Abstract
Purpose:
Evaluation of circumpapillary vessel density (VD) and perfusion density (PD) on optical coherence tomography angiography (OCTa) in mild-moderate glaucoma patients having unilateral visual field defects, with their fellow eyes and controls.
Methods:
Both eyes of 24 patients having a definitive nasal step or arcuate scotoma in one hemisphere of one eye only, and 24 controls, underwent OCTa.
Results:
In eyes with a superior field defect, the superior/inferior quadrant ratios, (SQ/IQ) of 3 mm scan of VD and PD were significantly higher in eyes with a superior arcuate scotoma than fellow eyes (P = 0.03,0.02) as also controls, (P = 0.004,0.001). The mean percentage loss of inferior quadrant VD between control to fellow eyes, and superior nasal step eyes were similar, 20.19%/19.57% respectively, P = 0.85, while a loss in arcuate scotoma eyes was 38.81% (P = 0.001). The percentage decrease in inferior quadrant PD in fellow eyes was 14.70%, superior nasal step 23.39%, and an arcuate scotoma 34.74% (P = 0.02). Eyes with a superior nasal step had significantly lower VD and PD absolute values in the inferior quadrant OCTa in 3 mm and 6 mm circle scan only as compared to control eyes, VD, P = 0.03,0.04/PD, P = 0.008,0.02. Fellow eyes of superior field defects had significantly lower VD and PD absolute values in the inferior quadrant in 3 mm and 6 mm circle scan as compared to control eyes, VD, P = 0.006,0.04/PD, P = 0.01,0.03. Eyes with an isolated inferior field defect in only one eye, showed a significant decrease in both VD and PD in all quadrants as compared to fellow eyes and control eyes. A significant positive correlation was found between VD and RNFL thickness in peripapillary superior unaffected quadrants in eyes with superior field defects and inferior unaffected quadrants in inferior defects (P = 0.001 and 0.01).
Conclusion:
There was a statistically significant increasing SQ/IQ ratio and percentage loss of vascular parameters from control to fellow eyes, those with a superior nasal step, and those with a superior arcuate scotoma. Inferior VFDs appeared to be associated with a more generalized circulatory loss. The asymmetry between hemispheres and between eyes could be used as a biomarker for early glaucomatous neuropathy.
Keywords: Fellow eyes, mild-moderate glaucoma, percentage decrease, superior/inferior quadrant ratios, Unilateral
Optical coherence tomography angiography (OCTa) is a non-invasive tool for evaluating retinal vasculature at different depths and has been used in various retinal diseases such as diabetic retinopathy and age-related macular degeneration.[1] Axonal stasis due to impaired vascular perfusion of the optic nerve head leads to a loss of retinal nerve fibers in glaucoma.[2]
This loss is clinically evident in the form of nerve fiber layer defects on red-free imaging. However, it is detectable on perimetry only after a significant loss of axons has occurred.[3] OCTa is therefore an exciting tool, as it may help to detect glaucoma at an early stage by looking for impaired vascular perfusion that could lead to loss of retinal nerve fibers.[4,5]
This study was performed to evaluate circumpapillary vascular density and perfusion density on OCTa in different severities of unilateral visual field defects (VFD) in one hemisphere, as compared to the uninvolved hemisphere, fellow eyes of the same glaucoma patients, and healthy controls, to help in detecting the presence and degree of change, which could be a biomarker for early detection of glaucomatous morbidity.
Methods
Study design
This was a cross-sectional study of consecutive primary open-angle glaucoma (POAG) patients from the Glaucoma service having a VFD in only one hemisphere of one eye only [Fig. 1], Institutional Ethics Board approval was obtained (Ref.No.:IEC-647/07.12.2018), and the study adhered to the tenets of Declaration of Helsinki. A total of 24 patients having a definitive nasal step/arcuate scotoma in one hemisphere of one eye only and 24 controls were included in the study.
Figure 1.
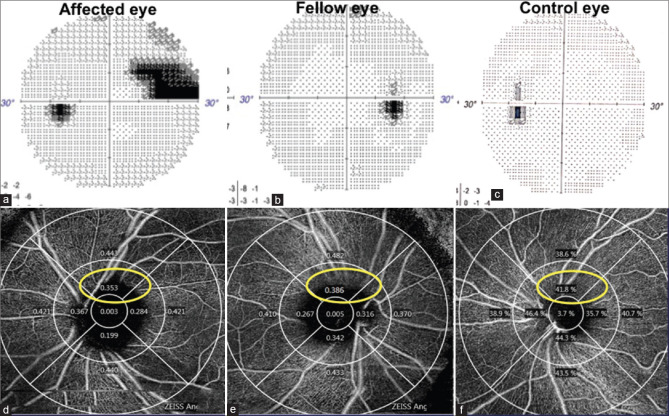
Top row: Humphrey’s visual field print out of a primary open angle glaucoma patient showing an affected eye with a superior nasal step in the left eye (a), a fellow eye with no visual field defect in the right eye (b) and control eye with no visual field defect in the left eye (c). Bottom row: OCTa Circumpapillary perfusion density map showing comparison of superior quadrants (corresponding to unaffected inferior hemisphere) in the affected eye (d), fellow eye (e), and control eye (f)
Inclusion/exclusion criteria
Inclusion criteria for POAG patients included a baseline IOP of >21 mmHg on more than 2 occasions, an open angle on gonioscopy, a definitive and reproducible glaucomatous VFD in one hemisphere of one eye only, mild-moderate glaucoma by Hodapp Parrish Anderson (HAP) criteria, with an intraocular pressure (IOP) controlled to <18 mmHg on medication. The fellow eyes of these glaucoma patients had no glaucomatous VFD on Humphrey visual field (HVF) but had to have a history of raised IOP and a suspicious optic disc with IOP <18 mmHg on glaucoma medications. Healthy subjects had to have no evidence of glaucoma on HVF or any other ocular/systemic pathology.
