Abstract
Purpose:
Earlier our group has demonstrated the drug reservoir function of the human amniotic membrane (HAM) using stable moxifloxacin and fortified cefazolin ophthalmic formulations and found it as a suitable tool to deliver drugs for an extended duration. The purpose of this study was to evaluate the extended-release kinetics of voriconazole from the impregnated human amniotic membrane (HAM) in vitro.
Methods:
HAM buttons were incubated with freshly prepared 1% topical ophthalmic formulation of voriconazole for 5 different exposure time to investigate the ideal exposure time for the extended-release of voriconazole from HAM. The drug release kinetics was studied in simulated tear fluid for 5 weeks and the amount of voriconazole released at different intervals was estimated using high-performance liquid chromatography (HPLC) with photodiode array (PDA) detector.
Results:
There was a marginal increase in drug entrapment efficiency with increased drug exposure time but neither the drug entrapment nor the drug release was found to be statistically significant (P ≥ 0.5). Voriconazole was detectable even at 5 weeks.
Conclusion:
A sustained release of voriconazole was achieved up to 5 weeks, when voriconazole was incubated with amniotic membrane for all the studied drug soaking times. Thus, voriconazole impregnated amniotic membrane can be considered for the sustained delivery for its in fungal keratitis.
Keywords: Amniotic membrane, drug reservoir, voriconazole and fungal keratitis
Fungal keratitis is one of the leading causes of corneal blindness worldwide, which can affect all age groups.[1] It accounts for nearly half of the infectious keratitis cases especially in tropical and sub-tropical countries such as South India, Nepal and Bangladesh.[2,3,4,5,6] In temperate regions such as North America, most keratitis are caused by bacteria; although the prevalence of fungal pathogens in infective keratitis cases has been reported to be 35% in southern Florida.[6,7] While Candida species tends to be predominant in temperate countries; the Fusarium species, Aspergillus species and Curvularia species, are more commonly, the cause for fungal keratitis cases that follow ocular trauma with vegetative matter, in tropical regions. Filament formation and biofilm formation are essential steps in the pathogenesis of these fungi.[8] Hence, the medical management of fungal keratitis has garnered particular attention due to the challenges that are posed in eradicating these fungi as a result of the limited availability of antifungals and their frequent application.
Topically applied drugs are greatly influenced by blinking, lacrimation, tear turnover rate, and absorption by non-productive adjacent tissues.[9] They have poor penetration, surface toxicity, and limited spectrum.[10] To overcome these problems, targeted drug delivery routes in the form of intracameral and intrastromal injection of antifungal agents has been explored with varying success.[10,11,12,13,14]
Human amniotic membrane (HAM) is a semi-transparent structure in the innermost layer of the placenta that is 0.02-0.05 mm thick.[15] It has found widespread ophthalmic usage in limbal stem cell deficiency, conjunctival reconstruction, persisting epithelial defects, perforating or non-perforating corneal ulcers, alkali burns, pterygium surgeries, band keratopathy, as a carrier for the ex vivo expansion of limbal epithelial cells, glaucoma surgeries and scleral melts.[16,17] Apart from these clinical applications, the drug reservoir function of HAM has been demonstrated previously by us and others with antibiotics and anti-viral drugs and utilized in cases of infective keratitis.[18,19,20,21,22]
Our group has earlier demonstrated the topical release kinetics of a single dose of 1% voriconazole in human eyes and found that “every 2 h dosing regimen” was sufficient enough to achieve the therapeutic concentration for all the causative fungal organisms.[23] In the present study, the extended-release kinetics of voriconazole loaded HAM has been investigated to check the suitability of voriconazole-laden HAM as a drug reservoir tool for the management of fungal keratitis.
Methods
The study was approved by the Institutional Review Board of our Institute (IR #: RES2015011BAS). The tissue was handled according to the tenets of the Declaration of Helsinki.
HAM organ culture, drug treatment and release kinetics
HAM was obtained by elective cesarean section at the Department of Gynecology, PAMC Hospital, Madurai after getting their informed consent and the HAM buttons were prepared for the experiment by the protocol as described earlier.[21,22] HAM Buttons (1Control, 5 Test) were incubated in a freshly prepared (1 ml) sterile solution of Voriconazole 1% (w/v) (Aurolab, India) for 3 h (Group I), 6 h (Group II), 12 h (Group III), 24 h (Group IV) and 48 h (Group V) in order to investigate the ideal drug soaking time.
