Abstract
The field of electroceuticals has attracted considerable attention over the past few decades as a novel therapeutic modality. The gastrointestinal tract (GIT) holds significant potential as a target for electroceuticals, as an intersection of neural, endocrine, and immune systems. We review recent developments in electrical stimulation of various portions of the GIT (including esophagus, stomach, small and large intestine) and nerves projecting to the GIT and supportive organs. This has been tested with varying degrees of success for a number of dysmotility, inflammatory, hormonal, and neurologic disorders. We outline a vision for the future of GI electroceuticals, building on advances in mechanistic understanding of GI physiology coupled with novel ingestible technologies. The next wave of electroceutical therapies will be minimally invasive and more targeted than current approaches, making them an indispensable tool in the clinical armamentarium.
Keywords: Gastrointestinal tract, electrical stimulation, electroceuticals
A brief history of electrotherapy
Electroceuticals aim to treat diseases through electrical stimulation, analogous to pharmaceuticals’ chemical stimulation. Attempts to modulate physiology through electrical stimulation span millennia. In the 4th century BC, Aristotle described electric rays that could produce numbness to treat pain[1]. Over the past century, devices have been developed for electrical stimulation of organs. Cardiac pacemakers delivering electrical stimuli to the heart were developed in the 1930s. These were refined as technology and understanding of cardiac pathophysiology progressed, forming the basis for today’s chronic pacemakers and closed-loop implantable cardioverter-defibrillators (ICDs). Electrical brain stimulation was developed as electro-convulsive therapy (ECT) and, more recently, deep brain stimulation (DBS) and commonly treat psychiatric and neurologic disorders. Similar to ICDs, DBS has also evolved towards less-invasive and more targeted therapy.
Electrotherapies have garnered significant public interest due to their ability to mediate organ function through nerves, rather than direct pharmacotherapy. The SPARC NIH initiative focuses on the use of electric neuromodulation to improve organ function. DARPA has emphasized electrical implants and neurotechnology through ElectRx, SUBNETS, HAPTIX, and TNT programs. Electrical stimuli can be applied directly to nerve bundles, as opposed to systemically-administered medication. Electrical signals occur over a matter of seconds, hence enabling finer temporal resolution than pharmacotherapies. In this review, we focus on electroceuticals targeting the gastrointestinal tract (GIT). We define GI electroceuticals as any approach utilizing electrical stimulation to treat GI disorders such as malabsorption and dysmotility, as well as GI-related disorders of metabolism, inflammation, and infection. The stimulus can be applied directly to GI tissue or to other tissues such as nerves.
GIT pathophysiology can arise from any component of its immune, metabolic, and neural interconnectivity (Box 1). Local muscle failure or aberrant signaling of the enteric nervous system (ENS) commonly result in dysmotility. ENS dysfunction can also impact cognitive status by affecting homeostatic control, leading to psychiatric and behavioral disorders. Dysfunction of digestive secretions from the biliary tree can affect digestion of nutrients, impacting nutritional status and leading to metabolic imbalances. Immune dysfunction, involving the GI-resident microbiome, can cause breakdown of barrier function, leading to infection, or chronic inflammation and autoimmunity in disorders such as inflammatory bowel disease (IBD). Perturbation of the microbiome can lead to a surge in pathogenic bacteria.
Text Box 1. Gastrointestinal Physiology [text box]
Overview
The GI tract can be separated at a macroscopic level into various specialized segments: mouth, esophagus, stomach, small intestine (composed of duodenum, jejunum, ileum), large intestine (or colon), and rectum. Ingestion and mechanical breakdown of food takes place in the mouth, chemical and further mechanical digestion in the stomach, nutrient uptake in the small intestine, water uptake and stool formation in the large intestine, and stool expulsion in the rectum. Food is propagated through the GI tract largely through peristaltic muscle contractions. Details of GI physiology have been published elsewhere[121].
The GI tract relies on a number of supportive organs such as the liver, bile ducts, and pancreas that secrete digestive juices. An extensive neuronal network in the GI, the enteric nervous system (ENS) (reviewed in[122, 123]), orchestrates the digestive process, senses the nature of nutrients absorbed, and relays this information to the brain for cognitive control of hunger and satiety (reviewed in[50]). Millions of microbes also reside in the GI tract, constituting the microbiome. The majority of these are symbiotic commensal bacteria although pathogenic microbes can also emerge, leading to disease. As such, there is a strong immune surveillance component in the GI tract which senses the microbiome and maintains barrier function to avoid infection.
Stomach
Food deposited in the stomach induces gastric accommodation, a reflex by which the stomach expands to larger volumes to receive food. Gastric accommodation is a fundamental part of the digestive process, as it also signals to the lower GI tract to prepare to receive nutrients, and the brain to begin to indicate satiety. Gastric digestion involves chemical degradation via enzymes released by specialized secretory cells in the mucosal lining, combined with mechanical mixing and crushing of food particles through synchronized contractions. Gastric contractions are similar to peristalsis but can take a wide variety of shapes and forms. A specialized set of pacemaker cells, called the interstitial cells of Cajal (ICC), exist in the gastric antrum control gastric contractions. As food is processed by the stomach, it slowly passes to the duodenum via the pyloric sphincter.
Small and Large Intestine
The small intestine (SI) receives food from the stomach and begins the process of extracting nutrients from the food. Bile ducts deposit bile acids from the liver, gallbladder, and pancreas to aid in the digestion and nutrient extraction. Mechanoreceptors in the SI signal to the stomach to slow down gastric motility and to distal portions of the GI tract to prepare for nutrient absorption. The SI is lined with enteroendocrine cells (EECs) which sense nutrient content. EECs are the initiators of multiple signaling cascades integrating satiety and metabolic hormones, as well as direct neural signals to modulate hunger and metabolism. The large intestine (colon) receives food from the ileum and is primarily responsible for water balance and stool formation. The colon also harbors the largest microbial population along the GI tract. As such, disorders associated with the colon primarily involve motility and inflammation.
GIT electrical stimulation has been attempted for over 40 years for the treatment of motility, metabolic, and immune disorders. These approaches largely re-purposed cardiac pacemakers, surgically implanting stimulators subcutaneously with electrode leads sutured to the serosa in various sections of the GIT. Nerve stimulation has also been tested, involving similarly implanted generators with electrode leads placed around or within nerves. Similar to the evolution of cardiac and brain stimulation, GI electroceuticals are poised to be revolutionized by enhanced understanding of physiology and advances in engineering, enabling endoscopic and ingestible platforms for non-invasive therapy with greater spatial and temporal resolution.
Electroceutical therapies of the GI tract
The majority of GI electrical stimulation treatments are performed through serosal musculature or nerves (Figure 1). We will first discuss recent advances in electroceuticals targeting GI tissue.
Figure 1.
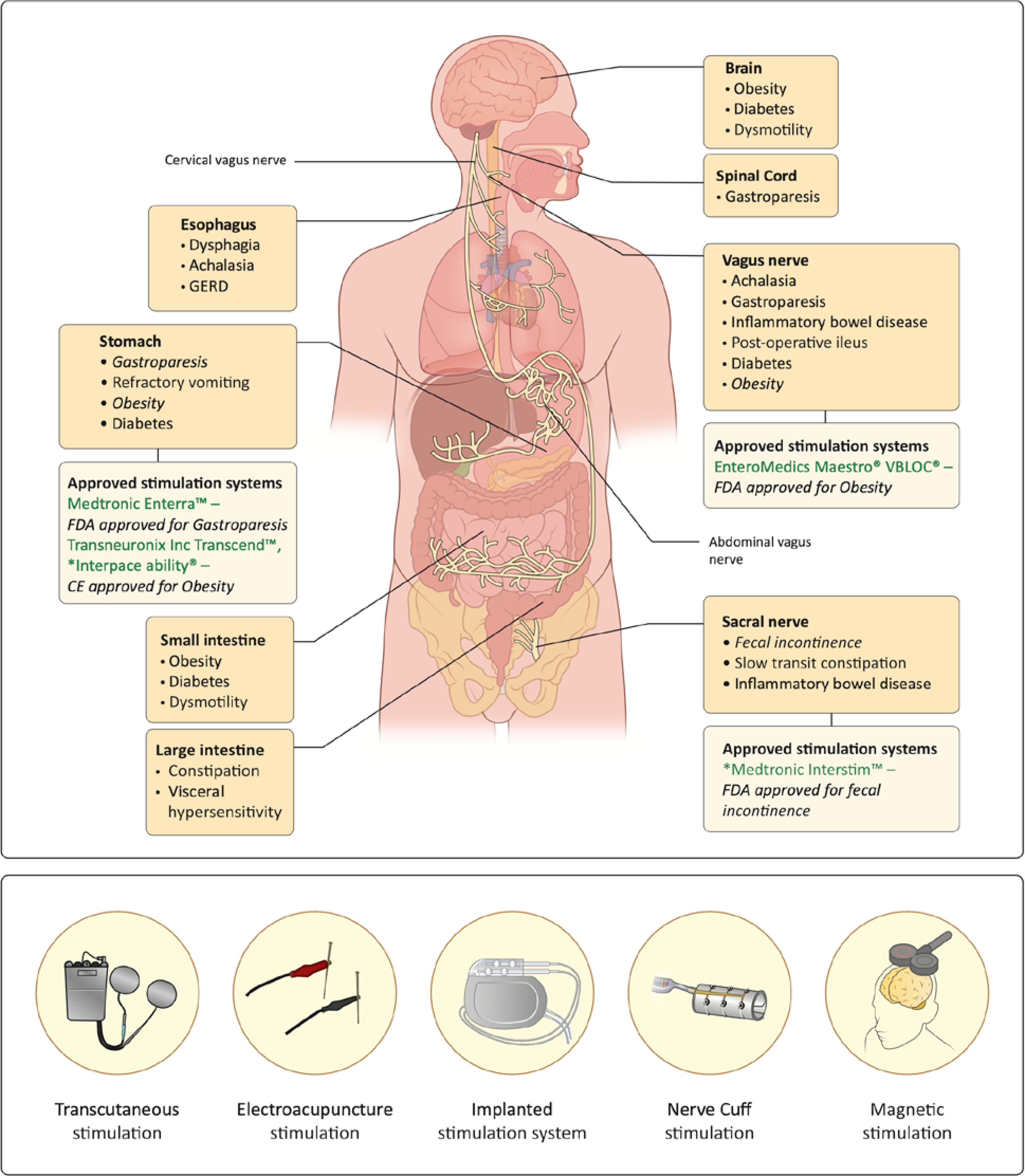
(A) Sites of electrical stimulation for therapy of gastrointestinal and related disorders. Each site is labeled with disorders it has been tested for. Also shown are Therapies for indications which are FDA and CE-approved stimulation sites are also shown. (B) Different forms of electroceutical stimulation.
