Abstract
Purpose:
To study topical timolol (0.5%) as a first-line treatment in ophthalmic pyogenic granuloma (PG) in terms of safety and efficacy.
Methods:
This was a prospective, interventional, single-arm study conducted at a tertiary eye care hospital in central India. Only new cases of PG were counseled to get enrolled in the study. A total of 40 patients were analyzed in the study. Topical timolol eye drop (0.5%) was started in each patient twice daily for 4–6 weeks duration. The patients were divided into five categories according to the percentage reduction in the size of PG as follows: i) 80–100% reduction - excellent responders, ii) 60–80% – good, iii) 40–60% – satisfactory, iv) 20–40% – poor, and v) <20% – very poor/nonresponder. After 6 months of starting treatment final evaluation was done.
Results:
The mean age of the patients was 23.5 ± 13.3 years. Etiology of the disease included chalazion (n = 11, 27.5%), trauma (n = 2, 5%), surgery (n = 7, 17.5%), foreign body (n = 2, 5%), and idiopathic (n = 18, 45%). An excellent response was achieved in 31 (77.5%) patients. Twenty-seven (67.5%) patients had complete resolution of lesions within 6 weeks. Recurrence of the lesion was not noticed in any patients.
Conclusion:
Timolol 0.5% in topical form is a good treatment option for ophthalmic PG in all age groups. The treatment has no adverse effects when given to suitable individuals for a limited period.
Keywords: Beta-blocker, pyogenic granuloma, timolol (0.5%) eye drop
Pyogenic granuloma (PG) is an acquired, benign vascular lesion that develops on cutaneous and mucosal surfaces. Ophthalmic PG usually occurs as sequelae of inflammation from ophthalmic surgery, trauma, or chalazia.[1] Histologically, PG is composed of capillaries and venules with plump endothelial cells separated into lobules by fibromyxoid stroma.[2] The lesions can grow on the palpebral or bulbar conjunctiva, which can cause foreign body sensation, spontaneous bleeding, and poor cosmesis. Usually, PG is a clinical diagnosis. Excisional biopsy may be required when the diagnosis is uncertain or the lesion is unremitting to more conservative treatment.
Treatment options for ophthalmic PG include topical steroids or surgical excision.[1] However, topical steroids can result in ocular hypertension, with 30% of patients experiencing a 6 to 15 mmHg rise in intraocular pressure (IOP) after 4 to 6 weeks of use,[3] and it may also lead to posterior subcapsular cataract formation. Besides, surgical intervention carries the risk of bleeding, infection, conjunctival scarring, and also increases the treatment cost.[4,5,6]
Recent reports have revealed that topical timolol (nonselective b-blocker) may be considered as an alternative, noninvasive treatment option for PG. Topical timolol 0.5%, 2 to 4 times daily has been used for variable durations with minimal adverse effects or recurrences.[7,8,9,10,11,12] Previous studies were limited by small sample size and retrospective design. There was no common consensus regarding dose frequency and duration of treatment. Our study aimed to prospectively evaluate the use of 0.5% topical timolol twice daily for 6 weeks as first-line treatment in cases of ophthalmic PG.
Methods
This was a single-arm, prospective, interventional, and analytical study conducted in patients presenting with ophthalmic PG. Consecutive patients were recruited from February 1, 2019 to January 31, 2020 at a tertiary eye care hospital in central India. All aspects of this research protocol adhered to the tenets of the Declaration of Helsinki. The institutional review board approved this study and informed written consent was taken from each patient.
The diagnosis was made clinically by observing a pink-red, fleshy, sessile or pedunculated, nontender conjunctival vascular mass which may or may not bleed to touch. Only new cases of PG were counseled for this treatment. The exclusion criteria were recurrent PG and patients who had already received some form of treatment. A minimum of 6 months of follow-up was necessary to qualify for the final analysis. Patients with conditions where timolol needs to be avoided like pregnancy, cardiac disease, or bronchial asthma were excluded. A total of 40 patients with clinical signs of ophthalmic PG were analyzed after considering inclusion and exclusion criteria.
