Abstract
Purpose:
The purpose of the study was to compare the choroidal thickness in normal population and hypertensive patients and to assess the possible effect of hypertension on choroidal thickness using Spectral Domain Optical Coherence Tomography (SD-OCT).
Methods:
This was a comparative cross-sectional study. A total of 68 eyes of 34 individuals in the age group of 40–60 years were included in both the hypertensive group and control group. Individuals with refractive error beyond ± 3 D and posterior segment pathology were excluded. The choroidal thickness was measured at the sub-foveal region, 500 μm nasal and 500 μm temporal to the fovea on SD-OCT with enhanced depth imaging (EDI) mode. Systolic blood pressure (SBP), Diastolic blood pressure (DBP), and Mean arterial pressure (MAP) were recorded in all individuals. Duration of hypertension was also noted in hypertensive individuals.
Results:
The choroidal thickness at all locations was significantly lower in the hypertensive group (subfoveal, nasal, temporal and mean choroidal thickness 253.24 ± 63.96 μm, 249.35 ± 63.57 μm, 250.01 ± 63.37 μm, 250.87 ± 63.38 μm, respectively) as compared to the control group (subfoveal, nasal, temporal and mean choroidal thickness 301.25 ± 55.79 μm, 298.97 ± 57.07 μm, 299.49 ± 55.06 μm, 299.90 ± 55.50 μm, respectively). The choroidal thickness in the hypertensive group also had a significant negative correlation with the SBP (Spearman correlation coefficient, rho = –0.35, P = 0.003) and the duration of hypertension (rho = -0.25, P = 0.037).
Conclusion:
The study demonstrated decreased choroidal thickness in systemic hypertensive subjects as compared to age-matched healthy individuals. The choroidal thickness in hypertensive subjects also had a significant but weak negative correlation with SBP and duration of hypertension.
Keywords: Choroid, choroidal thickness, enhanced depth imaging OCT, spectral-domain OCT, systemic hypertension
Collected data from different regions of the world have shown a rise in the prevalence of hypertension and estimated the global burden of hypertension to increase sharply.[1] A meta-analysis of hypertension in India reported an overall prevalence of 26.7–33%.[2]
The consequences of hypertension on ocular structures include hypertensive retinopathy, choroidopathy, optic neuropathy, retinal vascular occlusions, age-related macular degeneration, glaucoma, etc.[3] Although hypertensive retinopathy has been vastly studied in the past, data regarding the choroidal changes in systemic hypertension is still evolving.
The main function of the choroid is to provide vascular supply to the outer retinal layers, provide nourishment and oxygen, clear waste products, and help in temperature regulation of the outer coats of the eyeball. The choroidal blood flow is maintained by autoregulatory mechanism.[4] The choroidal arteries have a shorter course with fewer branches, and they supply the choriocapillaris at right angles. These unique features predispose the choroidal vasculature to systemic hypertension.[5] Compromised choroidal circulation can hamper the vital structures like photoreceptors, optic nerve, and retinal nerve fiber layer. Considering the blood supply to the retina is partly through the choroid, there could be a possibility of the choroid being affected earlier in systemic hypertension. Thus, studying the choroidal circulation and thickness can benefit in detecting early ocular changes in systemic hypertension.
Imaging of the choroid in vivo can be performed by various modalities including Fundus fluorescein angiography (FFA), Indocyanine green angiography (ICGA), Optical coherence tomography (OCT), ultrasonography, Magnetic resonance imaging (MRI).[6,7,8,9] Although each of these modalities has their own set of advantages and disadvantages, OCT has gained popularity in the recent era, as it is a rapid, non-invasive, repeatable and easily available investigative device with provision of quantitative estimation.[10] Newer deeper penetration OCT technologies like Enhanced depth Imaging (EDI) enabled Spectral Domain OCT (SD-OCT) or swept-source OCT (SS-OCT) have enabled better delineation of sclero-choroidal interface with precise measurement of choroidal thickness.
