, Stegodyphus dumicola (Eresidae). J Arachnol 28:195–200. [Google Scholar]
- Avilés L, Guevara J (2017) Sociality in Spiders. Cambridge University Press, Cambridge (UK). [Google Scholar]
- Barth FG (1982) Spiders and vibratory signals: sensory reception and behavioral significance. In: Witt PN, Rovner J (eds) Spider communication: mechanisms and ecological significance. Princeton University Press, Princeton, New Jersey, pp 67–120. [Google Scholar]
- Beleyur T, Bellur DU, Somanathan H (2015) Long-term behavioural consistency in prey capture but not in web maintenance in a social spider. Behav Ecol Sociobiol 69:1019–1028. [Google Scholar]
- Bradoo BL (1980) Feeding behaviour and recruitment display in the social spider Stegodyphus sarasinorum Karsch (Araneae,Eresidae). Tijdschr Entomol 123:89–104. [Google Scholar]
- Chacon P, Eberhard WG (1980) Factors affecting numbers and kinds of prey caught in artificial spider webs, with considerations of how orb webs trap prey. Bull Br Arachnol Soc 5:29–38. [Google Scholar]
- Creel S, Christianson D (2008) Relationships between direct predation and risk effects. Trends Ecol Evol 23:194–201. [DOI] [PubMed] [Google Scholar]
- Foster WA, Treherne JE (1981) Evidence for the dilution effect in the selfish herd from fish predation on a marine insect. Nature 293:466–467. [Google Scholar]
- Hunt ER, Mi B, Fernandez C, Wong BM, Pruitt JN, Pinter-Wollman N (2018) Social interactions shape individual and collective personality in social spiders. Proceedings of the Royal Society B: Biological Sciences 285:p.20181366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt ER, Mi B, Geremew R, Fernandez C, Wong BM, Pruitt JN, Pinter-Wollman N (2019) Resting networks and personality predict attack speed in social spiders. bioRxiv:p.591453. [DOI] [PMC free article] [PubMed]
- Keiser CN, Wright CM, Pruitt JN (2015) Warring arthropod societies: Social spider colonies can delay annihilation by predatory ants via reduced apparency and increased group size. Behav Processes 119:14–21. [DOI] [PubMed] [Google Scholar]
- Krafft B, Pasquet A (1991) Synchronized and rhythmical activity during the prey capture in the social spider Anelosimus eximius (Araneae, Theridiidae). Insectes Soc 38:83–90. [Google Scholar]
- Laskowski KL, Montiglio P, Pruitt JN (2016) Individual and group performance suffers from social niche disruption. Am Nat 187. [DOI] [PubMed] [Google Scholar]
- Lubin Y, Bilde T (2007) The evolution of sociality in spiders. Advances in the Study of Behavior, 37:83–145. [Google Scholar]
- Mielle D (1978) Contribution à l’étude du comportement prédateur et des mécanismes de tolérance dans le genre Tegenaria (Araneae, Agelenidae: ). In: (Vol 595, No M5). Universite de Nancy I. [Google Scholar]
- Modlmeier AP, Forrester NJ, Pruitt JN (2014a) Habitat structure helps guide the emergence of colony-level personality in social spiders. In, Behavioral Ecology and Sociobiology.