After drug treatment, HAM buttons were placed into 6-well plate containing 1 ml STF (without drug) and incubated at 37°C with relative humidity of 65% and 5% CO2. 100 ml of the STF was sampled out at different time intervals and replaced with equal volume of sterile STF in order to maintain the sink condition. The amount of drug released from the drug-laden HAM was studied for a period of 5 weeks, to assess the extended-release kinetics.
Estimation of voriconazole by HPLC
The amount of voriconazole released at different time intervals was quantified using a Shimadzu Prominence HPLC system with PDA detector (Shimadzu Corporation, Kyoto, Japan) by the method as described earlier.[23] The quantification of voriconazole was carried out at lMax of 272 nm and the spectral matching was done with an in-built library matching facility in the PDA detector.
Statistical analysis
The values are presented as mean ± SEM. Group means were compared by two-sample t-test. Differences with a P value <0.05 was considered statistically significant. All the statistical analysis was done using STATA ver. 14 (Texas, USA).
Results
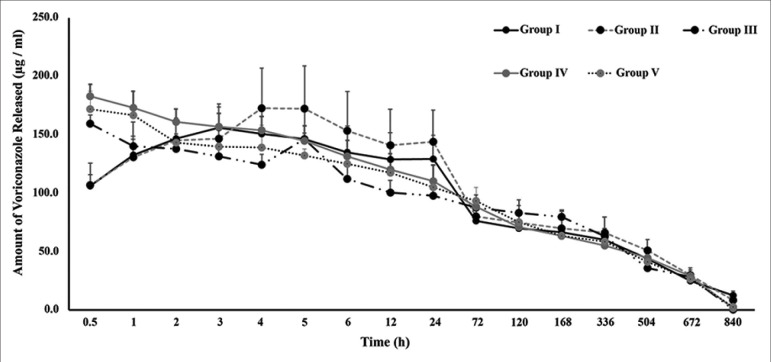
In this study, the extended-release kinetics of voriconazole loaded HAMs were investigated for different soaking periods. The amount of voriconazole released at each time point is represented in Fig. 1. The cumulative amount of voriconazole released over the study period is summarized in Table 1. In Group I-V, the cumulative amount of voriconazole released upto 5 weeks was found to be 1589.5, 1696.3, 1532.2, 1691.0 and 1605.6 mg/ml respectively. This indicates that there was a marginal increase in drug entrapment with increasing drug exposure time with HAMs but such increase was not found to be statistically significant (P = 0.6).
Figure 1.
Release Kinetics of Voriconazole from Drug-soaked HAM. The amount of voriconazole released from HAM buttons of different incubation periods (3h: Group I; 6h: Group II; 12h: Group III; 24h: Group IV; and 48h: Group V) for a period of 840 hours (5 weeks) is shown. No significant difference was observed between all groups studied (P ≥0.05)
Table 1.
Summary of Cumulative Release of Voriconazole From Drug Loaded HAM in Each Group
| Time (h) | Group I | Group II | Group III | Group IV | Group V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SEM (µg/ml) | Cumulative Amount (µg/ml) | Mean±SEM (µg/ml) | Cumulative Amount (µg/ml) | Mean±SEM (µg/ml) | Cumulative Amount (µg/ml) | Mean±SEM (µg/ml) | Cumulative Amount (µg/ml) | Mean±SEM (µg/ml) | Cumulative Amount (µg/ml) | |
| 0.5 | 106.6±9.4 | 106.6 | 107.1±18.8 | 107.1 | 159.7±7.4 | 159.7 | 183.3±10.2 | 183.3 | 172.1±15.5 | 172.1 |
| 1 | 132.9±13.4 | 239.5 | 131.1±29.8 | 238.2 | 140.5±8.5 | 300.2 | 173.3±14.0 | 356.6 | 166.9±7.1 | 339.0 |
| 2 | 146.7±13.7 | 386.2 | 145.3±19.2 | 383.5 | 138.1±12.9 | 438.3 | 161.1±11.3 | 517.7 | 143.5±4.7 | 482.5 |
| 3 | 156.3±17.8 | 542.6 | 146.7±29.8 | 530.3 | 131.8±11.3 | 570.1 | 157.0±11.4 | 674.7 | 140.0±5.7 | 622.5 |
