Abstract
Purpose:
Aerobic exercise (AE) has been reported to decrease intraocular pressure (IOP) in healthy subjects and there are concomitant morphological changes in the anterior segment of the eye including the Schlemm’s canal (SC). However, its effects on IOP and SC morphology in glaucoma patients had not been studied before. We aim to investigate the effect of AE on the IOP and SC dimension in both healthy and primary open-angle glaucoma (POAG) eyes.
Methods:
The area and diameter of SC and IOP were measured in 35 primary open-angle glaucoma (POAG) patients (59 eyes) and 36 healthy subjects (72 eyes) before and after performing moderate intensity of AE by running on a treadmill for 30 min. SC was imaged by swept-source optical coherence tomography (SS-OCT) for evaluation.
Results:
In comparison with baseline values, mean IOP decreased significantly following AE in both POAG and healthy eyes (both P < 0.001), in which POAG eyes showed a greater degree of reduction compared to healthy eyes (P = 0.002). In comparison with baseline values, in both POAG and healthy eyes, the average cross-sectional area (POAG: 80.48 +/- 59.54 vs. 99.20 +/- 54.87 pixels; healthy: 151.84 +/- 52.76 vs. 198.23 +/- 53.70 pixels; both P < 0.001) and diameter (POAG: 3.73 +/- 1.69 vs. 4.33 +/- 1.74 pixels; healthy: 5.61 +/- 1.02 vs. 6.47 +/- 1.20 pixels; both P < 0.001) of SC significantly increased after AE. In POAG, both treated and untreated with IOP-lowering medications, a significant reduction in mean IOP and increase in SC dimensions following AE were observed (all P < 0.05), and there were no significant differences of such measurements between the two subgroups (all P > 0.05).
Conclusion:
AE-induced reduction in IOP and an increase in SC dimensions in POAG eyes as in healthy eyes. Further studies to evaluate the long-term effect of AE on IOP control and SC morphology in POAG seem warranted.
Keywords: Aerobic exercise, intraocular pressure, Schlemm’s canal, primary open-angle glaucoma
Elevation of intraocular pressure (IOP) is a major risk factor of primary open-angle glaucoma (POAG), the commonest form of glaucoma worldwide.[1,2] To date, IOP reduction remains the mainstay of treatment for POAG to protect the optic nerve, attenuate degeneration of retinal ganglion cells and progression of visual field defects.[3] However, despite being prescribed maximal doses of antiglaucoma medications or having undergone multiple surgical procedures, some glaucoma patients still could not achieve satisfactory IOP control.[4,5] Furthermore, less invasive and conservative treatment options are often preferable from patients’ perspectives. Therefore, the identification of beneficial lifestyle modifications to control IOP in glaucoma is an area of clinical interest.
Aerobic exercise (AE) is known to reduce IOP in healthy eyes by various potential mechanisms.[6,7,8] Yan et al. recently observed that IOP was lowered while the dimension of Schlemm’s canal (SC) became significantly increased in healthy eyes after AE using ultrasound biomicroscopy (UBM) and swept-source optical coherence tomography (SS-OCT), respectively.[9,10] Prostaglandin (PG) analogs, trabeculectomy, and minimally invasive glaucoma surgery are all proven effective treatments for glaucoma with the common effects of facilitating aqueous outflow through the trabecular meshwork (TM) and SC to achieve IOP reduction.[11,12,13] Therefore, AE may potentially be a beneficial lifestyle modification in glaucoma management with its observed effects on IOP and SC. Nevertheless, previous studies on the effects of AE in IOP were mostly conducted in healthy eyes with limited evidence to support its role in POAG.[14,15] Furthermore, effects of AE in expanding SC dimension were only recently reported in healthy eyes.[9,10,16] Therefore, these effects of AE are yet to be comprehensively evaluated in POAG.
The morphology of SC in POAG eyes is grossly altered as compared to healthy eyes, and it is uncertain if the effects of AE in healthy eyes are retained and reproducible in POAG eyes.[17,18,19] Decreased dimension of SC in POAG was thought to be associated with impaired aqueous outflow and IOP elevation in POAG.[17] Furthermore, it has been shown that acute elevation of IOP caused the collapse of SC which could further increase the resistance of the aqueous outflow pathway and induce a vicious cycle of progressively increasing IOP.[20] In the presence of unfavorable SC morphology, further complicated by elevated IOP causing SC collapse in POAG eyes, it remains unknown if AE could achieve similar outcomes of increasing SC dimension and reducing IOP as in healthy eyes.
Imaging of the anterior segment has advanced. In recent studies, our group has extensively studied changes in SC morphology and anterior chamber in healthy eyes under various physiological conditions utilizing SS-OCT.[21,22] SS-OCT in current clinical practice allows noncontact, detailed, and high resolution in vivo imaging of the anterior segment and is a reliable SC measurement method.[13,23,24] During imaging by SS-OCT, patients remain in an upright sitting position, which maintains physiological aqueous fluid gravity. These properties of SS-OCT allowed noninvasive evaluation of the SC morphology in POAG eyes following AE in the most physiological setting with minimal influences of the eye by the imaging device.
The objectives of this study were to determine if AE retains similar effects of inducing IOP reduction and SC dimension increase as previously reported in healthy eyes and to compare such effects in different POAG treatment groups to evaluate the potential benefits of AE in POAG eyes.
Methods
Study design and subjects
This is an observational, cross-sectional study. A total of 35 POAG patients and 36 healthy volunteers were recruited from the Department of Glaucoma, Zhongshn Ophthalmic Center, and Sun Yat-sen University Medical School. This study was approved by the Ethics Review Committee of the Zhongshan Ophthalmic Center and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects before study participation. History of IOP-lowering medication use was recorded for all POAG patients.
All participants underwent a detailed ocular examination, which included measurements of best-corrected visual acuity, spherical equivalent (SE) by an autorefractor (KR-8900 version 1.07; Topcon Corporation, Tokyo, Japan), axial length (AL) (IOL Master, Carl Zeiss Meditec, LaJolla, CA, USA), slit-lamp biomicroscopy, gonioscopy, fundus examination by indirect ophthalmoscope and IOP measured by Goldmann applanation tonometry. Besides, all participants underwent examinations for measurement of blood pressure (BP) and heart rate (HR) with an Omron automatic BP instrument (Omron HEM-7200; Omron, Dalian, Liaoning, China). Subjects with unclear ocular media, coexisting ocular diseases other than POAG, history of ophthalmic surgeries or systemic diseases, and low-quality OCT images were excluded. In POAG patients, both eyes were included if they were diagnosed as POAG, otherwise, only the diseased eye was included. Both eyes of the healthy subjects were included.
POAG was diagnosed based on (1) drainage angle of Shaffer grade II or above on darkroom gonioscopy, (2) glaucomatous optic disc cupping and loss of neuroretinal rim, (3) typical glaucomatous visual field loss present on the 24–2 pattern Swedish interactive threshold algorithm (SITA) on the Humphrey Field Analyzer (Carl Zeiss Meditec Inc., Dublin, CA, USA) within 6 months of the study, and (4) absence of secondary causes for the glaucomatous optic neuropathy (e.g., previous trauma, use of steroids, uveitis, etc.). Healthy subjects had anterior chamber angle of Shaffer grade II or above, no optic disc abnormalities in clinical examination, no visual field abnormalities on visual field testing, and no history of glaucoma, or other ocular diseases except myopia or hyperopia of between -6.00 D to +3.00 D.