- Modlmeier AP, Laskowski KL, DeMarco AE, Coleman A, Zhao K, Brittingham HA, McDermott DR, Pruitt JN (2014b) Persistent social interactions beget more pronounced personalities in a desert-dwelling social spider. Biology Letters, pp 2014–19. [DOI] [PMC free article] [PubMed] [Retracted]
- Najm GM, Pe A, Pruitt JN, Pinter-Wollman N (2019) Physical and social cues shape nest site preference and prey capture behavior in social spiders. [DOI] [PMC free article] [PubMed]
- Parthasarathy B, Joshi CH, Kalyadan SS, Somanathan H (2019) Early ontogenic emergence of personality and its long-term persistence in a social spider. Behav Ecol Sociobiol 73:35. [Google Scholar]
- Parthasarathy B, Somanathan H (2018) A method for accurately estimating social spider numbers without colony damage. The Journal of Arachnology 46:373–376. [Google Scholar]
- Peake TM (2005) Eavesdropping in communication networks. In: McGregor P (ed) Animal Communication Networks. Cambridge University Press, Cambridge, UK, pp 13–37. [Google Scholar]
- Pinter-Wollman N, Fiore SM, Theraulaz G (2017a) The impact of architecture on collective behaviour. Nature Ecology and Evolution 1:1–2. [DOI] [PubMed] [Google Scholar]
- Pinter-Wollman N, Keiser CN, Wollman R, Pruitt JN (2016) The Eeffect of keystone individuals on collective outcomes can be mediated through interactions or behavioral persistence. Am Nat 188:240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter-Wollman N, Brian Mi, Pruitt JN (2017b) Replacing bold individuals has a smaller impact on group performance than replacing shy individuals. Behav Ecol.
- Powers KS, Aviles L (2007) The role of prey size and abundance in the geographical distribution of spider sociality. J Anim Ecol 76:995–1003. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Avilés L (2017) Social spiders: mildly successful social animals with much untapped research potential. Anim Behav.
- Pruitt JN, Wright CM, Keiser CN, DeMarco AE, Grobis MM, Pinter-Wollman N (2016) The Achilles’ heel hypothesis: mis-informed keystone individuals impair collective learning and reduce group success. Proceedings of the Royal Society B-Biological Sciences 283. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pruitt JN, Wright CM, Lichtenstein JLL, Chism GT, McEwen BL, Kamath A, Pinter-Wollman N (2018) Selection for collective aggressiveness favors social susceptibility in social spiders. Curr Biol 28:100–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf I, Lubin Y, Ovadia O (2011) Foraging decisions and behavioural flexibility in trap-building predators: a review. Biological Reviews 86:626–639. [DOI] [PubMed] [Google Scholar]
- Seeley TD (1982) Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol 11: 287–293. [Google Scholar]
- Seibt U, Wickler W (1990) The protective function of the compact silk nest of social Stegodyphus spiders (Araneae, Eresidae). Oecologia 82:317–321. [DOI] [PubMed] [Google Scholar]
- Seid MD, Traniello JF (2006) Age-related repertoire expansion and division of labor in Pheidole dentata (Hymenoptera: Formicidae): a new perspective on temporal polyethism and behavioral plasticity in ants. Behav Ecol Sociobiol 60:631–644. [Google Scholar]
- Treherne JE, Foster WA (1981) Group transmission of predator avoidance behaviour in a marine insect: the Trafalgar effect. Anim Behav, pp 911–917.
- Whitehouse MEA, Lubin Y (1999) Competitive foraging in the social spider Stegodyphus dumicola. Anim Behav 58:677–688. [DOI] [PubMed] [Google Scholar]
- Whitehouse MEA, Lubin Y (2005) The functions of societies and the evolution of group living: spider societies as a test case. Biological Reviews 80:347–361. [DOI] [PubMed] [Google Scholar]
- Wright CM, Keiser CN, Pruitt JN (2015) Personality and morphology shape task participation, collective foraging and escape behaviour in the social spider Stegodyphus dumicola. Anim Behav 105:47–54. [Google Scholar]
- Wright CM, Keiser CN, Pruitt JN (2016) Colony personality composition alters colony-level plasticity and magnitude of defensive behaviour in a social spider. Animal Behaviour, pp 175–183.
- Wright CM, Lichtenstein JLL, Montgomery GA, Luscuskie LP, Pinter-Wollman N, Pruitt JN (2017) Exposure to predators reduces collective foraging aggressiveness and eliminates its relationship with colony personality composition. 71:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip EC, Powers KS, Aviles L (2008) Cooperative capture of large prey solves scaling challenge faced by spider societies. Proceedings of the National Academy of Sciences of the United States of America 105:11818–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


