Abstract
Purpose:
The aim of this work was to study and document retinal changes in coronavirus disease-2019 (COVID-19) positive patients with nonsevere disease using a nonmydriatic handheld fundus camera.
Methods:
A cross-sectional observational study was conducted on patients affected by COVID-19 who were admitted at our center. Our study included patients with no, mild, and moderate symptoms (nonsevere cases). Intensive care unit (ICU)-admitted patients were excluded considering the difficulty in procuring the fundus image by the handheld camera due to patients positioning. Patients with systemic conditions (diabetes, hypertension, and severe anemia) known to cause retinopathy were also excluded. Bedside anterior segment examination, fundus examination using indirect ophthalmoscopy and fundus imaging of each patient using a nonmydriatic handheld fundus camera was done by a trained ophthalmologist posted for COVID duty.
Results:
In a cohort of 138 patients, 94 (68.1%) were men and 44 (31.9%) were women. A total of 276 eyes were evaluated. The mean age of the patients was 38.51 ± 14.4 years. Anterior segment evaluation showed no abnormality in any of the eyes. On fundus screening using nonmydriatic handheld camera, a single streak of superficial retinal hemorrhage was noted at the posterior pole of the fundus in the left eye of one patient (0.72%), which was away from fovea. Laboratory tests revealed low hemoglobin (between 10 and 10.9 g/dL falling under mild Anemia) in 12 patients, elevated total leucocyte count in 6 patients, raised LDH in majority of patients (323 ± 101 Units/L) and elevated CRP (14.6 ± 30.99 mg/L). Rest of the lab parameters were within the normal range.
Conclusion:
In our study, COVID patients with mild-to-moderate symptoms did not show any inflammatory/infective or vaso-occlusive lesions in the retina attributable to COVID-19 infection, except one patient who had a single streak hemorrhage in the macula away from fovea, probably incidental.
Keywords: COVID-19, fundus findings, ocular findings, retina
Coronavirus disease-2019 (COVID-19) is a novel disease whose outbreak emerged in Wuhan, China in December 2019, and became a pandemic soon.[1] As of September 29, India has reported 61,45,291 cases and 96,318 deaths in total attributed to COVID-19 which makes it the second highest affected country in the world.[1] COVID-19 is a highly contagious disease capable of causing severe dysfunction of respiratory, immune, and other organ systems resulting in significant morbidity and mortality.[2,3,4,5,6]
Conjunctivitis has been reported to be the primary ocular manifestation of COVID-19, with prevalence ranging from 0.8% to 31.6% of patients.[7] In addition, retinal disorders, such as retinal vasculitis,[8] retinal degeneration and blood–retinal barrier breakdown,[8] retinitis, and optic neuritis[8] have been demonstrated in experimental animal models of coronavirus infection.
Although recent data suggest that COVID-19 infection may be associated with changes in immune and coagulation systems and possible viral spread through the blood–brain barrier, with clinical and pathological findings of disseminated intravascular coagulopathy (DIC), the effects of these changes on the eye, especially retina, have never been studied.[9,10,11,12] We aim to report ocular findings and fundus changes if any in COVID-19 positive patients with no, mild, and moderate symptoms (nonsevere cases).
Methods
The observational cross-sectional study was conducted at COVID wards of our tertiary care hospital. A study was conducted on a patient cohort of SARS-CoV-2 RTPCR positives admitted between August 1, 2020 to August 31, 2020. Institutional Ethical Committee approval was obtained before the start of the study, and the study was conducted in accordance with the declaration of Helsinki. Informed consent was taken from all subjects who were willing to participate in the study.
The patients clinical history and investigations were noted. All symptomatic patients diagnosed with COVID-19 who were admitted in COVID wards, and who were willing to give consent for studying and allowing to record fundus images were included in the study. The exclusion criteria of the study were patients with hazy media/nonreadable image details due to cataract; patients with systemic conditions known to cause retinopathy like diabetes, hypertension, and severe anemia; ICU-admitted patients considering the difficulty in procuring the fundus image by the handheld camera due to patients positioning (COVID patients admitted in our ICU were given prone position for maximum hours).
