Abstract
The optimal timing of clamping and cutting the umbilical cord at birth among infants with congenital heart disease (CHD) remains a subject of controversy and debate. The benefits of delayed umbilical cord clamping (DCC) among term infants without CHD are well described, but the evidence base for DCC among infants with CHD has not been characterized adequately. The goals of the present review are to: 1) compare outcomes of DCC versus early cord clamping (ECC) in term (≥37 weeks of gestation) infants; 2) discuss potential risk/benefit profiles in applying DCC among term infants with CHD; 3) use rigorous systematic review methodology to assess the quality and quantity of published reports on cord clamping practices among term infants with CHD; 4) identify needs and opportunities for future research and interdisciplinary collaboration. Our systematic review shows that previous trials have largely excluded infants with CHD. Therefore, the supposition that DCC is advantageous because it is associated with improved neurologic and hematologic outcome is untested in the CHD population. Given that CHD is markedly heterogeneous, to minimize unnecessary and potentially harmful cord clamping practices, identification of subgroups (single-ventricle, cyanotic lesions) most likely to benefit from optimal cord clamping practices is necessary to optimize risk/benefit profiles. The available evidence base suggests that contemporary, pragmatic, randomized controlled trials comparing DCC with ECC among infants with CHD are needed.
Keywords: delayed cord clamping, congenital heart disease
Introduction:
Clamping and cutting of the umbilical cord at birth is likely the oldest and most widespread medical intervention in humans (1). The American College of Obstetrics and Gynecology (ACOG), American Academy of Pediatrics (AAP), and American College of Nurse-Midwives, recommend delayed cord clamping (DCC) for healthy term (≥37 weeks of gestation) infants, but identified the need for a better understanding of optimal cord clamping practices among higher-risk subgroups (2). One subgroup of infants for whom DCC could be advantageous, but also could be problematic, is infants with congenital heart disease (CHD). The primary goals of the present review are to: 1) appraise available evidence on cord clamping practices among healthy, term infants, including recent evidence on longer-term neurodevelopmental outcomes; 2) discuss potential risk/benefit profiles in applying DCC among infants with CHD; 3) use rigorous systematic review methodology to assess the quality and quantity of published reports on the timing of umbilical cord clamping among infants with CHD; 4) identify opportunities for future research and interdisciplinary collaboration.
Available evidence on cord clamping practices among healthy, term infants:
Prior to 2000, cutting the umbilical cord within seconds following delivery was considered standard practice (2). However, over the past 2 decades, health care providers have reconsidered optimal cord clamping practices. At term gestation, as much as 60 percent of the combined fetal-placental blood volume is in the placental circulation (3). Early cord clamping (ECC) is defined as cutting the umbilical cord within 60 seconds following delivery; in contrast, DCC by 2–3 minutes following delivery and lowering the infant below the perineum (vaginal delivery) or incision site (Cesarean delivery) at the time of birth increases transfer of blood from the placenta to the infant, leading to increases in circulating blood volumes by ~30–40% (25–30 ml/kg) (4).
Hematological status:
A 2007 systematic review and meta-analysis by Hutton and Hassan comparing ECC versus DCC included data from 15 controlled trials among 1912 term newborns (3). The authors reported that DCC provided improved hematological status, including greater hematocrits at 2 months (weighted mean difference [WMD], 3.7%, 95% confidence interval [CI], 2.0%–5.4%), serum ferritin at 2–3 months (WMD 17.9 μg/L; 95% CI 16.6–19.2) and stored iron at 6 months (WMD, 19.9 mg; 95% CI, 7.7–32.1) than ECC (3). Additionally, the authors noted lower risk of iron-deficiency anemia (relative risk, 0.50; 95% CI 0.40–0.70) following DCC than with ECC (3). These observations were consistent with a more contemporary review by McDonald et al, wherein DCC was associated with lower exposure to red blood cell (RBC) transfusions than with ECC (risk ratio 1.02, 95% CI 0.44–2.37) (5).
Neurodevelopmental sequelae:
More recently, evidence on the potential neurodevelopmental benefits of DCC among term infants have been explored. A study by Mercer et al. reported that singleton term infants receiving DCC had, based on magnetic resonance imaging, greater myelin contents in brain regions involved in motor, visual/spatial, and sensory functions than did infants following ECC (6). Evidence of improved neuroimaging is consistent with recent studies showing greater neurocognitive performance following DCC than following ECC. Rana et al. reported that, among 540 full-term infants at 12 months of life, those who received DCC had higher scores on the Ages and Stages Questionnaire (ASQ), caregiver-based assessment, than did infants receiving ECC, in the domains of communication (adjusted mean difference [AMD] 0.50, 95% CI 0.10–0.90), personal-social (AMD 1.0, 95% CI 0.30–1.7), and gross motor (AMD 1.4, 95% CI 0.30–2.6) (7). In a subsequent follow-up study of infants at 4 years of age (N=263) those in the DCC group exhibited higher scores on the personal-social (AMD 2.8, 95% CI 0.80–4.7; P<0.01) and fine motor (AMD 2.1, 95% CI 0.20–4.0; P=0.03) domains from the Ages and Stages Questionnaire than did infants in the ECC group (4). Moreover, the authors observed a potential gender-specific benefit of DCC at 4 years of age, noting that males receiving DCC had higher scores than did the respective ECC cohort in two ASQ domains; personal-social (AMD 4.9, 95% CI 1.6–8.3) and fine-motor development (AMD 4.7, 95% CI 1.0–8.4) (4). Fewer boys who received DCC scored below normative range in the Movement Assessment Battery for Children (ABC) Drawing Bicycling Trail Task (P<0.01) than did boys receiving ECC. Similar results were observed in the ASQ Fine Motor Score (P=0.03) (4). While the biological mechanisms for improvements in neurocognitive performance have not been elucidated, some authors speculate that the provision of greater iron stores following DCC, even among a cohort of infants at low-risk for iron deficiency anemia, may contribute to improved myelination and subsequent neurodevelopmental function (4).
Cardiopulmonary effects:
In an animal model, Bhatt et al. examined the effects of cord clamping practices before and after the onset of ventilation on cardiovascular function at birth. Compared to clamping prior to ventilation, DCC (3–4 minutes) until after ventilation provided a smoother cardiovascular transition at birth, wherein increased pulmonary blood flow led to decreased fluctuations in circulatory pressures, which could be particularly critical in the cerebrovascular bed (8). The authors speculated that, to mitigate risks of low cardiac output syndrome due to the abrupt increase in left ventricular afterload following removal of the low resistance placental circulation at birth, increases in pulmonary blood flow and left ventricular preload following DCC after ventilation may be paramount (8). In other words, the increase in blood flow though the lungs following DCC after ventilation augments venous return to the left atrium, which may help maintain left cardiac output and stabilize systemic blood pressure during the immediate transitional period at birth (9). Evidence of improved transition following DCC after ventilation is established has also been noted in large, observational studies of term infants. For example, a study of over 15,000 term infants showed that the risk of death or hospitalization decreased by 20% for every 10 second delay in clamping after the onset of spontaneous respirations at birth (9).