A detailed history was obtained and an ophthalmic examination was done for all the patients. The demographic details, the onset of a lesion, duration of symptoms, and any other causes related to the disease (like chalazion, trauma, surgery, foreign body) were documented. The etiology was idiopathic in patients who did not have any of these abovementioned factors. Appropriate treatment of the primary cause was also done whenever applicable. The foreign body was removed before commencing the treatment. Chalazion if remained was curetted after PG subsided with the treatment. Visual acuity and IOP were measured using the Snellen chart and Goldmann applanation tonometry, respectively. Slit-lamp biomicroscopy was done for anterior and posterior segment evaluation. The lesion was evaluated with the emphasis on size, site, clinical features, and morphology.
The size of the lesion was measured along its maximum vertical and horizontal dimensions using a caliper, and it was expressed in millimeter square.[13] Lesions more than 25 mm2 were considered “large.” Documentation of lesion was done by a single ophthalmologist who was masked regarding patient and treatment details.
The efficacy of treatment with timolol 0.5% eye drop was measured in terms of percentage reduction in the size of PG and patients were divided into five categories as follows: i) 80–100% reduction – excellent responders, ii) 60–80% – good, iii) 40–60% – satisfactory, iv) 20–40% – poor, and v) < 20% – very poor/nonresponder [Fig. 1].
Figure 1.
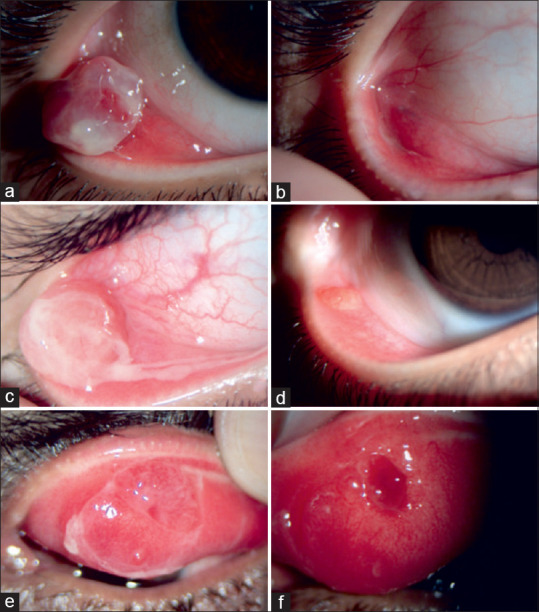
Patients showing excellent response before (a) and after (b); good response before (c) and after (d); satisfactory response before (e) and after (f) treatment
PG is not uncommon after eyelid and extraocular surgery. Thus, we have included a patient with eyelid skin involvement. The patient was advised application of cotton moistened with timolol over the lesion for a few minutes.
Treatment and follow-up protocol [Fig. 2]
Figure 2.