Treadmill exercise
All participants were requested to abstain from eating or drinking for at least 3 h before treadmill exercise. The prescribed HR range during exercise (between 60% and 80% of the estimated maximum HR [HRmax]) of each subject was calculated to monitor exercise intensity, where HRmax = 220 - age/minute.[25] All participants ran on a treadmill for 30 min, including a warm-up for 5 min. HR was tracked continuously throughout running with a Fitbit Charge 2™ wristband (Fitbit, San Francisco, CA, USA).[26] All participants were instructed to maintain personalized, moderate-intensity speed zones between 60% and 80% of their estimated HRmax throughout the exercise. The measurements of BP, HR, IOP, and imaging of SC were performed before and immediately after AE.
SS-OCT imaging acquisition and processing
Each subject was imaged by SS-OCT (CASIA SS-1000; Tomey, Nagoya Corp., Japan) before and immediately after AE in a dark room. Four quadrants of the corneoscleral limbus were captured with a three-dimensional imaging scan procedure with a 6 × 6 mm raster scan system, which comprised 256 B-scans, each consisting of 256 A-scans, with a total of 65,536 axial scans per volume. The participants were asked to stare at an external fixation target while scanning. The same position in crypts and furrows of the iris was referred to as the landmarks to scan SC images in the same subject [Fig. 1].
Figure 1.
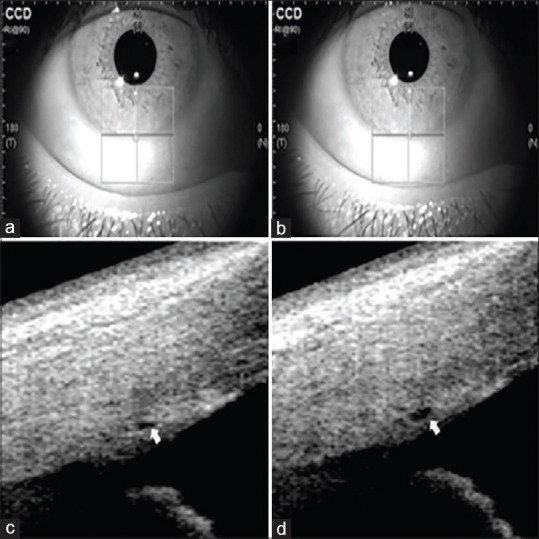
SS-OCT images of scanning position of SC in POAG eyes. The crypts and furrows of the iris are used to mark the scanning guidelines to ensure the same scanning cross-section of SC before (a) and after (b) aerobic exercise. Morphology of SC before (c) and after (d) aerobic exercise is marked by an arrow
ImageJ (http://imagej.nih.gov/ij/, National Institutes of Health, Bethesda, MD, USA) was used to measure the SC diameter and cross-sectional area. The diameter was defined as the average value of three measurements of the sagittal axial length of the thin, lucent space on the SS-OCT images [Fig. 1]. The cross-sectional area was drawn freehand twice by depicting the SC outline as described in our previous study.[27] The values of the SC parameters of superior, nasal, inferior, and temporal quadrants were included in the analysis. Two independent experienced technicians masked to the clinical data completed these measurements. The intra and interobserver reliability of the diameters and areas of SC was reported in Supplementary Tables S1 and S2. Average values of the two technicians’ measurements were recorded as the final SC measurements.
Supplementary Table S1.
Inter-observer reliability of SC dimension measurements
| Parameter | Observer | ICC | Mean | Difference | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| SC area | 1 | 0.991 | 244.1 | 168.45 | 0.985 | 0.994 |
| 2 | 0.999 | 220.5 | 141.8 | 0.999 | 1.000 | |
| SC diameter | 1 | 0.760 | 7.56 | 3.62 | 0.660 | 0.839 |
| 2 | 0.749 | 7.78 | 3.35 | 0.646 | 0.831 | |
CI = confidence interval, ICC = intrarclass correlation coefficient, SC = Schlemm’s canal.
Supplementary Table S2.
Intra-observer reliability of SC dimension measurements
| Parameter | ICC | Mean | Difference | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| SC area | 0.941 | 232.3 | 155.82 | 0.903 | 0.964 |
| SC diameter | 0.820 | 7.67 | 2.49 | 0.715 | 0.888 |
CI = confidence interval, ICC = intraclass correlation coefficient, SC = Schlemm’s canal.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software 24.0 (IBM Corp., Armonk, NY, USA). The means and standard deviations were calculated for all measured variables. The Chi-square test was used to compare the percentage of sections with observable SC before and after exercise. The nonparametric test (paired samples) was used for the comparison of the area and diameter of SC, IOP, and other parameters between baseline and post-exercise values, adjusting for age and sex using the analysis of covariance in all subjects. Analysis of covariance (ANCOVA) was performed to compare the changes of IOP and SC dimensions between POAG and healthy eyes and between POAG subgroups, adjusting for age, sex, and eye. Nonparametric Spearman’s correlation analysis was performed to statistically examine the relationships between the changes in IOP and SC parameters. Generalized estimating equations (GEEs) model, with 95% confidence intervals (CIs), was applied for the multivariable analysis. The statistical significance was defined as a P value of less than 0.05.
Results
A total of 59 eyes from 35 POAG patients and 72 eyes from 36 healthy volunteers were included in this study. The demographic and baseline ocular characteristics were reported in Table 1. The changes of HR and BP in all participants before and after AE were shown in Supplementary Table S3. The baseline value of IOP in eyes with POAG was statistically higher than that of normal eyes (P < 0.001).
Table 1.
Baseline Characteristics of POAG Patients and Healthy Subjects
| Variables | POAG Patients | Healthy Subjects | P† |
|---|---|---|---|
| Number of patients (eyes) | 35 (59) | 36 (72) | - |
| Mean age, years (SD) | 36.09 (10.53) | 31.97 (9.65) | 0.007 |
| Sex (male/female) | 31/4 | 15/21 | <0.001 |
| AL, mm (SD) | 25.06 (1.37) | 24.08 (0.92) | <0.001 |
| SE, diopter (SD) | -2.93 (2.11) | -1.97 (2.05) | 0.011 |
| IOP, mmHg (SD) | 16.8 (4.40) | 13.0 (2.31) | < 0.001 |
| Average SC area, pixels (SD) | 80.4 (59.54) | 151.8 (52.76) | < 0.001 |
| Average SC diameter, pixels (SD) | 3.73 (1.69) | 5.61 (1.02) | <0.001 |
AL = Axial length; IOP = Intraocular pressure; SC = Schlemm’s canal; SD = Standard deviation; SE = Spherical equivalent. †independent sample t-test was used for continuous variables; Chi-square test was used for categorical variables.