Multiple authors have studied the correlation of choroidal thickness with various parameters like age, gender, refractive status, axial length, and racial variation.[11,12] A conflicting data of association of the choroidal thickness with systemic hypertension was described in a handful number of reports in the literature. Few of these available studies are limited by absence of age-matched comparative arm. Correlation analysis of choroidal thickness with blood pressure in the hypertensive individuals was done in very few isolated reports.[13,14,15,16,17,18]
Hence, keeping these lacunae of literature in mind, we aimed to compare the choroidal thickness in hypertensive subjects with age-matched healthy individuals by EDI enabled SD-OCT and to look for any correlation between choroidal thickness and blood pressure parameters in hypertensive subjects.
Methods
This prospective comparative study was conducted at the ophthalmology department of a tertiary care institute in North India for 1 year from July 2018 to June 2019. The study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Ethics Coμmittee. The study group comprised 68 eyes of 34 adult individuals who were diagnosed with essential hypertension from the internal medicine department and life-style clinic of our institute. The cut-off value for hypertension was decided as systolic blood pressure (SBP) ≥140 mm Hg and diastolic blood pressure (DBP) ≥90 mm Hg.[19] However, any individual with SBP more than 180 mm Hg and/or DBP more than 110 mm Hg were excluded from the study.
The control group comprised 68 eyes of 34 age-matched normotensive individuals, selected from other patients visiting ophthalmology outpatient department or from healthy hospital staffs. The normotensive control individuals were categorized as group A and the hypertensive individuals were categorized as group B for the sake of description. Demographics of all the individuals including age, gender, and systemic disease status were noted for both the groups. An age limit of 40–60 years was applied for both groups. Individuals with clear optical media and good fixation were included in the study. The exclusion criteria of the study were individuals with ocular posterior segment pathology, history of ocular trauma or surgery, refractive error beyond +3.0 to –3.0 diopters, glaucoma, ocular hypertension, uveitis, and any neurodegenerative disorders.
Comprehensive ophthalmic evaluation including best-corrected visual acuity, refraction, intraocular pressure (IOP) by Goldman applanation tonometry, axial length (AL) by optical biometer (Lenstar LS 900, Haag-Streit, USA), slit-lamp biomicroscopic evaluation of anterior segment and fundus evaluation with 90D lens were performed on each individuals of both groups. The blood pressure (BP; μm Hg) measurements (SBP and DBP) were taken by mercury sphygmomanometers on the right upper arm with appropriate adult cuff size. Subjects were seated for at least ten minutes prior to BP measurement. An average of two BP measurements were noted. The mean arterial pressure (MAP, mm Hg) was calculated as follows: MAP = (SBP + 2DBP)/3.
All individuals in the study and control group were subjected to Spectral Domain Optical Coherence Tomography (SD-OCT; Cirrus™, Carl Zeiss Meditec, Dublin, CA) after adequate pupillary dilation. All images were taken by a single operator ensuring adequate signal strength (at least 8) in each of the scanned images. Measurements were performed in between 12 pm to 3 pm in all individuals to avoid any diurnal variation of choroidal thickness. Macular Line Raster scan protocol with enhanced depth imaging (EDI) mode was followed to measure the choroidal thickness at three locations in each eye (subfoveal, 500 μm nasal to fovea and 500 μm temporal to fovea) using the in-built calliper function. Both the eyes of each individual were subjected to scan sequentially. The choroidal thickness was measured as the perpendicular distance between the hyperreflective outer border of the retinal pigment epithelial–Bruch’s membrane layer (RPE-BM) and the sclero-choroidal interface by a single experienced observer blinded to the diagnosis of the individuals. A representative image of measurement of choroidal thickness in a normal and hypertensive subject was shown in [Fig. 1].
Figure 1.