Esophagus
Dysfunction of swallowing mechanisms, esophageal peristalsis, and the lower esophageal sphincter, are the most common pathologies of the esophagus, resulting in dysphagia, achalasia, and gastroesophageal reflux disease (GERD), respectively. All have been treated using electrical stimulation.
In a prospective non-placebo controlled study in healthy volunteers, transcutaneous neuromuscular stimulation of the upper esophagus (5ms pulse width, 10mA) improved swallowing by extending relaxation time of the upper esophageal sphincter[2]. In a non-placebo controlled trial, LES Stimulation (0.2ms, 3–8mA, 20Hz, 30min total) improved symptoms in patients with GERD over 3 years by increasing LES pressure[3]. Varying the parameters of esophageal electrical stimulation (10–50ms, 3–10mA, 10Hz – 50Hz) induced esophageal peristalsis in rabbits, a potential therapy for achalasia[4]. Esophageal stimulation therapies remain limited, in part due to difficulty accessing overlapping, relatively small regions of the oral cavity and upper esophagus. The burden of surgical implantation required in patients also prevents its use in all but the most extreme cases.
Stomach
Disorders of the stomach include impaired gastric accommodation and gastroparesis (GP) (Box 1). These are associated with dysfunction of gastric neural and hormonal signaling, with implications on neurological, psychiatric, and metabolic disorders[5].
Gastric electrical stimulation (GES) for GP has been attempted for over four decades and is effective in symptomatic improvement of nausea in patients, although it’s effect on gastric emptying is negligible[6]. The Enterra™ GES system is approved by the FDA (under humanitarian use exemption) for GP (0.33ms, 5mA, 14Hz). A randomized crossover study found that GES reduced the frequency refractory vomiting, although it did not accelerate gastric emptying[7].
Stimulation parameters directly determine GES’ mechanism of action. Short-pulse GES (pules width <1ms) activates neuronal pathways, resulting in symptomatic improvement with no influence on gastric contractions. Long-pulse GES (>100ms pulse width) induces muscle contraction[8], and can repair damaged ICC and antral smooth muscle cells in diabetic rats[9]. Combined short and long pulse stimulation in rats improved enteric neuronal survival[10]. Medium-pulse (2ms and 4ms) reduced food intake, inhibiting gastric motility and increasing gastric volume in dogs, and modulating neurohormonal peptides such as neuropeptide Y (NPY) and orexin in rats[11, 12]. In healthy dogs, GES (3–9mA, 1–10ms) significantly reduced high-fat, but not high-carbohydrate or balanced, diet consumption[13]. No significant fluctuation in hunger hormones was found, suggesting direct neuromodulation.
Electrode lead position also influences the efficacy of GES and mechanism of action. Identical GES parameters with different lead placement can completely abrogate the effects seen on gastric accommodation and motility in dogs[12]. GES applied to the distal stomach along lesser or greater curvatures exerted the largest effects while stimulation to the mid-body of the stomach had little effect. Electrical stimulation of locations more anatomically adjacent to certain nerves can facilitate neuromodulation[14]. One group reported no evidence of anti-emetic activity of GES on gastric distension-induced emesis[15]. This study used musk shrews, which unlike rodents possess an emetic reflex. It is possible that this model does not reflect GP pathophysiology. This disparity, however, suggests that GES might exert its anti-emetic effects through specific neural or hormonal pathways.
Given that GES yields symptomatic improvement of neurally-mediated symptoms like nausea and vomiting, but has no effect on gastric emptying, it is believed to act via neural mechanisms. This is reinforced by a retrospective finding that opioid administration before GES implantation procedure reduced clinical efficacy[16], and fMRI data showing activation of the amygdala by GES in dogs[17].
GES has also been utilized for treating obesity and diabetes, both conditions linked to GI function. Although early studies were inconsistent in demonstrating the efficacy of GES on obesity, a recent randomized non-placebo controlled, unblinded trial found that GES resulted in significant weight loss in obese patients[18]. These trials utilized closed-loop GES (CLGES) feedback to turn on GES after initial meal ingestion, detected by implanted antral electrodes sensing increase in gastric volume. CLGES also improved glucose control and Hba1c levels in type 2 diabetes in a randomized, blinded, cross-over trial, suggesting a possible alternative to insulin therapy[19]. The Transcend™ gastric stimulation treatment is available in Europe as treatment of obesity. An experimental third-generation gastric stimulation device (abiliti system) incorporates vagus nerve stimulation (VNS) through strategic electrode placement. CLGES systems enable patient-specific optimization of stimulation parameters from a wide range (0.1–2 ms, 4–30 mA, 40–120 Hz). A non-inferiority, randomized trial showed that CLGES was as effective in treating obesity as laparoscopic gastric banding after 12-months, and limited weight regain up to 24-months post-implantation[20, 21]. The extent to which behavioral changes might be affected by placebo are unknown as these trials lacked a control group. The exact mechanism by which CLGES generates fullness and satiety is not completely understood. Satiety is induced by neuronal and hormonal signals. CLGES could inhibit gastric motility, slowing gastric emptying, and prolonging satiety. It could also inhibit gastric distension, leading to stretch receptor activation at smaller gastric volumes, and leading to early fullness. Alternatively, electrical stimulation may directly modulate neurohomornal signaling, leading to satiety. Intermittent stimulation as in CLGES also avoids the development of tolerance, and the gradual loss of efficacy seen in persistent stimulation. Since the effects of electrical stimulation are multifold and non-specific, a variety of mechanisms for satiety modulation are possible.
GES studies frequently report large inter-subject variability of efficacy in preclinical and clinical trials[22]. This led to pilot studies using non-invasive temporary GES (tGES) to predict patient-specific response[23]. tGES involves determining symptomatic improvement following endoscopic implantation of a stimulator for one week. Electrical leads attached to the gastric mucosa are connected via nasogastric wires to an external stimulator worn by the patient. GES therapies stand to benefit significantly from more informed patient selection. Reliance on primarily functional and symptomatic metrics to diagnose and characterize gastric disorders, with limited knowledge of disease etiology, restricts patient stratification. Greater insight into disease physiology could allow for selection for those most likely to benefit from GES.
The next-generation of GES devices will be less-invasive, including ingestible, endoscopic or transcutaneous approaches. One randomized, double-blinded study used non-invasive transcutaneous electrical stimulation at acupoints (acustimulation) to increase gastric accommodation, reduce postprandial fullness and belching[24]. Chemotherapy-induced nausea and vomiting were reduced by similar acustimulation in another randomized, double-blinded study[25]. A deeper understanding of the differential effects of GES based on characteristics and position of stimulation is crucial to maximize benefit to patients.
Small Intestine
Small intestinal electrical stimulation (IES) arguably holds the greatest potential for regulation of digestion and metabolism. Duodenal stimulation (PW : 1ms – 3ms) accelerates SI transit time and delays gastric emptying in rats[26], and reduces food intake, elevates plasma GLP-1, and improves glucose tolerance and insulin resistance in obese and diabetic rats[26, 27]. The GLP-1-dependent hypoglycemic effect of IES is attributed to a protective effect over pancreatic β-cells in rats[28]. However, stimulation of the ileum more effectively regulates GLP-1 secretion in rats[29]. Studies using longer PW of up to 300ms reported an inhibition of small intestinal motility in dogs[30].
Certain challenges limit human trials of IES. Unlike tGES, access to the SI via endoscope remains challenging. Further, deciphering the effects of IES at various SI locations is particularly difficult given the sheer length of the SI (~7m), as well as subjective borders between the duodenum, ileum, and jejunum.
Large Intestine (Colon)
The colon receives food from the ileum and is responsible for water balance and stool formation. The colon also harbors the largest microbial population along the GI tract. As such, colonic disorders primarily involve motility and inflammation.
Colonic electrical stimulation (CES) via serosal electrodes (4ms pulse width) accelerates colonic transit time in dogs, a potential therapy for slow-transit constipation (STC)[31, 32]. The effect of CES, as with GES and IES, is location-dependent. Rectosigmoid CES directly induced bowel movements in dogs[33] while distal CES has a greater effect than proximal CES, suggesting that effects are mediated via reflexes unique to the distal colon[34]. The neural mechanism of action of CES is supported by studies showing that CES alleviates stress-induced visceral hypersensitivity in rats [35]. In a dog STC model, CES (1–5ms, 15–40Hz) regenerated myenteric plexus neurons of the ENS and enhanced motility, by reducing inflammation[31]. Colonic neuromodulation has been previously reviewed[14]. CES delivered via interferential electrical stimulation, a non-invasive approach, was used to treat constipation in postoperative Hirschsprung’s disease children in a randomized study[36].
The colon contains a high concentration of neuro-endocrine cells[37]. Thus, colonic stimulation holds significant potential for treating a wide variety of functional gastrointestinal disorders (FGIDs) and entero-endocrine disorders. An avenue for less invasive CES that has been clinically underexplored is rectally administered stimulation. Unlike the SI, the colon is readily accessible via colonoscopy. It is also more easily segmented into discrete sections (sigmoid, descending, transverse, ascending limbs). Non-invasive approaches for colonic stimulation could include colonoscopy or suppositories.
Gastrointestinal Neuromodulation
Neuromodulation of the GIT has seen significant advancement in the last few decades, despite limited mechanistic understanding (Figure 1). We discuss recent advances in GI neuromodulation, achieved through stimulation of various nerves within central and peripheral nervous systems.