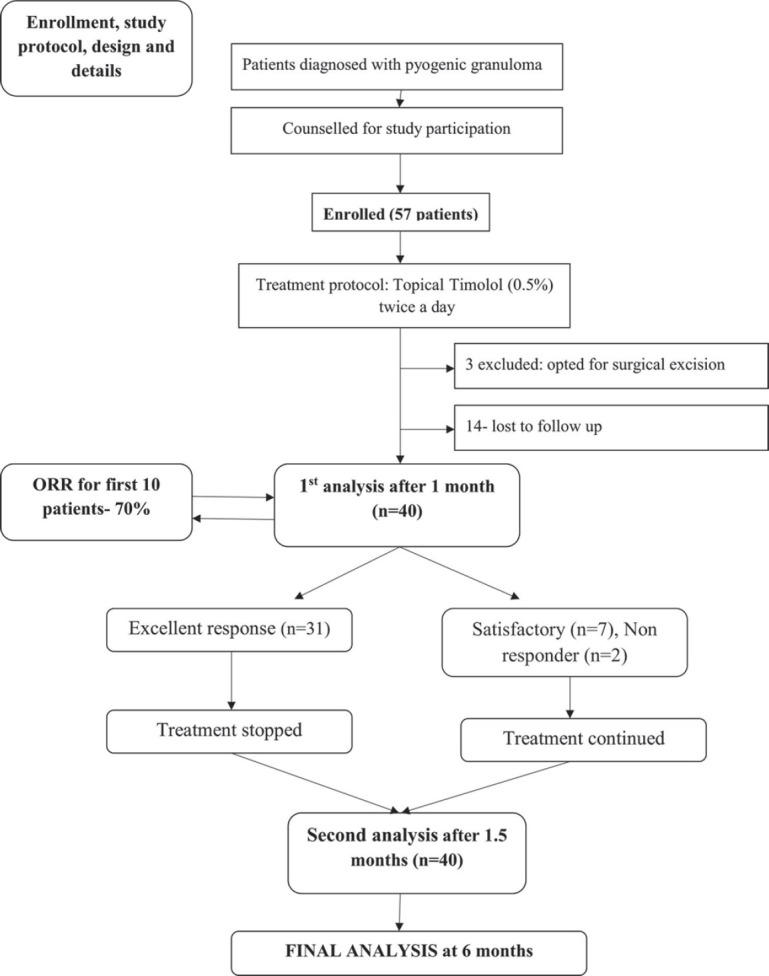
Treatment and follow-up protocol
A small pilot project was done in the first 10 cases of PG fulfilling the inclusion and exclusion criteria. We measured the objective response rate (ORR) i.e., the percentage of patients showing excellent response after 6 weeks of treatment. To continue this study, at least 60% of ORR was considered necessary.
Each patient was started on topical timolol 0.5% eye drop at a twice-daily dose for 4 weeks initially. The resolution of the lesion was measured after 4 weeks. If an excellent response was noted, then the treatment was stopped and patients were further followed weekly for another 2 weeks. In the rest of the patients, treatment was continued for another 2 weeks (total 6 weeks). The second analysis was done in all the patients at 6 weeks. Treatment was aborted after 6 weeks duration if there was no clinical resolution/improvement. All the patients were examined 6 weekly thereafter and the final evaluation was done at 6 months after starting the treatment.
Patients were also advised alternative/symptomatic treatment after 6 weeks in the form of steroid eye drop or surgical excision if no response was observed at all.
Treatment of recurrent PG/PG with inadequate response
The reappearance of the lesion within 6 weeks of stopping treatment was considered as recurrent PG in whom timolol was not restarted. Patients were followed up for at least 6 months in the hope of the late resolution of the lesion. If a satisfactory response was not achieved in the final analysis, then topical steroids or surgical excision was planned after consultation with the patient.
Statistical analysis
Data were collected and statistically analyzed by using Statistical Package for the Social Sciences (SPSS) version 20 (IBM Corp., Armonk, N.Y., USA). The sample size was calculated using the following parameters: type 1 error – 5%, power of the study –90%, the efficacy of the surgical excision – 95%, and the expected efficacy of the experimental approach with timolol –75%. The sample size thus calculated for a single-arm study having a dichotomous endpoint was 24. All qualitative data were presented in the form of frequency, percentages, and quantitative data were presented in the form of mean and standard deviation. Microsoft word (2010) and Excel (2010) were used to generate tables and graphs, respectively. X-Y scatter charts were generated along with the trend line to correlate outcomes with the age of the patient and the size of the lesion. Data were entered and analyzed in binary format to calculate any significance for not achieving an excellent outcome for various variables. The above was calculated using both bivariate and multivariate regression models. A P value of < 0.05 was considered statistically significant.
Results
A total of 57 patients with PG were enrolled in this study after considering the inclusion and exclusion criteria. All the patients were started with the proposed treatment. However, three patients after a week of starting treatment opted for surgical excision. Fourteen patients did not come for follow-up at all and could not be traced. Thus, 17 patients were excluded from the study and the final analysis could be done in 40 patients [Fig. 2].