Exclusion criteria included patients with visual acuity < 6/18, refractive error >±5D and astigmatism >3D, bilateral VFD, severe glaucoma, any other retinal/optic nerve disorder that could lead to VFDs, and patients who had undergone prior ocular surgery. Patients with a history of diabetes mellitus or hypertension were also excluded. Poor quality images were excluded based on poor fixation, motion artifacts, blink artifacts, media opacity, poor signal strength i.e <7, or segmentation errors in outlining vascular networks, which could affect vessel density quantification were also excluded.[6]
Study protocol
All patients underwent a comprehensive ocular examination, best-corrected-visual-acuity, applanation tonometry, gonioscopy, optic disc status, perimetry. The number of glaucoma medications used and systemic status were recorded. Perimetry was performed using the 30–2 SITA standard program of the Humphrey field analyzer, HFA™ II-i Series, (Zeiss, San Leandro, CA, USA) in perimetrically experienced patients. The severity of glaucoma was categorized according to Hodapp Parrish Anderson (HAP) criteria on at least 2 reproducible fields as having a nasal step, arcuate scotoma.[7] They also underwent OCTa and RNFL imaging after taking informed written consent. Age-matched healthy subjects were recruited as controls from the OPD with no ocular or systemic pathology.
Both eyes of glaucoma patients; (one eye with mild-moderate glaucomatous VFD in one hemisphere alone and the other eye with no VFD) and healthy subjects underwent OCTa imaging on Zeiss AngioPlex (Cirrus HD-OCT 5000, Zeiss Meditec. Inc.). ZEISS AngioplexTM OCTa has been prepared on the CIRRUS™ HD-OCT platform. Using the features of a high scanning rate of 68,000 A-scans per second and improved retinal tracking software (FastTracTM), it generates high-resolution three-dimensional maps of retinal and choroidal microvasculature with the inbuilt OCT microangiography-complex algorithm (OMAGC). OCTa enface images (6 X 6 mm) centered around the optic disc were acquired and analyzed. Software version (Angioplex, version-2017.1.0.144) was used for circumpapillary vascular density measurement, which removed large vessels from the field of interest, taking into account images with high quality. Three circle scans were graphed on the enface image with a diameter of 1 mm, 3 mm, and 6 mm. The 1 mm circle scan was for optic disc and the other two circle scans, 3 mm and 6 mm were divided into four sectors; superior, temporal, inferior, and nasal. Circumpapillary vessel density (VD) and circumpapillary perfusion density (PD) was calculated at these two circle scans in all four quadrants for the superficial retinal layer, which constituted of layers from the internal limiting membrane to the inner plexiform layer. VD is defined as the total length of perfused vasculature per unit area in the region of measurement. It is calculated by taking the mean of the skeletonized slab within a desired region of interest and scaling the result by the distance between pixels (in this case, 512 pixels per 3 mm). PD is defined as the total area of perfused vasculature per unit area in a region of measurement, calculated by taking the mean of the binary slab within a desired region of interest. The circumpapillary RNFL thickness was measured by using spectral-domain OCT (Spectralis, Heidelberg Engineering) with a circle of 3.46 mm diameter centered on the optic disc. All images were reviewed by two independent investigators (RS and SVA).
Statistical analysis
Paired t-test and ANOVA test were used for comparison of continuous variables. Post-hoc multiple comparisons were done by using the Rank sum test with Bonferroni correction. Chi-square and Fisher’s exact tests were used to compare categorical data. STATA 12.1 was used for statistical analysis (College Station, Texas, USA).