Supplementary Table S3.
HR and BP variations in healthy and POAG participants before and after exercise
| POAG patients | Healthy subjects | |||||
|---|---|---|---|---|---|---|
| Before Exercise | After Exercise | Pt | Before Exercise | After Exercise | Pt | |
| HR | 73.68±12.10bpm | 100.47±18.77bpm | <0.001* | 73.67±9.03bpm | 102.92±17.05bpm | <0.001* |
| SBP | 119.82±13.62mmHg | 122.65±12.69mmHg | 0.362 | 114.53±13.94mmHg | 119.08±14.58mmHg | 0.008* |
| DBP | 71.03±10.06mmHg | 71.12±8.49mmHg | 0.729 | 70.72±9.55mmHg | 71.50±7.85mmHg | 0.576 |
HR = heart rate, SBP = blood pressure, DBP = diastolic blood pressure.
† = Nonparametric test (Related samples).
Effect of AE on IOP and SC
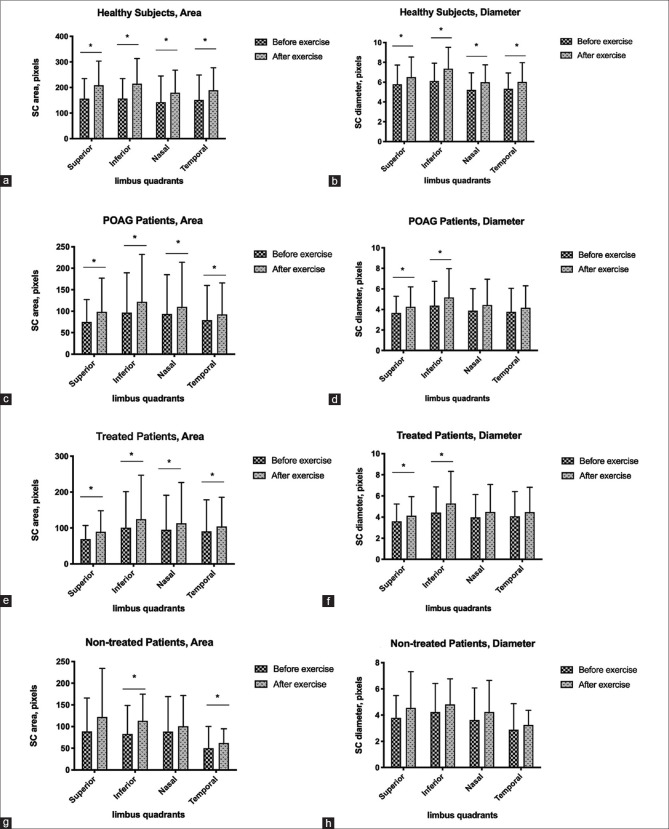
Mean IOP was significantly reduced after AE in both POAG (16.82 +/- 4.40 vs. 14.70 +/- 3.52 mmHg) and healthy (13.04 +/- 2.31 vs. 12.03 +/- 1.73 mmHg) eyes (both P < 0.001) [Table 2]. Average cross-sectional area and diameter of SC also significantly increased after AE in both POAG (cross-sectional area: 80.48 +/- 59.54 vs. 99.20 +/- 54.87 pixels; diameter: 3.73 +/- 1.69 vs. 4.33 +/- 1.74 pixels) and healthy (cross-sectional area: 151.84 +/- 52.76 vs. 198.23 +/- 53.70 pixels; diameter: 5.61 +/- 1.02 vs. 6.47 +/- 1.20 pixels) eyes (all P < 0.001) [Table 2 and Fig. 2]. Values of cross-sectional area and diameter of each quadrant of SC were also significantly increased (all P < 0.05) in both POAG and healthy eyes, except for the diameters of the nasal and temporal quadrants in POAG eyes (P = 0.063 and P = 0.077, respectively) [Table 2].
Table 2.
Changes in IOP and SC in POAG and Healthy Eyes Before and After Exercise
| POAG Eyes (Mean ± SD) | Healthy Eyes (Mean ± SD) | |||||
|---|---|---|---|---|---|---|
| Before Exercise | After Exercise | P† | Before Exercise | After Exercise | P† | |
| IOP, mmHg | 16.82 ± 4.40 | 14.70 ± 3.52 | <0.001 | 13.04 ± 2.31 | 12.03 ± 1.73 | <0.001 |
| Superior Area, pixels | 74.82 ± 52.29 | 98.62 ± 78.30 | 0.007 | 156.42 ± 78.58 | 208.67 ± 94.8 | <0.001 |
| Inferior Area, pixels | 96.53 ± 93.05 | 121.75 ± 110.37 | 0.001 | 156.89 ± 78.21 | 214.88 ± 98.64 | <0.001 |
| Nasal Area, pixels | 93.45 ± 91.54 | 110.07 ± 103.7 | 0.009 | 142.91 ± 102.35 | 179.93 ± 87.96 | <0.001 |
| Temporal Area, pixels | 79.34 ± 80.73 | 92.48 ± 73.17 | 0.009 | 151.14 ± 97.69 | 189.21 ± 88.18 | <0.001 |
| Superior Diameter, pixels | 3.65 ± 1.64 | 4.25 ± 1.95 | 0.014 | 5.79 ± 1.94 | 6.50 ± 2.04 | 0.002 |
| Inferior Diameter, pixels | 4.38 ± 2.36 | 5.17 ± 2.80 | 0.010 | 6.11 ± 1.81 | 7.36 ± 2.16 | <0.001 |
| Nasal Diameter, pixels | 3.88 ± 2.15 | 4.43 ± 2.52 | 0.063 | 5.22 ± 1.73 | 5.99 ± 1.76 | 0.002 |
| Temporal Diameter, pixels | 3.77 ± 2.29 | 4.15 ± 2.15 | 0.077 | 5.32 ± 1.61 | 6.02 ± 1.95 | <0.001 |
| Average Area, pixels | 80.48 ± 59.54 | 99.20 ± 54.87 | <0.001 | 151.84 ± 52.76 | 198.23 ± 53.70 | <0.001 |
| Average Diameter, pixels | 3.73 ± 1.69 | 4.33 ± 1.74 | <0.001 | 5.61 ± 1.02 | 6.47 ± 1.20 | <0.001 |
IOP = Intraocular pressure; POAG = Primary open angle glaucoma; SC = Schlemm’s canal; SD = Standard deviation. † = Nonparametric test (Related samples).
Figure 2.
Changes in cross-sectional area and diameter of SC in healthy subjects and POAG patients. Bar charts with error bar demonstrating a significant increase in cross-sectional area and diameter of SC after aerobic exercise in different quadrants (panels a and b) in healthy subjects and POAG patients (panels c-h)
We further compared the magnitude of changes in IOP and SC dimension between POAG and healthy eyes after AE [Supplementary Table S4]. Mean decrease in IOP after AE was significantly more in POAG eyes compared to healthy eyes (-2.12 +/- 0.25 vs. -1.04 +/- 0.22 mmHg; P = 0.002). Increase in average SC cross-sectional area was significantly less in POAG eyes compared to healthy eyes (15.90 +/- 6.55 vs. 47.60 +/- 5.80 pixels; P = 0.001). An increase in average SC diameter did not show significant differences between POAG and healthy eyes (P = 0.248).