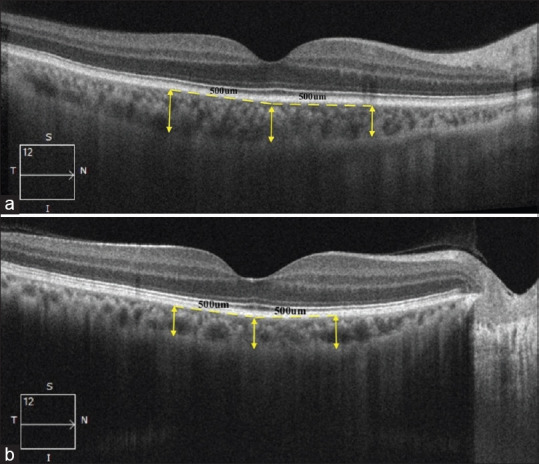
Representative image showing measurement of choroidal thickness at subfoveal location, 500 μm nasal to fovea and 500 μm temporal to fovea in a normal (a) and a hypertensive (b) subject
Data entry was done in a Microsoft Excel Spread Sheet (Microsoft Corporation, USA) and analyzed using Statistical Package for the Social Sciences (SPSS), version 23. Values were expressed as mean ± standard deviation (SD). Independent t-test, Chi-square test and Mann–Whitney U test were applied to the appropriate values after checking the normality of the data. Correlations between the variables were analyzed by Spearman’s correlation coefficient (rho). A value of P < 0.05 was considered as statistically significant.
Results
No statistically significant difference was found between the mean age, gender distribution, mean IOP and mean AL between the two groups. All the blood pressure (BP) parameters (SBP, DBP, MAP) were significantly higher in hypertensive group as compared to control group (P < 0.01). The mean duration of hypertension after diagnosis was 4.41 ± 2.87 years (range 1-10 years; median three years with interquartile range 2-7 years) in the hypertensive group. Twenty-three subjects (67.6%) were hypertensive for ≤5 years as compared to 11 subjects (32.4%) who were hypertensive for >5 years in the hypertensive group. The demographic data and clinical parameters of both groups were suμmarized in Table 1.
Table 1.
Comparison of demographic data, clinical and blood pressure parameters between Group A and Group B
| Parameters | Group | P | |
|---|---|---|---|
| A (Control) (n=68 eyes of 34 individuals) | B (Hypertensive) (n=68 eyes of 34 individuals) | ||
| Age (Years) | 50.24±5.86 | 51.59±7.07 | 0.1821 |
| Age | 0.7312 | ||
| 40-50 Years | 16 (47.1%) | 17 (50.0%) | |
| 51-60 Years | 18 (52.9%) | 17 (50.0%) | |
| Gender | 1.0002 | ||
| Male | 17 (50.0%) | 17 (50.0%) | |
| Female | 17 (50.0%) | 17 (50.0%) | |
| IOP (μmHg)† | 14.4±3.3 | 15.6±3.4 | 0.133, 0.153 |
| Axial Length (μm)† | 22.90±1.00 | 22.92±0.90 | 0.8833, 0.8723 |
| Duration of Hypertension (Years) | - | 4.41±2.87 | - |
| Duration of Hypertension | 1.0002 | ||
| ≤5 Years | 0 (0%) | 23 (67.6%) | |
| >5 Years | 0 (0%) | 11 (32.4%) | |
| Systolic BP (μmHg)* | 117.53±7.80 | 147.65±9.48 | <0.0011 |
| Diastolic BP (μmHg)* | 72.65±5.07 | 94.41±5.00 | <0.0011 |
| MAP (μmHg)* | 84.86±8.35 | 112.15±4.22 | <0.0011 |
*Significant at P<0.05, †Significance of P value did not change for these parameters when repeated measure analysis was considered (the former P value was obtained without considering repeated measures and the latter P value was obtained after considering repeated measures); 1: Wilcoxon-Mann-Whitney U Test, 2: Chi-Squared Test, 3: t-test
The mean choroidal thickness (mean of nasal, subfoveal and temporal regions) in control group was 299.90 ± 55.5 μm, which was significantly higher (P < 0.0001) than mean choroidal thickness in the hypertensive group (250.87 ± 63.38 μm). Similarly, the independent subfoveal, nasal and temporal choroidal thickness was also found to be statistically higher in control group as compared to hypertensive group (P < 0.001 for all locations; significance of P value did not alter when repeated measure analysis was considered). The subfoveal choroid was thicker than the nasal and the temporal choroid in both groups. The choroidal thickness values of both the groups at different locations were shown in Table 2.