Results
A total of 24 eyes of 24 consecutive POAG patients, having a single nasal step or arcuate scotoma in one hemisphere, 24 fellow eyes of these patients, and 24 control eyes of 24 healthy subjects who met all inclusion and exclusion criteria were analyzed. The mean age was 58.12 ± 7.51 years in VFD patients and 57.92 ± 6.83 years in controls respectively, P = 0.51. Glaucoma patients were evaluated in subgroups depending upon the pattern of VFD and hemisphere involved; superior nasal step (6), superior arcuate scotoma (11), inferior nasal step (2), and inferior arcuate scotoma (5). logMAR visual acuity was similar in all subgroups, being 0.04 ± 0.07 in controls, 0.07 ± 0.12 in fellow eyes, 0.06 ± 0.09 in superior nasal step, and 0.03 ± 0.07 in superior arcuate scotoma eyes, P = 0.83. IOP was 11.68 ± 1.24 mmHg in control, 13.5 ± 2.14 mmHg in fellow eyes, 13.33 ± 2.42 mmHg in superior nasal step, and 13.63 ± 2.50 mmHg in superior arcuate scotoma eyes. (P = 0.03, control v/s fellow eyes; 0.04, control v/s superior nasal step; 0.02, control v/s superior arcuate). The number of glaucoma medications was 1.62 ± 0.96 in fellow eyes, 2.0 ± 0.89 in superior nasal step, and 2.18 ± 0.75 in superior arcuate scotoma eyes, P = 0.41. The cup-disc-ratio was 0.32 ± 0.04 in control, 0.61 ± 0.08 in fellow eyes, 0.70 ± 0.06 in superior nasal step, and 0.86 ± 0.05 in superior arcuate scotoma eyes. (P < 0.001 for control v/s others, 0.02 for superior arcuate v/s fellow eyes).
The OCTa parameters in control eyes, fellow eyes, and affected eyes with a scotoma are provided in Tables 1-3.
Table 1.
Comparison of Superior quadrant/inferior quadrant ratio in 3 mm and 6 mm circle scan for controls, superior defects, and their fellow eyes
| Superior quadrant/ inferior quadrant ratio | Control eyes (n=24) A | Fellow eyes (n=17) B | Superior nasal step (n=6) C | Superior arcuate (n=11) D | P A v/s B | P A v/s C | P A v/s D | P B v/s C | P B v/s D | P C v/s D |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 mm circle | ||||||||||
| Vessel density | 1.04±0.18 | 1.18±0.40 | 1.15±0.18 | 1.47±0.36 | 0.39 | 0.35 | 0.004 | 1 | 0.03 | 0.68 |
| Perfusion density | 1.07±0.22 | 1.17±0.44 | 1.15±0.17 | 1.42±0.34 | 1 | 0.69 | 0.001 | 1 | 0.02 | 0.61 |
| 6 mm circle | ||||||||||
| Vessel density | 0.98±0.10 | 1.01±0.10 | 1.08±0.09 | 1.13±0.17 | 0.74 | 0.15 | 0.01 | 0.65 | 0.18 | 1 |
| Perfusion density | 0.99±0.11 | 1.02±0.11 | 1.09±0.10 | 1.13±0.18 | 0.79 | 0.23 | 0.02 | 0.84 | 0.25 | 1 |
P<0.05 is significant
Table 3.
Peripapillary vessel density and perfusion density in controls, eyes with inferior glaucomatous visual field defects, and fellow eyes at 3 mm and 6 mm circle scan
| Vessel density | Control eyes (n=24) A | Fellow eyes (n=7) B | Inferior visual field defects (n=7) C | P A v/s B | P A v/s C | P B v/s C |
|---|---|---|---|---|---|---|
| 3 mm circle | ||||||
| Superior | 18.27±1.85 | 15.45±1.20 | 12.54±2.78 | 0.03 | 0.0001 | 0.008 |
| Temporal | 17.16±2.86 | 14.50±3.03 | 11.41±1.65 | 0.02 | 0.0001 | 0.03 |
| Inferior | 17.83±2.19 | 14.34±2.66 | 11.64±3.45 | 0.0019 | 0.0001 | 0.001 |
| Nasal | 17.94±2.22 | 15.05±1.86 | 14.08±0.92 | 0.002 | 0.001 | 0.13 |
| Average vessel density | 17.56±1.83 | 14.70±2.80 | 12.54±1.45 | 0.002 | 0.001 | 0.02 |
| 6 mm circle | ||||||
| Superior | 18.11±1.43 | 18.0±1.42 | 15.32±0.96 | 0.92 | 0.0008 | 0.0009 |
| Temporal | 18.53±1.63 | 16.47±2.91 | 14.81±3.20 | 0.03 | 0.004 | 0.09 |
| Inferior | 18.42±1.59 | 18.17±1.92 | 16.50±1.76 | 0.58 | 0.01 | 0.07 |
