Abstract
Purpose:
To describe retinal manifestations seen in patients associated with COVID-19 infection at a multi-specialty tertiary care hospital in Southern India.
Methods:
In this retrospective chart review, all consecutive cases presenting to the Retina-Uveitis service from May 2020 to January 2021 with retinal manifestations associated with COVID-19 infection or its sequelae or as a result of treatment given for COVID-19 were included.
Results:
Of the 7 patients, 3 were female, and 4 were male. Four patients had onset of symptoms during the active phase of COVID-19 infection. Four had bilateral and three had unilateral involvement. The manifestations ranged from mild to vision threatening. Vision threatening manifestations included infections: endogenous endophthalmitis, candida retinitis and tubercular choroidal abscess and bilateral pre-foveal hemorrhages. Milder manifestations included paracentral acute middle maculopathy, central serous chorio-retinopathy and voriconazole induced visual symptoms. Final visual acuity was 6/36 or better in the four severe cases and 6/9 or better in the mild cases.
Conclusion:
This study highlights the retinal manifestations associated with COVID-19 infection and its sequelae. As these patients presented with an association with COVID-19 (either during or after recovery), ophthalmologists should be vigilant and screen for such entities in case of complaints of visual symptoms or in the presence of systemic sepsis. The outcomes can be good with prompt and aggressive management.
Keywords: COVID-associated mycoses, COVID 19, endophthalmitis, ocular manifestations, paracentral acute middle maculopathy, retinal manifestations
The coronavirus disease 2019 (COVID-19) which is caused by the newly identified severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a global pandemic.[1] Apart from severe systemic disease, various ophthalmic manifestations have been reported, ranging from anterior segment involvement such as conjunctivitis, severe keratitis and acute angle closure; retinal such as microangiopathy, cotton wool spots, hemorrhages and retinal vascular occlusions; and neuro-ophthalmic manifestations including optic neuritis, extra-ocular muscle palsies and idiopathic intracranial hypertension with papilledema.[1,2,3] These are attributed to: 1) a direct result of the viral infection and its cytopathological effects; 2) adverse effects of the pharmacological therapy administered for COVID-19 infection; 3) manifestations related to prolonged hospitalization and intensive care (such as nosocomial infections) 4) and lastly due to alterations in the host immune response. Systemic immunosuppression by decreased production of CD4 + T and CD8 + T cells and stimulation of cytokines is known to occur in COVID-19 infection thus predisposing to secondary opportunistic infections (especially fungal) during the middle and late stage of the disease.[4,5] This state of immunosuppression is further augmented by the use of systemic steroids and other immunosuppressives that are necessary in the management of COVID-19 infection.[6]
While previous publications have reported retinal involvement occurring mainly as vascular-related phenomenon,[7] with central retinal arterial occlusion and central retinal vein occlusion[8,9,10] being the severe ones reported so far, there are very few reports on other retinal findings. Herein we report a series of cases of retinal manifestations in patients associated with COVID-19 infection, ranging from mild to vision threatening conditions.
Methods
This was a retrospective case series, approved by the institutional review board and the research adhered to the tenets of the Declaration of Helsinki. Those patients presenting to either to the out-patient retina-uveitis department or were hospitalized and identified with retinal problems associated with COVID-19 infection or sequelae or treatment, seen between May 2020 to January 2021 were included. All patients had positive real-time reverse transcription-polymerase chain reaction (RT-PCR) for SARS-CoV-2 from a nasopharyngeal swab during the active phase of the COVID-19 infection. The details of the COVID-19 infection such as the use of systemic steroids and presence of co-morbid conditions as well as detailed ocular history were noted when available. Ambulatory patients underwent a complete eye examination which included visual acuity test, slit-lamp examination, non-contact tonometry and binocular indirect ophthalmoscopy and imaging as applicable {B-scan ultrasonography (B-scan), spectral domain optical coherence tomography (OCT) and Humphrey visual fields analysis (HVF)}. Cases where physical consultation or clinical documentation of the condition was not available (such as teleconsultation diagnosis) or poor quality of clinical photographs that would hinder the assessment of clinical course were excluded from this series.
Results
There were four male and three female patients, with age ranging from 23 to 75 years. Three of seven patients were admitted at initial examination, of which two were in intensive care. Diagnosis was divided as severe manifestations which included: fungal endophthalmitis, candida retinitis, tubercular choroiditis and pre-foveal hemorrhages and mild manifestations: acute macular neuro-retinopathy, central serous chorioretinopathy and voriconazole-induced transient visual disturbance. 4/7 had unilateral involvement. 3/7 developed symptoms during active COVID-19 infection and 4/7 received systemic steroids as part of therapy for COVID-19. Co-morbid conditions were present in 6/7 cases and included: steroid-induced diabetes mellitus in two patients, one patient had three factors: history of renal transplant, diabetes mellitus and systemic aspergillosis; candidemia with renal infection in one patient, Pseudomonas septicaemia with thrombocytopenia in one and splenic abscess in one. At last follow-up, all patients had improvement in visual recovery ranging from 6/36 to 6/9. Table 1 lists the demographic, co-morbid factors and ocular details of each patient.