Risks of DCC:
Despite the value of DCC in improving hematological, cardiopulmonary, and neurodevelopmental status, potential risks of the intervention must be considered carefully. For example, Hutton and Hassan observed higher blood viscosities following DCC at 2–4 hours and 5 days of life (WMD at 2–4 hours = 1.4, 95% CI 1.2–1.6; WMD at 5 days of age = 0.90, 95% CI 0.70–1.2), respectively, than following ECC (3). The data are mixed, but DCC has been associated with greater risks of higher bilirubin levels and neonatal jaundice (10). This may not be particularly concerning in centers with access to phototherapy, however higher bilirubin levels may have important implications on resource utilization and medical costs. Among term infants (n=699 infants), DCC was associated with a greater need for phototherapy than ECC (3.4% versus 1.9%, P=.2464) (3). Moreover, Hutton and Hassan reported a greater risk of polycythemia in DCC infants at both 7 hours and 24–48 hours after birth (relative risk [RR] at 7 hours: 3.4, 95% CI 1.3–9.5; RR at 24–48 hours: 3.8, 95% CI 1.1–13.2) (3).
Statement from governing bodies:
In 2017, following careful consideration of the risk/benefit profile, ACOG issued a statement endorsing the practice of DCC among healthy term infants (2). However, the governing bodies noted the urgent need for consideration of optimal cord clamping practices among high-risk infants. One subgroup where DCC could provide value, but also could be problematic, is infants with congenital heart disease (CHD).
Considerations of optimal cord clamping practices among infants with CHD
Congential heart disease (CHD) is the most common congenital structural defect, occurring in 10 out of 1,000 live births. Over the past 2 decades, tremendous progress has been made in the survival of infants with CHD (11). However, among survivors, major early morbidities include hematological perturbations, cardiac disturbances (e.g. cardiac arrest) and neurologic injury (12–16). While the benefits of DCC have been demonstrated among healthy term infants, as outlined below, the unique risk/benefit profiles of infants with CHD warrant separate consideration. Given that CHD is markedly heterogenous, to minimize unnecessary and potentially harmful cord clamping practices, identification of subgroups (single-ventricle, cyanotic lesions, CHD requiring neonatal surgery) most likely to benefit from optimal cord clamping practices is necessary to optimize risk/benefit profiles.
Potential benefits of DCC among infants with CHD
A. Minimizing exposure to red blood cell transfusions:
The primary purpose of giving a red blood cell (RBC) transfusion is to increase oxygen-carrying capacity, and in certain clinical scenarios, represents a life-saving treatment modality (17). While the increases in red cell mass derived from RBC transfusions are beneficial, potential adverse consequences include a myriad of transfusion-related inflammatory responses, including capillary leak syndrome, generalized edema, heart failure, and multiple organ dysfunction. Stored blood products trigger unbalanced electrolytes and metabolic acidosis, with evidence that transfusions increase risks for health-care associated infections (18, 19). Most importantly, RBC transfusions have been associated increasingly with worse clinical outcomes in children with heart disease (20). Since infants with CHD receive the greatest number of RBC transfusions among all pediatric patients, strategies that can safely improve blood volume status and limit exposure to homologous RBC transfusions are needed (21).
In a recent study, Kartha et al. reported that, in the first 30 days of life, infants with CHD undergoing early cardiac surgery, defined as critical CHD (CCHD), require RBC transfusions in 99.7% of cases (interquartile range [IQR]: 91.3%–100%) (21). Moreover, in the absence of optimal transfusion thresholds, infants with CHD, particularly those with cyanotic lesions, frequently receive RBC transfusions to maintain elevated hemoglobin (Hb) concentrations, sometimes as high as 14 g/dL (22).
Previous investigators have shown, among a subgroup of infants with CCHD, that DCC is associated with lower likelihood to receive RBC transfusions than following ECC (23). Moreover, the greater oxygen-carrying capacities and higher blood volumes following DCC should improve tissue oxygenation, which becomes critically important among infants with cyanotic heart disease, in whom oxygen saturation and systemic blood flow are suboptimal. These anticipated benefits make DCC attractive as a strategy to limit exposure to RBC transfusion, and optimize oxygen-carrying capacity, among infants with CCHD. However, significant heterogeneity in cardiac disease, including anatomic lesions (cyanotic and acyanotic), cardiac physiologies, and antenatal risk factors (e.g. infant of diabetic mother) for polycythemia warrant careful consideration prior to widespread acceptance of this practice.
B. Reducing iron deficiency anemia:
Iron deficiency is frequently found in children with CHD (24). For example, a study by Puri et al. showed that, among 107 pediatric patients with heart failure, over half (56%) were iron deficient (25). Moreover, among infants with single or biventricular cardiac physiology, the authors observed those with iron deficiency anemia were at least twice as likely to experience an adverse cardiac event, (need for ventricular assist device implantation, heart transplantation, or death) than those without iron deficiency (25). Given that DCC has been shown consistently to reduce the risk of iron deficiency anemia, even among term infants at low risk, potential applications of DCC among infants with CHD are attractive and warrant investigation.
C. Improving neurodevelopmental outcomes among infants with CHD:
Over half of surviving requiring cardiac surgery demonstrate motor and visuospatial neurocognitive deficits that that impede their progress in school and result in social and economic challenges to patients and families (26–28). Not surprisingly, children with CHD are at higher risk of cognitive deficits and poorer school outcomes than are children without CHD, with many infants with CHD requiring special educational services. Therefore, the neuroprotective effects of DCC in other clinical scenarios may be relevant to infants with CHD. However, no studies to date have shown an improvement in longer-term outcomes, including neurocognitive function, among infants with CHD following DCC.
Theoretical risks of DCC among infants with CHD
A. Maintaining shunt patency:
While DCC provides higher hematocrits than does ECC, secondary increases in blood viscosity may be detrimental, particularly among infants with palliative shunts, such as modified Blalock-Taussig shunts. As demonstrated by Sahoo et al., lowering hematocrits to 40–45% (compared to a common, standard hematocrit of ~55%) increased shunt patency from 84% to 100%. This is compounded by findings that observed greater risks of shunt thrombosis with higher pre-operative hemoglobin levels (>18g/dL), particularly among infants weighing less than 3kg (29). Additionally, increased viscosities associated with higher hematocrits and volume overloads could be detrimental in scenarios of CHD associated with depressed heart function.