| Nasal | 17.46±2.31 | 17.55±1.12 | 15.72±2.31 | 0.61 | 0.02 | 0.12 |
| Average vessel density | 18.10±1.29 | 17.53±1.39 | 16.08±0.96 | 0.48 | 0.001 | 0.01 |
| Perfusion density | ||||||
| 3 mm circle | ||||||
| Superior | .474±.064 | .315±.066 | .274±.079 | 0.04 | 0.0001 | 0.01 |
| Temporal | .401±.057 | .340±.088 | .265±.047 | 0.002 | 0.0001 | 0.04 |
| Inferior | .449±.065 | .364±.087 | .264±.046 | 0.002 | 0.0004 | 0.02 |
| Nasal | .465±.058 | .376±.055 | .252±.034 | 0.001 | 0.0002 | 0.22 |
| 6 mm circle | ||||||
| Superior | .468±.047 | .450±.043 | .383±.037 | 0.40 | 0.0005 | 0.008 |
| Temporal | .454±.045 | .397±.012 | .388±.080 | 0.09 | 0.04 | 1 |
| Inferior | .476±.045 | .453±.057 | .414±.056 | 0.22 | 0.02 | 0.24 |
| Nasal | .447±.068 | .442±.071 | .389±.069 | 0.19 | 0.02 | 0.25 |
P<0.05 is significant
Superior quadrant to inferior quadrant OCTa ratios (SQ/IQ)
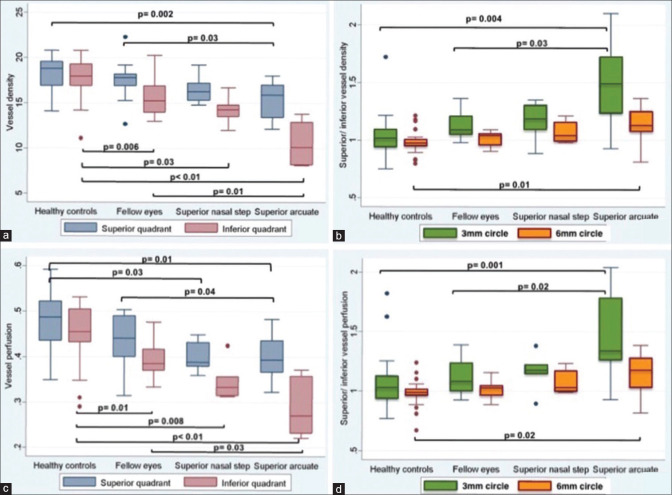
In control eyes, SQ/IQ for VD and PD were 1.04 ± 0.18 and 1.07 ± 0.22 respectively for 3 mm circle scan values, and 0.98 ± 0.10 and 0.99 ± 0.11 for the 6 mm circle scan. The SQ/IQ ratios in eyes with superior arcuate defects were statistically higher, 1.47 ± 0.36 (VD) and 1.42 ± 0.34 (PD) respectively as compared to control eyes in 3 mm and 6 mm scan, as also fellow eyes in the 3 mm circle scan. On comparing the different superior VFDs, a gradual increase in SQ/IQ ratio from controls to fellow eyes to superior nasal step to superior arcuate scotoma eyes, for both VD and PD in 3 mm circle scan, and to some extent the 6 mm circle scan was seen. [Table 1 and Fig. 2b, Fig. 2d] There was no significant difference in ratios between eyes with a superior nasal step as compared to control or fellow eyes or eyes with a superior arcuate scotoma. The SQ/IQ ratio for the 7 eyes with inferior VFDs did not show any significant difference when compared with controls and their fellow eyes.
Figure 2.
(a and c): Boxplots showing the comparative evaluation of circumpapillary vessel density and circumpapillary perfusion density in between healthy controls, fellow eyes, superior nasal step, and superior arcuate for superior and inferior quadrants. (b and d): Box plots showing the comparative evaluation of superior quadrant/inferior quadrant ratio for vessel density and perfusion density in between healthy controls, fellow eyes, superior nasal step, and superior arcuate for 3 mm and 6 mm circle scan
The percentage loss of inferior quadrant vessel density on OCTa between control to fellow eyes, and control compared to eyes with a superior nasal step was not significant, 20.19%/19.57% respectively, P = 0.85. Between control and fellow eyes to those with an arcuate scotoma, the percentage difference was 38.81%, P = 0.001. The percentage decrease in inferior quadrant perfusion density in fellow eyes was 14.70%, eyes with a superior nasal step 23.39% and in eyes with an arcuate scotoma 34.74%, P = 0.02.
OCTa - Absolute values
Eyes with a superior nasal step had significantly lower VD and PD in the inferior quadrant OCTa in 3 mm and 6 mm circle scan only as compared to control eyes, VD, P = 0.03,0.04/PD, P = 0.008,0.02. The fellow eyes of superior defects had significantly lower VD and PD absolute values in the inferior quadrant in the 3 mm and 6 mm circle scan as compared to control eyes, VD, P = 0.006,0.04/PD, P = 0.01,0.03. No difference was noted between eyes having a superior nasal step v/s fellow eyes or eyes having a superior arcuate scotoma. [Table 2]
Table 2.