Table 2.
Comparison of Choroidal thickness measurements between Group A and Group B
| Parameters | Group | P | |
|---|---|---|---|
| A (Control) (n=68) | B (Hypertensive) (n=68) | ||
| Subfoveal CT (µm)* | 301.25±55.79 | 253.24±63.96 | <0.0011 |
| Nasal CT (µm)* | 298.97±57.07 | 249.35±63.57 | <0.0013 |
| Temporal CT (µm)* | 299.49±55.06 | 250.01±63.37 | <0.0011 |
| Mean CT (µm)* | 299.90±55.50 | 250.87±63.38 | <0.0011 |
*Significant at P<0.05, 1: Wilcoxon-Mann-Whitney U Test, 3: t-test; CT: Choroidal thickness; Significance of P value did not change for none of these parameters when repeated measure analysis was considered
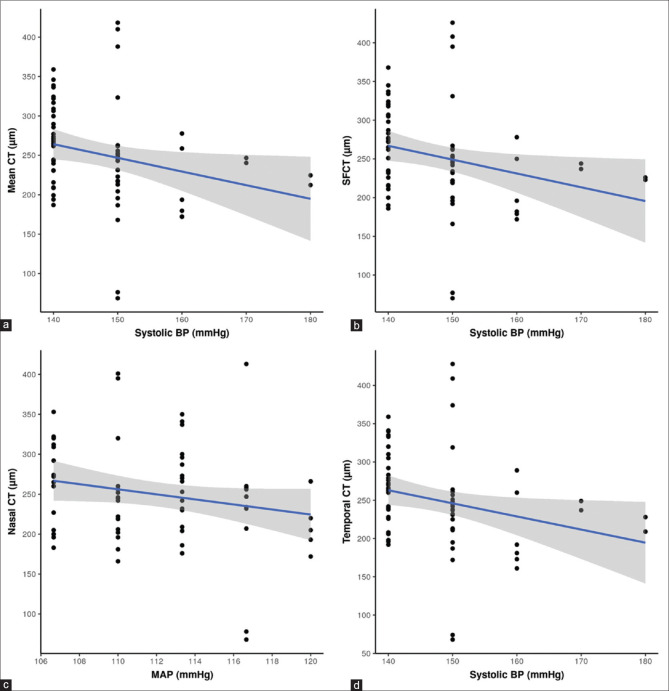
A statistically significant negative correlation was found between the choroidal thickness at all locations and the SBP in the hypertensive group (subfoveal rho = -0.37, P = 0.002; nasal rho = -0.35, P = 0.003; temporal rho = -0.36, P = 0.003). However, the correlation of choroidal thickness with DBP and MAP were statistically insignificant in the hypertensive group (rho = 0.04, P = 0.737 and rho = -0.02, P = 0.102, respectively). The correlation analysis between the choroidal thickness at all locations and SBP in the hypertensive group was depicted in multiple scatterplot diagrams in [Fig. 2]. None of the BP parameters (SBP, DBP, and MAP) had any statistically significant correlation with choroidal thickness at any location (subfoveal, nasal and temporal) in the control group. Correlation analyses between the choroidal thickness and BP parameters (SBP, DBP, MAP) in the hypertensive group were suμmarized in Table 3.
Figure 2.
Scatterplot presentation of correlation analysis between the systolic blood pressure and mean choroidal thickness (a), subfoveal choroidal thickness (b), nasal choroidal thickness (c) and temporal choroidal thickness (d) in Group B. The blue lines represent the general trend of correlation between the two variables. The shaded grey areas represent the 95% confidence interval of these trendlines
Table 3.