Table 1.
Clinical characteristics of the seven patients
| Case | Age/Gender | Eye | COVID Infection timeline | Use of steroids | Systemic disease, status, investigations | Onset and duration of symptoms | Vision | Examination | Diagnosis | Treatment | Final outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42/M | RE | 6 weeks prior to ocular symptoms | Intravenous steroids for five days | Steroid induced diabetes | Onset: while on COVID treatment 4 weeks duration | Hand movements | Conjunctival congestion, cells 3+, hypopyon, dense vitreous exudates, fungal balls | Fungal endophthalmitis (microbiology negative) | Pars plana vitrectomy + intravitreal antifungals followed by oral antifungals for 6 weeks | Resolved with 6/9 vision |
| 2 | 75/M | RE | Not available | Not available | Candida septicemia, and candida renal infection during COVID 19, blood and urine cultures positive. Generalized compromised status | Not noticed by the patient | Counting fingers at 2 meters | 2+cells, 1+vitreous cells three retinitis lesions | Candida retinitis | Oral fluconazole and voriconazole for 6 weeks, followed by intravitreal Amphotericin B and voriconazole | 6/36, slow response |
| 3 | 59/F | RE | COVID-19 infection two months prior | Oral steroids used for 4 weeks | Nocturnal pyrexia, whole body imaging revealed splenic abscesses | 4 weeks | 6/9 | 1+ vit cells, choroidal abscess with scarring of edges and miliary lesions | Intraocular tuberculosis | Anti-tubercular therapy, lesions resolving over 6 weeks | 6/6 |
| 4 | 32/M | Both eyes | COVID 4 months ago | No | Nil | 3 days | 6/6 | Bilateral greyish white retinal lesions | Acute Macular Neuroretinopathy (AMN) and Paracentral Acute Middle Maculopathy (PAMM) | Observation | 6/6 |
| 5 | 27.F | LE | COVID-19 infection two weeks prior | On oral steroids for one week | Nil | One week | 6/18 | Serous detachment of macula | CSCR | NIL | 6/6 |
| 6 | 32/F | BES | COVID infection 6 weeks prior | Yes | Pseudomonas sepsis and thrombocytopenia, admitted in intensive care unit | One month | Counting fingers at 4 meters | Bilateral prefoveal hemorrhages | Retinal hemorrhages | NIL | 6/18 and 6/36 |
| 7 | 40/M | BES | COVID infection 6 weeks prior | Yes | Intensive care unit admission for systemic aspergillosis, status post renal transplant and Diabetes mellitus | 3 weeks | 6/6 | Normal examination, visual fields: normal | Voriconazole induced transient visual disturbance | Decreasing the dose of voriconazole, recovery for one week followed by relapse after resuming therapy | 6/6 |
Case Reports
Case 1: Presumed fungal endogenous endophthalmitis
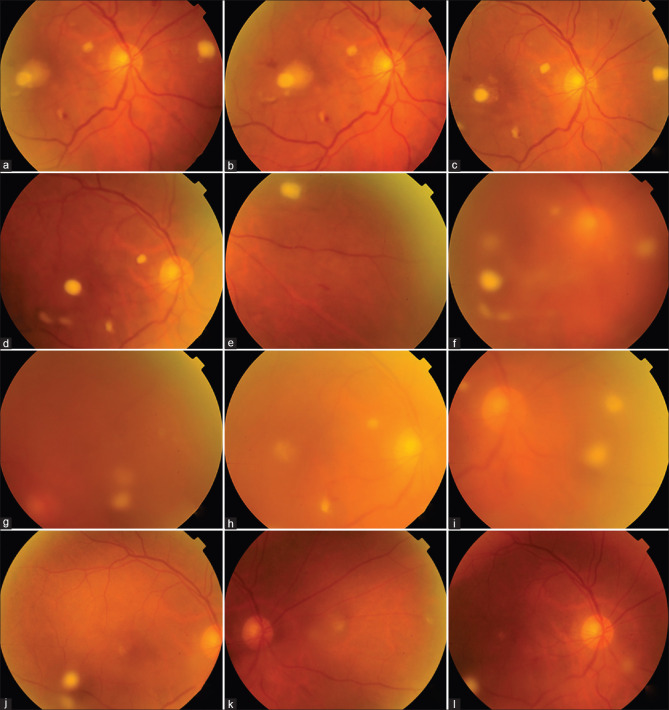