The mean age of patients was 23.50 ± 13.37 years (range from 2–65 years). There was no significant gender predilection with the male/female ratio of 1.22:1 [Table 1].
Table 1.
Baseline and demographic characteristics
| Parameter | Number |
|---|---|
| Total patients | 40 |
| Total eyes | 40 |
| Number of lesions | 54 |
| Age (mean±SD) | 23.3+13 |
| <16 years | 12 |
| 16-30 years | 21 |
| >30 years | 7 |
| Gender (ratio) | 1.22:1 |
| Male | 22 |
| Female | 18 |
| Eye involved | |
| Right | 26 |
| Left | 14 |
Table 2 shows the etiology and characteristics of the lesions. Associations with past surgeries (n = 7) included dacryocystorhinostomy with intubation in one, evisceration in three, and incision and curettage in three.
Table 2.
Characteristics of lesions
| Parameter | Number | Percentage |
|---|---|---|
| Etiology | ||
| Chalazion | 11 | 27.5 |
| Foreign body | 2 | 5 |
| Surgery | 7 | 17.5 |
| Trauma | 2 | 5 |
| Idiopathic | 18 | 45 |
| Location | ||
| UPC | 16 | 40 |
| LPC | 18 | 45 |
| BC | 4 | 10 |
| Punctum | 1 | 2.5 |
| Skin | 1 | 2.5 |
| Morphology | ||
| Pedunculated | 15 | 37.5 |
| Sessile | 24 | 60 |
| Mixed | 1 | 2.5 |
| Lesion in an eye (n) | ||
| 1 | 34 | 85 |
| 2 | 3 | 7.5 |
| 3 | 1 | 2.5 |
| 4 | 1 | 2.5 |
| 7 | 1 | 2.5 |
| Duration since onset | ||
| Mean duration (weeks) | 8.1+8.8 | 62.5 |
| Recent (<4 weeks) | 25 | 20 |
| Intermediate (4-12 weeks) | 8 | 17.5 |
| Old (>12 weeks) | 7 |
UPC - Upper Palpebral Conjunctiva, LPC - Lower Palpebral Conjunctiva, BC - Bulbar Conjunctiva
As mentioned in the method section, ORR was counted in the first 10 patients. We found an excellent response after 6 weeks in 7/10 (70%) patients. Then, the study proceeded as per protocol. At the last follow-up, the excellent response was achieved in 31 (77.5%) patients. Recurrence of the lesion was not noticed in any patient. At the first and second follow-ups, a satisfactory response was achieved in seven patients, while two were nonresponders. At the last follow-up, further resolution of the lesion was noticed in 2/7 patients who had a satisfactory response initially. Apart from those two cases, further change in the size of the lesions was not noticed between the first and last follow-up.
Table 3 shows an analysis of subgroups using regression models. A significant association was not observed in any category. Treatment success was observed in old lesions (93.3%), large lesions (73.3%) as well as in multiple lesions (83%).
Table 3.
Analysis of possible factors associated with outcomes
| Parameter | Number (%) | Excellent response (%) | Bivariate | Multivariate |
|---|---|---|---|---|
| Age (years) | ||||
| <16 | 12 (30) | 9 (75) | 0.83 | 0.46 |
| 16-30 | 21 (52.5) | 16 (76.2) | ||
| >30 | 7 (17.5) | 6 (85.7) | ||
| Sex | ||||
| Male | 22 (55) | 16 (72.7) | 0.42 | 0.66 |
| Female | 18 (45) | 15 (83.3) | ||
| Etiology | ||||
| Chalazion | 11 (27.5) | 10 (90.9) | 0.23 | >0.05 |
| Surgery | 7 (17.5) | 6 (85.7) | 0.57 | |
| Idiopathic | 18 (45) | 12 (66.6) | 0.24 | |
| Duration (old) | ||||
| Present | 15 (37.5) | 14 (93.3) | 0.09 | 0.21 |
| Absent | 25 (62.5) | 17 (68) | ||
| Size (large) | ||||
| Present | 15 (37.5) | 11 (73.3) | 0.62 | 0.72 |
| Absent | 25 (62.5) | 20 (80) | ||
| Morphology | ||||
| Pedunculated | 15 (37.5) | 11 (73.3) | 0.62 | 0.76 |
| Sessile | 25 (62.5) | 20 (80) | ||
| Location | ||||
| LPC | 18 (45) | 12 (66.7) | 0.14 | 0.17 |
| UPC | 16 (40) | 14 (87.5) | ||
| Multiple lesions | ||||
| Present | 6 (15) | 5 (83) | 0.88 | 0.42 |
| Absent | 34 (85) | 26 (76.5) |
LPC - Lower Palpebral Conjunctiva, UPC - Upper Palpebral Conjunctiva
A total of 12/18 (66.6%) lesions affecting lower palpebral conjunctiva (LPC) did not show an excellent response. A significant statistical association was not seen in any of the subtypes. X-Y scatter graphs showed no adverse response concerning the size of the lesions. A trend towards less favorable outcomes was seen in the younger age group [Fig. 3].