Vagus Nerve
The vagus nerve innervates several organs with afferent and efferent fibers. Therefore, vagus nerve stimulation (VNS), can modulate a wide variety of homeostatic processes ([38, 39]). VNS entails implanted cuff electrodes encapsulating the vagus nerve connected to a subcutaneously-implanted stimulator, and is currently FDA-approved for epilepsy and depression, and in trials for autoimmune disorders. Here we discuss VNS therapies of GI disorders.
Motility
Bilateral cervical VNS restored esophageal motility in opossum models of achalasia (0.1–5ms, 1–20Hz, 0.1–10 seconds)[40]. Cervical VNS (0.36ms, 0.6mA, 10Hz, 20s on, 40s off) promoted gastric emptying in rats by increasing pyloric opening as assessed by MRI[41]. Noninvasive cervical VNS (1ms, 5kHz) successfully relieved symptoms in patients with drug-refractory GP in an open-label proof of concept study. This effect, while significant, was only seen in 40% of patients, suggesting a need for patient stratification[42]. One option is to stratify by disease etiology.
Inflammatory Bowel Disease
The anti-inflammatory effects of VNS could treat IBD. Abdominal VNS reduced inflammation in rats (0.2ms)[43], and counteracted LPS-induced inflammation (1ms, 1mA, 10Hz) in pigs by reducing pro-inflammatory cytokine levels, suggesting a possible therapy for post-operative ileus [44]. A 2016 open-label pilot study showed that VNS (0.5ms) in Crohn’s disease patients reduced inflammation over 6-months[45]. Another open-label, non-randomized trial found that VNS also decreased electroencephalography (EEG)-recorded alpha waves elevated in Crohn’s, and correlated with clinical improvement[46]. This suggests that the anti-inflammatory effect of VNS in IBD is at least partially mediated through vagal afferents.
Metabolism
Short pulse (0.5ms) VNS corrected glucose metabolism that is aberrant in obese mini-pigs[47]. In obese and diabetic rats, VNS was neuroprotective and reversed depressive-like behavior, hyperglycemia, and brain insulin receptor expression[48]. This study found that effects of VNS are pulse-width dependent. Short pulses (0.3ms) reduced blood glucose, whereas longer pulses (3ms) did not, consistent with previous findings that shorter pulses stimulate neurons to a larger extent than longer pulses, which activate smooth muscle cells. This effect was seen with 5Hz stimulation, but not at higher frequencies of 14 or 40Hz. The glucose-lowering effect was completely abrogated by a GLP-1 antagonist, suggesting that VNS activates efferent fibers to induce SI release of GLP-1.
Abdominal VNS (0.5ms, 5mA, 30Hz, 30s on, 5min off) reduced daily food intake and increased resting energy expenditure in obese mini-pigs[49]. Brain PET and fMRI found concomitant increases in extracellular dopamine and decrease in serotonin. Both dopamine and serotonin are implicated in feeding circuits and their involvement indicates a centrally-mediated effect of VNS in appetite regulation[50]. In contrast, one group reported that cervical VNS with similar parameters (1ms pulse width) can impair glucose tolerance and suppress insulin release in rats[51].
Another emerging clinical therapy is vagal block (VBLOC), which uses high frequency (5000Hz, 3–8mA) stimulation to interrupt vagal signaling. A prospective, open-label, single arm study found VBLOC sustained weight loss and glycemic control in type 2 diabetic patients up to 2 years[52]. With a less invasive approach, transcutaneous auricular (taVNS) conferred neuroprotection in diabetic rats[53]. taVNS (0.25ms, 10mA, 25 Hz) in patients during open laparotomy successfully increased vagal efferent activity as measured by gastrin as well as gastric muscle activity[54]. taVNS (25Hz, 30:30s on:off) also reduced gastric contraction frequency in healthy adults in a randomized, cross-over study[55]. Acustimulation (0.2ms, 2mA, 15min) in rats treated post-operative ileus through vagal mechanisms[56].
In addition to less-invasive techniques, VNS would benefit from improved targeting of specific vagal fibers. Vagal innervation of numerous organs results in severe VNS side-effects of cardiac and respiratory dysfunction[57]. Approaches targeting abdominal branches, as opposed to cervical, decrease off-target effects (Figure 1). Another approach differentially stimulates either efferent or afferent nerve bundles using directional stimulation. Such directional stimulation could play a critical role to decipher differential effects of VNS on insulin secretion and blood glucose regulation[58]. Knowledge of electrophysiological properties of each type of vagal fiber (e.g. myelinated A- and B-fibers vs unmyelinated C-fibers[38, 39]) could inform new approaches for selective stimulation or blocking, using different stimulation parameters for each fiber type. For example, peripheral nerve block has been achieved using frequencies as high as 100 kHz. Optogenetic approaches in preclinical models allow for even greater molecular specificity in targeting. A non-viral technique with similar molecular specificity for targeted VNS would enable more precise control of efferent or afferent signaling, respectively, to or from, discrete organs.
Sacral nerve
The sacral nerves are a set of 5 spinal nerves projecting to the pelvic floor, carrying afferent and efferent fibers for communication between pelvic organs and the central nervous system (CNS).
Sacral nerve stimulation (SNS) is effective for primary fecal incontinence (FI) in humans[59]. Medtronic’s Interstim™ system is FDA-approved for bladder and bowel control (0.21ms, 15Hz)[60, 61], believed to act on spinal afferents in the anal canal. An open-label study found SNS was ineffective for FI in patients with systemic sclerosis, an autoimmune disorder associated with neuropathy[62]. Specificity of stimulation is important, and a randomized, single-blinded study reported that bilateral SNS was not more efficacious that unilateral SNS[63]. As such, optimizing lead placement in patients using non-invasive imaging and custom fabricated guides can improve efficacy[64, 65].
SNS does not appear to have similar efficacy for slow-transit constipation (STC). Randomized controlled trials failed to show effect of SNS (0.2–0.5ms, 10–14Hz) on increasing bowel movements[66, 67], although a minority of patients did see a sustained benefit. One case report found that when SNS was used to treat FI in a patient with overlapping FI and STC, both conditions improved, suggesting a unique etiology[68]. Pilot studies found that SNS is effective in treating children with constipation due to slow transit, anatomical malformations, and Hirschsprung’s disease[69, 70]. A significant placebo effect was noted in all trials[67], and in non-responders, modifying stimulation parameters did not alter efficacy[69]. Similar to the large inter-subject variability of GES, this placebo effect highlights the importance of patient stratification by disease etiology. FGIDs like constipation and dysmotility can arise from different conditions. Knowledge of the pathophysiology of each can better inform SNS. Using temporary less-invasive SNS as a predictor for implanted SNS, much like tGES, may also benefit patients.
In addition to its effect on motility, SNS can activate spinal afferents and vagal efferents[71]. SNS (0.2ms, 14Hz) increased gastric accommodation[72] and improved barrier function, reducing inflammation, in rats[73]. SNS was used to treat ulcerative proctitis and low anterior resection syndrome in open-label pilot studies[74, 75]. A randomized, controlled trial showed that SNS reduced irritable bowel syndrome (IBS) symptoms by more than 50% after 3 years[76].
A significant drawback of SNS is loss of efficacy over time. This can occur due to scar tissue formation and subsequent increase in tissue impedance surrounding the implant and the development of physiologic tolerance. This is treated by modifying lead placement, replacing leads, or increasing stimulus amplitude. For example, increasing frequency from 14 to 31 Hz restored efficacy in patients implanted with SNS[77]. Device replacement and revisions are also common, including removal for MRI scans[78]. Decreasing the invasiveness of SNS could decrease scarring and improve outcomes. New rechargeable, remote-controlled systems aim to increase lifespan beyond the current SNS system’s approximately 9 years[79]. Axonics has developed a wirelessly-rechargeable, MRI-compatible, SNS system for FI with functional life of 15 years. Non-invasive approaches for SNS include transcutaneous interferential electrical nerve stimulation (TIENS), which an open-label pilot study reported to treat STC in children. Such techniques are valuable alternatives to surgically implanted SNS[80].
Spinal cord
Spinal cord stimulation (SCS) is FDA-approved for pain management, and holds potential for GI Modulation. While there exist limited studies on the role of SCS for GI modulation, a recent report highlighted that SCS (0.2 and 0.5ms, 20 Hz, 2s on, 3s off) at the T10 vertebra in rats increased gastric motility by reversing autonomic dysfunction, inhibiting sympathetic activity and enhancing vagal activity[81]. Access to spinal nerves could enable neuromodulation complementary to VNS. Similar to VNS, less invasive access to the spinal cord and techniques to stimulate specific nerve fiber would significantly enhance relevance as therapy for GI disorders.
Deep brain stimulation
Brain stimulation can modulate motility and metabolic circuits to treat GI and GI-related disorders. In obese rats, deep brain stimulation (DBS; 0.1ms, 150μA, 130Hz, 1hr/day, 15 days) of the nucleus accumbens (NAc-DBS) and hypothalamus (Hyp-DBS), respectively, reduced food intake and increased resting energy expenditure[82]. NAc-DBS (90μs, 0.5mA, 130Hz) in diet-induced obese rats increased dopamine levels and reduced energy intake and weight gain[83].
DBS (60–90μs, 60Hz) of the lateral hypothalamus (LH-DBS) in two obese individuals refractory to bariatric surgery increased resting metabolic rate[84]. An ongoing open-label trial is using DBS of the ventromedial hypothalamus (VMH-DBS, 90μs, 50Hz) in obese individuals for weight loss and basal metabolism modification[85]. LH-DBS (91μs, 3.5mA, 130Hz) was not successful in treating patients with genetic obesity, however, as seen in Prader-Willi syndrome [86]. This highlights that DBS acts through specific neurohormonal pathways that may not be relevant for some disease etiologies. Similar to GES and SNS, patient stratification is critical.