Supplementary Table S4.
Changes in IOP and SC dimension in POAG and healthy eyes after exercise
| POAG Eyes | Healthy Eyes | P† | |
|---|---|---|---|
| IOP, mmHg | -2.12±0.25 | - 1.04±0.22mmHg | 0.002 |
| SC Area, pixels | 15.90±6.55 | 47.60±5.80 pixels | 0.001 |
| SC Diameter, pixels | 0.61±1.53 pixels | 0.86±1.37 pixel | 0.248 |
IOP = intraocular pressure; POAG = primary open angle glaucoma; SC = Schlemm’s canal.
† = analysis of covariance (ANCOVA).
The SC was observable in 84.3% of POAG eyes before exercise and 87.3% after exercise. However, the difference in such values was not significant (X = 0.852, P = 0.356; Table 3). The SC was observable in 97.9% of healthy eyes both before and after exercise with no statistical difference (X = 0.000, P = 1.000).
Table 3.
Proportion of POAG and Healthy Eyes with an Observable Schlemm’s Canal Before and After Aerobic Exercise
| Quadrants | POAG Eyes with Observable SC | Healthy Eyes with Observable SC | ||||||
|---|---|---|---|---|---|---|---|---|
| Before AE | After AE | χ2 | P(two tail)† | Before AE | After AE | χ2 | P(two tail)† | |
| Total, n (%) | 199/236 (84.3%) | 206/236 (87.3) | 0.852 | 0.356 | 141/144 (97.9) | 141/144 (97.9) | 0.000 | 1.000 |
| Superior Region, n (%) | 53/59 (89.8) | 53/59 (89.8) | 0.000 | 1.000 | 36/36 (100) | 36/36 (100) | 0.000 | 1.000 |
| Inferior Region, n (%) | 51/59 (86.4) | 52/59 (88.1) | 0.076 | 0.782 | 35/36 (97.2) | 35/36 (97.2) | 0.000 | 1.000 |
| Nasal Region, n (%) | 43/59 (72.9) | 48/59 (81.4) | 1.201 | 0.273 | 35/36 (97.2) | 35/36 (97.2) | 0.000 | 1.000 |
| Temporal Region, n (%) | 52/59 (88.1) | 53/59 (89.8) | 0.086 | 0.769 | 35/36 (97.2) | 35/36 (97.2) | 0.000 | 1.000 |
| P (among four quadrants) | 0.045 | 0.458 | - | - | 0.796 | 0.796 | - | - |
AE = Aerobic exercise; SC = Schlemm’s canal. † = χ2 test.
The POAG eyes were further stratified into eyes treated with topical prostaglandin analogues (PG) (19 patients, 33 eyes) and eyes treated by other topical IOP-lowering medications (non-PG) (6 patients, 8 eyes) according to the history of use of IOP-lowering medications for analysis of effects of AE on IOP and SC dimension. IOP decreased significantly after AE in both PG (16.50 +/- 4.75 vs. 14.00 +/- 3.79 mmHg; P < 0.001) and non-PG groups (17.26 +/- 3.93 vs. 15.22 +/- 3.01 mmHg; P < 0.001). Average area and diameter of SC significantly increased after AE in both PG (area: 93.26 +/- 69.12 vs. 107.53 +/- 62.39 pixels; diameter: 4.07 +/- 1.85 vs. 4.55 +/- 1.97 pixels; P = 0.012 and P = 0.015, respectively) and non-PG (area: 64.26 +/- 40.24 vs. 88.62 +/- 42.35 pixels; diameter: 3.92 +/- 1.37 vs. 4.04 +/- 1.39 pixels; P = 0.001 and P = 0.004, respectively) groups [Table 4].
Table 4.
Changes in IOP and SC Parameters in POAG Subgroups Before and After Aerobic Exercise
| Parameters | PG (Mean ± SD) | Non-PG (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| Before Exercise | After Exercise | P* | Before Exercise | After Exercise | P* | |
| IOP, mmHg | 16.50 ± 4.75 | 14.00 ± 3.79 | <0.001 | 17.26 ± 3.93 | 15.22 ± 3.01 | <0.001 |
| Superior Area, pixels | 75.51 ± 36.63 | 98.97 ± 62.29 | 0.026 | 73.95 ± 68.03 | 98.18 ± 96.31 | 0.114 |
| Inferior Area, pixels | 109.13 ± 110.66 | 132.18 ± 133.31 | 0.018 | 80.11 ± 69.06 | 100.22 ± 65.65 | 0.010 |
| Nasal Area, pixels | 103.60 ± 105.78 | 117.55 ± 126.10 | 0.258 | 93.45 ± 91.54 | 110.07 ± 103.7 | 0.005 |
| Temporal Area, pixels | 95.98 ± 94.72 | 102.59 ± 84.83 | 0.213 | 54.38 ± 44.95 | 77.30 ± 49.18 | 0.008 |
| Superior Diameter, pixels | 3.87 ± 1.58 | 4.46 ± 1.88 | 0.041 | 3.38 ± 1.71 | 3.98 ± 2.04 | 0.131 |
| Inferior Diameter, pixels | 4.53 ± 2.73 | 5.43 ± 3.27 | 0.029 | 4.15 ± 1.71 | 4.78 ± 1.88 | 0.171 |
| Nasal Diameter, pixels | 4.09 ± 2.32 | 4.30 ± 2.79 | 0.925 | 3.61 ± 2.09 | 4.59 ± 2.17 | 0.009 |
| Temporal Diameter, pixels | 4.14 ± 2.48 | 4.37 ± 2.34 | 0.459 | 3.17 ± 1.85 | 3.80 ± 1.81 | 0.049 |
| Average Area, pixels | 93.26 ± 69.12 | 107.53 ± 62.39 | 0.012 | 64.26 ± 40.24 | 88.62 ± 42.35 | 0.001 |
| Average Diameter, pixels | 4.07 ± 1.85 | 4.55 ± 1.97 | 0.015 | 3.92 ± 1.37 | 4.04 ± 1.39 | 0.004 |
IOP = Intraocular pressure; PG = Prostaglandin analogues; SC = Schlemm’s canal; SD = Standard deviation; * = Wilcoxon test (paired samples)
Furthermore, the magnitude of reduction of IOP and increase in SC dimension showed no significant differences between treated and PG and non-PG groups (all P > 0.05) [Supplementary Table S5].
Supplementary Table S5.
Mean change of IOP, SC parameters in PG and non-PG groups after exercise.
| PG group | Non-PG group | P† | |
|---|---|---|---|
| IOP | -2.41 ± 0.38mmHg | -1.80 ± 0.46 mmHg | 0.327 |
| SC area | 9.65 ± 5.31 pixels | 3.14 ± 6.05pixels | 0.440 |
| SC diameter | 0.49 ± 1.15 pixel | 0.75 ± 1.12 pixel | 0.328 |
IOP= intraocular pressure, SC=Schlemm’s canal;
† = Wilcoxon test (paired samples).