Figure 3.
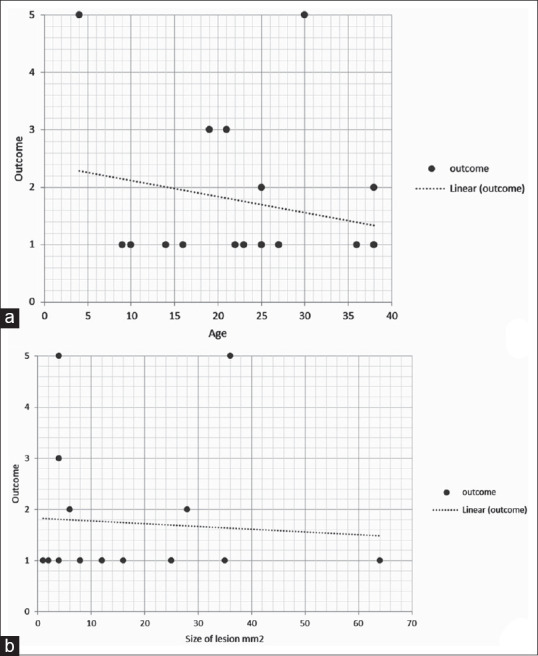
X-Y scatter graphs along with trend lines to correlate treatment outcomes. The X-axis represents age in years (a) and size of the lesion in mm2 (b). The Y-axis represents treatment outcome among five categories, where one indicates excellent response and five indicates nonresponder
Details of patients who could not achieve excellent results are shown in Table 4.
Table 4.
Details of patients who could not achieve excellent results
| Age/Sex | Duration (weeks) | Description of the lesion (size in mm2, etiology, location, morphology) | Treatment Response |
|---|---|---|---|
| 19/F | 12 | 4, Idiopathic, LPC, Sessile | Satisfactory |
| 38/M | 2 | 6, Surgery, LPC, Sessile | Good |
| 21/F | 4 | 4, Chalazion, LPC, Sessile | Satisfactory |
| 25/M | 4 | 28, Idiopathic, LPC, Pedunculated | Good |
| 12/M | 4 | 25, Idiopathic, UPC, Pedunculated | Satisfactory |
| 8/M | 4 | 4, Trauma, Skin, Sessile | Satisfactory |
| 22/M | 4 | 49, Idiopathic, UPC, Pedunculated | Satisfactory |
| 30/F | 4 | 4, Idiopathic, LPC, Sessile | No response |
| 4/M | 2 | 36, Idiopathic, LPC, Pedunculated | No response |
UPC - Upper Palpebral Conjunctiva, LPC - Lower Palpebral Conjunctiva
Discussion
We used topical timolol (0.5%) eye drop as the first-line treatment option for PG considering various advantages like a nonsurgical child-friendly approach with ease of administration. Complete resolution was achieved in 31 (77%) cases without any noticeable adverse event. Only two (5%) patients failed to show any improvement for which alternative treatment in the form of topical steroids was used.