The effects of DBS of the subthalamic nucleus (STN-DBS) on GI physiology has largely been studied in the context of Parkinson’s disease (PD) patients, who often suffer from GI dysmotility. STN-DBS enhanced esophageal motility and LES opening[87], and restored voluntary control of anorectal motility[88] in randomized cross-over trials, and improve gastric motility in a pilot study[89]. While these studies provide insight into brain-GI interconnectivity, they should be interpreted with caution as treatment options for non-PD patients, given complexities of PD pathophysiology that could influence GI motility.
The invasiveness of DBS precludes its use for most GI disorders. However, non-invasive (not skin or skull penetrating) modalities for DBS could also treat obesity by modulating behavior. In a randomized, double-blind, sham-controlled trial, direct transcranial magnetic stimulation (dTMS) of the prefrontal cortex and insula (18Hz) over a year enhanced weight loss in obese individuals when combined with physical exercise and diet[90]. dTMS is FDA-approved for the treatment of depression and OCD. Transcranial direct current stimulation (tDCS) (2mA) reduced food consumption and enhanced weight loss in obese females, one randomized double blinded, sham-controlled trial found[91]. This effect, however, was more prevalent in individuals with certain psychological traits, and another study illustrated a significant placebo effect when participants were told about possible effects of tDCS in reducing intake[92]. Unlike dTMS, tDCS remains an experimental therapy in the US, although it is approved in Europe and other countries for depression and pain.
Next-generation GI electroceuticals
Electroceuticals offers an attractive alternative to traditional pharmacotherapy, often with greater spatiotemporal resolution. They are easily transferrable between different organs, unlike new drugs, which require significant resources to optimize for specific targets. We describe crucial milestones that must be achieved for the widespread implementation of GI electrotherapy.
Towards a Mechanistic understanding
Understanding Interconnected Networks
The pathophysiology of GI disease is complex, involving multiple feedback loops under neuronal and hormonal control, and is not yet fully understood. In the last decade, tools such as optogenetics have allowed for dissection of neural circuits and led to a significantly more nuanced understanding of the role of the brain in motility and metabolic disorders, as well as the role of the GI tract in the pathogenesis of neurologic disorders. Specific brain regions express hormonal receptors, suggesting that gut-brain communication is both neuronal and hormonal[93]. Intestinal cells directly synapse onto the central nervous system to transmit information about ingested food[94]. Involvement of higher order dopaminergic reward circuits in the brain during eating has led us to re-think the neurologic basis for metabolic disorders[95].
Tailoring Therapy by Patient Stratification
Improving our understanding of disease will allow us to better stratify patients. Studies have shown that despite similar symptoms in gastroparesis (GP), for example, different etiologies can distinguish success rates[96, 97]. Thus, knowledge of patient-specific pathophysiology would allow us to stratify patients by etiology (e.g. post-surgical, diabetic, or idiopathic GP). This is challenging as FGIDs are diagnosed based on functional tests such as gastric emptying and intestinal transit time studies, and rated based on subjective, symptomatic scores (e.g. Gastroparesis Cardinal Symptom Index (GCSI)). New tools are needed to more directly evaluate functional tissue pathology in these conditions.
Optimizing electrical stimulation parameters
Understanding how electrical stimuli travel and influence specific cells and circuits would better inform selection of stimulation sites, which is done empirically. Computation and in silico models and simulations are beneficial here[98]. In addition, while we have a generalized understanding of the physiological effects of electrical stimulation parameters (pulse width, shape, amplitude, total stimulation time), greater empirical studies utilizing quantitative metrics are required. The sheer number of permutations render this challenging. For example, few studies have attempted non-square electrical pulses for GI stimulation, despite potential benefit of other shapes in delivering the same total energy with less potential for over-stimulation[99]. Techniques to enable quick, quantitative feedback on efficacy could enable patient-specific parameter tuning.
Assessing efficacy through quantitative metrics
How might we evaluate functionality and efficacy in vivo without relying on long-term symptomatic metrics? Minimally invasive recording electrodes, for example, could quickly indicate if a treatment is working and therapeutic fibers are being adequately stimulated or blocked, and if not, adjust parameters accordingly. From this we could also detect instances of device failure, placement inaccuracies, or excessive scarring impeding functionality, and address the large variations in human studies. Devices for more accurate neural recordings would also aid in de-coupling interdependent neuronal and muscle signals in the GI tract[100]. Tools that can evaluate gut motility non-invasively would help in assessing the effects of various interventions[101]. Direct assessment of peristalsis or muscle contraction would be beneficial in combination with currently-used metrics of transit times as proxies of motility.
Such approaches would also be beneficial in preclinical studies, where subjective self-evaluation of subjects is not possible. Selection of ideal animal models for GI diseases is outside the scope of this review. However, larger animals are beneficial in avoiding engineering challenges of scaling down technologies smaller than needed for human use.
Overcoming power barriers
Developing high-energy, miniaturized stimulators remains a primary limitation to long pulse-width stimulation. Energy harvesting and wireless charging techniques in development aim to address this[102]. Power-harvesting ingestible devices with piezoelectric, triboelectric, and wireless charging capabilities have been developed by various groups, utilizing advances in materials and ultrathin electronics[103, 104]. Although chemical batteries that operate on gastric acid and intestinal juices[105] have been developed, none show adequate power harvesting for electrical stimulation. While wireless charging is used to power some clinical devices, none have been reported with sufficient capacity to power high energy electrical stimulators in a reasonable time frame and form factor for regular patient use.
Microbiome electrotherapeutics
One area that has remained largely unstudied to date is the impact of electrical stimulation on the intestinal microbiome. The microbiome can directly influence physiology through neuronal communication[106], and plays an important role in priming of the immune system. The microbiome also affects intestinal gases, which can influence gastrointestinal motility and disease[107]. The effects of electrical stimulation on the GI microbiome are likely to be mediated through motility, immune, and neuronal mechanisms. Unraveling these could enable electroceuticals to act as a microbiome therapeutic complementary to probiotics and orally ingested bacteria.
Reduced invasiveness with increased specificity
Efficacious electrotherapy requires tissue specificity. This makes invasive implantation of stimulators requisite, presenting a barrier to widespread adoption and implementation.
Ingestible Electronics
Oral access to the GIT has given rise to the field of ingestible electronics, swallowable capsules containing electronics (reviewed in[108]), for sensing or drug delivery. Ingestibles could be used to deliver electrical stimulation, obviating the need for invasive surgeries for implantation. Alternatively, endoscopic delivery of devices presents another non-invasive option, similar to tGES[23], without forgoing tissue-specificity.
Ingestible devices would need to target and anchor to different portions of the GI tract, depending on indication. The GI tract is specifically tailored to process and excrete ingested materials. As such, achieving residency of an ingested device in specific portions without endoscopically-placed clips is extremely difficult. Gastric and intestinal-resident devices utilize a variety of mechanically-unfolding shapes, chemical adhesives, or biomimetic designs to attach to the mucosa[108, 109]. Navigation, sampling, and actuation capabilities have also been incorporated onto capsule endoscopes[110]. These techniques could be used for detection of medications, temperature, gases and delivery of drugs[108]. The varying pH environments in the GIT could be leveraged to aid device deployment in a specific region.
Electronic-enabled therapies
We have limited our definition of electroceuticals to electrical stimuli, however, electrical functionalities in ingestibles also present a huge potential for multi-modal functionality and combination therapies. Electrically-actuated payload release could tailor the location of drug delivery, or perhaps increase local permeability of the intestine to enhance drug uptake[111]. Electrically-powered phototherapy is efficacious in the treatment of Helicobacter pylori infection, and could allow for optogenetic ENS excitation[112]. Ingestible electronic capsules can remotely sense various gases in the gut[113]. Combining electronics with synthetic biology is also a powerful combination to make use of customs biologic agents as sensors, to enable closed-loop electrical stimulation and enhancing temporal specificity[114]. While such ingestible devices may not be able to directly stimulate non-GI tissues or nerves, they would permit access to the entire length of the GIT.
Targeted Neuromodulation
Neuromodulation specificity can also be significantly improved. Despite VNS’ successes, off-target effects remain and will doubtlessly be uncovered as long-term follow-up studies on patients receiving VNS continue. Use of subdermal electrodes for VNS can minimize invasiveness. Applying neuromodulation to smaller nerve branches can increase specificity while decreasing toxicity[115]. Modulating organ-specific nerves can affect organ function. Stimulation of the pancreatic nerve, for example, can inhibit autoimmune diabetes[116]. Optogenetic approaches can target nerves and tissues with even greater specificity by targeting individual fibers (reviewed in [117]). This necessitates, however, viral transfection, a major limitation to clinical translation.
Non-invasive approaches for neuromodulation are being tested in numerous clinical trials, and could increase accessibility to treatment (Table 1). Auricular VNS, delivered through an ear-mounted device, is being tested for brain and peripheral neuromodulation[118], and is FDA-approved for opioid withdrawal. Noninvasive ultrasound could be directed at specific organs such as the spleen, for example, to decrease inflammation as therapy for rheumatoid arthritis[119, 120]. Recent clinical trials are testing transcutaneous stimulation and electroacupuncture using subdermal electrodes for FGIDs and inflammatory conditions (Table 1).
Table 1.