In healthy eyes, increase of SC area in the temporal quadrant and diameter in the inferior quadrant showed a positive correlation with the decrease of IOP in both univariable (temporal area: r = 0.274, P = 0.022 and inferior diameter: r = 0.294, P = 0.014) and multivariable (temporal area: b = 0.004, P = 0.026 and inferior diameter: b = 0.170, P = 0.034) analyses. In POAG eyes, the increase in inferior SC area was negatively correlated with the decrease of IOP in univariable analysis (r = -0.295, P = 0.046). However, no significant associations in multivariable analysis (b = -0.182, P = 0.127) were observed [Table 5]. In the POAG subgroup analysis, no significant correlations and associations were observed between SC dimension and IOP [Supplementary Table S6].
Table 5.
Association Analysis of IOP Changes in Healthy and Overall POAG Patients
| Variables | Healthy Eyes | POAG Eyes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||||
| r | P | β | 95%CI | P | r | P | β | 95%CI | P | |
| Eyes | -0.109 | 0.368 | - | - | - | -0.029 | 0.827 | - | - | - |
| Age | 0.279 | 0.020* | 0.030 | -0.003, 0.063 | 0.079 | 0.093 | 0.485 | - | - | - |
| Sex | -0.082 | 0.498 | - | - | - | -0.176 | 0.182 | - | - | - |
| SE | -0.062 | 0.611 | - | - | - | 0.037 | 0.783 | - | - | - |
| AL | 0.095 | 0.435 | - | - | - | -0.052 | 0.703 | - | - | - |
| δ SBP | -0.013 | 0.940 | - | - | - | 0.124 | 0.357 | - | - | - |
| δ DBP | 0.131 | 0.452 | - | - | - | 0.055 | 0.686 | - | - | - |
| δ HR | -0.118 | 0.500 | - | - | - | -0.270 | 0.043* | -0.042 | -0.108, 0.023 | 0.205 |
| δ Average area | 0.117 | 0.335 | - | - | - | -0.025 | 0.853 | - | - | - |
| δ Average diameter | 0.081 | 0.507 | - | - | - | -0.167 | 0.222 | - | - | - |
| δ superior area | -0.160 | 0.186 | - | - | - | 0.080 | 0.576 | - | - | - |
| δ inferior area | 0.229 | 0.057 | - | - | - | -0.179 | 0.218 | - | - | - |
| δ nasal area | -0.070 | 0. 568 | - | - | - | -0.074 | 0.637 | - | - | - |
| δ temporal area | 0.274 | 0.022 | 0.004 | 0.000, 0.007 | 0.026 | -0.142 | 0.334 | - | - | - |
| δ superior diameter | -0.043 | 0.721 | - | - | - | 0.049 | 0.741 | - | - | - |
| δ inferior diameter | 0.294 | 0.014 | 0.170 | 0.013, 0.326 | 0.034 | -0.295 | 0.046* | -0.182 | -0.414, 0.052 | 0.127 |
| δ nasal diameter | -0.154 | 0.204 | - | - | - | -0.262 | 0.103 | - | - | - |
| δ temporal diameter | 0.003 | 0.982 | - | - | - | -0.198 | 0.192 | - | - | - |
IOP = Intraocular pressure, SC = Schlemm’s canal, SE = Spherical equivalent, AL = Axial length, systolic SBP = Systolic blood pressure, DBP = Diastolic blood pressure, HR = Heart rate, δ = The change of the variables after exercise. The variables without δ were baseline data. CI = Confidence interval. The multivariable analysis adjusted for age, sex, eye, AL, SE
Supplementary Table S6.
Association analysis of IOP changes in POAG subgroups
| Parameters | PG | Non-PG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||||
| r | P | β | 95% CI | P | r | P | β | 95%CI | P | |
| Eyes | -0.030 | 0.865 | - | - | - | -0.022 | 0.915 | - | - | - |
| Age | 0.036 | 0.840 | - | - | - | 0.046 | 0.825 | - | - | - |
| Sex | - | - | - | - | - | -0.249 | 0.229 | - | - | - |
| SE | -0.107 | 0.555 | - | - | - | 0.316 | 0.124 | - | - | - |
| AL | 0.109 | 0.540 | - | - | - | -0.358 | 0.093 | - | - | - |
| δ SBP | 0.188 | 0.287 | - | - | - | 0.245 | 0.260 | - | - | - |
| δ DBP | 0.196 | 0.267 | - | - | - | 0.173 | 0.429 | - | - | - |
| δ HR | -0.191 | 0.280 | - | - | - | 0.395 | 0.062 | |||
| δ Average area | -0.136 | 0.450 | - | - | - | 0.005 | 0.980 | - | - | - |
| δ Average | -0.151 | 0.425 | - | - | - | -0.214 | 0.305 | - | - | - |
| diameter | ||||||||||
| δ superior area | 0.154 | 0.425 | - | - | - | 0.060 | 0.791 | - | - | - |
| δ inferior area | -0.382 | 0.037* | - | - | - | 0.000 | 0.999 | - | - | - |
| δ nasal area | -0.023 | 0.543 | - | - | - | -0.050 | 0.845 | - | - | - |
| δ temporal area | -0.116 | 0494 | - | - | - | -0.033 | 0.897 | - | - | - |
| δ superior diameter | 0.039 | 0.849 | - | - | - | -0.110 | 0.627 | - | - | - |
| δ inferior diameter | -0.320 | 0.103 | - | - | - | -0.312 | 0.193 | - | - | - |
| δ nasal diameter | -0.096 | 0.671 | - | - | - | -0.165 | 0.514 | - | - | - |
| δ temporal | 0.028 | 0.891 | - | - | - | -0.324 | 0.190 | - | - | - |
| diameter | ||||||||||
IOP=intraocular pressure, SC=Schlemm’s canal, SE=spherical equivalent, AL=axial length, systolic SBP=blood pressure, DBP=diastolic blood pressure, HR=heart rate, δ,the change of the variables after exercise. The variables without δ were baseline data. CI=confidence interval, β/P value: regression coefficient and P values (two tails) of the independent variables in the generalized estimating equations. The influence factors as age, sex, eyes, AL, SE, have been adjusted. * means P has significant difference (P <0.05).
Discussion
In this study, we evaluated the effect of AE on the IOP and SC dimension in both POAG and healthy eyes. We further compared such effect between POAG and healthy eyes, and between POAG subgroups receiving different IOP-lowering pharmacological treatments. Our results demonstrated that short-term AE induced IOP reduction immediately in both POAG and healthy eyes, regardless of history of IOP-lowering medication use, which reproduced and corroborated findings in previous studies conducted in only healthy eyes.[9,10] Results of this study supported that AE could be of a potentially beneficial role to POAG eyes and further considered as a lifestyle modification in POAG patients for achieving satisfactory IOP control.