Ocular examination for anterior segment redness/conjunctivitis was carried out at the bedside using torchlight and fundus images were taken using a nonmydriatic handheld fundus camera (Non Mydriatic Fundus On Phone [NMFOP] Remidio Innovative Solutions Pvt. Ltd., Bengaluru, India), with the patient in sitting position [Fig. 1a]. NMFOP device has a static field of view of 40°. Fundus screening was done to the extent possible using indirect ophthalmoscopy (IDO) on all patients for any changes in the peripheral retina. For every patient included in the study, we collected demographic data, systemic and ocular history, laboratory test results (Spo2, hemoglobin [Hb], total leucocyte count [TLC], LDH, C-reactive proteins [CRP], and serum ferritin levels), and ocular findings.
Figure 1.
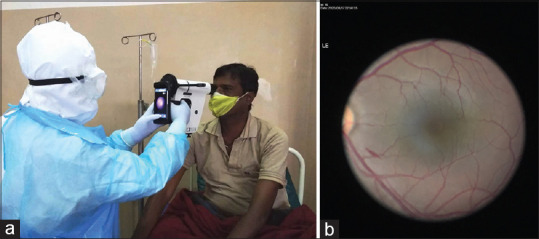
(a). Recording fundus image using a nonmydriatic handheld fundus camera in COVID ward. (b). Showing single streak hemorrhage in the posterior pole in left eye. The peripheral retina of this patient on IDO was unremarkable
Statistical analysis
Data were entered into the Excel spreadsheet. SPSS (Statistical Package for Social Sciences) version 20. (IBM SPSS statistics [IBM, Armonk, New York released 2011]) was used to perform the statistical analysis. Descriptive statistics of the explanatory and outcome variables were calculated by frequency and percentage for qualitative data. Mean and standard deviation were calculated for quantitative data.
Results
The study included 276 eyes of 138 COVID patients diagnosed by RTPCR for SARS-CoV-2. Of these patients 94 (68.1%) were men and 44 (31.9%) were women. Six (4.34%) of them were health-care workers of our institute who acquired the infection while on COVID duty. The mean age of the patients was 38.51 ± 14.4 years. All patients were stratified into mild or moderate symptoms.[13] Two (1.45%) of patients had anosmia, one (0.72%) had a loss of taste, 39 (28.26%) had fever, 4 (2.92%) had myalgia and body ache, 2 (1.45%) patients had headache, 5 (3.62%) had sore throat, 3 (2.17%) had malaise, and 17 (12.5%) had cough with breathlessness [Table 1]. A total of 65 patients had no systemic symptoms on day of evaluation, 56 had mild symptoms, and 17 had moderate symptoms [Table 2]. None of the patients had previous eye disease. None of them had any ocular complaints at the time of examination. The patients had been hospitalized after a median of 3 days (range 0–8 days) from COVID-19 symptoms onset and ophthalmological screening was performed after a median of 6 days (range 1–9 days).
Table 1.
Showing different systemic symptoms and retinal findings
| Systemic symptoms | Haemorrhage | Nil retinal changes | Total | |
|---|---|---|---|---|
| Frequency | Frequency | Frequency | Percentage | |
| Anosmia | 0 | 1 | 1 | 0.72 |
| Cough with breathlessness | 0 | 17 | 17 | 12.32 |
| Fever | 0 | 39 | 39 | 28.26 |
| Headache | 0 | 2 | 2 | 1.45 |
| loss of smell | 0 | 2 | 2 | 1.45 |
| Malaise | 0 | 3 | 3 | 2.17 |
| Myalgia | 0 | 4 | 4 | 2.90 |
| Sore throat | 0 | 5 | 5 | 3.62 |
| NIL | 1 | 64 | 65 | 47.10 |
| Total | 1 | 137 | 138 | 100.00 |
Table 2.