Recent and ongoing clinical trials involving electrical stimulation for gastrointestinal and related disorders. All trials registered with ClinicalTrials.gov with unique National Clinical Trial (NCT) number.
| Stimulation Site | Conditions | NCT | Intervention |
|---|---|---|---|
| Mouth | Gingival Recession | 2987231 | Vestibular Gingival Stimulation |
| Esophagus | Malnutrition | 2515123 | Esophageal Stimulation (E-Motion System) |
| GERD | 2749071 | Lower Esophageal Sphincter Stimulation (Endostim® System) | |
| Dysphagia | 3476265 | Lower Esophageal Sphincter Stimulation | |
| GERD | |||
| Stomach | Abdominal Pain | 4121325 | Gastric Electrical Stimulation (GES) (Enterra™ System) |
| Gastroparesis | 3123809 | Gastric Electrical Stimulation (GES) (Enterra™ System) | |
| Obesity | 3734081 | ODC System (Type 1 Capsule)|Device: ODC System (Type 2 Capsule) | |
| Abdomen | Neurogenic Bowel | 4307303 | Abdominal Functional Electrical Stimulation |
| GERD | 2500264 | Transcutaneous Electrical Nerve Stimulation (TENS) | |
| Dysmotility | 3316105 | Transcutaneous Electrical Nerve Stimulation (TENS) (Elira System) | |
| Obesity | |||
| Acustimulation | Gastroparesis | 4349891 | Transcutaneous Electroacupuncture |
| Postoperative Ileus | 3222557 | Transcutaneous Electroacupuncture | |
| Postoperative Intestinal Obstruction | 3086304 | Transcutaneous Electroacupuncture | |
| Scleroderma | 3294616 | Transcutaneous Electroacupuncture | |
| Gastroparesis | |||
| Vagus Nerve | Inflammatory Bowel Disease | 3953768 | Vagal Nerve Stimulation (VNS) |
| Inflammatory Bowel Disease | 3863704 | Transcutaneous Electrical Nerve Stimulation (TENS) | |
| Dyspepsia | 3603730 | Transcutaneous Auricular Vagal Nerve Stimulation (taVNS) | |
| Gastroparesis | |||
| Irritable Bowel Syndrome | 2420158 | Transcutaneous Vagal Nerve Electrical Stimulation | |
| Gastroparesis | 3120325 | ||
| Functional Gastrointestinal Disorders | 3675321 | Active Auricular Neurostimulation | |
| Functional Gastrointestinal Disorders | 4247100 | Transcutaneous Electrical Nerve Stimulation (TENS) | |
| Brain | Functional Dyspepsia | 3869372 | Repetitive Transcranial Magnetic Stimulation (rTMS) |
| Irritable Bowel Syndrome | |||
| Obesity | 3943979 | Transcranial Direct-Current Stimulation (tDCS) | |
| Diabetes | |||
| Vestibular Nerve | Obesity | 3138382 | Vestibular Nerve Stimulator |
| Metabolic Syndrome |
Concluding Remarks
The rise of electroceuticals as a new therapeutic modality could revolutionize GI therapies. The use of electrical, as opposed to chemical, stimuli enables high spatial (organ-specific) and temporal (seconds to minutes) control. Achieving full translational potential will involve further development and collaborations across multiple fields from gastroenterology, neuroscience, and engineering in tackling issues that remain (see Outstanding Questions). These include gaining mechanistic insight to guide optimal stimulation parameters and placement, developing new non-invasive approaches to target specific nerve bundles in tissues with fewer off-target effects, and using improved metrics to stratify patients most likely to benefit from electroceutical therapies. Electroceuticals could soon establish a new paradigm in medicine, and a vital toolkit for the research and treatment of diverse diseases and disorders.
Outstanding questions.
What is the optimal placement and parameter for electrical stimulation to selectively modulate neural, hormonal, and muscle signaling along the gastrointestinal tract?
How can we reduce the invasiveness of current stimulation therapies?
What platforms might we develop that allow for orally ingested electrical pills, and how do we power such pills appropriately?
What is the effect of electrical stimulation on the GI microbiome?
How can we enhance the spatial resolution of non-invasive, transcutaneous stimulation approaches of nerve and tissue?
How can we target specific bundles within the vagus nerve to reduce off-target effects?
What are the various circuits of the enteric nervous system and how might we target circuits specifically?
What metrics can we use to better stratify patients with GI disease to predict electroceutical therapy efficacy?
Text Box 2. Electrical Stimulation Parameters [text box]
Electrical stimuli of tissue can take various forms (Box 1, Figure 1A). The basic elements of an electrical stimulus are amplitude, pulse width, pulse shape, frequency, and duty cycle (Box 1, Figure 1B). Stimulation can be current-controlled or voltage-controlled. Amplitude is determined by the maximum current or voltage applied. Pulse width is the length of time the pulse is applied. The vast majority of electrical stimulation utilizes square pulses. Frequency and duty cycle together determine the timing and number of pulses delivered. Varying these parameters can influence the effect of the electrical stimulus. Short pulse widths (up to 1ms) can be used for neuronal excitation while longer pulses widths (up to 1 sec) can induce muscle contractions[124]. Higher amplitude pulses (>10mA) are often used for cauterizing procedures, as they can lesion tissue. Varying frequency and duty cycle can determine the periodicity of stimulation, and allow for a refractory period where target tissues can reset prior to re-excitation. In nerve block therapy, high frequency stimulation is intentionally used to block signal transmission through nerve bundles.
Other factors that determine stimulation parameters are the location of stimulation and delivery mechanism. Most electrode leads for the GI tract are directly implanted into muscle tissue. Intraneural electrodes interface with nerves, reducing the amount of tissue stimuli must traverse. This enables excitation at lower amplitudes. How the stimulation is applied is influenced by the power supply available, conductivity of the electrodes, impedance of the tissue, anatomical access, and voltage compliance of the stimulator. Most implanted pulse generators are expected to function over 5+ years. However, current battery technologies do not allow for always-on long pulse width stimulation over such a long lifetime. Acute stimulation electrodes can be connected to benchtop power supplies, eliminating battery restrictions on stimulation.
Box 2, Figure 1.
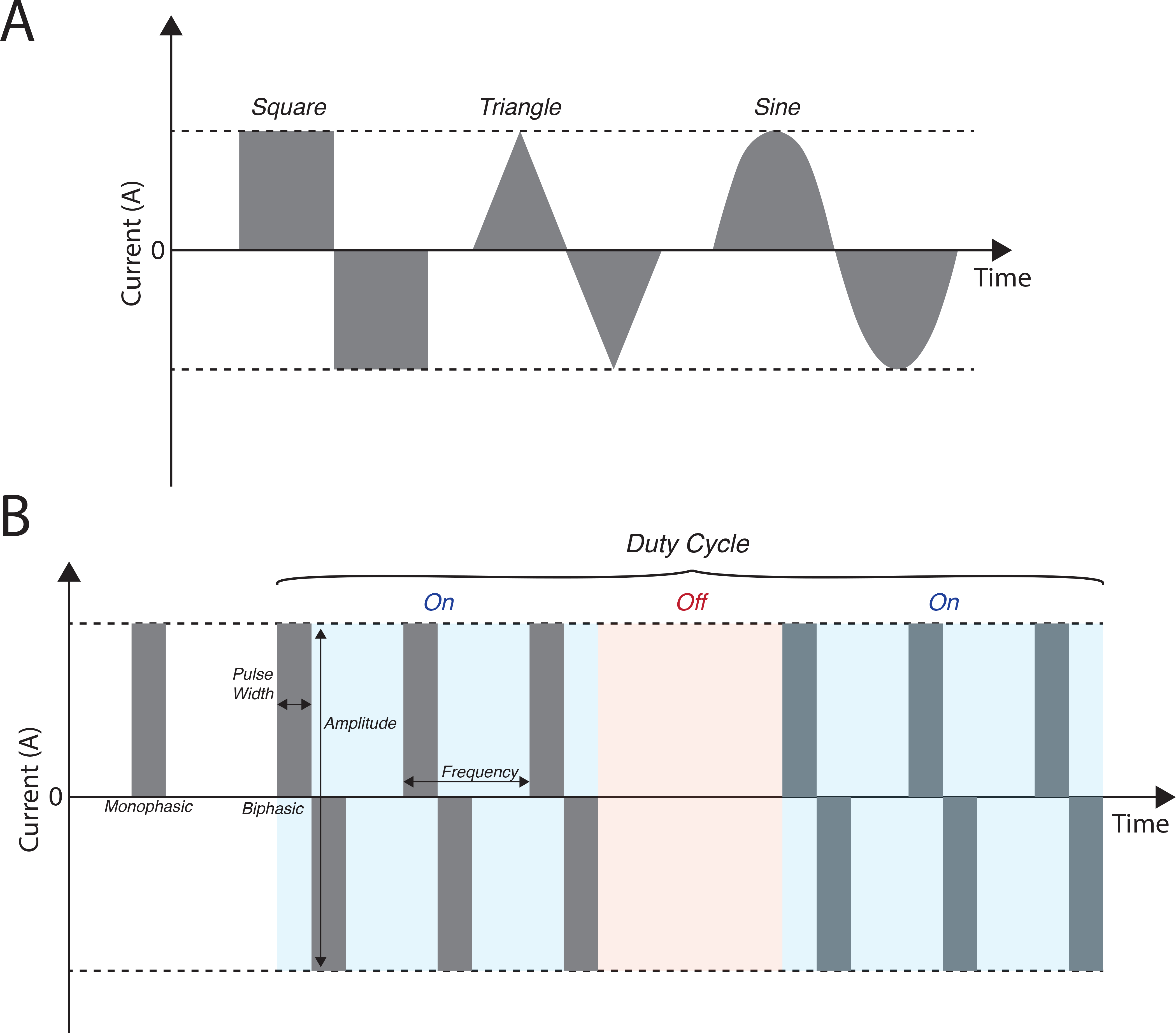
Illustration of electrical pulse stimuli and related parameters. (A) Square, triangle, and sine wave formats. (B) Parameters of electrical stimuli that can be tuned.
Acknowledgements
K.B.R. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under Award Number F32DK122762. S.S. was supported by the Schmidt Science Fellows program.