Previous studies demonstrated grossly altered SC morphology in glaucomatous eyes as compared to healthy eyes, which could impair aqueous drainage and result in a vicious cycle of IOP elevation.[17,18,19] The dimension of SC in POAG eyes in our current study was also smaller than that in healthy eyes at baseline, which was following the results of previous literature.[19,28] In our current study, following AE, IOP in both POAG and healthy eyes was significantly reduced. Meanwhile, there was a significant increase in average cross-sectional area and diameter of the SC after AE in both POAG and healthy eyes. These results suggested the utility of AE in promoting the expansion of SC in POAG eyes, despite altered and less favorable baseline SC morphology, in addition to healthy eyes as reported in previous studies.[9,10] The enlargement of SC likely facilitated aqueous outflow and drainage through it into episcleral veins, in turn, lowering the IOP.[17]
We further compared the magnitude of changes in SC dimensions in POAG and healthy eyes following AE. In comparison with healthy eyes, the extent of increase in SC area after AE in POAG eyes was less. However, despite a lesser degree of SC expansion, POAG eyes achieved a greater degree of IOP reduction after AE. These findings suggested that POAG eyes have retained reactiveness in SC in response to AE as healthy eyes, and the same dose of exercise induces more effect in IOP reduction in POAG eyes despite a less favorable baseline SC dimension and a lesser degree of SC expansion following AE. Vahabikashi et al. found that tissues from glaucomatous eyes were stiffer than normal eyes, with greatly increased stiffness residing within the inner-wall surface of SC.[29] In addition, SC also exhibited less favorable morphology at baseline in POAG eyes compared to healthy eyes.[10,17,18,20] These previous findings may explain the less reactive SC in POAG to AE as evidenced by the smaller proportion of POAG eyes with observable SC both before and after AE, and the lesser SC expansion following AE compared to healthy eyes. Nonetheless, the greater degree of IOP reduction in POAG with a modest expansion in SC following AE proved that AE could still be beneficial for IOP reduction in POAG patients. Furthermore, these findings suggest that the increase in aqueous outflow facility and SC expansion may not be in a linear relationship, which may, therefore, explain the insignificant relationships between the increase in SC dimensions and decrease in IOP in regression analyses of the current study and previous studies conducted in healthy eyes.[10] The exact mechanisms and full picture of AE leading to SC expansion and IOP reduction are, therefore, yet to be completely elucidated. Further studies in this direction are warranted.
Among 25 patients (41 eyes) receiving prior treatments, 19 patients (33 eyes) patients are prescribed topical PG analogs. PG analogs facilitate aqueous outflow through both the conventional and uveoscleral pathways.[30,31] In the conventional pathway, Chen et al. have reported that PG analogs led to IOP reduction and SC expansion.[12] Following prior treatment by PG analogs, significant IOP reduction and SC expansion following AE were still observed in the PG-treated POAG eyes, and the changes of IOP and SC dimension following AE did not differ significantly between POAG eyes receiving PG analogs and other classes of IOP-lowering medications. This was an interesting observation and revealed that following prior pharmacological treatment which targeted SC for IOP reduction, effects of AE on both IOP reduction and SC expansion were still retained. We have previously further stratified all POAG eyes into treated (25 patients, 41 eyes) and newly diagnosed nontreated (10 patients, 18 eyes) groups and found that both groups also showed similar responses to AE [Supplementary Tables S7 and S8]. To our knowledge, this observation has not been reported in the existing literature. There are various implications. Firstly, this is evidence to further support the use of AE as a lifestyle modification for IOP reduction in patients already on prior pharmacological treatment. Besides, many patients with IOP refractory to pharmacological treatments undergo laser treatments or glaucoma surgeries targeting the TM or SC to reduce IOP by facilitating aqueous outflow through such pathway. AE may also retain its effects and can be recommended as a lifestyle modification for IOP reduction in this group of patients who have undergone invasive interventions in addition to pharmacological treatments. Lastly, it indicates that the regulation of SC is likely due to a multitude of mechanisms. Previous studies have reported the role of sympathetic activation which might explain the effect of AE to induce further changes in SC following treatment by PG analogs.[32,33,34] The current observation suggests these regulatory pathways act synergistically in expanding SC. Further studies to evaluate the effect of AE on POAG eyes with prior interventions targeting SC, the exact mechanisms of SC regulation, and methods to achieve maximal SC activation for IOP reduction are warranted.
Supplementary Table S7.
Changes in IOP and SC dimension in POAG treatment subgroups before and after exercise.
| Treated group | Non-Treated group | |||||
|---|---|---|---|---|---|---|
| Before Exercise Mean±SD | After Exercise Mean±SD | P† | Before Exercise Mean±SD | After Exercise Mean±SD | P† | |
| IOP, mmHg | 16.60±4.70 | 14.06±3.80 | <0.001 | 17.33±3.71 | 15.56±2.50 | 0.004 |
| Superior Area, pixels | 69.23±37.79 | 89.10±58.99 | 0.027 | 88.58±77.46 | 122.10±112.06 | 0.100 |
| Inferior Area, pixels | 100.78±100.54 | 124.43±122.39 | 0.006 | 83.04±65.65 | 113.23±61.47 | 0.034 |
| Nasal Area, pixels | 95.22±95.88 | 113.13±113.38 | 0.031 | 88.16±81.12 | 100.89±70.60 | 0.131 |
| Temporal Area, pixels | 90.68±87.85 | 104.36±81.06 | 0.045 | 50.16±50.11 | 61.91±33.04 | 0.048 |
| Superior Diameter, pixels | 3.60±1.64 | 4.12±1.82 | 0.028 | 3.79±1.70 | 4.54±2.77 | 0.140 |
| Inferior Diameter, pixels | 4.42±2.44 | 5.29±3.03 | 0.027 | 4.24±2.18 | 4.81±1.96 | 0.155 |
| Nasal Diameter, pixels | 3.97±2.17 | 4.49±2.59 | 0.153 | 3.63±2.44 | 4.24±2.41 | 0.182 |
| Temporal Diameter, pixels | 4.08±2.34 | 4.47±2.35 | 0.236 | 2.89±1.98 | 3.25±1.11 | 0.152 |
| Average Area, pixels | 86.85±64.05 | 103.59±57.96 | 0.001 | 65.97±46.03 | 89.18±47.06 | 0.016 |
| Average Diameter, pixels | 3.95±1.73 | 4.52±1.82 | 0.001 | 3.22±1.51 | 3.89±1.49 | 0.039 |
IOP = intraocular pressure; SC = Schlemm’s canal; SD = standard deviation.
†= Nonparametric test (Related samples).
Supplementary Table S8.
Changes in IOP and SC dimension in treated and non-treated groups of POAG patients after exercise.
| Treated group | Non-treated group | Pt | |
|---|---|---|---|
| IOP, mmHg | -2.52±0.36 mmHg | -1.28±0.60 mmHg | 0.109 |
| SC Area, pixels | 19.65±6.19 pixel | 23.97±9.87 pixel | 0.731 |
| SC Diameter, pixels | 0.55±0.19 pixel | 0.71±0.31 pixel | 0.697 |
IOP = intraocular pressure, SC = Schlemm’s canal.