Peripapillary vessel density and perfusion density in controls, eyes with superior glaucomatous visual field defects, and fellow eyes at 3 mm and 6 mm circle scan
| Vessel density | Control eyes (n=24) A | Fellow eyes (n=17) B | Superior nasal step (n=6) C | Superior arcuate (n=11) D | P A v/s B | P A v/s C | P A v/s D | P B v/s C | P B v/s D |
|---|---|---|---|---|---|---|---|---|---|
| 3 mm circle | |||||||||
| Superior | 18.27±1.85 | 17.19±2.30 | 16.36±1.70 | 15.42±2.13 | 0.22 | 0.10 | 0.002 | 0.93 | 0.03 |
| Temporal | 17.16±2.86 | 14.48±4.13 | 13.34±4.83 | 13.02±2.55 | 0.03 | 0.20 | 0.01 | 1 | 1 |
| Inferior | 17.83±2.19 | 14.23±2.76 | 14.34±1.73 | 10.91±2.45 | 0.006 | 0.03 | 0.001 | 1 | 0.01 |
| Nasal | 17.94±2.22 | 15.82±1.87 | 15.98±1.01 | 14.74±2.39 | 0.003 | 0.14 | 0.001 | 1 | 1 |
| Average vessel density | 17.56±1.83 | 15.50±1.97 | 14.94±1.43 | 13.87±1.32 | 0.003 | 0.03 | 0.001 | 1 | 0.14 |
| 6 mm circle | |||||||||
| Superior | 18.11±1.43 | 18.21±1.62 | 17.45±1.38 | 18.02±1.45 | 1 | 1 | 0.69 | 0.9 | 0.66 |
| Temporal | 18.53±1.63 | 17.72±2.63 | 18.02±1.22 | 18.37±1.53 | 0.27 | 0.95 | 1 | 1 | 1 |
| Inferior | 18.42±1.59 | 16.98±1.69 | 16.58±1.24 | 15.70±2.33 | 0.04 | 0.04 | 0.003 | 0.24 | 0.02 |
| Nasal | 17.46±2.31 | 17.22±1.77 | 16.92±2.13 | 16.63±2.86 | 1 | 0.17 | 0.85 | 0.50 | 1 |
| Average vessel density | 18.10±1.29 | 17.82±1.26 | 17.12±0.81 | 17.03±1.05 | 0.80 | 0.17 | 0.02 | 0.68 | 0.25 |
| Perfusion density | |||||||||
| 3 mm circle | |||||||||
| Superior | .474±.064 | .429±.065 | .393±.033 | .335±.068 | 0.09 | 0.03 | 0.01 | 0.63 | 0.04 |
| Temporal | .401±.057 | .325±.089 | .268±.080 | .303±.054 | 0.003 | 0.001 | 0.04 | 0.43 | 1 |
| Inferior | .449±.065 | .383±.065 | .344±.046 | .293±.062 | 0.01 | 0.008 | 0.001 | 0.60 | 0.03 |
| Nasal | .465±.058 | .398±.058 | .385±.044 | .374±.065 | 0.001 | 0.03 | 0.001 | 1 | 1 |
| 6 mm circle | |||||||||
| Superior | .468±.047 | .458±.039 | .448±.029 | .450±.034 | 0.68 | 0.45 | 0.33 | 1 | 1 |
| Temporal | .454±.045 | .421±.048 | .422±.065 | .323±.014 | 0.31 | 0.22 | 1 | 1 | 0.28 |
| Inferior | .476±.045 | .422±.049 | .413±.033 | .404±.064 | 0.03 | 0.02 | 0.008 | 0.44 | 0.04 |
| Nasal | .447±.068 | .431±.052 | .399±.056 | .423±.076 | 0.31 | 0.08 | 0.67 | 0.68 | 1 |
P<0.05 is significant
In eyes with a superior arcuate VFD, 3 mm and 6 mm scan VD and PD values in the inferior quadrant on OCTa, were significantly lower than control and fellow eyes, VD, P = 0.001,0.003 and 0.01,0.02: PD, P = 0.001,0.008 and 0.03,0.04. Fig.1 shows an example of a comparison between a glaucoma eye with superior VFD with its fellow eye and control. To determine differences in circumpapillary parameters on OCTa of possible pre-perimetric changes in patients with superior VFDs, the OCTa values of the superior optic nerve head quadrant, subserving the inferior perimetrically normal visual field, and thought to be most predisposed to future glaucomatous change, were compared with control eyes and fellow eyes. The VD and PD values in 3 mm circle scan of this superior quadrant (perimetrically normal inferior hemisphere), showed a significant difference in comparison to controls, as well as fellow eyes, VD, P = 0.002/0.03/PD, P = 0.01/0.04, again suggesting a gradual loss of microcirculation to the optic nerve head. [Table 2 and Fig. 2a, c].