G.T. was supported in part by the Karl van Tassel (1925) Career Development Professorship, MIT, Department of Mechanical Engineering, MIT, a grant from Novo Nordisk, NIH Grant EB000244, and Grant INV-002177 from the Bill & Melinda Gates Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary
- Achalasia
Dysfunction of esophageal peristalsis
- Deep Brain Stimulation (DBS)
A technique for stimulation of brain regions through chronically implanted electrodes. DBS is FDA-approved for Parkinson’s disease, dystonia, obsessive-compulsive disorder, and epilepsy
- Deep Transcranial Magnetic Stimulation (dTMS)
A technique for brain stimulation using a sequence of applied magnetic field pulses
- Dysphagia
Difficulty swallowing foods or liquids
- Electroencephalography (EEG)
A method for recording brain waves through a series of acutely placed scalp electrodes
- Enteric nervous system (ENS)
The collection of neural circuits within the GI tract
- Gastroesophageal reflux disease (GERD)
Abnormal backflow of acidic contents of the stomach into the esophagus where it damages the mucosal lining, commonly due to relaxation of the esophageal sphincter
- Gastroparesis (GP)
A condition arising from delayed gastric emptying of contents to the duodenum. GP can result in bloating, infection, and recurrent nausea and vomiting. While the pathophysiology of GP is not understood, it is more common in diabetics, suggesting a role for neuropathy in its etiology. GP can also arise from malignancies, surgeries, or be idiopathic
- GLP-1
A insulinotropic hormone produced by intestinal enteroendocrine cells and neurons in the nucleus tractus solitarii (NTS). GLP-1 acts to decrease blood glucose through enhancing insulin secretion, and has been shown to suppress food intake and induce weight loss in humans
- Hirschsprung’s disease
A congenital disorder characterized by lack of adequate neural innervation of the colon, resulting in constipation, vomiting, abdominal pain, diarrhea
- Ileus
A disorder that results in intestinal dysmotility, common after abdominal or pelvic surgery
- Inflammatory bowel disease (IBD)
A group of disorders of the GI tract characterized by chronic inflammation. These include ulcerative colitis and Crohn’s disease
- Interferential Electrical Stimulation
A non-invasive method of electrical stimulation achieved by application of paired electrodes that deliver alternating current of medium (150 Hz) and high (4,000 Hz) frequencies
- Irritable bowel syndrome (IBS)
A chronic disorder of the large intestine that is characterized by symptoms including cramping, abdominal pain, bloating, gad, diarrhea, and constipation
- Lower anterior resection syndrome
A constellation of symptoms that may arise after low anterior resection surgery. These include fecal incontinence, constipation, frequency, urgency, and pain
- Neuropeptide Y (NPY)
A neuropeptide secreted by neurons of the sympathetic nervous system and hypothalamus, believed to regulate food intake, metabolism, anxiety, blood pressure, and circadian rhythm
- Orexin
A neuropeptide secreted by the hypothalamus and perifornical area of the brain, that regulates appetite, arousal, and wakefulness
- Piezoelectricity
The accumulation of electrical charge that occurs in certain materials due to applied mechanical stress
- Prader-Willi syndrome
A genetic disorder that leads to obesity and slow cognitive and physical development
- Transcutaneous direct current stimulation (tDCS)
A technique for neuromodulation utilizing low currents delivered through scalp electrodes
- Vagus Nerve
A primary conduit of neuronal signaling between organs and the brain. (Also known as Cranial Nerve X
- Vagus nerve stimulation (VNS)
Electrical stimulation of the vagus nerve. VNS can be transcutaneous, or invasive using implanted cuff electrodes around the vagus nerve. Different branches of the vagus nerve can be targeted (e.g. cervical, abdominal, auricular)
Footnotes
Disclaimer Statement
G.T. is a co-founder and has a financial interest in Lyndra Therapeutics, Celero Systems, Suono Bio, which are biotechnology companies developing a broad set of systems for the gastrointestinal tract for sensing and drug delivery applications which in some embodiments include electronics. All other authors declare no competing interests. Complete details of all relationships for profit and not for profit for G.T. can be found at the following link: https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0
Resources
References
- 1.Aristotle, History of Animals. 1993: Harvard University Press. [Google Scholar]
- 2.Jungheim M, et al. , Impact of neuromuscular electrical stimulation on upper esophageal sphincter dynamics: a high-resolution manometry study. Ann Otol Rhinol Laryngol, 2015. 124(1): p. 5–12. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez L, et al. , Electrical stimulation therapy of the lower esophageal sphincter is successful in treating GERD: long-term 3-year results. Surg Endosc, 2016. 30(7): p. 2666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, et al. , Study on effects of electrical stimulation on rabbit esophageal body motility in vivo. Physiol Res, 2018. 67(2): p. 275–282. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE, et al. , Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol, 2015. 13(3): p. 466–476.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abell TL, et al. , Effectiveness of gastric electrical stimulation in gastroparesis: Results from a large prospectively collected database of national gastroparesis registries. Neurogastroenterol Motil, 2019. 31(12): p. e13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ducrotte P, et al. , Gastric Electrical Stimulation Reduces Refractory Vomiting in a Randomized Crossover Trial. Gastroenterology, 2020. 158(3): p. 506–514 e2. [DOI] [PubMed] [Google Scholar]
- 8.Schiemer JF, et al. , Five-fold Gastrointestinal Electrical Stimulation With Electromyography-based Activity Analysis: Towards Multilocular Theranostic Intestinal Implants. J Neurogastroenterol Motil, 2019. 25(3): p. 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, et al. , Long-Pulse Gastric Electrical Stimulation Repairs Interstitial Cells of Cajal and Smooth Muscle Cells in the Gastric Antrum of Diabetic Rats. Gastroenterol Res Pract, 2018. 2018: p. 6309157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang N, et al. , Gastric electrical stimulation improves enteric neuronal survival. Int J Mol Med, 2017. 40(2): p. 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan Y, et al. , Chronic gastric electrical stimulation leads to weight loss via modulating multiple tissue neuropeptide Y, orexin, alpha-melanocyte-stimulating hormone and oxytocin in obese rats. Scand J Gastroenterol, 2016. 51(2): p. 157–67. [DOI] [PubMed] [Google Scholar]
- 12.Song GQ, et al. , Gastric electrical stimulation optimized to inhibit gastric motility reduces food intake in dogs. Obes Surg, 2015. 25(6): p. 1047–55. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, et al. , The effects of individualized gastric electrical stimulation on food craving and gastrointestinal peptides in dogs. Neuromodulation, 2014. 17(5): p. 483–8; discussion 488–9. [DOI] [PubMed] [Google Scholar]
- 14.Payne SC, Furness JB, and Stebbing MJ, Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nature Reviews Gastroenterology & Hepatology, 2019. 16(2): p. 89–105. [DOI] [PubMed] [Google Scholar]
- 15.Horn CC, et al. , Impact of electrical stimulation of the stomach on gastric distension-induced emesis in the musk shrew. Neurogastroenterology and Motility, 2016. 28(8): p. 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boules M, et al. , Pre-operative opioid analgesia reduces clinical success of laparoscopic gastric electrical stimulation placement in patients with gastroparesis. Surg Endosc, 2015. 29(4): p. 805–9. [DOI] [PubMed] [Google Scholar]
- 17.Yu X, et al. , Antiemesis effect and brain fMRI response of gastric electrical stimulation with different parameters in dogs. Neurogastroenterol Motil, 2014. 26(7): p. 1049–56. [DOI] [PubMed] [Google Scholar]
- 18.Alarcon Del Agua I, et al. , Post-implant Analysis of Epidemiologic and Eating Behavior Data Related to Weight Loss Effectiveness in Obese Patients Treated with Gastric Electrical Stimulation. Obes Surg, 2017. 27(6): p. 1573–1580. [DOI] [PubMed] [Google Scholar]
- 19.Lebovitz HE, et al. , Gastric electrical stimulation treatment of type 2 diabetes: effects of implantation versus meal-mediated stimulation. A randomized blinded cross-over trial. Physiol Rep, 2015. 3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horbach T, et al. , Closed-loop gastric electrical stimulation versus laparoscopic adjustable gastric band for the treatment of obesity: a randomized 12-month multicenter study. Int J Obes (Lond), 2016. 40(12): p. 1891–1898. [DOI] [PubMed] [Google Scholar]
- 21.Morales-Conde S, et al. , Implanted Closed-Loop Gastric Electrical Stimulation (CLGES) System with Sensor-Based Feedback Safely Limits Weight Regain at 24 Months. Obes Surg, 2018. 28(6): p. 1766–1774. [DOI] [PubMed] [Google Scholar]
- 22.Yao S, et al. , Visceral sensitivity to gastric stimulation and its correlation with alterations in gastric emptying and accommodation in humans. Obes Surg, 2005. 15(2): p. 247–53. [DOI] [PubMed] [Google Scholar]
- 23.Corvinus FM, et al. , Minimally-invasive temporary gastric stimulation: A pilot study to predict the outcome of electronic gastric stimulation with the Enterra (TM) system. Digestive and Liver Disease, 2018. 50(10): p. 1030–1034. [DOI] [PubMed] [Google Scholar]
- 24.Hu YD, et al. , Ameliorating Effects and Autonomic Mechanisms of Transcutaneous Electrical Acustimulation in Patients With Gastroesophageal Reflux Disease. Neuromodulation, 2019: p. 8. [DOI] [PubMed] [Google Scholar]
- 25.Guo WC and Wang F, Effect of nerve electrical stimulation for treating chemotherapy-induced nausea and vomiting in patients with advanced gastric cancer: A randomized controlled trial. Medicine (Baltimore), 2018. 97(51): p. e13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S and Chen JD, Pulse Width-Dependent Effects of Intestinal Electrical Stimulation for Obesity: Role of Gastrointestinal Motility and Hormones. Obes Surg, 2017. 27(1): p. 70–77. [DOI] [PubMed] [Google Scholar]