† = analysis of covariance (ANCOVA).
After adjusting for age and sex, we only observed a positive association between an increase of SC area in the temporal quadrant and SC diameter in the inferior quadrant with IOP reduction in healthy eyes. No statistically significant associations were found in changes in SC dimension in other quadrants with IOP reduction. In POAG eyes, no statistically significant associations were observed between changes in SC dimension and IOP reduction. Such findings were similar to results from Yan et al.[10] AE is well known to reduce IOP in healthy eyes in existing literature, and previous studies had proposed various mechanisms to explain such a phenomenon.[14,35] To date, the precise mechanisms of AE leading to IOP reduction remain unknown. Various mechanisms including sympathetic activation, dehydration, increased plasma osmolarity, and decreased blood pH has been suggested to contribute to the post-exercise IOP reduction.[14,35,36] SC expansion following AE, therefore, may only have a modest role and does not fully account for the IOP reduction. Furthermore, similar to prior studies, the current study which focused on SC morphology did not directly measure the increase in aqueous outflow through the expanded SC following AE.[9,10,16] We hypothesize that direct measurements of such increased outflow which results in IOP reduction and of episcleral venous pressure which increases after increased aqueous outflow through TM and SC may better account for and correlate with IOP reduction as compared to the measurement of changes in physical dimensions of SC. Future studies of such design are warranted so that the precise effects of AE-induced SC enlargement on IOP reduction can be evaluated.
To our knowledge, this is the first study conducted on POAG patients to demonstrate the utility of AE both in inducing SC expansion and IOP reduction. The American Academy of Ophthalmology Preferred Practice Pattern recommends that the IOP of glaucoma patients should achieve the target pressure at which visual field is unlikely to progress over their lifetime,[37] and physical activity is reported to be associated with a significantly lower risk of glaucoma.[38] The results of this current study provide evidence to support that AE may be a potentially beneficial lifestyle modification in both treatment naïve eyes and eyes treated with IOP-lowering medications to achieve satisfactory IOP control for prevention of visual field progression.
Limitations
Limitations of our study should be noted. Firstly, it is a cross-sectional study. The long-term impact of AE on IOP in POAG eyes cannot be ascertained. Secondly, we did not follow the subjects to evaluate their SC morphology and IOP at other time-points following AE to look for recovery of SC and IOP to baseline values to ascertain the sustainability of AE’s effect on these two outcomes. A recent study reported that SC dimensions and IOP in healthy individuals recovered at 15 and 60 min following AE, respectively.[16] Further studies are warranted to evaluate the long-term effects of AE on SC morphology and IOP in POAG eyes, and longitudinal studies are necessary to evaluate the potential benefits of AE in lowering the risk of POAG development and progression.
Conclusion
This study revealed AE-induced SC expansion and reduced IOP in both POAG and healthy eyes. Our results showed that AE retained its effects to induce SC enlargement and IOP reduction in POAG eyes despite the worse baseline SC morphology in POAG eyes compared to healthy eyes. Besides, prior use of IOP-lowering medications did not attenuate AE-induced SC and IOP changes in POAG eyes. These results laid down some groundwork for the possibility of the potential use of AE as a beneficial lifestyle modification for IOP reduction in POAG patients. However, further longitudinal studies with larger sample sizes are warranted to evaluate the long-term impacts of AE on IOP control, SC morphology, and disease progression in POAG patients to whether AE has an adjunctive role in the management of POAG.
Financial support and sponsorship
This work was supported by the National key research and development project (2018YFC010302), Science and Technology Planning Projects of Guangdong Province (2017B030314025), and Science and Technology Program of Guangzhou, China (201803010066).
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Ms. Li Mian Xiao and the Guangzhou TianPeng Computer Technology Co. Ltd for their support in statistical analysis.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JWY, Chan PP, Zhang X, Chen LJ, Jonas JB. Latest developments in normal-pressure glaucoma:Diagnosis, epidemiology, genetics, etiology, causes and mechanisms to management. Asia Pac J Ophthalmol (Phila) 2019;8:457–68. doi: 10.1097/01.APO.0000605096.48529.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin LA. Neuroprotection in optic neuropathy. Asia Pac J Ophthalmol (Phila) 2018;7:246–50. doi: 10.22608/APO.2018299. [DOI] [PubMed] [Google Scholar]
- 4.Nassiri N, Kamali G, Rahnavardi M, Mohammadi B, Nassiri S, Rahmani L, et al. Ahmed glaucoma valve and single-plate Molteno implants in treatment of refractory glaucoma:A comparative study. Am J Ophthalmol. 2010;149:893–902. doi: 10.1016/j.ajo.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman PL, Mohr ME, Riccomini SP, Rasmussen CA. Glaucoma drugs in the pipeline. Asia Pac J Ophthalmol (Phila) 2018;7:345–51. doi: 10.22608/APO.2018298. [DOI] [PubMed] [Google Scholar]
- 6.Langham ME, Rosenthal AR. Role of cervical sympathetic nerve in regulating intraocular pressure and circulation. Am J Physiol. 1966;210:786–94. doi: 10.1152/ajplegacy.1966.210.4.786. [DOI] [PubMed] [Google Scholar]
- 7.Gungor K, Beydagi H, Bekir N, Arslan C, Suer C, Erbagci I, et al. The impact of acute dynamic exercise on intraocular pressure:Role of the beta 2-adrenergic receptor polymorphism. J Int Med Res. 2002;30:26–33. doi: 10.1177/147323000203000105. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Li F, Zhou R, Gao K, Liang L, Zhang X. Aerobic Exercise Increases Tear Secretion and Decreases Inflammatory Cytokines in Healthy Subjects. Asia Pac J Ophthalmol (Phila) 2020;9:404–11. doi: 10.1097/APO.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 9.Yan X, Li M, Zhang H. Relationship between post-exercise changes in the lens and Schlemm's canal:A swept-source optical coherence tomography study. Curr Eye Res. 2018;43:1351–6. doi: 10.1080/02713683.2018.1498523. [DOI] [PubMed] [Google Scholar]
- 10.Yan X, Li M, Song Y, Guo J, Zhao Y, Chen W, et al. Influence of exercise on intraocular pressure, Schlemm's canal, and the trabecular meshwork. Invest Ophthalmol Vis Sci. 2016;57:4733–9. doi: 10.1167/iovs.16-19475. [DOI] [PubMed] [Google Scholar]