Correlation analysis between the choroidal thickness and the blood pressure parameters in Group B (hypertensive)
| Location | Correlation Coefficient with P | SBP | DBP | MAP | Duration of hypertension |
|---|---|---|---|---|---|
| Mean CT | rho | -0.35 | 0.04 | -0.02 | -0.25 |
| P | 0.003 | 0.737 | 0.102 | 0.037 | |
| Subfoveal CT | rho | -0.37 | 0.05 | -0.20 | -0.27 |
| P | 0.002 | 0.660 | 0.108 | 0.025 | |
| Nasal CT | rho | -0.35 | 0.05 | -0.19 | -0.24 |
| P | 0.003 | 0.673 | 0.124 | 0.047 | |
| Temporal CT | rho | -0.36 | 0.03 | -0.21 | -0.25 |
| P | 0.003 | 0.807 | 0.082 | 0.039 |
CT: Choroidal thickness; rho: Spearman’s correlation coefficient
Further, correlation analysis between the choroidal thickness and the duration of hypertension (years) revealed that the mean choroidal thickness had a significant negative correlation with duration of hypertension (rho = -0.25, P = 0.037) in hypertensive group.
Discussion
The choroidal thickness in different posterior segment ocular disorders have been studied by various authors in the past. The thickness of choroid is found to be increased in polypoidal choroidal vasculopathy, central serous chorioretinopathy and Vogt-Koyanagi- Harada syndrome.[20,21,22,23] Whereas, studies performed on disorders like macular hole, high myopia, chorioretinal atrophies have reported a thinner choroid.[24,25,26] Other than these posterior segment ocular disorders, conflicting association of choroidal thickness with systemic hypertension has been reported in few studies in the literature.[13,14,15,16,17,18] The objective of the current study was to evaluate the choroidal thickness in normotensive as well as hypertensive individuals in the absence of any other ocular disorders and also to look for any correlation of choroidal thickness with blood pressure in hypertensive individuals.
In our study, both the groups were comparable with regard to age, axial length, refractive error, and intra ocular pressure, thus reducing any possible impact of these parameters on the measurement of choroidal thickness. Moreover, the choroidal thickness was also measured between 12 pm to 3 pm in all individuals to avoid any diurnal variation.
Few studies are available regarding the choroidal thickness in healthy individuals. A pilot study to evaluate the choroidal thickness using EDI tool of OCT was conducted by Margolis et al. on 54 healthy eyes with mean age group of 50.4 years. They observed a topographic variation in the choroidal thickness within the posterior pole. They found the choroid to be thickest at the sub-foveal region (mean 287 ± 76 μm) compared to the nasal and temporal region and hypothesized that sub-foveal region is relatively thicker due to a greater metabolic demand as compared to other regions.[9] Another study on the choroidal thickness profile in 211 eyes of healthy Indian subjects by Chhabalani et al. also revealed that choroidal thickness varies according to the location. They also found thinner choroid in the nasal region and a thicker choroid in the sub-foveal region.[27] Measurement values of sub-foveal choroidal thickness and its topographical variations nasally and temporally in both normotensive and hypertensive groups of our study are consistent with the above mentioned studies.