B. Infants with CHD with the perceived need for immediate resuscitation:
The risk of poor transition to extrauterine life and cardiopulmonary instability among infants with CHD is largely dependent upon the following factors: i) type and severity of underlying cardiac defect; ii) changes in systemic and pulmonary blood flow and resistance; iii) patency of fetal shunts, including the foramen ovale and ductus arteriosus. The overwhelming majority of infants with CHD, even those with critical cardiac disease who are likely to require intervention in the first 28 days of life, will not require specialized resuscitative efforts beyond those provided in the Neonatal Resuscitation Program (NRP). Several lesions are at higher risk of early cardiovascular compromise at birth: i) D-transposition of the great arteries with a restrictive/intact atrial septum; ii) hypoplastic left heart syndrome (HLHS) with a restrictive/intact atrial septum; iii) obstructed total anomalous pulmonary venous return (TAPVR). However, even in these settings, infants will continue to receive oxygenated blood from the placenta, making ongoing hypoxia unlikely. Prenatal consultation and education of maternal-fetal-medicine (MFM) specialists is critical in planning on the optimal mode and timing of delivery, including the potential need for delivery via a planned induction or cesarean section at a center capable of addressing all aspects of maternal, fetal, and neonatal cardiac care. As part of those prenatal discussions, optimal timing of cord clamping practices should be considered. While data on optimal cord clamping practices among infants with CHD are not available, an Italian task force provided the following recommendations (Table 1) (30).
Table 1:
Recommendations on timing of cord clamping based on heart disease severity. Data from Ghirardello S., et al., Frontiers in Pediatrics, 2018:6;372 (30).
| Type of Congenital Heart Disease | Examples of cardiac defect | Recommendation |
|---|---|---|
| Low likelihood of cardiovascular instability at delivery |
|
|
| Moderate likelihood of cardiovascular instability at delivery |
|
|
| High likelihood of cardiovascular instability at delivery |
|
|
D-TGA = D-Transposition of the Great Arteries; HLHS = Hypoplastic left heart syndrome; RAS = Restrictive atrial septum; TAPVR = Total anomalous pulmonary venous return
Even among higher-risk subgroups, the initial steps of neonatal resuscitation (drying, stimulation) can be offered with the umbilical cord intact, providing a source of oxygen to the infant following delivery. However, we acknowledge that certain lesions (e.g. d-TGA with intact atrial septum, HLHS with intact atrial septum) at higher risk for immediate cardiovascular instability warrant careful consideration of optimal cord clamping practices. One technique that may be a reasonable alternative to DCC is umbilical cord milking (UCM) (31). Health care providers perform UCM by grasping the unclamped umbilical cord and pushing (milking) blood towards the infant 2–4 times prior to clamping. While providing a similar placental transfusion to DCC, UCM takes 15–20 seconds to perform. Despite being more efficient than DCC, evidence of worse outcomes among preterm infants (<32 weeks of gestation) has diminished enthusiasm for this approach (32).
Systematic review on umbilical cord clamping practices among infants with CHD:
One subgroup of infants in whom DCC could provide value, but also could be problematic, is infants with CHD. Thus, we performed a systematic review on the available evidence on cord clamping practices among infants with CHD. We conducted a literature search using PubMed/Medline database, using combinations of the relevant medical subject heading terms, key words, and word variants are shown in Figure 1. The electronic search was conducted on June 15th, 2020, and then updated on August 5th, 2020; the search was limited to reports published after January 1st, 2000 and prior to June 1st, 2020. The reference lists of relevant articles and reviews were searched by hand for additional reports. Randomized controlled trials that compared outcomes of DCC versus ECC among term infants were included. One reviewer (B.R.) independently assessed the methodological quality of included studies. Studies that were identified were evaluated using a modified Delphi Scale (33, 34). No studies were excluded based on quality.
Figure 1:
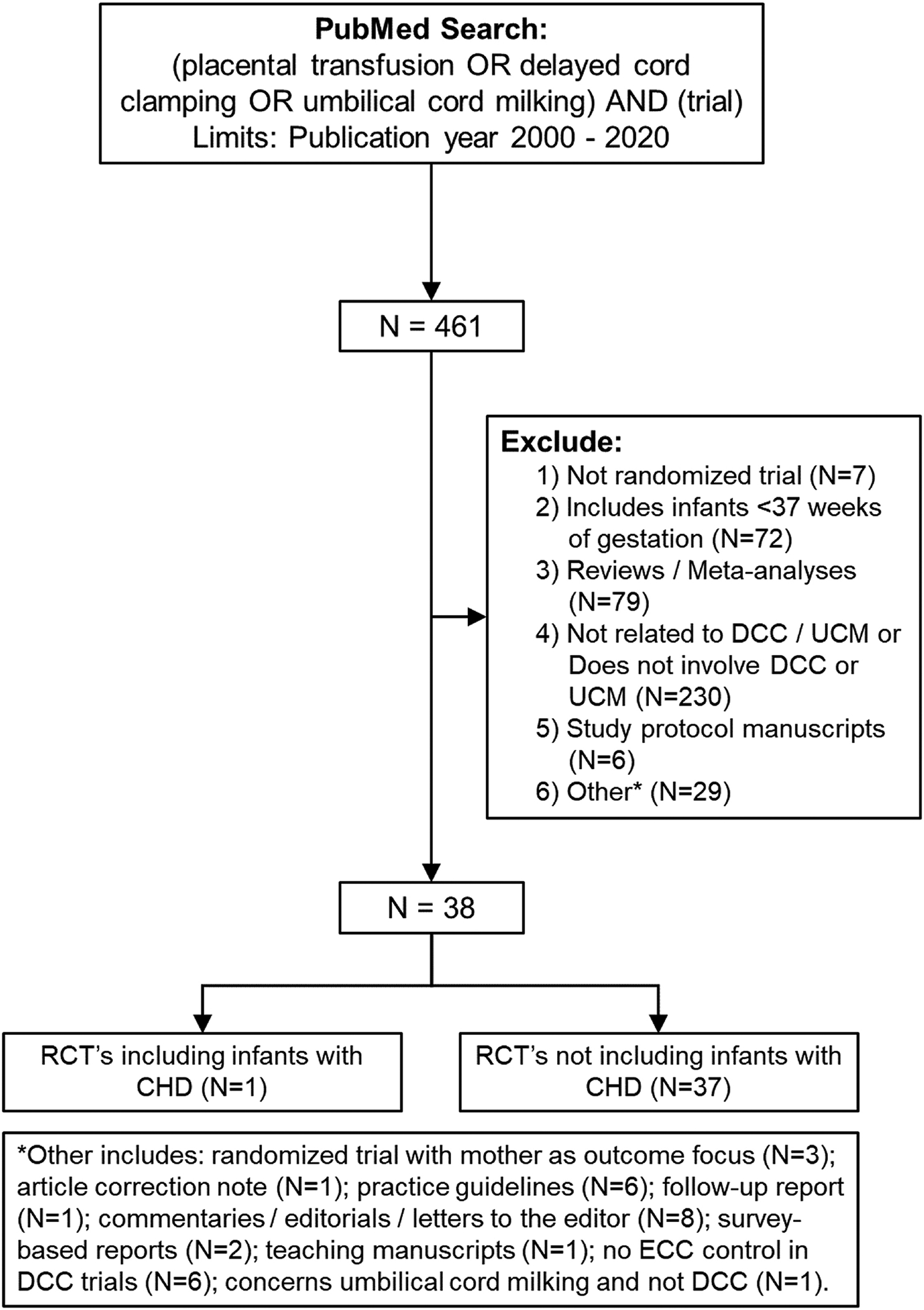
Terms used in PubMed/Medline search and manuscript selection flowchart for systematic review.