A 46-year-old man presented with a 4-week history of right eye (RE) ocular pain, redness and visual loss. He was diagnosed and treated for COVID-19 infection two weeks before onset of symptoms and had received home therapy of intravenous steroids for five days as a result of which he had developed steroid-induced diabetes. At presentation, visual acuity was hand motions; examination showed ciliary congestion, anterior chamber showed 2 + cells and 1 mm hypopyon and there were dense vitreous exudates [Fig. 1a]. B-scan revealed vitreous echoes and attached retina. A diagnosis of endogenous endophthalmitis was made and he underwent pars plans vitrectomy with injection of intravitreal vancomycin 1.0 mg, amikacin 300 ug, amphotericin-B 5 ug and voriconazole 100 ug. On table the vitreous exudates appeared dry with snowball like accumulations suggesting fungal infection [Fig. 1b and c]. Thick sheets of pre-retinal exudates were seen in the inferior periphery. The vitreous biopsy samples were sent for microbiological processing for smears (Grams and KOH), and aerobic and anerobic cultures by plating on blood and chocolate agar and fungal cultures by plating on Sabouraud dextrose agar. All were negative. Oral voriconazole was continued for 6 weeks. The infection resolved rapidly with visual recovery to 6/9 six weeks later [Fig. 1d].
Figure 1.
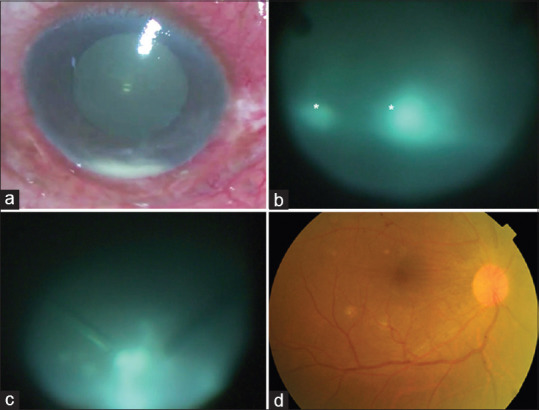
(a) Intraoperative photograph of the right eye shows severe ciliary congestion and hypopyon. (b) Intra-operative photograph during pars plana vitrectomy shows dense and dry vitreous lesions (asterisk) (c) Snowball like opacities noted in the inferior periphery (d) Same eye shows compete resolution with clear media and normal fundus at 6 weeks
Case 2: Candida retinitis
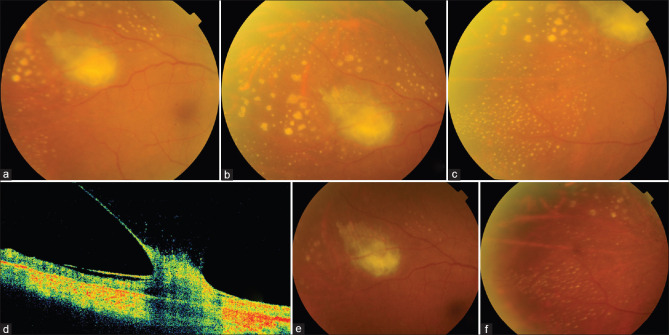
A 57-year-old male, diabetic and hypertensive, was hospitalized for severe Candida septicemia, with renal infection during COVID-19 infection. Blood and urine cultures were positive for Candida tropicalis. The patient did not complain of loss of vision but on testing, his visual acuity had dropped to counting fingers at 2 meters in his RE. LE was normal. RE showed 2 + cells and flare and 1+ vitreous cells and three well-circumscribed retinitis lesions in his RE, one involving the center of macula [Fig. 2a and b]. He was switched from systemic fluconazole to intra-venous voriconazole 200 mg twice daily due to the superior ocular bioavailability of the latter. After two weeks, the retinitis appeared to be resolving [Fig. 2c] but subsequently worsened [Fig. 2d and e]. Intravitreal amphotericin-B 5 ug and voriconazole 100 ug were given. Ten days later, there was increase in the vitreous haze, but the original retinitis lesions were resolving [Fig. 2f and g]. As he was not systemically fit to undergo surgery, intervention was deferred and the ocular condition was closely monitored. Four weeks after the intravitreal injection, the vitreous inflammation persisted but retinitis showed early signs of resolution [Fig. 2h and i]. Systemic voriconazole was continued for the renal and ocular infection and 3 months following the intravitreal injection his visual acuity improved to 6/36 with complete resolution of the vitreous inflammation and retinitis [Fig. 2j-l]. He was advised to continue oral voriconazole for a further 4 weeks.