| 4 | 151.2±19.3 | 693.8 | 173.1±34.1 | 703.4 | 124.4±9.2 | 694.5 | 154.0±11.9 | 828.8 | 139.4±18.9 | 762.0 |
| 5 | 146.5±23.7 | 840.3 | 172.7±36.6 | 876.1 | 146.5±5.1 | 841.0 | 144.8±13.2 | 973.6 | 132.4±5.7 | 894.3 |
| 6 | 135.0±22.8 | 975.3 | 153.8±33.4 | 1029.8 | 112.5±14.6 | 953.5 | 131.9±13.4 | 1105.5 | 125.4±12.1 | 1019.8 |
| 12 | 129.1±22.9 | 1104.4 | 141.1±31.0 | 1170.9 | 100.9±10.3 | 1054.4 | 120.5±13.3 | 1226.1 | 117.9±4.4 | 1137.6 |
| 24 | 129.4±20.4 | 1233.8 | 144.3±27.0 | 1315.2 | 98.2±10.0 | 1152.7 | 110.5±13.8 | 1336.6 | 105.3±6.2 | 1242.9 |
| 72 | 76.5±10.4 | 1310.3 | 80.2±15.8 | 1395.4 | 88.0±10.7 | 1240.7 | 88.5±7.1 | 1425.1 | 93.7±11.3 | 1336.7 |
| 120 | 70.2±11.9 | 1380.5 | 75.0±14.5 | 1470.4 | 83.4±11.2 | 1324.1 | 71.2±6.0 | 1496.3 | 75.4±6.6 | 1412.0 |
| 168 | 66.8±12.5 | 1447.4 | 70.2±14.6 | 1540.6 | 79.8±6.1 | 1403.9 | 63.8±4.0 | 1560.1 | 63.8±2.0 | 1475.8 |
| 336 | 60.3±9.6 | 1507.6 | 66.2±13.4 | 1606.8 | 63.4±0.4 | 1467.3 | 55.3±3.0 | 1615.4 | 58.9±2.4 | 1534.7 |
| 504 | 44.1±6.3 | 1551.7 | 51.1±9.4 | 1658.0 | 35.9±8.1 | 1503.2 | 44.6±3.7 | 1660.0 | 41.4±4.0 | 1576.2 |
| 672 | 25.2±4.8 | 1577.0 | 30.1±6.0 | 1688.0 | 28.5±5.9 | 1531.7 | 29.3±1.2 | 1689.3 | 26.6±5.7 | 1602.8 |
| 840 | 12.5±3.6 | 1589.5 | 8.3±0.4 | 1696.3 | 0.5±0.1 | 1532.2 | 1.7±0.3 | 1691.0 | 2.8±1.8 | 1605.6 |
Different groups represent different drug soaking times with 1% voriconazole. Group I (3 h); Group II (6 h); Group III (12 h); Group IV (24 h); and Group V (48 h). No significant difference was observed with different drug soaking times. Bold values represent the total cumulative amount of drug released up to the study period
Discussion
HAM has been termed as a ‘biological bandage’ due to its myriad clinical applications. The anti-infective properties of HAM are not considered to be potent enough to treat infective keratitis.[24] Hence, a strategy to fortify it with antimicrobial drugs was investigated for the first time in 2001.[18] They proved that HAM can be used as a slow-release drug reservoir by investigating the level of ofloxacin in HAM, tear film, corneal, and aqueous levels in rabbit eyes. Subsequently, the drug reservoir function of HAM has been demonstrated with netilmicin antibiotic and found that HAM can absorb and release the antibiotic in a dose-dependent manner and antibacterial effect was present in the elution media for at least 3 days after treatment.[19] This was succeeded by in vitro studies with antiviral-treated HAM that also proved to be successful in inhibiting viral replication.[20]
Our group has also demonstrated the drug reservoir function of HAM with stable moxifloxacin and fortified cefazolin topical formulations and found that HAM is not only capable of releasing moxifloxacin for up to 7 weeks and but also suitable to release the fortified formulation of cefazolin without compromising its stability.[21,22] Therefore, in this study, we have chosen to study the extended release kinetics of an antifungal drug i.e., voriconazole from drug-loaded HAM over a period of 5 weeks duration.