Despite more than 35 RCTs among healthy term infants, we identified only one small, single-center, pilot study that included infants with CHD (Table 2). In fact, in this single study, only infants with critical CHD, defined as the need for surgical or catheter-based intervention within the first 28 days of life, were included. The pilot study (N=32) reported that DCC is safe and feasible among infants with critical CHD, with no evidence of increased risk for polycythemia, hyperviscosity, or derangements to cardiopulmonary blood flow. The authors observed that infants receiving DCC had higher hematocrits during the first 72 hours of life and received fewer red blood cell transfusions (2.2 ± 1.4 versus 3.4 ± 1.6, P=.05; Figure 2) than did infants receiving ECC (23). However, the pilot study was not designed to detect longer-term differences in safety and efficacy between the two groups.
Table 2:
Published RCT’s on DCC versus ECC
| First Author (Year) | Placental Transfusion Method(s) | Modified Delphi Scorea | Primary Findings | Inclusion of Infants with CHDb |
|---|---|---|---|---|
| Mercer JS, et al. (‘20)c (6) | DCC, UCMd | 7 | DCC infants had greater myelin content in brain regions of motor function, visual/spatial, and sensory processing than ECC infants. | No |
| Mercer JS, et al. (‘18)c (35) | DCC, UCMd | 7 | DCC infants had increased ferritin levels and increased brain myelin than ECC infants. | No |
| Mercer JS, et al. (‘17) (36) | DCC, UCMd | 7 | DCC infants had increased neonatal hemoglobin and hematocrit levels at 24 to 48 hours of age with no differences on peak bilirubin levels than ECC infants. | No |
| Berglund SK, et al. (‘20)e (37) | DCC | 5 | Hepicidin can be used to evaluate for iron deficiency in early infancy. | No |
| Andersson O, et al. (‘15)c (4) | DCC | 7 | DCC infants had higher scores on personal- social and fine-motor development at 4 years, particularly in boys, than ECC infants. | No |
| Andersson O, et al. (‘14)c (38) | DCC | 7 | Compared to ECC, DCC at birth did not affect iron status or neurodevelopment at age 12 months in a selected population of healthy term-born infants. | No |
| Andersson O, et al. (‘13)c (39) | DCC | 7 | No differences between infants receiving DCC versus ECC on early development. | No |
| Andersson O, et al. (‘13)c (40) | DCC | 7 | DCC did not have a significant effect on the maternal outcomes postpartum hemorrhage or need for blood transfusions than ECC. | No |
| Andersson O, et al. (‘11) (41) | DCC | 7 | DCC improved ferritin levels and reduced the prevalence of iron deficiency at 4 months of age than ECC. | No |
| Songthamwat M, et al. (‘20) (42) | DCC | 5 | Incidence of neonatal anemia decreased with the longer timing of DCC. | No |
| De Bernardo G, et al. (‘20) (43) | DCC | 3 | DCC infants had increased hematocrit and bilirubin levels, but without need of phototherapy, than ECC infants. | No |
| Rana N, et al. (‘19)c (7) | DCC | 7 | DCC (3 minutes) is associated with an improvement of the neurodevelopment at 12 months of age compared to ECC. | No |
| Kc A, et al. (‘19)c (44) | DCC | 6 | DCC increased oxygen saturation and lowers heart rate compared to ECC; time of first breath/regular breathing was earlier in infants with cord clamping > 180 seconds. | No |
| Andersson O, et al. (‘19)c (45) | DCC | 6 | Resuscitation with an intact umbilical cord did not increase safety concerns and was associated with a better recovery than routine resuscitation after clamping and cutting the cord. | No |
| Kc A, et al. (‘17) (46) | DCC | 7 | DCC for 180 seconds lowered iron-deficiency anemia at 8 and 12 months of age than ECC. | No |
| Purisch SE, et al. (‘19) (47) | DCC | 5 | In cesarean deliveries, compared to ECC, DCC does not increase the risk of maternal hemorrhage. | No |
| Cavallin F, et al. (‘19) (48) | DCC | 6 | Among infants born by elective Cesarean- section, DCC >60 seconds increases hematocrit at day 2 of life than ECC. | No |
| Chen X, et al. (‘18) (49) | DCC | 7 | In full-term infants, DCC > 60 s increases hematocrit levels at 24 h after birth, without any harmful effects on infants and mothers, than ECC | No |
| Nouraie, S, et al. (‘19) (50) | DCC | 4 | DCC increases the risk of neonatal jaundice compared to ECC. | No |
| Vatansever B, et al. (‘18) (51) | DCC, UCMf | 4 | ECC results in increased oxidation reactions compared to DCC/UCM. | No |
| Katheria AC, et al. (‘17) (52) | DCC | 5 | DCC (5 min) could be accomplished safely without compromising the ability to perform resuscitation and was associated with trends for less resuscitation and improved Apgar scores than ECC. | No |