Figure 2.
(a-k) Creamy-white candida retinitis lesions at presentation (a), macular involvement (b) Two weeks after voriconazole (c) Six weeks later: increasing activity (d and e) 10 days after intravitreal injections new vitreous exudates are noted (f); older retinitits lesions show some resolution (g)The lesions and vitreous exudates persist with some resolution after 4 weeks of injections. 3 months review: complete resolution of vitreous inflammation and retinitis, (j-l) with scarring noted at the site of the original lesions
Case 3: Choroidal abscess
A 59-year-old lady presented with complaint of RE visual deterioration and nocturnal low-grade fever for 4 weeks. Symptoms had started 2 months ago during COVID-19 infection, for which systemic steroids were given for 4 weeks. Locally she had undergone Magnetic Resonance Imaging (MRI) of the whole body for her pyrexia, which had revealed abdominal abscesses and she had received systemic antibiotics for the same with no response. On examination, visual acuity was 6/9 and 6/6 in the RE and LE respectively. LE was normal. RE showed 1 + vitreous cells and a large choroidal abscess supero-temporal to the macula [Fig. 3a]. The abscess showed central activity, scarring at its edges and multiple discrete yellow miliary lesions around it [Fig. 3b and c]. OCT revealed vitreous traction over the lesion [Fig. 3d]. Serological investigations to look for possible etiological causes which included: complete blood picture, Mantoux skin test, Quantiferon-TB gold test, Venereal disease research laboratory test, Treponema pallidum hemagglutinin assay and blood and urine cultures, were negative. Whole body Positive Emission Tomography/Computed tomography (PET/CT) scan revealed multiple hypodense lesions in the spleen, the largest being 43 millimeters in diameter, low for hypermetabolic activity. Based on the clinical appearance of the chorio-retinal lesions, a provisional diagnosis of tuberculosis was considered. After discussion with her internist, a therapeutic trial of 4-drug anti-tubercular therapy (ATT) was started, with the intention of considering vitreous biopsy in case of worsening. She was closely followed up every week and the lesions showed improvement. Six-weeks after initiating ATT the choroidal abscess and the miliary lesions had resolved significantly [Fig. 3e and f]. The splenic abscesses had also started showing resolution. She was continued on 4-drug ATT for the first two months with the intention to continue 2-drug ATT for 10 months.
Figure 3.
(a) Large choroidal abscess with active appearing center and resolving activity at edges supero-temporal to the right macula. (b and c) Multiple miliary lesions around the choroidal abscess in temporal and inferotemporal fundus. Lesions are larger and irregular closer to the abscess, becoming smaller, punctate with smoother edges further from it. (d) OCT showing epiretinal membrane and vitreous traction over the choroidal abscess. (e and f) Significant resolution of choroidal abscess and miliary lesions 6 weeks after initiating anti-tubercular therapy
Case 4. Acute macular neuroretinopathy (AMN) and paracentral acute middle maculopathy (PAMM)
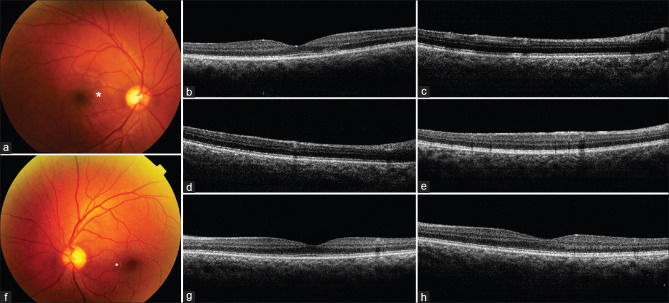
A 32-year-old man presented with 3-day onset of paracentral and triangular negative scotoma in RE infero-temporal visual field. He had recovered from COVID-19 infection 4 months prior. LE was asymptomatic. He had undergone a complete health check-up including serological investigations a month prior which were normal. RE fundus revealed a triangular deeper retinal greyish-white lesion located supero-nasal to center of macula [Fig. 4a]. OCT over the corresponding area showed disruption in the outer retinal layers [Fig. 4b], hyper-reflective lesions in superficial retinal layers with shadowing of deeper retina [Fig. 4c and d]. The entire inner retinal surface just inferior to foveal center showed hyper-reflectivity [Fig. 4e]. LE fundus revealed small greyish-white lesion nasal to the foveal center and multiple similar lesions inferonasal and temporal to the center [Fig. 4f]. OCT revealed a single hyper-reflective lesion in the superficial retina with underlying shadowing temporal to the center [Fig. 4g] and hyper-reflectivity of the entire inner retinal surface [Fig. 4h]. Based on the characteristic symptoms, the fundus lesions and OCT findings he was diagnosed as post COVID-19 RE symptomatic AMN, and bilateral asymptomatic PAMM. He was placed under observation.