Spontaneous regression of PG is very rare and seen in less than 5% of cases. The majority of the cases require some kind of treatment.[1,14,15,16,17,18] Surgical options available include surgical excision, cryotherapy, curettage, electrocautery, and laser ablation (which includes continuous, pulsed CO2, Nd YAG, and pulsed dye), and sclerotherapy.[19,20] Surgical interventions are usually considered most effective but general anesthesia may be required in the younger patients. Adverse sequelae after surgery include scarring, risk of infection, and recurrence.[21]
These disadvantages have led the researchers to use alternative approaches. Topical steroid eye drops/ointment have been popular not only among ophthalmologists but also with dermatologists to treat skin PG lesions. In a series of 10 patients of PG (developed secondary to strabismus surgery) when treated with topical steroids, a resolution was noticed in 90% of cases. In the same article, seven patients of PG failed to show improvement and further required surgical excision.[9]
Recently, topical beta-blockers have been tried as the first line of treatment in PG.[1] These medications block the beta receptors in the lesion causing vasoconstriction.[6] Immediate effects may be observed in the form of reduced bleeding. Over the long term, apoptosis is induced in the tissues leading to gradual regression of the lesion.[6] Beta-blockers target angiogenic factors like vascular endothelial growth factors and fibroblast growth factors, which play an important role in the growth of PG. The resolution of the lesions is usually noted within 1–6 months.[22,23,24] Beta-blockers have an excellent safety profile in the majority of the cases even when used for years, like in glaucoma cases.
The utility of beta-blocker in ocular PG was first demonstrated by Del Pozzo-Magana and Lara-Corrales in 2014. Timolol (0.5%) gel in BID dosage was used in a 3-year-old child and the resolution was noted after 6 months.[25] Oke et al. also demonstrated successful treatment with timolol (0.5% BID, gel/solution) in four pediatric cases.[1] DeMaria et al. noted the efficacy of topical timolol in the largest study till date involving 17 patients. Treatment was given in BID dosage for 6 weeks. A successful resolution was achieved in 15/17 (88%) cases. Two cases (18 mm size) failed to achieve resolution in which surgical excision had to be done.[26] All the previous studies were limited by small numbers and/or retrospective design.
In our study, we tried to address some of the issues by using a prospective design and enrolling in more cases. Still, we had considerable attrition of 17/57 cases, which may have had a significant impact on the outcomes. We treated patients maximally for 6 weeks based on the previous reports.
The mean age of all the patients in our study was 23.3 + 13 years (range 2–65 years). Twelve patients belonged to the pediatric age group and 33 (82.5%) patients overall were younger than 30 years of age. A similar result was also observed in a study done by DeMaria et al., where the mean age of the 17 patients was 23 years.[26]
Our results were better in the older age groups. A good response was obtained in all the etiologies. Success was lesser in PG involving LPC (12/18, 66.7%), though statistically insignificant. The exact reason for this could not be identified. Large lesions in our study also showed good response in contrast to the study of DeMaria et al.[26] The trend analysis in our study showed that the size of the lesion did not impact the overall outcomes. Better results in old age groups may be due to better compliance with the treatment, but the same cannot be said conclusively.
In 31 cases with an excellent response, complete resolution was achieved in 27 cases within 6 weeks only. The rest four patients also showed complete resolution within 6 months of the study period. Further, success was maintained thereafter. Recurrence or any adverse effects were not noted in any case. Timolol eye drop may cause abnormalities of the tear film and ocular surface. Such adverse effects are usually seen after long-term use of multiple antiglaucoma medications in glaucoma patients.[27] Though we did not evaluate tear film objectively, such adverse effects were not observed clinically. At the end of 1 month, seven had a satisfactory response while two did not respond at all. Two out of seven satisfactory cases further improved and achieved a good response at the end. Thus, it is worthwhile to note that the delayed response could be obtained in PG.