- 11.Hong J, Yang Y, Wei A, Deng SX, Kong X, Chen J, et al. Schlemm's canal expands after trabeculectomy in patients with primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2014;55:5637–42. doi: 10.1167/iovs.14-14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Huang H, Zhang S, Chen X, Sun X. Expansion of Schlemm's canal by travoprost in healthy subjects determined by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:1127–34. doi: 10.1167/iovs.12-10396. [DOI] [PubMed] [Google Scholar]
- 13.Fuest M, Kuerten D, Koch E, Becker J, Hirsch T, Walter P, et al. Evaluation of early anatomical changes following canaloplasty with anterior segment spectral-domain optical coherence tomography and ultrasound biomicroscopy. Acta Ophthalmol. 2016;94:e287–92. doi: 10.1111/aos.12917. [DOI] [PubMed] [Google Scholar]
- 14.Risner D, Ehrlich R, Kheradiya NS, Siesky B, McCranor L, Harris A. Effects of exercise on intraocular pressure and ocular blood flow:A review. J Glaucoma. 2009;18:429–36. doi: 10.1097/IJG.0b013e31818fa5f3. [DOI] [PubMed] [Google Scholar]
- 15.Zhu MM, Lai JSM, Choy BNK, Shum JWH, Lo ACY, Ng ALK, et al. Physical exercise and glaucoma:A review on the roles of physical exercise on intraocular pressure control, ocular blood flow regulation, neuroprotection and glaucoma-related mental health. Acta Ophthalmol. 2018;96:e676–91. doi: 10.1111/aos.13661. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Yan X, Luo Z, Zhang H. Postexercise recovery of Schlemm's canal and intraocular pressure in healthy individuals:An observational study using swept-source optical coherence tomography. J Ophthalmol. 2018;2018:8513760. doi: 10.1155/2018/8513760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allingham RR, de Kater AW, Ethier CR. Schlemm's canal and primary open angle glaucoma:Correlation between Schlemm's canal dimensions and outflow facility. Exp Eye Res. 1996;62:101–9. doi: 10.1006/exer.1996.0012. [DOI] [PubMed] [Google Scholar]
- 18.Hong J, Xu J, Wei A, Wen W, Chen J, Yu X, et al. Spectral-domain optical coherence tomographic assessment of Schlemm's canal in Chinese subjects with primary open-angle glaucoma. Ophthalmology. 2013;120:709–15. doi: 10.1016/j.ophtha.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Yan X, Li M, Chen Z, Zhu Y, Song Y, Zhang H. Schlemm's canal and trabecular meshwork in eyes with primary open angle glaucoma:A comparative study using high-frequency ultrasound biomicroscopy. PLoS One. 2016;11:e0145824. doi: 10.1371/journal.pone.0145824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–83. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Li H, Yang J, Liu J, Aung T, Zhang X. Upside-down position leads to choroidal expansion and anterior chamber shallowing:OCT study. Br J Ophthalmol. 2020;104:790–4. doi: 10.1136/bjophthalmol-2019-314418. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Wang W, Chen S, Huang W, Liu Y, Wang J, et al. Effects of valsalva maneuver on anterior chamber parameters and choroidal thickness in healthy Chinese:An AS-OCT and SS-OCT study. Invest Ophthalmol Vis Sci. 2016;57 doi: 10.1167/iovs.15-18449. OCT189-95. [DOI] [PubMed] [Google Scholar]
- 23.Mastropasqua L, Agnifili L, Salvetat ML, Ciancaglini M, Fasanella V, Nubile M, et al. In vivo analysis of conjunctiva in canaloplasty for glaucoma. Br J Ophthalmol. 2012;96:634–9. doi: 10.1136/bjophthalmol-2011-301058. [DOI] [PubMed] [Google Scholar]
- 24.McKee H, Ye C, Yu M, Liu S, Lam DS, Leung CK. Anterior chamber angle imaging with swept-source optical coherence tomography:Detecting the scleral spur, Schwalbe's line, and Schlemm's canal. J Glaucoma. 2013;22:468–72. doi: 10.1097/IJG.0b013e31824485fa. [DOI] [PubMed] [Google Scholar]
- 25.Kiuchi Y, Mishima HK, Hotehama Y, Furumoto A, Hirota A, Onari K. Exercise intensity determines the magnitude of IOP decrease after running. Jpn J Ophthalmol. 1994;38:191–5. [PubMed] [Google Scholar]
- 26.Nogic J, Thein PM, Cameron J, Mirzaee S, Ihdayhid A, Nasis A. The utility of personal activity trackers (Fitbit Charge 2) on exercise capacity in patients post acute coronary syndrome [UP-STEP ACS Trial]:A randomised controlled trial protocol. BMC Cardiovasc Disord. 2017;17:303. doi: 10.1186/s12872-017-0726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao K, Li F, Aung T, Zhang X. Diurnal variations in the morphology of Schlemm's canal and intraocular pressure in healthy Chinese:An SS-OCT study. Invest Ophthalmol Vis Sci. 2017;58:5777–82. doi: 10.1167/iovs.17-22019. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Shi G, Li X, Lu J, Ding Z, Sun X, et al. Comparison of Schlemm's canal's biological parameters in primary open-angle glaucoma and normal human eyes with swept source optical. J Biomed Opt. 2012;17:116008. doi: 10.1117/1.JBO.17.11.116008. [DOI] [PubMed] [Google Scholar]
- 29.Vahabikashi A, Gelman A, Dong B, Gong L, Cha EDK, Schimmel M, et al. Increased stiffness and flow resistance of the inner wall of Schlemm's canal in glaucomatous human eyes. Proc Natl Acad Sci U S A. 2019;116:26555–63. doi: 10.1073/pnas.1911837116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler NS, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocul Pharmacol Ther. 2014;30:102–9. doi: 10.1089/jop.2013.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47(Suppl 1):S53–64. doi: 10.1016/s0039-6257(02)00306-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhou EH, Krishnan R, Stamer WD, Perkumas KM, Rajendran K, Nabhan JF, et al. Mechanical responsiveness of the endothelial cell of Schlemm's canal:Scope, variability and its potential role in controlling aqueous humour outflow. J R Soc Interface. 2012;9:1144–55. doi: 10.1098/rsif.2011.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akagi Y, Ibata Y, Sano Y. The sympathetic innervation of the ciliary body and trabecular meshwork of the cat. Fluorescence histochemistry and electron microscopy. Cell Tissue Res. 1976;173:261–9. doi: 10.1007/BF00221379. [DOI] [PubMed] [Google Scholar]
- 34.Jampel HD, Lynch MG, Brown RH, Kuhar MJ, De Souza EB. Beta-adrenergic receptors in human trabecular meshwork. Identification and autoradiographic localization. Invest Ophthalmol Vis Sci. 1987;28:772–9. [PubMed] [Google Scholar]
- 35.Martin B, Harris A, Hammel T, Malinovsky V. Mechanism of exercise-induced ocular hypotension. Invest Ophthalmol Vis Sci. 1999;40:1011–5. [PubMed] [Google Scholar]
- 36.Ashkenazi I, Melamed S, Blumenthal M. The effect of continuous strenuous exercise on intraocular pressure. Invest Ophthalmol Vis Sci. 1992;33:2874–7. [PubMed] [Google Scholar]
- 37.Chauhan BC, Garway-Heath DF, Goni FJ, Rossetti L, Bengtsson B, Viswanathan AC, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–73. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meier NF, Lee DC, Sui X, Blair SN. Physical activity, cardiorespiratory fitness, and incident glaucoma. Med Sci Sports Exerc. 2018;50:2253–8. doi: 10.1249/MSS.0000000000001692. [DOI] [PMC free article] [PubMed] [Google Scholar]