Figure 4.
(a) Triangular greyish-white lesion (asterisk) of AMN (b) Corresponding OCT shows disruption of external retinal layers (c and d) Focal areas of hyper-reflectivity with deeper shadowing of PAMM. (e) OCT right eye: diffuse hyper-reflectivity of entire inner retinal surface (f) Small greyish-white lesions in superficial retina nasal, inferonasal and temporal to the foveal center in left eye. (g) Corresponding OCT shows hyper-reflective lesion with underlying shadowing (h) OCT left eye showing diffuse hyper-reflectivity of the inner retinal surface
Case 5: Central serous chorioretinopathy (CSCR)
A 27-year-old female patient presented with central vision deterioration in LE of 10-days duration. She had recovered from COVID-19 infection two weeks prior for which she had been on oral steroids. RE was normal; left eye visual acuity was 6/18. Examination showed no signs of inflammation. Fundus examination revealed serous detachment of the macula and was confirmed by OCT [Fig. 5]. She was observed and the symptoms resolved spontaneously a few weeks later.
Figure 5.
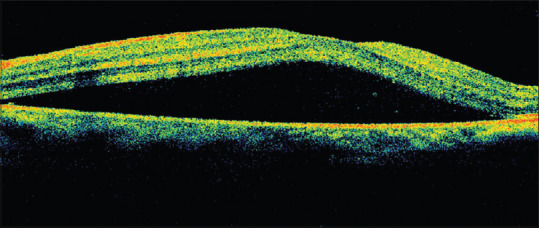
OCT left eye of a young female treated with oral steroids for COVID-19 showing serous macular detachment
Case 6: Post-COVID-19 sepsis related bilateral pre-foveal hemorrhages
A 32-year-old lady in the ICU complained of rapid loss of central vision in both eyes (one month after COVID-19 infection). She was admitted with fever and Pseudomonas sepsis while still on treatment for COVID-19 infection. She had severe anemia (hemoglobin 6.8 gm%), leukocytosis (total leucocyte count 13000/mm3) and thrombocytopenia (platelets 72,000/mm3). Vision was counting fingers at 4 meters. Fundus examination showed bilateral pre-foveal hemorrhages. Four weeks later she followed up in the outpatient department and reported no improvement. Her visual acuity was recorded as 6/18 in and 6/36 in the RE and LE respectively. Bilateral retinal hemorrhages were noted as before. The corresponding OCT showed pre-foveal location with underlying shadowing [Fig. 6a-d]. A month later her visual acuity had improved to 6/6 and 6/9 in the RE and LE respectively. The pre-foveal heme had resolved almost completely [Fig. 6e and f] although the RE OCT showed residual paracentral pre-foveal heme and the LE OCT now revealed some intraretinal heme [Fig. 6g and h]. She was advised to follow-up after 3 months.
Figure 6.
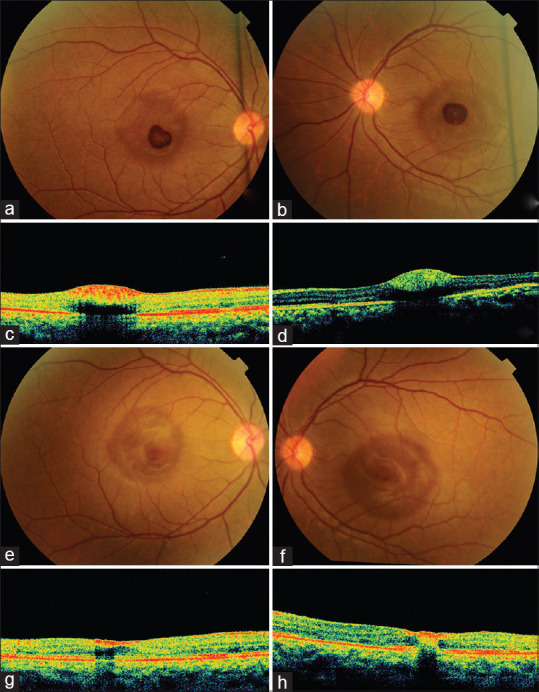
Bilateral pre-foveal hemorrhage of the right (a) and left eye (b) in a young lady with post COVID-19 sepsis. OCT of the right eye (c) and left eye (d) showing pre-foveal location of the hemorrhage with underlying shadowing. One month later, there is almost complete resolution of the pre-foveal heme in the RE (e) and LE (f) Corresponding optical coherence tomography scans show residual paracentral pre-foveal heme in the RE (g) and intra-retinal heme in the LE (h)
Case 7: Voriconazole-induced transient visual disturbance
A 40-year-old gentleman complained of multiple non-specific but distressing visual symptoms including severe visual blur and extreme brightness and flashes of light for 3 weeks. He was admitted in ICU with invasive systemic aspergillosis and was on intravenous voriconazole (300 mg twice daily) for 4 weeks as microbiology isolates had shown sensitivity only to voriconazole. The systemic aspergillosis had been diagnosed during his COVID-19 infection, which he had developed 6 weeks prior. History was significant for renal transplant 3 years back and diabetes mellitus for 2 months (onset after recovery from COVID-19 infection). He was also on tablet augmentin 1 gram twice daily; oral mycophenolate mofetil 360 mg twice daily; oral prednisolone 5 mg daily; tacrolimus 0.5 mg twice daily; and subcutaneous insulin. The BCVA was 6/6 BES. Color vision (using Ishihara charts), pupil response to light, anterior segment and fundus evaluation were within normal limits in both eyes each eye. Perimetry (Humphrey’s visual field analysis central 24-2 program) was normal. A review of all the medications that the patient was on was done and based on the onset of his symptoms, a possibility of voriconazole-induced visual symptoms was made. The dose of voriconazole was reduced with improvement in symptoms within a week. However, the drug had to be stepped up in view of his systemic condition of invasive aspergillosis and the patient again reported recurrence of his visual symptoms.
Discussion