7 eyes with an isolated inferior field defect in only one eye, showed a significant decrease in both VD and PD in all quadrants as compared to fellow eyes and control eyes. For a 3 mm circle scan, both VD and PD of fellow eyes was significantly less than controls in all quadrants. Also, there was a significant difference between inferior defect eyes and their fellow eyes in peripapillary superior, temporal, and inferior quadrants OCTa. [Table 3] In eyes with an inferior VFD, a generalized reduction of circumpapillary vascularity was seen.
The superior quadrant RNFL thickness was 114.7 ± 6.27 mm in controls, 106.1 ± 13.4 mm in fellow eyes, and 83.6 ± 12.3 mm in superior VFD. (p = 0.0004 for control and superior defects). The inferior quadrant RNFL thickness was 97.8 ± 4.45 mm in controls, 88.3 ± 6.6 mm in fellow eyes, and 65.7 ± 11.4 mm in superior VFD. (p = 0.001 for control and superior defects). No difference was found between fellow eyes and controls. The SQ/IQ for RNFL thickness was 1.17 ± 0.02 in controls, 1.20 ± 0.17 in fellow eyes, and 1.28 ± 0.16 in superior defects (p = 0.04 for control and superior defect). On comparing the correlation of OCTa values with RNFL data, a significant positive correlation was found between VD and RNFL thickness in the superior quadrant for patients with unaffected inferior hemisphere and also in the inferior quadrant for patients with unaffected superior hemisphere (p = 0.001 and 0.01). There was no significant correlation between SQ/IQ of VD and mean RNFL thickness (p = 0.52).
Discussion
OCTa studies in glaucoma generate many numbers, with much inter-individual variability and differing degrees of significance. The diagnostic accuracy of circumpapillary microcirculation measurements for glaucoma is better than for the macular region.[8] Primary open angle glaucoma is usually bilateral, but the presentation is often asymmetric or one eye follows the other, providing a platform for evaluating differential loss of neuroretinal rim (NRR), retinal nerve fiber loss, and microcirculation concerning differences in VFDs in the same patient.[9] Hou et al. found a greater asymmetry in vessel density between the 2 eyes on OCTa in glaucoma suspects.[10] Few studies available in glaucomatous eyes with hemifield defects found reduced vessel density in healthy, perimetrically intact regions of glaucoma eyes, but they did not classify the type of VFD or compare the affected eye with the fellow pre-perimetric eye.[11,12,13,13] Recently, OCTa has shown abnormal vasculature in the unaffected fellow eyes of unilateral VFD POAG patients when compared to healthy controls.[14] But a quadrantic evaluation of vessel density in the fellow eye was suggested for detecting an upcoming VFD. Also, the absolute values of OCTa for various circumpapillary parameters could vary for a person when measured at different times of a day, with different machines, and together with a known inter-individual variability.
Therefore, a ratio applicable at all instances, e.g., SQ/IQ and percentage loss in circumpapillary OCTa parameters, maybe better biomarkers for early/moderate glaucomatous change. These could use the known asymmetry in glaucoma and may permit an earlier, more reproducible, objective diagnosis of glaucomatous changes by OCTa.
This study looked at absolute values, ratios, and percentage loss on OCTa in eyes with early definitive glaucomatous VFDs–nasal steps and single arcuate scotomas in only one hemisphere of one eye, and compared them with the fellow eye having no scotomas but evidence of glaucomatous optic nerve head changes and a raised IOP, therefore possibly pre-perimetric glaucoma, and age-matched controls. It also looked for the most affected quadrant in fellow eyes, which would give us a biomarker of future VFD damage.
In control eyes, both vessel density and perfusion density, were generally similar in the superior and inferior quadrants, with a ratio of around 1. As control eyes had an SQ/IQ ratio for both VD and VP of approximately 1, an increase in SQ/IQ would signify a loss of inferior NRR, thereby a superior VFD. Similarly, a decrease in SQ/IQ could denote a significant superior NRR loss suggesting an inferior VFD. The SQ/IQ ratios in eyes with superior arcuate defects (inferior damage to the optic nerve) were significantly higher, 1.47 ± 0.36 (VD) and 1.42 ± 0.34 (PD) respectively as compared to control eyes, as also fellow eyes in the 3 mm and 6 mm circle scan. However, a statistically significant difference was noted only between eyes having superior arcuate defects and fellow and control eyes. There was no significant difference in SQ/IQ ratios between eyes with a superior nasal step as compared to control or fellow eyes, or eyes with a superior arcuate scotoma, despite differences in mean absolute values, probably because of small numbers studied and interindividual variability. The SQ/IQ for the 7 eyes with inferior VFDs did not show any significant difference when compared with controls and their fellow eyes, suggesting possibly a more generalized loss in these eyes. Chen et al. looked at macular ganglion cell asymmetry for diagnosis of pre-perimetric glaucoma and found the log IT/SN index had the largest AUROC (0.734), followed by the log IT/IN index (0.725).[15]