Showing patients with no, mild and moderate symptoms
| Symptoms on day of fundus evaluation | No of patients. |
|---|---|
| No symptoms | 65 |
| Mild symptoms (URTI, fever, cough, malaise, no evidence of hypoxia, SPO2>94%) | 56 |
| Moderate symptoms (RR -24-26/min, SPO2 90-94% on room air) | 10 |
| Moderate symptoms (dyspnoea, RR-26-30/min; High Flow Nasal Oxygen 40-60 L/min with FiO2 60-80% requirement) | 7 |
None of the eyes showed any abnormality on anterior segment evaluation with IDO and magnifying +20D lens and all eyes had normal pupillary reaction. Fundus screening using a nonmydriatic handheld camera showed a single streak of superficial hemorrhage in left eye of a patient (0.72%) at the posterior pole of the fundus, 2 disc diameter away from the fovea inferiorly adjacent to major vascular arcade [Fig. 1b]. Peripheral fundus examination by IDO was unremarkable for the patient and others. Laboratory tests revealed low hemoglobin (between 10 and 10.9 g/dL, thus mild Anemia) in 12 patients, elevated total leucocyte count in 6 patients, raised LDH in majority of patients (323 ± 101) and elevated CRP (14.6 ± 30.99). Rest of the lab parameters were within the normal range [Table 3]. However, none of the patients with Lab tests derangement showed any retinal changes in this study.
Table 3.
Showing different lab parameters
| Parameter | n | Mean | Std. Deviation |
|---|---|---|---|
| age | 138.00 | 38.51 | 14.40 |
| spO2 | 138.00 | 97.96 | 0.81 |
| Hb | 138.00 | 13.57 | 2.23 |
| TC | 138.00 | 7615 | 3486 |
| LDH | 138.00 | 323 | 101 |
| D-Dimer | 138.00 | 0.42 | 0.46 |
| CRP | 138.00 | 14.60 | 30.99 |
| Ferritin | 138.00 | 220.97 | 205.03 |
Discussion
COVID-19 is a novel disease caused by SARS CoV 2 RNA virus. COVID-19 is known to affect various organ systems in the human body with clinical presentations ranging from asymptomatic disease to severe respiratory failure and untimely death. Thus, eye involvement can be expected in the spectrum of COVID-19 manifestations. Currently, our understanding of the possible ocular complications of SARS-CoV-2 infection is very limited. Known ocular manifestations include conjunctivitis,[14] conjunctival hyperemia, and cotton wool spots.[15] The virus is known to spread primarily through respiratory droplets produced when an infected person coughs or sneezes. Previous studies have reported the presence of the virus in tear samples of COVID patients suggesting tears can be a source of transmission of virus.[16.17] This knowledge is essential amongst the front-line workers to triage any patient with suspected conjunctivitis and attend with proper personal protective equipment and sanitization measures. Therefore proper eye protection with goggles/shield along with N95 mask is recommended when caring for patients potentially infected with COVID-19.
Genomic and structural analyses have shown that the SARS-CoV-2 has a similar receptor-binding-motif as SARS-CoV, which allows it to infect host cells via the Angiotensin-Converting Enzyme 2 (ACE2).[18] The Human eye has its own intraocular RAS (renin-angiotensin system). Given that ACE2 has also been detected in the aqueous humor,[9] human retina, retinal pigment epithelium, choroid, and conjunctival epithelia, further clinical studies are needed more to fully evaluate the clinical spectrum of ocular diseases that can be caused by SARS-CoV-2 infection[19] and if it can cross the blood–retinal barrier to infect the posterior segment.