Voriconazole is a triazole antifungal agent. It inhibits cytochrome P450 demethylase to alter fungal cell membrane permeability and to arrest growth. In vitro studies have shown promising results with voriconazole and was found to have a broad spectrum of action against Aspergillus species (MIC90 for A. flavus and A. fumigatus: 0.5 mg/ml), Blastomyces dermatitidis (MIC90: 0.25 mg/ml), Candida species (MIC90: C.albicans 0.06; C.parapsilosis: 0.12-0.25; C. tropicalis: 0.25 to >16.0 mg/ml), Coccidioides immitis (MIC90: 0.25 mg/ml), Cryptococcus neoformans (MIC90: 0.06-0.25 mg/ml), Curvularia species (MIC90: 0.06-0.25 mg/ml), Fusarium species (MIC90: 2-8 mg/ml), Histoplasma capsulatum (MIC90: 0.25 mg/ml), Paecilomyces lilacinus (MIC90: 0.5 mg/ml), Penicillium species (MIC90: 0.03 mg/ml), Scedosporium species (MIC90: 0.5 mg/ml), and others.[25] Topically administered 1% voriconazole eye drops have been documented to achieve good intraocular penetration in non-inflamed and inflamed eyes.[23,26,27,28] It is evident from these studies that 1% voriconazole eye drops offered sufficient voriconazole concentrations in the aqueous humor which is above the MIC90 (0.06 – 8 mg/L) for most fungal species.[29] However, a large filamentous fungal susceptibility study using ocular isolates from keratitis cases in South India showed that MIC90 of voriconazole for the Fusarium isolates were in the range of 0.13 to >64 mg/ml as compared to natamycin whereas the in vitro activity of voriconazole against A. flavus isolates were in the range of 0.13 -8 mg/ml.[30] It is very clear from these studies that Fusarium isolates were less susceptible to voriconazole and A. flavus isolates appeared to have lower susceptibility to natamycin compared to other organisms. Therefore, the treatment with topical voriconazole may be relevant in cases of fungal keratitis caused by Aspergillus isolates.
In this study, the extended-release kinetics of voriconazole from HAM was investigated for 5 different soaking times to check the ideal exposure time for voriconazole for better release. It is found that the drug entrapment efficiency was increased with increase in drug exposure time but not the release from the membrane. This is in agreement with the previous observation by us and others that HAM may not need longer exposure time to completely fill up the membrane.[21,31] However, a detectable amount of voriconazole was observed even at 5 weeks. This clearly indicates the reservoir function of HAM for a variety of drugs.
Two hourly dosing regimen offered concentrations that were sufficient to eradicate keratitis caused by Aspergillus and Candida species but not Fusarium species. Such poorer clinical resolution with topical 1% voriconazole has been reported for keratitis caused by Fusarium species in patients.[6,32,33] In the previous study, it is found that Aspergillus flavus isolates had decreased susceptibility to natamycin.[30] In MUTT I, Aspergillus cases had better clinical outcomes with voriconazole treatment than natamycin treatment, though this was not significant. Other studies have also shown that voriconazole treatment is efficacious against Aspergillus ulcers, whereas natamycin treatment had poor efficacy.[34] By comparing the efficacy of both natamycin and voriconazole, it is found that there was no difference between voriconazole and natamycin in 3-month best spectacle-corrected visual activity or in proportion of cases perforating.[35] Thus, the role of voriconazole in the treatment of fungal keratitis still remains relevant.
The limitation of this study includes the anti-fungal activity of the effluent media collected during the release kinetics was not investigated. Since, the previous studies demonstrated the superiority of 5% natamycin over 1% voriconazole in treating fungal keratitis, this study investigated only voriconazole. A systematic study to show how the natamycin is released from HAM may provide choice for the clinician to choose between voriconazole and natamycin based on the causative fungal species.
Conclusion
In conclusion, this study once again demonstrated the reservoir function of HAM using newer anti-fungal agent, voriconazole. HAM is capable of releasing voriconazole for the extended duration. Hence, drug-loaded HAM as a biological bandage can be considered for clinical application. However, its efficacy in the sustained drug delivery of voriconazole, its destructive effects on the viability of the HAM, its interaction with fungal species, as well as the factors that influence its binding capacity to HAM, need further investigations.
Financial support and sponsorship
Aravind Eye Hospital, Madurai – Intramural Grant
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors acknowledge Aravind Eye hospital, Madurai (Intramural Grant), for financial assistance and Mrs. Ishwarya for Statistical analysis. The authors acknowledge the Rotary Aravind International Eye bank, AEH, Madurai, and Aurolab, Madurai, for providing HAM tissues.