The percentage decrease in inferior quadrant perfusion density in fellow eyes, eyes with a superior nasal step and with an arcuate scotoma, had a graded loss with increasing visual field loss. The percentage loss of inferior quadrant vessel density between control and fellow eyes, and control compared to eyes with a superior nasal step was similar about 20%, as compared to eyes with an arcuate scotoma where it was almost double. Richter et al. showed a stepwise loss of microcirculation across glaucoma severity with inferior and superior quadrants being most significantly affected.[16] Ghahari et al. reported that circumpapillary vessel density was associated with the severity of visual field damage, even in advanced POAG.[17]
There was a graduated decrease in absolute values of VD and PD in all quadrants from controls, to fellow eyes, to superior nasal step, and finally eyes with a superior arcuate scotoma. Fellow eyes of superior defect patients had a significantly lower perfusion density in all quadrants of 3 mm circle scan as compared to control eyes, suggesting pre-perimetric loss in the fellow eyes. There was no significant difference between eyes having a superior nasal step and fellow eyes, possibly due to a very localized loss causing the nasal step. The 3 mm circle again showed more statistically significant differences between eyes having an arcuate scotoma, the probably pre-perimetric fellow eyes, and control eyes, suggesting that initial microcirculatory dysfunction was better recorded near the optic nerve. Chang et al. also found that parameters from 4.5 mm scan outperformed 6.0 mm ones.[18] OCTa macular 6 × 6 mm scan for vessel density was reported to be better than 3 × 3 mm macular scan for differentiating healthy and mild glaucoma eyes.[19]
There was a significant difference between control eyes and all other superior defect eyes and fellow eyes for vessel density, however, there was no significant difference between eyes with a nasal step compared to fellow eyes or those with an arcuate scotoma. Thus, it appears that eyes with a superior nasal step have a localized decrease in vessel density in the inferior quadrant, while arcuate scotomas, even if only superior, have a generalized decrease in vessel density in all quadrants. Miguel et al. found a significant reduction in mean circumpapillary vessel density reported in glaucomatous eyes for many OCTa devices.[20] Moghimi et al. were able to show a correlation between vessel density and visual field MD, even in advanced glaucoma.[21] Nascimento et al. demonstrated a reduced deep circumpapillary macro vasculature in POAG eyes.[22] Lommatzsch et al. found circumpapillary VD to be significantly reduced in glaucomatous eyes.[23] Kurysheva et al. were able to distinguish early POAG from moderate to severe by values of the inferotemporal VD.[24]
Eyes with an isolated inferior field defect in only one eye, showed a significant decrease in both VD and PD in all quadrants as compared to fellow eyes and control eyes, suggesting a generalized reduction of circumpapillary vascularity.
Limitations of our study were the small number of eyes in each group, as only eyes with a field defect in one quadrant of one eye were studied. Only the superficial microcirculation around the optic nerve head was evaluated as quantitative data of the deeper layers was not validated in this OCTa machine. A more detailed, quantitative analysis of superficial and deep microcirculation in a larger number of patients would be ideal.
Conclusion
In conclusion, an increasing SQ/IQ ratio and percentage loss of vascular parameters from control to fellow eyes, those with a superior nasal step, and those with a superior arcuate scotoma could be seen. Inferior VFDs appeared to be associated with a more generalized circulatory loss. The asymmetry between hemispheres and between eyes could be used as a biomarker for early glaucomatous neuropathy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge our statistician Mrs Veena Pandey, BSc, Department of Biostatistics, All India Institute of Medical Sciences, New Delhi.
References
- 1.Onishi AC, Fawzi AA. An overview of optical coherence tomography angiography and the posterior pole. Ther Adv Ophthalmol. 2019;11:1–16. doi: 10.1177/2515841419840249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan KKW, Tang F, Tham CCY, Young AL, Cheung CY. Retinal vasculature in glaucoma:A review. BMJ Open Ophthalmol. 2017;1:32. doi: 10.1136/bmjophth-2016-000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 4.Rao HL, Pradhan ZS, Weinreb RN, Reddy HB, Riyazuddin M, Dasari S, et al. Regional comparisons of optical coherence tomography angiography vessel density in primary open-angle glaucoma. Am J Ophthalmol. 2016;171:75–83. doi: 10.1016/j.ajo.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Jia Y, Takusagawa HL, Pechauer AD, Edmunds B, Lombardi L, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133:1045–52. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao HL, Pradhan ZS, Weinreb RN, Reddy HB, Riyazuddin M, Sachdeva S, et al. Determinants of peripapillary and macular vessel densities measured by optical coherence tomography angiography in normal eyes. J Glaucoma. 2017;26:491–7. doi: 10.1097/IJG.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 7.Hodapp E, Parrish RK, Anderson DR. St. Louis: C. V. Mosby; 1993. Clinical Decisions in Glaucoma; pp. 52–61. [Google Scholar]