| Sun M, et al. (‘17) (53) | DCC | 3 | Compared to ECC, DCC did not increase hemoglobin and hematocrit levels, highest bilirubin, Apgar scores, or hyperbilirubinemia. | No |
| Withanathantrige M and Goonewardene I. (‘17) (54) | DCC | 6 | During antepartum lower segment caesarean section, DCC is feasible and safe, and is not associated with any differences in the risk of postoperative hemorrhage or maternal or neonatal morbidity, compared to ECC. | -- |
| De Paco C, et al. (‘16) (55) | DCC | 4 | DCC does not affect the time of the third stage of labor compared to ECC. | No |
| Backes CH, et al. (‘15) (23) | DCC | 4 | Among infants with critical CHD, DCC is safe, feasible and lowers the proportion of infants exposed to RBC transfusion during hospitalization, than ECC. | Yes |
| Nesheli HM, et al. (‘14) (56) | DCC | 3 | DCC increases 6-month hematological indices (hematocrit, hemoglobin, serum iron) compared to ECC. | No |
| Li N, et al. (‘12) (57) | DCC | 4 | DCC >1 minute improves iron stores of breastfed infants at 4 months compared to ECC. | -- |
| Al-Talwil MM, et al. (‘12) (58) | DCC | 6 | DCC increases serum ferritin and reduces rates of iron deficiency at 3–5 months of age, compared to ECC. | No |
| De Paco C, et al. (‘11) (59) | DCC | 4 | Among vaginally delivered neonates, DCC infants had higher mean umbilical artery pO2 values than ECC infants. | No |
| Ceriani Cernadas JM, et al. (‘10)c (60) | DCC | 7 | At 6 months of age, DCC at 3 minutes had higher plasma ferritin levels than ECC (15 seconds). | No |
| Ceriani Cernadas JM, et al. (‘06) (61) | DCC | 7 | DCC at 3 minutes after birth increases hematocrit levels and decreases neonatal anemia than DCC at 1 minute after birth. | No |
| Jaleel R, et al. (‘09) (62) | DCC | 3 | DCC results in increase in hemoglobin levels, without adverse events, than ECC. | No |
| Jahazi A, et al. (‘08) (63) | DCC | 3 | Hematocrit and polycythemia levels did not differ between infants receiving DCC versus ECC. | No |
| Chaparro CM, et al. (‘07)c (64) | DCC | 7 | ECC leads to higher infant blood lead concentrations at 6 months of age among infants with higher postnatal lead exposure than DCC | No |
| Chaparro CM, et al. (‘06) (65) | DCC | 7 | DCC (2 minutes) increases iron status at 6 months of age (ferritin) mean corpuscular volume, and total body iron, than ECC. | No |
| Van Rheenen P, et al. (‘07) (66) | DCC | 5 | Among infants living at risk for malaria, DCC increases hematological status (hemoglobin) and decreased risk of anemia compared to ECC. | No |
| Emhamed MO, et al. (‘04) (67) | DCC | 4 | DCC increases red cell mass compared to ECC. | No |
| Gupta R and Ramji S (‘02) (68) | DCC | 5 | Iron stores and Hb levels during infancy can be increased in neonates born to anemic mothers by DCC versus ECC | No |
CHD = Congenital Heart Disease; DCC = Delayed cord clamping; ECC = Early cord clamping; UCM = Umbilical cord “milking”; -- = Unclear/not included
Referred to as “modified” as two categories had constant scores: the care provided (DCC or ECC) could not be blinded due to the intervention and the all patients (newborn infants) could be considered as “blinded” upon receipt of intervention due to age and lack of visual acumen. Thus, the maximal score is “7”, rather than “9” (34).
Determination of non-inclusion of infants with CHD was based on review of inclusion/exclusion criteria and published results; no clear evidence of inclusion was deemed non-inclusion.
Follow-up cohort
Intent of the study was to compare DCC to ECC. Authors note, “If unable to carry out the DCC protocol, or if delivery was by cesarean section, the cord was milked five times before being clamped (n=11).” (35)
Sub-analysis of data collected during previously-conducted trial (41).
Included three independent study groups: DCC versus ICC versus UCM.
Figure 2:
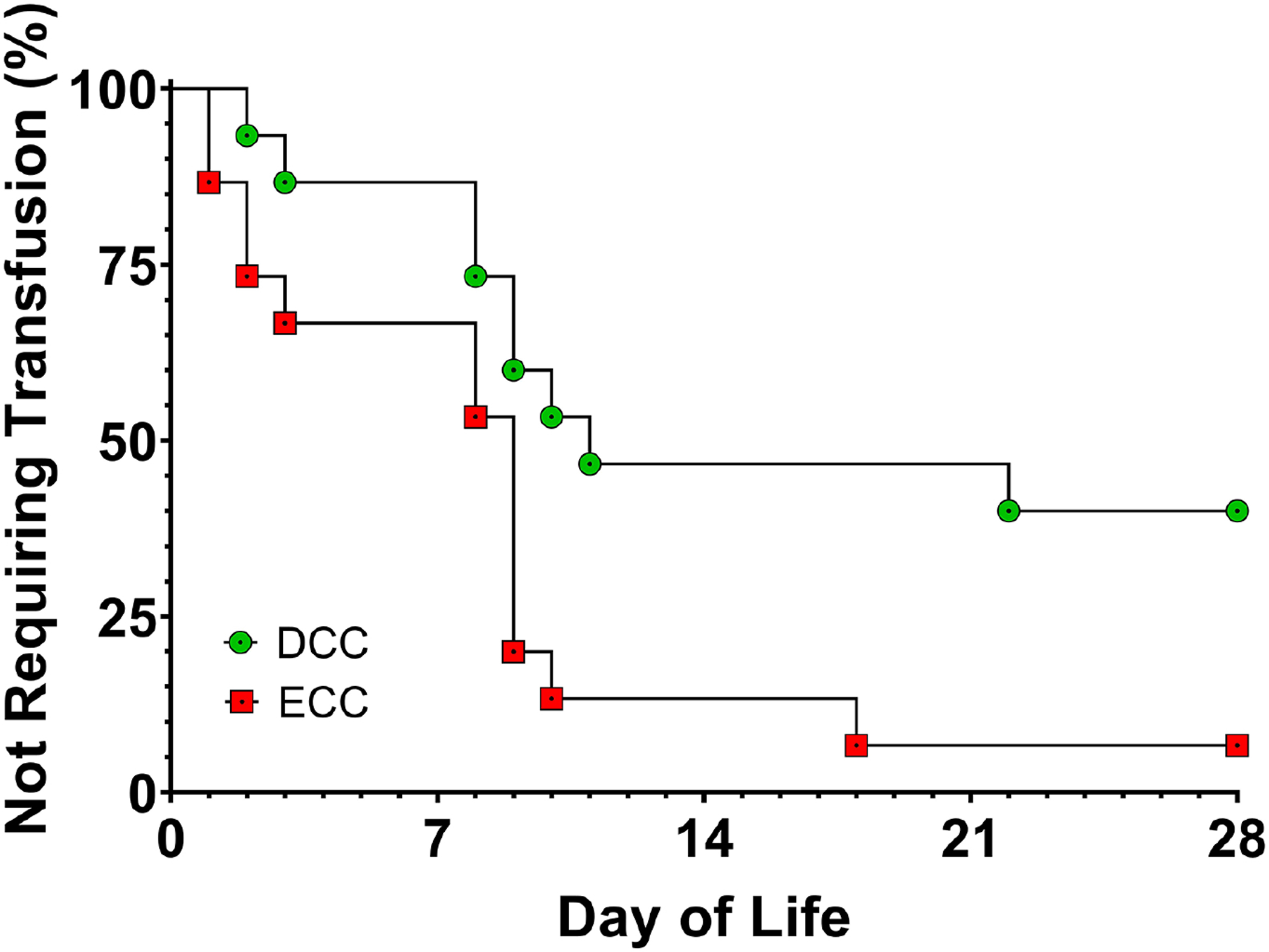
Time-to-event curve showing the need for a transfusion during the first 28 days of life among preterm infants with congenital heart disease receiving delayed cord clamping (DCC) or early cord clamping (ECC) at birth. Log-rank (Mantel-Cox) comparison demonstrates significant differences in the curves (P=0.02). The corresponding Mantel-Haenszel hazard ratio (HR) for transfusion need in the ECC group versus the DCC group is 3.1 with a 95% confidence interval of 1.2 – 7.9. (Reproduced from Backes CH, et al., Journal of Perinatology, 2015:35;826–831.)”