An important differential diagnosis of the PG includes lymphangioma, capillary hemangioma, and Kaposi’s sarcoma.[28] Hence, we advised the surgical excision of the mass after 6 weeks to two nonresponders. At that time both the patients denied surgery due to the younger age of one patient and the small size of the lesion in the other case. We then prescribed steroid eye drops (prednisolone acetate, one drop four times a day in tapering dosage) and observed resolution of the lesion within 6 weeks.
An important limitation of the present study was the lack of another treatment arm for comparison. Besides, 17 patients had to be excluded due to various reasons mentioned earlier. That could have improved or lowered the outcomes. Though we lost many patients during follow-up, still we could achieve the desired number of patients calculated to maintain the power of the study. It is also unclear, whether prolonged treatment (beyond 6 weeks) would have improved the final results or not. However, the present study was conducted prospectively using a rigid treatment and follow-up protocol. The total number of patients studied was also larger than similar studies conducted previously. The masking of the observer also helped to minimize the biases related to the results. The size of the lesions and their reduction were studied for accurate quantification of the outcomes. Further studies are necessary using a comparative arm to decide the optimal dosage, frequency, and duration of the treatment.
Conclusion
To conclude, topical timolol (0.5%) is a good nonsurgical treatment modality for patients having PG. The treatment is practically devoid of any adverse effects when given to suitable individuals for a limited period. The treatment can be successfully adopted in all age groups. Variation in the etiology, chronicity, size, or locations is not detrimental to the treatment outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Oke I, Alkharashi M, Petersen RA, Ashenberg A, Shah AS. Treatment of ocular pyogenic granuloma with topical timolol. JAMA Ophthalmol. 2017;135:383–5. doi: 10.1001/jamaophthalmol.2017.0110. [DOI] [PubMed] [Google Scholar]
- 2.Wollina U, Langner D, França K, Gianfaldoni S, Lotti T, Tchernev G. Pyogenic granuloma - A common benign vascular tumor with variable clinical presentation:New findings and treatment options. Open Access Maced J Med Sci. 2017;5:423–6. doi: 10.3889/oamjms.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feroze KB, Khazaeni L. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2021. Jan, Steroid Induced Glaucoma. 2020 Jul 21; pp. 2–653. [PubMed] [Google Scholar]
- 4.Niiyama S, Amoh Y, Katsuoka K. Pyogenic granuloma that responded to local injection of steroid. J Plast Reconstr Aesthet Surg. 2009;62:e153–4. doi: 10.1016/j.bjps.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Tursen U, Demirkan F, Ikizoglu G. Giant recurrent pyogenic granuloma on the face with satellitosis responsive to systemic steroids. Clin Exp Dermatol. 2004;29:40–1. doi: 10.1111/j.1365-2230.2004.01451.x. [DOI] [PubMed] [Google Scholar]
- 6.Wine Lee L, Goff KL, Lam JM, Low DW, Yan AC, Castelo-Soccio L. Treatment of pediatric pyogenic granulomas using b-adrenergic receptor antagonists. Pediatr Dermatol. 2014;31:203–7. doi: 10.1111/pde.12217. [DOI] [PubMed] [Google Scholar]
- 7.Ji Y, Chen S, Li K, Xiao X, Zheng S, Xu T. The role of b-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells. Cell Div. 2013;8:1. doi: 10.1186/1747-1028-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagliai KA, Cohen BA. Pyogenic granuloma in children. Pediatr Dermatol. 2004;21:10–3. doi: 10.1111/j.0736-8046.2004.21102.x. [DOI] [PubMed] [Google Scholar]