- 27.Ye F, et al. , Hypoglycemic Effects of Intestinal Electrical Stimulation by Enhancing Nutrient-Stimulated Secretion of GLP-1 in Rats. Obes Surg, 2018. 28(9): p. 2829–2835. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang X, et al. , Intestinal electrical stimulation attenuates hyperglycemia and prevents loss of pancreatic beta cells in type 2 diabetic Goto-Kakizaki rats. Nutr Diabetes, 2019. 9(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval D, et al. , Impact of intestinal electrical stimulation on nutrient-induced GLP-1 secretion in vivo. Neurogastroenterol Motil, 2013. 25(8): p. 700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, et al. , Diffused and sustained inhibitory effects of intestinal electrical stimulation on intestinal motility mediated via sympathetic pathway. Neuromodulation, 2014. 17(4): p. 373–79; discussion 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, et al. , Colonic electrical stimulation promotes colonic motility through regeneration of myenteric plexus neurons in slow transit constipation beagles. Biosci Rep, 2019. 39(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, et al. , Implantable Colonic Electrical Stimulation Improves Gastrointestinal Transit and Defecation in a Canine Constipation Model. Neuromodulation, 2016. 19(1): p. 108–15. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, et al. , Effects of colonic electrical stimulation using different individual parameter patterns and stimulation sites on gastrointestinal transit time, defecation, and food intake. Int J Colorectal Dis, 2016. 31(2): p. 429–37. [DOI] [PubMed] [Google Scholar]
- 34.Bourbeau D, et al. , Electrical Colon Stimulation Reflexively Increases Colonic Activity. Neuromodulation, 2019. [DOI] [PubMed] [Google Scholar]
- 35.Qin XR, Tan Y, and Sun XN, Effect of retrograde colonic electrical stimulation on colonic transit and stress-induced visceral hypersensitivity in rats with irritable bowel syndrome. Asian Pac J Trop Med, 2017. 10(8): p. 827–832. [DOI] [PubMed] [Google Scholar]
- 36.Ladi-Seyedian SS, et al. , A comparative study of transcutaneous interferential electrical stimulation plus behavioral therapy and behavioral therapy alone on constipation in postoperative Hirschsprung disease children. J Pediatr Surg, 2017. 52(1): p. 177–183. [DOI] [PubMed] [Google Scholar]
- 37.Jensen AB, et al. , Increase in clinically recorded type 2 diabetes after colectomy. eLife, 2018. 7: p. e37420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan H and Silberstein SD, Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache, 2016. 56(1): p. 71–8. [DOI] [PubMed] [Google Scholar]
- 39.Yuan H and Silberstein SD, Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part II. Headache, 2016. 56(2): p. 259–66. [DOI] [PubMed] [Google Scholar]
- 40.Khajanchee YS, et al. , Electrical stimulation of the vagus nerve restores motility in an animal model of achalasia. J Gastrointest Surg, 2003. 7(7): p. 843–9; discussion 849. [DOI] [PubMed] [Google Scholar]
- 41.Lu KH, et al. , Vagus nerve stimulation promotes gastric emptying by increasing pyloric opening measured with magnetic resonance imaging. Neurogastroenterology and Motility, 2018. 30(10): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulon E, et al. , Proof of concept: short-term non-invasive cervical vagus nerve stimulation in patients with drug-refractory gastroparesis. Frontline Gastroenterol, 2017. 8(4): p. 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payne SC, et al. , Anti-inflammatory Effects of Abdominal Vagus Nerve Stimulation on Experimental Intestinal Inflammation. Frontiers in Neuroscience, 2019. 13: p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stakenborg N, et al. , Abdominal vagus nerve stimulation as a new therapeutic approach to prevent postoperative ileus. Neurogastroenterology and Motility, 2017. 29(9): p. 11. [DOI] [PubMed] [Google Scholar]
- 45.Bonaz B, et al. , Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil, 2016. 28(6): p. 948–53. [DOI] [PubMed] [Google Scholar]
- 46.Kibleur A, et al. , Electroencephalographic correlates of low-frequency vagus nerve stimulation therapy for Crohn’s disease. Clin Neurophysiol, 2018. 129(5): p. 1041–1046. [DOI] [PubMed] [Google Scholar]
- 47.Malbert CH, et al. , Obesity-Associated Alterations in Glucose Metabolism Are Reversed by Chronic Bilateral Stimulation of the Abdominal Vagus Nerve. Diabetes, 2017. 66(4): p. 848–857. [DOI] [PubMed] [Google Scholar]
- 48.Li S, et al. , Therapeutic effect of vagus nerve stimulation on depressive-like behavior, hyperglycemia and insulin receptor expression in Zucker fatty rats. PLoS One, 2014. 9(11): p. e112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malbert CH, et al. , Chronic abdominal vagus stimulation increased brain metabolic connectivity, reduced striatal dopamine transporter and increased mid-brain serotonin transporter in obese miniature pigs. Journal of Translational Medicine, 2019. 17: p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andermann ML and Lowell BB, Toward a Wiring Diagram Understanding of Appetite Control. Neuron, 2017. 95(4): p. 757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stauss HM, et al. , Cervical vagal nerve stimulation impairs glucose tolerance and suppresses insulin release in conscious rats. Physiol Rep, 2018. 6(24): p. e13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shikora SA, et al. , Intermittent Vagal Nerve Block for Improvements in Obesity, Cardiovascular Risk Factors, and Glycemic Control in Patients with Type 2 Diabetes Mellitus: 2-Year Results of the VBLOC DM2 Study. Obes Surg, 2016. 26(5): p. 1021–8. [DOI] [PubMed] [Google Scholar]
- 53.Chunchai T, et al. , Vagus Nerve Stimulation Exerts the Neuroprotective Effects in Obese-Insulin Resistant Rats, Leading to the Improvement of Cognitive Function. Sci Rep, 2016. 6: p. 26866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong GS, et al. , Effect of transcutaneous vagus nerve stimulation on muscle activity in the gastrointestinal tract (transVaGa): a prospective clinical trial. International Journal of Colorectal Disease, 2019. 34(3): p. 417–422. [DOI] [PubMed] [Google Scholar]
- 55.Teckentrup V, et al. , Non-invasive stimulation of vagal afferents reduces gastric frequency. Brain Stimulation, 2020. 13(2): p. 470–473. [DOI] [PubMed] [Google Scholar]
- 56.Fang JF, et al. , Electroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammation. Scientific Reports, 2017. 7: p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Ferrari GM, et al. , Long-term vagal stimulation for heart failure: Eighteen month results from the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) trial. Int J Cardiol, 2017. 244: p. 229–234. [DOI] [PubMed] [Google Scholar]
- 58.Meyers EE, et al. , Contrasting effects of afferent and efferent vagal nerve stimulation on insulin secretion and blood glucose regulation. Physiol Rep, 2016. 4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Altomare DF, et al. , Long-term outcomes of sacral nerve stimulation for faecal incontinence. Br J Surg, 2015. 102(4): p. 407–15. [DOI] [PubMed] [Google Scholar]
- 60.Wexner SD, et al. , Sacral Nerve Stimulation for Fecal Incontinence: Results of a 120-Patient Prospective Multicenter Study. Annals of Surgery, 2010. 251(3): p. 441–449. [DOI] [PubMed] [Google Scholar]
- 61.Haas S, et al. , Does Sacral Nerve Stimulation Improve Continence Through Enhanced Sensitivity of the Anal Canal? A Pilot Study. Dis Colon Rectum, 2016. 59(11): p. 1039–1046. [DOI] [PubMed] [Google Scholar]
- 62.Butt SK, et al. , Lack of effect of sacral nerve stimulation for incontinence in patients with systemic sclerosis. Colorectal Dis, 2015. 17(10): p. 903–7. [DOI] [PubMed] [Google Scholar]
- 63.Duelund-Jakobsen J, et al. , Bilateral compared with unilateral sacral nerve stimulation for faecal incontinence: results of a randomized, single-blinded crossover study. Colorectal Dis, 2015. 17(12): p. 1085–93. [DOI] [PubMed] [Google Scholar]
- 64.Rubio-Perez I and Diaz Lantada A, Surgical Planning of Sacral Nerve Stimulation Procedure in Presence of Sacral Anomalies by Using Personalized Polymeric Prototypes Obtained with Additive Manufacturing Techniques. Polymers (Basel), 2020. 12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castillo J, et al. , Sacral nerve stimulation lead implantation in partial sacral agenesis using intra-operative computerized tomography. Colorectal Dis, 2016. 18(9): p. O330–3. [DOI] [PubMed] [Google Scholar]
- 66.Dinning PG, et al. , Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two-phase, double-blind randomized controlled crossover study. Am J Gastroenterol, 2015. 110(5): p. 733–40. [DOI] [PubMed] [Google Scholar]
- 67.Zerbib F, et al. , Randomized clinical trial of sacral nerve stimulation for refractory constipation. Br J Surg, 2017. 104(3): p. 205–213. [DOI] [PubMed] [Google Scholar]
- 68.Sreepati G and James-Stevenson T, Use of Sacral Nerve Stimulation for the Treatment of Overlapping Constipation and Fecal Incontinence. Am J Case Rep, 2017. 18: p. 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu PL, et al. , Sacral nerve stimulation for constipation and fecal incontinence in children: Long-term outcomes, patient benefit, and parent satisfaction. Neurogastroenterol Motil, 2018. 30(2). [DOI] [PubMed] [Google Scholar]
- 70.Besendörfer M, et al. , A Pilot Study of Non-invasive Sacral Nerve Stimulation in Treatment of Constipation in Childhood and Adolescence. Frontiers in Pediatrics, 2020. 8(169). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tu L, et al. , Anti-inflammatory effects of sacral nerve stimulation: a novel spinal afferent and vagal efferent pathway. Am J Physiol Gastrointest Liver Physiol, 2020. 318(4): p. G624–G634. [DOI] [PubMed] [Google Scholar]
- 72.Ye F, et al. , Sacral nerve stimulation increases gastric accommodation in rats: a spinal afferent and vagal efferent pathway. Am J Physiol Gastrointest Liver Physiol, 2020. 318(3): p. G574–G581. [DOI] [PubMed] [Google Scholar]
- 73.Guo J, et al. , Sacral nerve stimulation improves colonic inflammation mediated by autonomic-inflammatory cytokine mechanism in rats. Neurogastroenterol Motil, 2019. 31(10): p. e13676. [DOI] [PubMed] [Google Scholar]