In a study by Sansom et al., a modest negative correlation was found between subfoveal choroidal thickness and SBP in normal individuals. However, this study did not include any hypertensive individual.[28]
In our study, we found significantly decreased choroidal thickness in the hypertensive group as compared to the control group. This is in agreement with a study by Akay et al., who in their prospective, case-control study involving 80 hypertensive patients and 80 control individuals also found decreased choroidal thickness in systemic arterial hypertension. However, this study was done in a relatively younger age group (20-29 years) as compared to our study and included patients with clinically visible changes of hypertensive retinopathy also (upto grade two hypertensive retinopathy according to Keith-Wagner classification). In our study, we also found significant negative correlation of choroidal thickness with SBP and duration of hypertension, contrary to the finding reported by Akay et al. who did not find any such correlation.[13]
In another prospective study by Ellakwa et al. comprising 50 hypertensive and 50 normotensive subjects, significantly decreased choroidal thickness was found in the hypertensive group as compared to normotensive group. The age range in this study was almost similar (50-70 years) to our study population (40-60 years), although the authors did not perform any correlation analysis between the choroidal thickness and blood pressure among the hypertensive individuals.[14]
A retrospective study done by Masis et al. involving 112 hypertensive patients and 15 healthy individuals also revealed significant choroidal thinning in hypertensive subjects.[15]
Few hypothesis have been postulated to explain the above mentioned choroidal thinning in chronic hypertensive individuals. Choriocapillaris receive rich sympathetic innervations. In response to raised blood pressure, the aggravated sympathetic stimulus causes vasoconstriction of the vessels which in turn can lead to the thinning of the choroidal layer in hypertensive subjects. Geraci et al. studied choroidal thickness and renal hemodynamic changes based on renal resistive index in subjects with essential hypertension. They explained that the pathophysiological mechanism of arterial stiffening leads to increased hemodynamic pressure on the choroidal circulation causing vascular damage and reduced choroidal thickness. Also, endothelial dysfunction increases the passage of strong vasoconstrictors which further increases the vasoconstriction eventually leading to ischemia which may also contribute to thinning of the choroidal layer further.[29]
Contrary to the above studies and hypothesis, few authors did not find any difference in the choroidal thickness in chronic hypertensive patients. Gok et al. in their prospective, cross-sectional study involving 116 hypertensive patients and 116 control subjects, did not reveal any significant difference in subfoveal choroidal thickness among the two groups. The authors in this study postulated that the choroidal vasculature remains relatively protected even in the setting of chronic hypertension due to it’s intrinsic property, as this choroidal network has a critical role in retinal nourishment and has to maintain a optimal perfusion pressure. This might be the reason of unaltered choroidal thickness in hypertensive individuals.[17]
Balmforth et al. in their three-arm comparative study involving patients with hypertension, chronic kidney disease (CKD) and healthy control group demonstrated reduced choroidal thickness in CKD patients as compared to hypertensive and normal control group. But, no difference of choroidal thickness was seen in between hypertensive and normal individuals.[16] Niknam et al. used laser doppler flowmetry to determine the choroidal haemodynamics in systemic hypertensive patients and concluded systemic hypertension does not have any large effect on the choroidal circulation in hypertensives.[18]
Subfoveal choroidal thickness was reported to be increased in acute hypertension due to accumulation of interstitial fluid as a result of hypertensive choroidopathy.[30] Acute rise in blood pressure may cause breakdown of autoregulatory mechanism, leading to choroidal ischemia with subsequent choriocapillaris and RPE necrosis.[31] This in turn leads to increased permeability of choriocapillaris and interstitial fluid accumulation.[32] However, the sequence of pathophysiologic events is different in acute rise of blood pressure as compared to chronic essential hypertension, which might explain the observed differences in choroidal thickness in these two scenarios.
Our study has few limitations too. The sample size was relatively small. Measurement of choroidal thickness was performed by a single observer manually. For precise measurements, additional image analysis software can be used to avoid variations in measurements. Early treatment of Diabetic Retinopathy Study (ETDRS) grid defined sectoral choroidal volume assessment could have given more holistic representation of the total choroidal thickness instead of measurement of point choroidal thickness. This study was a cross-sectional study and did not have long term follow-up of hypertensive or control group to study the longitudinal effect of hypertension on choroidal morphology. Further, correlation analysis of choroidal thickness and blood pressure measurements were based on fixed time measurement of blood pressure instead of 24-hour ambulatory blood pressure measurement.
Most of the patients in our hypertensive group were on anti-hypertensive medications, which might have contributed to the choroidal flow alteration, ultimately impacting choroidal thickness. However, few authors did not find any significant change in choroidal blood flow with antihypertensive medications,[18] justifying the rationale of incorporating patients with anti-hypertensive medications in the hypertensive arm of our study.