- 9.Espinoza GM, Lueder GT. Conjunctival pyogenic granulomas after strabismus surgery. Ophthalmology. 2005;112:1283–6. doi: 10.1016/j.ophtha.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 10.Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema-McKenney AE. b-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446–51. doi: 10.1038/modpathol.2012.108. [DOI] [PubMed] [Google Scholar]
- 11.Patrizi A, Gurioli C, Dika E. Pyogenic granulomas in childhood:New treatment modalities. Dermatol Ther. 2015;28:332. doi: 10.1111/dth.12237. [DOI] [PubMed] [Google Scholar]
- 12.Ferry AP. Pyogenic granulomas of the eye and ocular adnexa:A study of 100 cases. Trans Am Ophthalmol Soc. 1989;87:327–43. [PMC free article] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Lubahn JG, Lee RK, Karp CL. Resolution of conjunctival sessile hemangioma with topical timolol. Cornea. 2014;33:99–100. doi: 10.1097/ICO.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–51. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 16.Chakkittakandiyil A, Phillips R, Frieden IJ, Siegfried E, Lara-Corrales I, Lam J, et al. Timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas:A retrospective, multicenter, cohort study. Pediatr Dermatol. 2012;29:28–31. doi: 10.1111/j.1525-1470.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics. 2013;131:1739–47. doi: 10.1542/peds.2012-3828. [DOI] [PubMed] [Google Scholar]
- 18.Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma:A brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163–7. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 19.Ghodsi SZ, Raziei M, Taheri A, Karami M, Mansoori P, Farnaghi F. Comparison of cryotherapy and curettage for the treatment of pyogenic granuloma:A randomized trial. Br J Dermatol. 2006;154:671–5. doi: 10.1111/j.1365-2133.2005.06923.x. [DOI] [PubMed] [Google Scholar]
- 20.Sud AR, Tan ST. Pyogenic granuloma-treatment by shave-excision and/or pulsed-dye laser. J Plast Reconstr Aesthet Surg. 2010;63:1364–8. doi: 10.1016/j.bjps.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Baek YS, Kwon SH, Jeon J. Combination of ligation and timolol before surgical excision of pyogenic granuloma. J Am Acad Dermatol. 2018;78:e141–2. doi: 10.1016/j.jaad.2017.12.065. [DOI] [PubMed] [Google Scholar]
- 22.Piraccini BM, Alessandrini A, Dika E, Starace M, Patrizi A, Neri I. Topical propranolol 1% cream for pyogenic granulomas of the nail:Open-label study in 10 patients. J Eur Acad Dermatol Venereol. 2016;30:901–2. doi: 10.1111/jdv.13071. [DOI] [PubMed] [Google Scholar]
- 23.Mashiah J, Hadj-Rabia S, Slodownik D, Harel A, Sprecher E, Kutz A. Effectiveness of topical propranolol 4% gel in the treatment of pyogenic granuloma in children. J Dermatol. 2019;46:245–8. doi: 10.1111/1346-8138.14740. [DOI] [PubMed] [Google Scholar]
- 24.Neri I, Baraldi C, Balestri R, Piraccini BM, Patrizi A. Topical 1% propranolol ointment with occlusion in treatment of pyogenic granulomas:An open-label study in 22 children. Pediatr Dermatol. 2018;35:117–20. doi: 10.1111/pde.13372. [DOI] [PubMed] [Google Scholar]
- 25.Del Pozzo-Magana B, Lara-Corrales I. Topical Timolol for pyogenic granuloma in a child:A case report and literature review. Adv Pediatr Res. 2014;1:5. [Google Scholar]
- 26.DeMaria LN, Silverman NK, Shinder R. Ophthalmic pyogenic granulomas treated with topical timolol-clinical features of 17 cases. Ophthalmic Plast Reconstr Surg. 2018;34:579–82. doi: 10.1097/IOP.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 27.Wong ABC, Wang MTM, Liu K, Prime ZJ, Danesh-Meyer HV, Craig JP. Exploring topical anti-glaucoma medication effects on the ocular surface in the context of the current understanding of dry eye. Ocul Surf. 2018;16:289–93. doi: 10.1016/j.jtos.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Shields JA, Mashayekhi A, Kligman BE, Kunz WB, Criss J, Eagle RC, Jr, et al. Vascular tumors of the conjunctiva in 140 cases. Ophthalmology. 2011;118:1747–53. doi: 10.1016/j.ophtha.2011.04.034. [DOI] [PubMed] [Google Scholar]