References
- 1.Zidan G, Rupenthal ID, Greene C, Seyfoddin A. Medicated ocular bandages and corneal health:Potential excipients and active pharmaceutical ingredients. Pharm Dev Technol. 2018;23:255–60. doi: 10.1080/10837450.2017.1377232. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh AK, Gupta A, Rudramurthy SM, Paul S, Hallur VK, Chakrabarti A. Fungal keratitis in North India:Spectrum of agents, risk factors and treatment. Mycopathologia. 2016;181:843–50. doi: 10.1007/s11046-016-0042-3. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15:321–7. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness:A global perspective. Bull World Health Organ. 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81:965–71. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, Das S, Virdi A, Fernandes M, Sahu SK, Koday NK, et al. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br J Ophthalmol. 2015;99:1190–5. doi: 10.1136/bjophthalmol-2014-306485. [DOI] [PubMed] [Google Scholar]
- 7.Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis:An analysis of the peer-reviewed literature. Br J Ophthalmol. 2011;95:762–7. doi: 10.1136/bjo.2009.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmoudi S, Masoomi A, Ahmadikia K, Tabatabaei SA, Soleimani M, Rezaie S, et al. Fungal keratitis:An overview of clinical and laboratory aspects. Mycoses. 2018;61:916–30. doi: 10.1111/myc.12822. [DOI] [PubMed] [Google Scholar]
- 9.Lee VHL, Robinson JR. Topical ocular drug delivery:Recent developments and future challenges. J Ocul Pharmacol. 1986;2:67–108. doi: 10.1089/jop.1986.2.67. [DOI] [PubMed] [Google Scholar]
- 10.Konar P, Joshi S, Mandhare S, Thakur R, Deshpande M, Dayal A. Intrastromal voriconazole:An adjuvant approach for recalcitrant mycotic keratitis. Indian. J Ophthalmol. 2020;68:35–8. doi: 10.4103/ijo.IJO_378_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakash G, Sharma N, Goel M, Titiyal JS, Vajpayee RB. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146:56–9. doi: 10.1016/j.ajo.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Kalaiselvi G, Narayana S, Krishnan T, Sengupta S. Intrastromal voriconazole for deep recalcitrant fungal keratitis:A case series. Br J Ophthalmol. 2015;99:195–8. doi: 10.1136/bjophthalmol-2014-305412. [DOI] [PubMed] [Google Scholar]
- 13.Sharma N, Agarwal P, Sinha R, Titiyal JS, Velpandian T, Vajpayee RB. Evaluation of intrastromal voriconazole injection in recalcitrant deep fungal keratitis:Case series. Br J Ophthalmol. 2011;95:1735–7. doi: 10.1136/bjo.2010.192815. [DOI] [PubMed] [Google Scholar]
- 14.Sharma N, Sankaran P, Agarwal T, Arora T, Chawla B, Titiyal JS, et al. Evaluation of intracameral amphotericin B in the management of fungal keratitis:Randomized controlled trial. Ocul Immunol Inflamm. 2016;24:493–7. doi: 10.3109/09273948.2015.1057597. [DOI] [PubMed] [Google Scholar]
- 15.Dua HS, Rahman I, Miri A, Said DG. Variations in amniotic membrane:Relevance for clinical applications. Br J Ophthalmol. 2010;94:963–4. doi: 10.1136/bjo.2009.157941. [DOI] [PubMed] [Google Scholar]
- 16.Jirsova K, Jones GLA. Amniotic membrane in ophthalmology:Properties, preparation, storage and indications for grafting—a review. Cell Tissue Bank. 2017;18:193–204. doi: 10.1007/s10561-017-9618-5. [DOI] [PubMed] [Google Scholar]
- 17.Aykut V, Celik U, Celik B. The destructive effects of antibiotics on the amniotic membrane ultrastructure. Int. Ophthalmol. 2015;35:381–5. doi: 10.1007/s10792-014-9959-z. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Sah WJ, Kim YJ, Kim JC, Hahn TW. Amniotic membrane, tear film, corneal, and aqueous levels of ofloxacin in rabbit eyes after amniotic membrane transplantation. Cornea. 2001;20:628–34. doi: 10.1097/00003226-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Mencucci R, Menchini U, Dei R. Antimicrobial activity of antibiotic-treated amniotic membrane:An in vitro study. Cornea. 2006;25:428–31. doi: 10.1097/01.ico.0000214207.06952.23. [DOI] [PubMed] [Google Scholar]