We have described a series of cases with retinal manifestations, which were associated with COVID-19 infection, with symptoms that started either during the infection or soon after resolution. Of the three cases of intra ocular infection in our series, in the case of presumed endogenous endophthalmitis the systemic focus of infection could not be detected. The patient had a complete recovery with surgery and antifungals. We postulate that the severe infection in our otherwise healthy patient could be as a result of immunosuppression caused by COVID-19 infection, and the steroids used for its management.
The second case which was retinal candidiasis in COVID-19 infection has not yet been reported. Contrary to the first case, the source was obvious as this patient had invasive candidemia. The patient was also diabetic, which no doubt contributed to the risk. It is recommended that surveillance for systemic fungal infections should be done in severe COVID-19 infection.[4,5,11]
Our third case of intra-ocular infection was a diagnostic challenge. Systemic tuberculosis associated with COVID-19 has been reported,[12,13,14] however, choroidal abscess due to tuberculosis, in association with recent COVID-19 has not. Our patient tested negative for both Mantoux test and interferon gamma assay which could indicate miliary tuberculosis. The value of PET scan imaging in tuberculosis has been well documented. It has been used to detect tubercular granulomas and assess disease activity.[15] In our case, while the splenic lesions could be ruled as non-malignant, confirmatory diagnosis for tuberculosis would necessitate a tissue biopsy. On the other hand, ocular choroidal lesions with their pattern of peripheral scarring in our case leaned towards a diagnosis of ocular tuberculosis.[16] The patient was not keen to undergo any invasive diagnostic evaluation either systemically or ocular and thus we resorted to a therapeutic trial, following which the patient had improvement in both the ocular and systemic condition. One more feature in our case was the presence of yellowish inflammatory deposits around the chorio-retinal lesions, which have been described with syphilitic retinitis.[17] However, the relevant investigations were negative for syphilis and the clinical picture appeared to be consistent with tuberculosis.
We had a case of strikingly remarkable pre-foveal hemorrhages. This was secondary to thrombocytopenia in systemic Pseudomonas sepsis. Sepsis and signs of multi-organ injury typical of sepsis occur in approximately 2-5% of those with COVID-19 after approximately 8-10 days.[18,19] Thrombocytopenia is a known complication of sepsis.[20] While bilateral simultaneous pre-foveal hemorrhage as a complication of post COVID-19 sepsis has not been reported, bilateral premacular sub-hyaloid hemorrhage unmasking COVID-19 infection has been reported.[21]
We had one case of PAMM/AMN. These are rare conditions characterized by the sudden onset of single or multiple paracentral scotomas, which persist indefinitely or may resolve partially over months.[22,23] Most scotomas spare the fixation by being perifoveal and often do not cause significant visual loss in the affected eye.[24] The exact etiology is not clear although microvascular abnormality of the retina is hypothesized as well as altered coagulation status[22,24] We did not advice inflammatory markers or coagulation profile markers due to affordability for our patient. There was a typical abrupt onset of a paracentral scotoma corresponding to a triangular greyish-white lesion in the RE fundus and an area of disruption of the external retinal layers on OCT suggesting symptomatic AMN. In addition, presence of other asymptomatic parafoveal greyish lesions in left eye fundus and bilateral OCT features of hyper- reflective lesions in superficial retinal layers suggested bilateral asymptomatic PAMM. Both PAMM and AMN have been reported following viral illness in as high as 47.5%. of cases[22] In association with COVID-19, there are only two reports so far, one representing post-infectious complication[25] and another where PAMM was associated with acute myeloid leukemia and co-existing COVID-19 infection.[26]
We also had retinal manifestations due to the adverse effects of medication used for the management of COVID-19 infection or its sequelae. CSCR is a recognized complication of steroid use[27] and hence its association with COVID-19 management is of course anticipated. However, there are no specific case reports of CSCR in patients with COVID-19 infection.