- 8.Richter GM, Chang R, Situ B, Chu Z, Burkemper B, Reznik A, et al. Diagnostic performance of macular versus peripapillary vessel parameters by optical coherence tomography angiography for glaucoma. Transl Vis Sci Technol. 2018;7:21. doi: 10.1167/tvst.7.6.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Lee EK, Park KH, Kim DM, Jeoung W. Asymmetry analysis of macular inner retinal layers for glaucoma diagnosis :Swept-Source Optical Coherence Tomography Study. PLoS One. 2016;11:0164866. [Google Scholar]
- 10.Hou H, Moghimi S, Zangwill LM, Shoji T, Ghahari E, Manalastas PIC, et al. Inter-eye asymmetry of optical coherence tomography angiography vessel density in bilateral glaucoma, glaucoma suspect, and healthy eyes. Am J Ophthalmol. 2018;190:69–77. doi: 10.1016/j.ajo.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pradhan ZS, Dixit S, Sreenivasaiah S, Rao HL, Venugopal JP, Devi S, et al. A sectoral analysis of vessel density measurements in perimetrically intact regions of glaucomatous eyes. J Glaucoma. 2018;27:525–31. doi: 10.1097/IJG.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 12.Chen CL, Bojikian KD, Wen JC, Zhang Q, Xin C, Mudumbai RC, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in eyes with glaucoma and single-hemifield visual field loss. JAMA Ophthalmol. 2017;135:461–8. doi: 10.1001/jamaophthalmol.2017.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarmohammadi A, Zangwill LM, Diniz-Filho A, Saunders LJ, Suh MH, Wu Z, et al. Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology. 2017;124:709–19. doi: 10.1016/j.ophtha.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarmohammadi A, Zangwill LM, Manalastas PIC, Fuller NJ, Diniz-Filho A, Saunders LJ, et al. Peripapillary and macular vessel density in patients with primary open-angle glaucoma and unilateral visual field loss. Ophthalmology. 2018;125:578–87. doi: 10.1016/j.ophtha.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MJ, Yang HY, Chang YF, Hsu CC, Ko YC, Liu CJL. Diagnostic ability of macular ganglion cell asymmetry in Preperimetric Glaucoma. BMC Ophthalmol. 2019;19:1–9. doi: 10.1186/s12886-018-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter GM, Sylvester B, Chu Z, Burkemper B, Madi I, Chang R, et al. Peripapillary microvasculature in the retinal nerve fiber layer in glaucoma by optical coherence tomography angiography:Focal structural and functional correlations and diagnostic performance. Clin Ophthalmol. 2018;12:2285–96. doi: 10.2147/OPTH.S179816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghahari E, Bowd C, Zangwill LM, Proudfoot J, Hasenstab KA, Hou H, et al. Association of macular and circumpapillary microvasculature with visual field sensitivity in advanced glaucoma. Am J Ophthalmol. 2019;204:51–61. doi: 10.1016/j.ajo.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang R, Chu Z, Burkemper B, Lee GC, Fard A, Durbin MK, et al. Effect of scan size on glaucoma diagnostic performance using OCT angiography en face images of the radial peripapillary capillaries. J Glaucoma. 2019;28:465–72. doi: 10.1097/IJG.0000000000001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penteado RC, Bowd C, Proudfoot JA, Moghimi S, Manalastas PIC, Ghahari E, et al. Diagnostic ability of optical coherence tomography angiography macula vessel density for the diagnosis of glaucoma using difference scan sizes. J Glaucoma. 2020;29:245–51. doi: 10.1097/IJG.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 20.Miguel AIM, Silva AB, Azevedo LF. Diagnostic performance of optical coherence tomography angiography in glaucoma:A systematic review and meta-analysis. Br J Ophthalmol. 2019;103:1677–84. doi: 10.1136/bjophthalmol-2018-313461. [DOI] [PubMed] [Google Scholar]
- 21.Moghimi S, Bowd C, Zangwill LM, Penteado RC, Hasenstab K, Hou H, et al. Measurement floors and dynamic ranges of OCT and OCT angiography in glaucoma. Ophthalmology. 2019;126:980–8. doi: 10.1016/j.ophtha.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nascimento e Silva R, Chiou CA, Wang M, Wang H, Shoji M, Chou J, et al. Microvasculature of the optic nerve head and peripapillary region in patients with primary open-angle glaucoma. J Glaucoma. 2019;28:281–8. doi: 10.1097/IJG.0000000000001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lommatzsch C, Rothaus K, Koch JM, Heinz C, Grisanti S. Vessel density in OCT angiography permits differentiation between normal and glaucomatous optic nerve heads. Int J Ophthalmol. 2018;11:835–43. doi: 10.18240/ijo.2018.05.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurysheva NI, Maslova EV, Zolnikova IV, Fomin AV, Lagutin MB. A comparative study of structural, functional and circulatory parameters in glaucoma diagnostics. PLoS One. 2018;13:0201599. doi: 10.1371/journal.pone.0201599. doi:10.1371/journal.pone. 0201599. [DOI] [PMC free article] [PubMed] [Google Scholar]