Needs and opportunities for future research and interdisciplinary collaborations
No studies have yet addressed whether DCC among infants with CHD improves short and longer-term outcomes. Likewise, the notion that DCC is advantageous because it is associated with improved neurologic and hematologic outcome is untested. Given the paucity of available data to guide evidence-based medicine, the need for well-designed, pragmatic, randomized controlled trials to determine what cord clamping strategies best improve patient outcomes is realized. Given the large number of patient (gestational age, cardiac lesion), procedural (bypass time, general anesthesia), and institutional (operator experience)-level factors that contribute to patient outcomes, such trials will need to be meticulously designed and appropriately powered to evaluate the risks and benefits of DCC versus ECC in this unique subgroup of patients.
In the design of these necessary studies, we emphasize the following: 1) clear evidence that obstetrical providers have equipoise and are willing to recruit and randomize patients; 2) evidence that caregivers are willing to consent to proposed studies; 3) to allow prioritization of research efforts and minimize unnecessary and potentially harmful cord clamping practices, identification of subgroups (single-ventricle, cyanotic lesions) most likely to benefit from optimal cord clamping practices; 4) rather than short-term, surrogate markers, use of robust, longer-term outcomes meaningful to families and clinically relevant. with an emphasis on longer-term neurocognitive performance; 5) emphasis on multicenter and multidisciplinary (obstetricians, pediatric cardiologists, and neonatologists) collaboration.
Conclusion:
Evidence of hematological, cardiopulmonary, and neurodevelopmental benefits following DCC among term infants with CHD makes the practice a potentially attractive one for infants with CHD. However, our review of the available literature shows that, to date, infants born with CHD have been largely excluded from clinical trials of cord clamping practices. Therefore, the supposition that DCC is advantageous among infants with CHD is untested. Given that CHD is markedly heterogenous, to minimize unnecessary and potentially harmful cord clamping practices, the need is clear for more robust, evidence-based data on optimal cord clamping practices in this high-risk subgroup of infants.
Highlights.
Delayed cord clamping (DCC) is common practice among healthy term infants
Although unproven, infants with congenital heart disease may benefit from DCC
Compared to healthy infants, CHD infants may have unique DCC risk/benefit profiles
Existing evidence on the potential value of DCC among infants with CHD is limited
Further research regarding cord clamping practices among infants with CHD is needed
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Declarations of interest: none.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no commercial of financial conflicts of interest to disclose.
References:
- (1).Hutton EK, Stoll K, Taha N. An observational study of umbilical cord clamping practices of maternity care providers in a tertiary care center. Birth. 2013;40:39–45. [DOI] [PubMed] [Google Scholar]
- (2).Committee on Obstetric P. Committee Opinion No. 684: Delayed Umbilical Cord Clamping After Birth. Obstet Gynecol. 2017;129:e5–e10. [DOI] [PubMed] [Google Scholar]
- (3).Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297:1241–52. [DOI] [PubMed] [Google Scholar]
- (4).Andersson O, Lindquist B, Lindgren M, Stjernqvist K, Domellof M, Hellstrom-Westas L. Effect of Delayed Cord Clamping on Neurodevelopment at 4 Years of Age: A Randomized Clinical Trial. JAMA Pediatr. 2015;169:631–8. [DOI] [PubMed] [Google Scholar]
- (5).McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013:CD004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mercer JS, Erickson-Owens DA, Deoni SCL, Dean Iii DC, Tucker R, Parker AB, et al. The Effects of Delayed Cord Clamping on 12-Month Brain Myelin Content and Neurodevelopment: A Randomized Controlled Trial. Am J Perinatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rana N, Kc A, Malqvist M, Subedi K, Andersson O. Effect of Delayed Cord Clamping of Term Babies on Neurodevelopment at 12 Months: A Randomized Controlled Trial. Neonatology. 2019;115:36–42. [DOI] [PubMed] [Google Scholar]
- (8).Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. 2013;591:2113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ersdal HL, Linde J, Mduma E, Auestad B, Perlman J. Neonatal outcome following cord clamping after onset of spontaneous respiration. Pediatrics. 2014;134:265–72. [DOI] [PubMed] [Google Scholar]
- (10).Yang S, Duffy JY, Johnston R, Fall C, Fitzmaurice LE. Association of a Delayed Cord-Clamping Protocol With Hyperbilirubinemia in Term Neonates. Obstet Gynecol. 2019;133:754–61. [DOI] [PubMed] [Google Scholar]
- (11).Stasik CN, Gelehrter S, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006;131:412–7. [DOI] [PubMed] [Google Scholar]
- (12).Badhwar V, Rankin JS, Thourani VH, D’Agostino RS, Habib RH, Shahian DM, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Research: Outcomes Analysis, Quality Improvement, and Patient Safety. Ann Thorac Surg. 2018;106:8–13. [DOI] [PubMed] [Google Scholar]
- (13).Jacobs JP, He X, Mayer JE Jr., Austin EH 3rd, Quintessenza JA, Karl TR, et al. Mortality Trends in Pediatric and Congenital Heart Surgery: An Analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2016;102:1345–52. [DOI] [PubMed] [Google Scholar]
- (14).Jacobs JP, Mayer JE Jr., Mavroudis C, O’Brien SM, Austin EH 3rd, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2016 Update on Outcomes and Quality. Ann Thorac Surg. 2016;101:850–62. [DOI] [PubMed] [Google Scholar]