Voriconazole is known to cause reversible visual symptoms in 18-30% patients including altered light perception, photopsia, photophobia, blurred vision, or color vision changes[28] but this often remains a diagnosis of exclusion. The retina is believed to be the site of the visual disturbances because reversible decreases in the amplitude of the electroretinogram (ERG) have been documented in voriconazole-treated humans.[29] Fortunately, these effects are completely reversible, and are not reported with the other azoles such as fluconazole and itraconazole.[28] In our patient, the best corrected visual acuity, color vision, ocular findings and perimetry were normal ruling out any ocular or visual pathways lesion accounting for the visual symptoms. Considering the association of systemic fungal infections with COVID-19 infection, voriconazole-induced visual symptoms must remain as one of the possible causes of inexplicable visual symptoms in a setting of normal ocular examination in a COVID-19 patient, as it is the easiest to reverse by simply decreasing or stopping the medication.
Conclusion
While there are isolated case reports of COVID-19 related retinal manifestations, this is the first single large case series of diverse retinal manifestations of COVID-19 infection, some of these not reported, ranging from medication related mild adverse effects and post viral complications like PAMM and pre-foveal hemorrhages to grave sight-threatening ocular infections such as endophthalmitis, candida retinitis & tubercular choroidal abscess. Even in the severe cases, we could institute therapy promptly and our patients were able to recover well, suggesting an overall good prognosis in this subset of patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Funding provided by Hyderabad Eye Research Foundation, Hyderabad, India for Dr Somasheila I. Murthy. The funders had no role in the preparation, review or approval of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bertoli F, Veritti D, Danese C, Samassa F, Sarao V, Rassu N, et al. Ocular findings in COVID-19 patients:A review of direct manifestations and indirect effects on the eye. J Ophthalmol. 2020;2020:4827304. doi: 10.1155/2020/4827304. doi:10.1155/2020/4827304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas KA, Douglas VP, Moschos MM. Ocular manifestations of COVID-19 (SARS-CoV-2):A critical review of current literature. In Vivo. 2020;34(3 Suppl):1619–28. doi: 10.21873/invivo.11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lani-Louzada R, Ramos CdVF, Cordeiro RM, Sadun AA. Retinal changes in COVID-19 hospitalized cases. PLoS One. 2020;15:e0243346. doi: 10.1371/journal.pone.0243346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic:A clinical and diagnostic perspective from China. Mycopathologia. 2020;7:1–8. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei Y, Song Y, Shu Y, Zhao Y, Huo X, Wang H, et al. Fungal antigenemia in patients with severe Coronavirus disease 2019 (COVID-19):The facts and challenges. J Microbiol Immunol Infect. 2020;53:657–9. doi: 10.1016/j.jmii.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19):A review. JAMA. 2020;323:1824–36. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 7.Pereira LA, Soares LC, Nascimento PA, Cirillo LR, Sakuma HT, da Veiga GL, et al. Retinal findings in hospitalized patients with severe COVID-19. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-317576. doi:10.1136/bjophthalmol-2020-317576. [DOI] [PubMed] [Google Scholar]
- 8.Montesel A, Bucolo C, Mouvet V, Moret E, Eandi CM. Case report:Central retinal artery occlusion in a covid-19 patient. Front Pharmacol. 2020;11:588384. doi: 10.3389/fphar.2020.588384. doi:10.3389/fphar. 2020.588384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. ID Cases. 2020;21:e00867. doi: 10.1016/j.idcr.2020.e00867. doi:10.1016/j.idcr. 2020.e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yahalomi T, Pikkel J, Arnon R, Pessach Y. Central retinal vein occlusion in a young healthy COVID-19 patient:A case report. Am J Ophthalmol Case Rep. 2020;20:100992. doi: 10.1016/j.ajoc.2020.100992. doi:10.1016/j.ajoc. 2020.100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia:An increased risk for candidemia? Autoimmun Rev. 2020;19:102564. doi: 10.1016/j.autrev.2020.102564. doi:10.1016/j.autrev. 