Compared with the adenovirus and influenza virus, which can frequently cause kerato-conjunctivitis or conjunctivitis, ocular diseases caused by coronavirus are relatively rare.[16] However, conjunctivitis has been reported in the patients infected with SARS-CoV-2.[20] The novel coronavirus RNA was also detected in tears and conjunctival samples from the infected individual.[16,17]
Animal studies have demonstrated the occasional onset of uveitis or retinitis in feline and murine models. This has been described and linked to an underlying autoimmune process inducing vasculitis or to a viral-mediated inflammation.[9,20,22,23] Recent clinical and histopathological studies have attributed the endothelial damage as one of the most prominent causes of the systemic vascular thrombo-embolic and/or inflammatory manifestations of COVID-19. In this setting, the retina as a privileged tissue for noninvasive and in vivo evaluation of systemic diseases may reveal alterations such as vascular occlusion related to the thrombotic susceptibility and chorioretinitis or vasculitis directly mediated by the virus.[24,25,26,27]
At present, there are only a few case reports which described retinal findings in asymptomatic COVID patients. In a small cohort of 12 patients, Marinho et al.[27] reported hyperreflective lesions at the level of ganglion cell and inner plexiform layers more prominently in papillomacular bundles in both eyes on optical coherence tomography (OCT) in four patients. However, optical coherence tomography angiography (OCTA) and Ganglion cell complex were normal. But it remains uncertain whether these hyperreflective lesions are due to viral infections or secondary to the vascular complications/vascular occlusive events of COVID-19. We, unfortunately, could not subject our patients for OCT evaluation.
Chen et al. studied the ocular manifestations in COVID-19 patients who presented with red-eye during his course of illness. In their case report, they reported bilateral acute follicular conjunctivitis. RT-PCR assay demonstrated the presence of viral RNA in the conjunctival specimen and the nonmydriatic fundus camera revealed unremarkable findings. Macular ultra-structure and thickness measured on OCT were within the normal ranges.[19] Although animal models suggest retinal lesions like retinitis and optic neuritis, there are no such reports in humans to date.
Emmanuel Bettach et al. have reported a case of bilateral anterior uveitis in a 54 year old female suspected of multisystem inflammatory syndrome because of COVID-19-related cytokine storm. Though they have not specifically proven the etiology of uveitis with paracentesis, it is still wise to emphasize that we should be aware of possible ocular inflammation secondary to covid-19 infection.[28]
We found unremarkable fundus changes in our cohort except single streak of hemorrhage in one eye. Streak hemorrhage occurs due of blockage of superficial retinal capillary plexus. As our patient did not have any systemic illness/comorbidity or deranged blood parameters, hemorrhage in One case may be unrelated to COVID and may be coincidental. Our study included COVID positive patients with no symptoms, mild and moderate symptoms, in whom viral load may be lesser than those admitted in intensive care units. This remains a limitation of our study.
Conclusion
Ocular evaluation was near normal in our study except for a small capillary blockage resulting in superficial retinal hemorrhage seen on fundus. COVID-19 is thus not a threat to retina in mild-to-moderate cases (nonsevere disease).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. WHO COVID-19 dashboard. Available from: https://COVID-19.who.int .