A recent review on choroidal thickness in patients with cardiovascular diseases have suggested that the choroidal thickness and blood flow may be linked to smoking, hyperlipidaemia and coronary artery disease also.[33] As many of these parameters often co-exist in hypertensives, choroidal thickness depends upon interplay of these various associated parameters. Most of the available studies in the literature including ours have not taken into account of these parameters while assessing choroidal thickness in hypertensives. Future studies should address this shortcoming and also try to investigate the role of choroidal thickness as a potential biomarker for various cardiovascular diseases.
The OCT device gives quantitative and qualitative estimate of static choroidal structure rather than the exact choroidal blood flow. Interpretation of choroidal changes due to hypertension is based on assumptions of alterations in choroidal thickness secondary to hemodynamic alteration at microstructural level. Hence, the pathophysiology behind choroidal changes in hypertension should additionally be investigated by a flow-based study rather than only structural assessment. Swept-source OCT and OCT-angiography, which are recent additions to the armamentarium of choroidal imaging may provide novel insights regarding choroidal changes in hypertensive individuals in future larger prospective comparative studies.
Conclusion
In conclusion, we found choroidal thickness is decreased significantly in hypertensive subjects as compared to normal individuals. The choroidal thickness in the hypertensive subjects also had a significant negative correlation with SBP and the duration of hypertension. Thus, measurement of choroidal thickness by OCT can be used as a non-invasive biomarker to assess the effect of systemic hypertension on the eye before other clinically detectable fundus changes of hypertension become appreciable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kearney P, Whelton M, Reynolds K, Muntner P, Whelton P, He J. Global burden of hypertension:Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Anchala R, Kannuri N, Pant H, Khan H, Franco O, Di Angelantonio E, et al. Hypertension in India. J Hypertens. 2014;32:1170–7. doi: 10.1097/HJH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong T, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–7. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman P, Alm A, Adler F, Levin L. Adler's Physiology of the Eye (Physiology of the Eye) Elsevier Health Sciences. 2011 [Google Scholar]
- 5.Niknam R, Schocket L, Metelitsina T, DuPont JC, Grunwald JE. Effect of hypertension on foveolar choroidal haemodynamics. Br J Ophthalmol. 2004;88:1263–5. doi: 10.1136/bjo.2003.038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R, Invernizzi A, Agarwal A, Kumari N, Gupta A. Enhanced depth imaging spectral domain optical coherence tomography versus ultrasonography b-scan for measuring retinochoroidal thickness in normal eyes. Retina. 2015;35:250–6. doi: 10.1097/IAE.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Nateras O, Peng Q, Kuranov R, Harrison J, Milner T, et al. Lamina-specific anatomic magnetic resonance imaging of the human retina. Invest Ophthalmol Vis Sci. 2011;52:7232–7. doi: 10.1167/iovs.11-7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman W. Simultaneous indocyanine green and fluorescein angiography using a confocal scanning laser ophthalmoscope. Arch Ophthalmol. 1998;116:455–63. doi: 10.1001/archopht.116.4.455. [DOI] [PubMed] [Google Scholar]
- 9.Margolis R, Spaide R. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Spaide R, Koizumi H, Pozonni M. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Tuncer I, Karahan E, Zengin M, Atalay E, Polat N. Choroidal thickness in relation to sex, age, refractive error, and axial length in healthy Turkish subjects. Int Ophthalmol. 2014;35:403–10. doi: 10.1007/s10792-014-9962-4. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Cano A, Orduna E, Segura F, Lopez C, Cuenca N, Abecia E, et al. Choroidal thickness and volume in healthy young white adults and the relationships between them and axial length, aμmetropy and sex. Am J Ophthalmol. 2014;158:574–83.e1. doi: 10.1016/j.ajo.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Akay F, Gundogan F, Yolcu U, Toyran S, Uzun S. Choroidal thickness in systemic arterial hypertension. Eur J Ophthalmol. 2015;26:152–7. doi: 10.5301/ejo.5000675. [DOI] [PubMed] [Google Scholar]