- (15).Jacobs JP, Mayer JE Jr., Mavroudis C, O’Brien SM, Austin EH 3rd, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2017 Update on Outcomes and Quality. Ann Thorac Surg. 2017;103:699–709. [DOI] [PubMed] [Google Scholar]
- (16).Jacobs JP, Mayer JE Jr., Pasquali SK, Hill KD, Overman DM, Louis JD, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2019 Update on Outcomes and Quality. Ann Thorac Surg. 2019;107:691–704. [DOI] [PubMed] [Google Scholar]
- (17).Guzzetta NA. Benefits and risks of red blood cell transfusion in pediatric patients undergoing cardiac surgery. Paediatr Anaesth. 2011;21:504–11. [DOI] [PubMed] [Google Scholar]
- (18).Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Zhang DS, Huang YS, Xie DK, He N, Dong WB, Lei XP. [Effect of red blood cell storage duration on the clinical effect of exchange transfusion and internal environment in neonates with hyperbilirubinemia]. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21:635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Cholette JM, Willems A, Valentine SL, Bateman ST, Schwartz SM, Pediatric Critical Care T, et al. Recommendations on RBC Transfusion in Infants and Children With Acquired and Congenital Heart Disease From the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19:S137–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kartha VM, Jacobs JP, Vener DF, Hill KD, Goldenberg NA, Pasquali SK, et al. National Benchmarks for Proportions of Patients Receiving Blood Transfusions During Pediatric and Congenital Heart Surgery: An Analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2018;106:1197–203. [DOI] [PubMed] [Google Scholar]
- (22).Tremblay-Roy JS, Poirier N, Ducruet T, Lacroix J, Harrington K. Red Blood Cell Transfusion in the Postoperative Care of Pediatric Cardiac Surgery: Survey on Stated Practice. Pediatr Cardiol. 2016;37:1266–73. [DOI] [PubMed] [Google Scholar]
- (23).Backes CH, Huang H, Cua CL, Garg V, Smith CV, Yin H, et al. Early versus delayed umbilical cord clamping in infants with congenital heart disease: a pilot, randomized, controlled trial. J Perinatol. 2015;35:826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).West DW, Scheel JN, Stover R, Kan J, DeAngelis C. Iron deficiency in children with cyanotic congenital heart disease. J Pediatr. 1990;117:266–8. [DOI] [PubMed] [Google Scholar]
- (25).Puri K, Price JF, Spinner JA, Powers JM, Denfield SW, Cabrera AG, et al. Iron Deficiency Is Associated with Adverse Outcomes in Pediatric Heart Failure. J Pediatr-Us. 2020;216:58–+. [DOI] [PubMed] [Google Scholar]
- (26).Bellinger DC, Bernstein JH, Kirkwood MW, Rappaport LA, Newburger J. Visual-spatial skills in children after open-heart surgery. J Dev Behav Pediatr. 2003;24:169–79. [DOI] [PubMed] [Google Scholar]
- (27).Miatton M, De Wolf D, Francois K, Thiery E, Vingerhoets G. Neurocognitive consequences of surgically corrected congenital heart defects: A review. Neuropsychol Rev. 2006;16:65–85. [DOI] [PubMed] [Google Scholar]
- (28).Miatton M, De Wolf D, Francois K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr. 2007;151:73–8, 8 e1. [DOI] [PubMed] [Google Scholar]
- (29).Sahoo TK, Chauhan S, Sahu M, Bisoi A, Kiran U. Effects of hemodilution on outcome after modified Blalock-Taussig shunt operation in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 2007;21:179–83. [DOI] [PubMed] [Google Scholar]
- (30).Ghirardello S, Di Tommaso M, Fiocchi S, Locatelli A, Perrone B, Pratesi S, et al. Italian Recommendations for Placental Transfusion Strategies. Front Pediatr. 2018;6:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Fuwa K, Tabata N, Ogawa R, Nagano N, Yamaji N, Ota E, et al. Umbilical cord milking versus delayed cord clamping in term infants: a systematic review and meta-analysis. J Perinatol. 2020:[ePub ahead of print]. DOI: 10.1038/s41372-020-00825-6 [DOI] [PubMed] [Google Scholar]
- (32).Katheria A, Reister F, Essers J, Mendler M, Hummler H, Subramaniam A, et al. Association of Umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA. 2019;322(19):1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- (34).Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–41. [DOI] [PubMed] [Google Scholar]
- (35).Mercer JS, Erickson-Owens DA, Deoni SCL, Dean DC 3rd, Collins J, Parker AB, et al. Effects of Delayed Cord Clamping on 4-Month Ferritin Levels, Brain Myelin Content, and Neurodevelopment: A Randomized Controlled Trial. J Pediatr. 2018;203:266–72 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mercer JS, Erickson-Owens DA, Collins J, Barcelos MO, Parker AB, Padbury JF. Effects of delayed cord clamping on residual placental blood volume, hemoglobin and bilirubin levels in term infants: a randomized controlled trial. J Perinatol. 2017;37:260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Berglund SK, Chmielewska AM, Domellof M, Andersson O. Hepcidin is a relevant iron status indicator in infancy: results from a randomized trial of early vs. delayed cord clamping. Pediatr Res. 2020. [DOI] [PubMed] [Google Scholar]
- (38).Andersson O, Domellof M, Andersson D, Hellstrom-Westas L. Effect of delayed vs early umbilical cord clamping on iron status and neurodevelopment at age 12 months: a randomized clinical trial. JAMA Pediatr. 2014;168:547–54. [DOI] [PubMed] [Google Scholar]
- (39).Andersson O, Domellof M, Andersson D, Hellstrom-Westas L. Effects of delayed cord clamping on neurodevelopment and infection at four months of age: a randomised trial. Acta Paediatr. 2013;102:525–31. [DOI] [PubMed] [Google Scholar]
- (40).Andersson O, Hellstrom-Westas L, Andersson D, Clausen J, Domellof M. Effects of delayed compared with early umbilical cord clamping on maternal postpartum hemorrhage and cord blood gas sampling: a randomized trial. Acta Obstet Gynecol Scand. 2013;92:567–74. [DOI] [PubMed] [Google Scholar]