- 2.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillaine-Barrésyndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–6. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST-segment elevation in patients with COVID-19-A case series. N Engl J Med. 2020;382:2478–80. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Jun IS, Anderson DE, Zheng Kang AE, Wang L, Rao P, Young BE, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127:977–9. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabi YM, Murthy S, Webb S. COVID-19:A novel coronavirus and a novel challenge for critical care. Intensive Care Med. 2020;46:833–6. doi: 10.1007/s00134-020-05955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong RLM, Ting DSW, Wan KH, Lai KHW, Ko CN, Ruamviboonsuk P, et al. COVID-19:Ocular manifestations and the APAO prevention guidelines for ophthalmic practices. Asia Pac J Ophthalmol (Phila) 2020;9:281–4. doi: 10.1097/APO.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin MS, Hooper LC, Hooks JJ, Detrick B. Identification of a-fodrin as an autoantigen in experimental coronavirus retinopathy (ECOR) J Neuroimmunol. 2014;273:42–50. doi: 10.1016/j.jneuroim.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes?A review of coronaviruses and ocular implications in humans and animals. OculImmunolInflamm. 2020;28:391–5. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harenberg J, Favaloro E. COVID-19:Progression of disease and intravascular coagulation –present status and future perspectives. Clin Chem Lab Med. 2020;58:1029–36. doi: 10.1515/cclm-2020-0502. [DOI] [PubMed] [Google Scholar]
- 11.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–40. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voves C, Wuillemin WA, Zeerleder S. International Society on Thrombosis and Haemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patients. Blood Coagul Fibrinolysis. 2006;17:445–51. doi: 10.1097/01.mbc.0000240916.63521.2e. [DOI] [PubMed] [Google Scholar]
- 13.Clinical management protocol:COVID-19. Government of India. Ministry of Health and Family Welfare. Directorate General of Health Services. Version 3 13.06.20. :4–5. [Google Scholar]
- 14.Gandhi A, Sahu Y, Narula Association of Covid-19 with conjunctivitis. J Ophthalmic Clin Res. 2020;7:068. [Google Scholar]
- 15.Oncul H, Oncul FY, Alakus MF, Çaglayan M, Dag U. Ocular findings in patients with coronavirus disease 2019 (COVID-19) in an outbreak hospital. J Med Virol. 2020;93:1126–32. doi: 10.1002/jmv.26412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–94. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar K, Prakash AA, Gangasagara SB, Rathod SB, Ravi K, Rangaiah A, et al. Presence of viral RNA of SARS-CoV-2 in conjunctival swab specimens of COVID-19 patients. Indian J Ophthalmol. 2020;68:1015–7. doi: 10.4103/ijo.IJO_1287_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan:An analysis based on decade-long structural studies of SARS. J Virol. 2020;94:e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020;104:748–751. doi: 10.1136/bjophthalmol-2020-316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty MJ. Ocular manifestations of feline infectious peritonitis. J Am Vet Med Assoc. 1971;159:417–24. [PubMed] [Google Scholar]
- 21.Hök K. Morbidity, mortality and coronavirus antigen in previously coronavirus free kittens placed in two catteries with feline infectious peritonitis. Acta Vet Scand. 1993;34:203–10. doi: 10.1186/BF03548211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins SG, Detrick B, Hooks JJ. Retinopathy following intravitreal injection of mice with MHV strain JHM. Adv Exp Med Biol. 1990;276:519–24. doi: 10.1007/978-1-4684-5823-7_72. [DOI] [PubMed] [Google Scholar]
- 23.Shindler KS, Kenyon LC, Dutt M, Hingley ST, Das Sarma J. Experimental optic neuritis induced by a demyelinating strain of mouse hepatitis virus. J Virol. 2008;82:8882–6. doi: 10.1128/JVI.00920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease:Implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–73. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.England JT, Abdulla A, Biggs CM, Lee AYY, Hay KA, et al. Weathering the COVID-19 storm:Lessons from hematologic cytokine syndromes. Blood Rev. 2021 Jan;45:100707. doi: 10.1016/j.blre.2020.100707. doi:10.1016/j.blre.2020.100707. Epub 2020 May 15. 32425294. 227559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copin MC, Parmentier E, Duburcq T, Poissy J, Mathieu D Lille COVID-19 ICU and Anatomopathology Group. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46:1124–6. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marinho PM, Marcos AA, Romano AC, Nascimento H, Belfort R. Retinal findings in patients with COVID-19. The Lancet. 2020;23:395(10237), 1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettach E, Zadok D, Weill Y, Brosh K, Hanhart J. Bilateral anterior uveitis as a part of a multisystem inflammatory syndrome secondary to COVID-19 infection. J Med Virol. 2021;93:139–40. doi: 10.1002/jmv.26229. [DOI] [PMC free article] [PubMed] [Google Scholar]