- 14.Yousef A, Ellakwa A, Ibraheem A. The effect of hypertension on choroidal thickness measured by optical coherence tomography. Menoufia Med J. 2019;32:678–82. [Google Scholar]
- 15.Masís M, Hernandez E, Wu L. Choroidal thickness in patients with systemic hypertension. Invest Ophthalmol Vis Sci. 2011;52:5296. [Google Scholar]
- 16.Balmforth C, van Bragt J, Ruijs T, Cameron J, Kiμmitt R, Moorhouse R, et al. Chorioretinal thinning in chronic kidney disease links to inflaμmation and endothelial dysfunction. JCI Insight. 2016;1:e89173. doi: 10.1172/jci.insight.89173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gok M, Karabas V, Emre E, Aksar A, Aslan M, Ural D. Evaluation of choroidal thickness via enhanced depth-imaging optical coherence tomography in patients with systemic hypertension. Indian J Ophthalmol. 2015;63:239–43. doi: 10.4103/0301-4738.156928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niknam R. Effect of hypertension on foveolar choroidal haemodynamics. Br J Ophthalmol. 2004;88:1263–5. doi: 10.1136/bjo.2003.038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Hiμmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults:A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 20.Tan C, Ouyang Y, Ruiz H, Sadda S. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Opthalmol Vis Sci. 2012;53:261–6. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 21.Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31:1603–8. doi: 10.1097/IAE.0b013e31820f4b39. [DOI] [PubMed] [Google Scholar]
- 22.Fong A, Li K, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt–Koyanagi–Harada disease. Retina. 2011;31:502–9. doi: 10.1097/IAE.0b013e3182083beb. [DOI] [PubMed] [Google Scholar]
- 23.Chung S, Kang S, Lee J, Kim Y. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118:840–5. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara T, Imamura Y, Margolis R, Slakter J, Spaide R. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Spaide R. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147:801–10. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Kubota T, Jonas J, Naumann G. Decreased choroidal thickness in eyes with secondary angle closure glaucoma. An aetiological factor for deep retinal changes in glaucoma? Br J Ophthalmol. 1993;77:430–2. doi: 10.1136/bjo.77.7.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chhablani J, Rao PS, Venkata A, Rao HL, Rao BS, Kumar U, et al. Choroidal thickness profile in healthy Indian subjects. Indian J Ophthalmol. 2014;62:1060–6. doi: 10.4103/0301-4738.146711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sansom L, Suter C, McKibbin M. The association between systolic blood pressure, ocular perfusion pressure and subfoveal choroidal thickness in normal individuals. Acta Ophthalmologica. 2015;94:e157–8. doi: 10.1111/aos.12794. [DOI] [PubMed] [Google Scholar]
- 29.Geraci G, Maria Zaμmuto M, Vadal M, Mattina A, Castellucci M, Guarrasi G, et al. Choroidal thickness is associated with renal hemodynamics in essential hypertension. J Clin Hypertens. 2020;22:245–53. doi: 10.1111/jch.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velazquez-Villoria D, Marti Rodrigo P, DeNicola M, Zapata Vitori M, Segura García A, García-Arumí J, et al. Swept source optical coherence tomography evaluation of chorioretinal changes in hypertensive choroidopathy related to HELLP syndrome. Retin Cases Brief Rep. 2019;13:30–3. doi: 10.1097/ICB.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 31.Tso M, Jampol L. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132–45. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- 32.Ahn S, Woo S, Park K. Retinal and choroidal changes with severe hypertension and their association with visual outcome. Invest Ophthalmol Vis Sci. 2014;55:7775–85. doi: 10.1167/iovs.14-14915. [DOI] [PubMed] [Google Scholar]
- 33.Yeung SC, You Y, Howe KL, Yan P. Choroidal thickness in patients with cardiovascular disease:A review. Surv Ophthalmol. 2020;65:473–86. doi: 10.1016/j.survophthal.2019.12.007. [DOI] [PubMed] [Google Scholar]