- 74.Bregeon J, et al. , Improvement of Refractory Ulcerative Proctitis With Sacral Nerve Stimulation. J Clin Gastroenterol, 2015. 49(10): p. 853–7. [DOI] [PubMed] [Google Scholar]
- 75.Eftaiha SM, et al. , Sacral nerve stimulation can be an effective treatment for low anterior resection syndrome. Colorectal Dis, 2017. 19(10): p. 927–933. [DOI] [PubMed] [Google Scholar]
- 76.Fassov J, et al. , Three-year follow-up of sacral nerve stimulation for patients with diarrhoea-predominant and mixed irritable bowel syndrome. Colorectal Dis, 2017. 19(2): p. 188–193. [DOI] [PubMed] [Google Scholar]
- 77.Duelund-Jakobsen J, et al. , Improved longevity and efficacy of sacral nerve stimulation by simple adjustments at follow-up. Colorectal Dis, 2020. 22(3): p. 310–318. [DOI] [PubMed] [Google Scholar]
- 78.Gevelinger MM, et al. , Evaluation of Sacral Nerve Stimulation Device Revision and Explantation in a Single Center, Multidisciplinary Study. Neuromodulation, 2019. [DOI] [PubMed] [Google Scholar]
- 79.Langlois L, et al. , Development of a Remote-Controlled Implantable Rat Sacral Nerve Stimulation System. Neuromodulation, 2019. 22(6): p. 690–696. [DOI] [PubMed] [Google Scholar]
- 80.Yik YI, Hutson J, and Southwell B, Home-Based Transabdominal Interferential Electrical Stimulation for Six Months Improves Paediatric Slow Transit Constipation (STC). Neuromodulation, 2018. 21(7): p. 676–681. [DOI] [PubMed] [Google Scholar]
- 81.Song GQ, et al. , Therapeutic potential of spinal cord stimulation for gastrointestinal motility disorders: a preliminary rodent study. Neurogastroenterol Motil, 2014. 26(3): p. 377–84. [DOI] [PubMed] [Google Scholar]
- 82.Casquero-Veiga M, et al. , Stimulating the nucleus accumbens in obesity: A positron emission tomography study after deep brain stimulation in a rodent model. PLoS One, 2018. 13(9): p. e0204740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang C, et al. , Deep brain stimulation of the nucleus accumbens shell induces anti-obesity effects in obese rats with alteration of dopamine neurotransmission. Neurosci Lett, 2015. 589: p. 1–6. [DOI] [PubMed] [Google Scholar]
- 84.Whiting AC, et al. , Deep Brain Stimulation of the Hypothalamus Leads to Increased Metabolic Rate in Refractory Obesity. World Neurosurg, 2019. 121: p. e867–e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Salles AAF, et al. , An Open-Label Clinical Trial of Hypothalamic Deep Brain Stimulation for Human Morbid Obesity: BLESS Study Protocol. Neurosurgery, 2018. 83(4): p. 800–809. [DOI] [PubMed] [Google Scholar]
- 86.Franco RR, et al. , Assessment of Safety and Outcome of Lateral Hypothalamic Deep Brain Stimulation for Obesity in a Small Series of Patients With Prader-Willi Syndrome. JAMA Netw Open, 2018. 1(7): p. e185275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Derrey S, et al. , Impact of deep brain stimulation on pharyngo-esophageal motility: a randomized cross-over study. Neurogastroenterol Motil, 2015. 27(9): p. 1214–22. [DOI] [PubMed] [Google Scholar]
- 88.Gourcerol G, et al. , Does Bilateral Deep Brain Stimulation of the Subthalamic Nucleus Modify Ano-Rectal Motility in Parkinson’s Disease? Results of a Randomized Cross-Over Study. Neuromodulation, 2019. 22(4): p. 478–483. [DOI] [PubMed] [Google Scholar]
- 89.Krygowska-Wajs A, et al. , The effect of subthalamic deep brain stimulation on gastric motility in Parkinson’s disease. Parkinsonism Relat Disord, 2016. 26: p. 35–40. [DOI] [PubMed] [Google Scholar]
- 90.Ferrulli A, et al. , Weight loss induced by deep transcranial magnetic stimulation in obesity: A randomized, double-blind, sham-controlled study. Diabetes Obes Metab, 2019. 21(8): p. 1849–1860. [DOI] [PubMed] [Google Scholar]
- 91.Usanos CA, et al. , Neuromodulation of the prefrontal cortex facilitates diet-induced weight loss in midlife women: a randomized, proof-of-concept clinical trial. International Journal of Obesity, 2020. 44(3): p. 568–578. [DOI] [PubMed] [Google Scholar]
- 92.Ray MK, et al. , The effect of expectation on transcranial direct current stimulation (tDCS) to suppress food craving and eating in individuals with overweight and obesity. Appetite, 2019. 136: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fortin SM, et al. , GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Science Translational Medicine, 2020. 12(533): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaelberer MM, et al. , A gut-brain neural circuit for nutrient sensory transduction. Science, 2018. 361(6408). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han W, et al. , A Neural Circuit for Gut-Induced Reward. Cell, 2018. 175(3): p. 665–678 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shah H, et al. , Treating an oft-unrecognized and troublesome entity: using gastric electrical stimulation to reduce symptoms of malignancy-associated gastroparesis. Support Care Cancer, 2017. 25(1): p. 27–31. [DOI] [PubMed] [Google Scholar]
- 97.Shen S, et al. , Gastric peroral endoscopic pyloromyotomy versus gastric electrical stimulation in the treatment of refractory gastroparesis: a propensity score-matched analysis of long term outcomes. Endoscopy, 2020. [DOI] [PubMed] [Google Scholar]
- 98.Barth BB, et al. , Electrical stimulation of gut motility guided by an &ITin silico &ITmodel. Journal of Neural Engineering, 2017. 14(6): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang JS and Paydarfar D, Optimizing stimulus waveforms for electroceuticals. Biological Cybernetics, 2019. 113(1–2): p. 191–199. [DOI] [PubMed] [Google Scholar]
- 100.Sperry ZJ, et al. , Flexible microelectrode array for interfacing with the surface of neural ganglia. Journal of Neural Engineering, 2018. 15(3): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berry R, et al. , A novel retractable laparoscopic device for mapping gastrointestinal slow wave propagation patterns. Surgical Endoscopy and Other Interventional Techniques, 2017. 31(1): p. 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang R, et al. , A Miniature Configurable Wireless System for Recording Gastric Electrophysiological Activity and Delivering High-Energy Electrical Stimulation. Ieee Journal on Emerging and Selected Topics in Circuits and Systems, 2018. 8(2): p. 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nadeau P, et al. , Prolonged energy harvesting for ingestible devices. Nature Biomedical Engineering, 2017. 1(3): p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bettinger CJ, Materials Advances for Next-Generation Ingestible Electronic Medical Devices. Trends in Biotechnology, 2015. 33(10): p. 575–585. [DOI] [PubMed] [Google Scholar]
- 105.Huang XY, et al. , A Fully Biodegradable Battery for Self-Powered Transient Implants. Small, 2018. 14(28): p. 8. [DOI] [PubMed] [Google Scholar]
- 106.Obata Y, et al. , Neuronal programming by microbiota regulates intestinal physiology. Nature, 2020. 578(7794): p. 284–289. [DOI] [PubMed] [Google Scholar]
- 107.Kalantar-Zadeh K, et al. , Intestinal gases: influence on gut disorders and the role of dietary manipulations. Nature Reviews Gastroenterology & Hepatology, 2019. 16(12): p. 733–747. [DOI] [PubMed] [Google Scholar]
- 108.Steiger C, et al. , Ingestible electronics for diagnostics and therapy. Nature Reviews Materials, 2019. 4(2): p. 83–98. [Google Scholar]
- 109.Xie WC, Kothari V, and Terry BS, A bio-inspired attachment mechanism for long-term adhesion to the small intestine. Biomedical Microdevices, 2015. 17(4): p. 9. [DOI] [PubMed] [Google Scholar]
- 110.Wu ZG, et al. , A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Science Robotics, 2019. 4(32): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu WY, et al. , A Smart Capsule With GI-Tract-Location-Specific Payload Release. Ieee Transactions on Biomedical Engineering, 2015. 62(9): p. 2289–2295. [DOI] [PubMed] [Google Scholar]
- 112.Tortora G, et al. , An Ingestible Capsule for the Photodynamic Therapy of Helicobacter Pylori Infection. Ieee-Asme Transactions on Mechatronics, 2016. 21(4): p. 1935–1942. [Google Scholar]
- 113.Kalantar-Zadeh K, et al. , A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nature Electronics, 2018. 1(1): p. 79–87. [Google Scholar]
- 114.Mimee M, et al. , An ingestible bacterial-electronic system to monitor gastrointestinal health. Science, 2018. 360(6391): p. 915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thompson N, Mastitskaya S, and Holder D, Avoiding off-target effects in electrical stimulation of the cervical vagus nerve: Neuroanatomical tracing techniques to study fascicular anatomy of the vagus nerve. Journal of Neuroscience Methods, 2019. 325: p. 108325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guyot M, et al. , Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nature Biotechnology, 2019. 37(12): p. 1446–+. [DOI] [PubMed] [Google Scholar]
- 117.Boesmans W, Hao MM, and Vanden Berghe P, Optogenetic and chemogenetic techniques for neurogastroenterology. Nature Reviews Gastroenterology & Hepatology, 2018. 15(1): p. 21–38. [DOI] [PubMed] [Google Scholar]
- 118.Frangos E, Ellrich J, and Komisaruk BR, Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimulation, 2015. 8(3): p. 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zachs DP, et al. , Noninvasive ultrasound stimulation of the spleen to treat inflammatory arthritis. Nature Communications, 2019. 10: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cotero V, et al. , Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation. Nature Communications, 2019. 10: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barrett KE, Gastrointestinal Physiology. Lange Medical Books. 2013: McGraw-Hill Education / Medical. 336. [Google Scholar]
- 122.Spencer NJ and Hu H, Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nature Reviews Gastroenterology & Hepatology, 2020. 17(6): p. 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Furness JB, The enteric nervous system and neurogastroenterology. Nature Reviews Gastroenterology & Hepatology, 2012. 9(5): p. 286–294. [DOI] [PubMed] [Google Scholar]
- 124.Lin Z and Chen JDZ, Developments in Gastrointestinal Electrical Stimulation. Crit Rev Biomed Eng, 2017. 45(1–6): p. 263–301. [DOI] [PubMed] [Google Scholar]