- 20.Mencucci R, Paladini I, Menchini U, Gicquel JJ, Dei R. Inhibition of viral replication in vitro by antiviral-treated amniotic membrane. Possible use of amniotic membrane as drug-delivering tool. Br J Ophthalmol. 2011;95:28–31. doi: 10.1136/bjo.2010.179556. [DOI] [PubMed] [Google Scholar]
- 21.Yelchuri ML, Madhavi B, Gohil N, Sajeev HS, Prajna NV, Srinivasan S. In vitro evaluation of the drug reservoir function of human amniotic membrane using moxifloxacin as a model drug. Cornea. 2017;36:594–9. doi: 10.1097/ICO.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 22.Sara S, Prajna N, Senthilkumari S. Human amniotic membrane as a drug carrier-An in-vitro study using fortified cefazolin ophthalmic solution. Indian. J Ophthalmol. 2019;67:472–5. doi: 10.4103/ijo.IJO_1336_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senthilkumari S, Lalitha P, Prajna NV, Haripriya A, Nirmal J, Gupta P, et al. Single and multidose ocular kinetics and stability analysis of extemporaneous formulation of topical voriconazole in humans. Curr Eye Res. 2010;35:953–60. doi: 10.3109/02713683.2010.506968. [DOI] [PubMed] [Google Scholar]
- 24.Sangwan VS, Basu S. Antimicrobial properties of amniotic membrane. Br J Ophthalmol. 2011;95:1–2. doi: 10.1136/bjo.2010.184259. [DOI] [PubMed] [Google Scholar]
- 25.Vemulakonda GA, Hariprasad SM, Mieler WF, Prince RA, Shah GK, Van Gelder RN. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Arch Ophthalmol. 2008;126:18–22. doi: 10.1001/archophthalmol.2007.8. [DOI] [PubMed] [Google Scholar]
- 26.Lau D, Fedinands M, Leung L, Fullinfaw R, Kong D, Davies G, et al. Penetration of voriconazole, 1%, eyedrops into human aqueous humor:A prospective open-label study. Arch Ophthalmol. 2008;126:343–6. doi: 10.1001/archophthalmol.2007.71. [DOI] [PubMed] [Google Scholar]
- 27.Neoh CF, Leung L, Chan E, Al-Badriyeh D, Fullinfaw RO, Jhanji V, et al. Open-label study of absorption and clearance of 1% voriconazole eye drops. Antimicrob Agents Chemother. 2016;60:6896–8. doi: 10.1128/AAC.00683-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiel MA, Zinkernagel AS, Burhenne J, Kaufmann C, Haefeli WE. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob Agents Chemother. 2007;51:239–44. doi: 10.1128/AAC.00762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis:A 10-year review at a referral eye care center in South India. Cornea. 2002;21:555–9. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Lalitha P, Sun CQ, Prajna NV, Karpagam R, Geetha M, O'Brien KS, et al. In vitro susceptibility of filamentous fungal isolates from a corneal ulcer clinical trial. Am J Ophthalmol. 2014;157:318–26. doi: 10.1016/j.ajo.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resch MD, Resch BE, Csizmazia E, Imre L, Németh J, Szabó-Révész P, et al. Drug reservoir function of human amniotic membrane. J Ocul Pharmacol Ther. 2011;27:323–6. doi: 10.1089/jop.2011.0007. [DOI] [PubMed] [Google Scholar]
- 32.Prajna NV, Mascarenhas J, Ravindranath Reddy TPR, Prajna L, Srinivasan M, Vaitilingam CM, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128:672–8. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkatesh Prajna N, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, et al. The mycotic ulcer treatment trial:A randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131:422–9. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pradhan L, Sharma S, Nalamada S, Sahu SK, Das S, Garg P. Natamycin in the treatment of keratomycosis:Correlation of treatment outcome and in vitro susceptibility of fungal isolates. Indian J Ophthalmol. 2011;59:512–4. doi: 10.4103/0301-4738.86328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prajna VN, Lalitha PS, Mascarenhas J, Krishnan T, Srinivasan M, Vaitilingam CM, et al. Natamycin and voriconazole in Fusarium and Aspergillus keratitis:Subgroup analysis of a randomised controlled trial. Br J Ophthalmol. 2012;96:1440–1. doi: 10.1136/bjophthalmol-2012-301825. [DOI] [PMC free article] [PubMed] [Google Scholar]