2020.102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. 2020;56:2001708. doi: 10.1183/13993003.01708-2020. doi:10.1183/13993003.01708-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadolini M, Codecasa LR, García-García JM, Blanc FX, Borisov S, Alffenaar JW, et al. Active tuberculosis, sequelae and COVID-19 co-infection:First cohort of 49 cases. Eur Respir J. 2020;56:2001398. doi: 10.1183/13993003.01398-2020. doi:10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crisan-Dabija R, Grigorescu C, Pavel CA, Artene B, Popa, Cernomaz A, et al. Tuberculosis and COVID-19:Lessons from the past viral outbreaks and possible future outcomes. Can Respir J. 2020;2020:1401053. doi: 10.1155/2020/1401053. doi:10.1155/2020/1401053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ankrah AO, van der Werf TS, de Vries EF, Dierckx RA, Sathekge MM, Glaudemans AW. PET/CT imaging of Mycobacterium tuberculosis infection. Clin Transl Imaging. 2016;4:131–44. doi: 10.1007/s40336-016-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23:7–13. doi: 10.3109/09273948.2014.967358. [DOI] [PubMed] [Google Scholar]
- 17.Pathengay A, Kaza H, Tyagi M, Patel A, Pappuru RR, Agrawal H. Miliary retinal lesions in ocular syphilis:Imaging characteristics and outcomes. Ocul Immunol Inflamm. 2019;5:1–5. doi: 10.1080/09273948.2019.1659830. [DOI] [PubMed] [Google Scholar]
- 18.Colantuoni A, Martini R, Caprari P, Ballestri M, Capecchi PL, Gnasso A, et al. COVID-19 sepsis and microcirculation dysfunction. Front Physiol. 2020;11:747. doi: 10.3389/fphys.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltrán-García J, Osca-Verdegal R, Pallardó FV, Ferreres J, Rodríguez M, Mulet S, et al. Sepsis and coronavirus disease 2019:Common features and anti-inflammatory therapeutic approaches. Crit Care Med. 2020;48:1841–4. doi: 10.1097/CCM.0000000000004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis:Incidence, risk factors, and its association with clinical outcome. J Intensive Care. 2013;1:9. doi: 10.1186/2052-0492-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Kumar P, Singh A, Srujana D, Kaushik J. Bilateral premacular sub-hyaloid hemorrhage-unmasking COVID-19 induced pancytopenia. J Med Virol. 2020;12:1–5. doi: 10.1002/jmv.26752. [DOI] [PubMed] [Google Scholar]
- 22.Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, et al. Acute macular neuroretinopathy:A comprehensive review of the literature. Surv Ophthalmol. 2016;61:538–65. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Casalino G, Arrigo A, Romano F, Munk MR, Bandello F, Parodi MB. Acute macular neuroretinopathy:Pathogenetic insights from optical coherence tomography angiography. Br J Ophthalmol. 2019;103:410–4. doi: 10.1136/bjophthalmol-2018-312197. [DOI] [PubMed] [Google Scholar]
- 24.Sarraf D, Rahimy E, Fawzi AA, Sohn E, Barbazetto I, Zacks DN, et al. Paracentral acute middle maculopathy:A new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131:1275–87. doi: 10.1001/jamaophthalmol.2013.4056. [DOI] [PubMed] [Google Scholar]
- 25.Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye. 2020;34:2352–3. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamani G, Ataei Azimi S, Aminizadeh A, Shams Abadi E, Kamandi M, Mortazi H, et al. Acute macular neuroretinopathy in a patient with acute myeloid leukemia and deceased by COVID-19:A case report. J Ophthalmic Inflamm Infect. 2021;10:39. doi: 10.1186/s12348-020-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Deng T, Zhang J. Risk factors for central serous chorioretinopathy:A systematic review and meta-analysis. Retina. 2016;36:9–19. doi: 10.1097/IAE.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 28.Eiden C, Peyrière H, Cociglio M, Djezzar S, Hansel S, Blayac JP, et al. Adverse effects of voriconazole:Analysis of the French pharmacovigilance database. Ann Pharmacother. 2007;41:755–63. doi: 10.1345/aph.1H671. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita J, Iwata N, Ohba M, Kimotsuki T, Yasuda M. Mechanism of voriconazole-induced transient visual disturbance:Reversible dysfunction of retinal ON-bipolar cells in monkeys. Invest Ophthalmol Vis Sci. 2011;52:5058–63. doi: 10.1167/iovs.11-7183. [DOI] [PubMed] [Google Scholar]