- (41).Andersson O, Hellstrom-Westas L, Andersson D, Domellof M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ. 2011;343:d7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Songthamwat M, Witsawapaisan P, Tanthawat S, Songthamwat S. Effect of Delayed Cord Clamping at 30 Seconds and 1 Minute on Neonatal Hematocrit in Term Cesarean Delivery: A Randomized Trial. Int J Womens Health. 2020;12:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).De Bernardo G, Giordano M, De Santis R, Castelli P, Sordino D, Trevisanuto D, et al. A randomized controlled study of immediate versus delayed umbilical cord clamping in infants born by elective caesarean section. Ital J Pediatr. 2020;46:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kc A, Singhal N, Gautam J, Rana N, Andersson O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth - randomized clinical trial. Matern Health Neonatol Perinatol. 2019;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Andersson O, Rana N, Ewald U, Malqvist M, Stripple G, Basnet O, et al. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 minutes of birth (Nepcord III) - a randomized clinical trial. Matern Health Neonatol Perinatol. 2019;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Kc A, Rana N, Malqvist M, Jarawka Ranneberg L, Subedi K, Andersson O. Effects of Delayed Umbilical Cord Clamping vs Early Clamping on Anemia in Infants at 8 and 12 Months: A Randomized Clinical Trial. JAMA Pediatr. 2017;171:264–70. [DOI] [PubMed] [Google Scholar]
- (47).Purisch SE, Ananth CV, Arditi B, Mauney L, Ajemian B, Heiderich A, et al. Effect of Delayed vs Immediate Umbilical Cord Clamping on Maternal Blood Loss in Term Cesarean Delivery: A Randomized Clinical Trial. JAMA. 2019;322:1869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Cavallin F, Galeazzo B, Loretelli V, Madella S, Pizzolato M, Visentin S, et al. Delayed Cord Clamping versus Early Cord Clamping in Elective Cesarean Section: A Randomized Controlled Trial. Neonatology. 2019;116:252–9. [DOI] [PubMed] [Google Scholar]
- (49).Chen X, Li X, Chang Y, Li W, Cui H. Effect and safety of timing of cord clamping on neonatal hematocrit values and clinical outcomes in term infants: A randomized controlled trial. J Perinatol. 2018;38:251–7. [DOI] [PubMed] [Google Scholar]
- (50).Nouraie S, S AMA, Vameghi R, Akbarzade Baghban A. The Effect of the Timing of Umbilical Cord Clamping on Hemoglobin Levels, Neonatal Outcomes and Developmental Status in Infants at 4 Months Old. Iran J Child Neurol. 2019;13:45–55. [PMC free article] [PubMed] [Google Scholar]
- (51).Vatansever B, Demirel G, Ciler Eren E, Erel O, Neselioglu S, Karavar HN, et al. Is early cord clamping, delayed cord clamping or cord milking best? J Matern Fetal Neonatal Med. 2018;31:877–80. [DOI] [PubMed] [Google Scholar]
- (52).Katheria AC, Brown MK, Faksh A, Hassen KO, Rich W, Lazarus D, et al. Delayed Cord Clamping in Newborns Born at Term at Risk for Resuscitation: A Feasibility Randomized Clinical Trial. J Pediatr. 2017;187:313–7 e1. [DOI] [PubMed] [Google Scholar]
- (53).Sun M, Song X, Shi W, Li Y, Shan N, Zhang H. Delayed umbilical cord clamping in cesarean section reduces postpartum bleeding and the rate of severe asphyxia. Clin Exp Obstet Gynecol. 2017;44:14–6. [PubMed] [Google Scholar]
- (54).Withanathantrige M, Goonewardene I. Effects of early versus delayed umbilical cord clamping during antepartum lower segment caesarean section on placental delivery and postoperative haemorrhage: a randomised controlled trial. Ceylon Med J. 2017;62:5–11. [DOI] [PubMed] [Google Scholar]
- (55).De Paco C, Herrera J, Garcia C, Corbalan S, Arteaga A, Pertegal M, et al. Effects of delayed cord clamping on the third stage of labour, maternal haematological parameters and acid-base status in fetuses at term. Eur J Obstet Gynecol Reprod Biol. 2016;207:153–6. [DOI] [PubMed] [Google Scholar]
- (56).Nesheli HM, Esmailzadeh S, Haghshenas M, Bijani A, Moghaddams TG. Effect of late vs early clamping of the umbilical cord (on haemoglobin level) in full-term neonates. J Pak Med Assoc. 2014;64:1303–5. [PubMed] [Google Scholar]
- (57).Li N, Yang LC, Wu Q, Han CC, Wang L, Rong L, et al. [The effects of iron stores and growth of delayed umbilical cord clamp timing on term breastfed infants at 4 months]. Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:303–6. [PubMed] [Google Scholar]
- (58).Al-Tawil MM, Abdel-Aal MR, Kaddah MA. A Randomized Controlled Trial on Delayed Cord Clamping and Iron Status at 3–5 Months in Term Neonates Held at the Level of Maternal Pelvis. J Neonatal Perinatal Med. 2012;5:319–326. [Google Scholar]
- (59).De Paco C, Florido J, Garrido MC, Prados S, Navarrete L. Umbilical cord blood acid-base and gas analysis after early versus delayed cord clamping in neonates at term. Arch Gynecol Obstet. 2011;283:1011–4. [DOI] [PubMed] [Google Scholar]
- (60).Ceriani Cernadas JM, Carroli G, Pellegrini L, Ferreira M, Ricci C, Casas O, et al. [The effect of early and delayed umbilical cord clamping on ferritin levels in term infants at six months of life: a randomized, controlled trial]. Arch Argent Pediatr. 2010;108:2018. [DOI] [PubMed] [Google Scholar]
- (61).Ceriani Cernadas JM, Carroli G, Pellegrini L, Otano L, Ferreira M, Ricci C, et al. The effect of timing of cord clamping on neonatal venous hematocrit values and clinical outcome at term: a randomized, controlled trial. Pediatrics. 2006;117:e779–86. [DOI] [PubMed] [Google Scholar]
- (62).Jaleel R, Deeba F, Khan A. Timing of umbilical cord clamping and neonatal haematological status. J Pak Med Assoc. 2009;59:468–70. [PubMed] [Google Scholar]
- (63).Jahazi A, Kordi M, Mirbehbahani NB, Mazloom SR. The effect of early and late umbilical cord clamping on neonatal hematocrit. J Perinatol. 2008;28:523–5. [DOI] [PubMed] [Google Scholar]
- (64).Chaparro CM, Fornes R, Neufeld LM, Tena Alavez G, Eguia-Liz Cedillo R, Dewey KG. Early umbilical cord clamping contributes to elevated blood lead levels among infants with higher lead exposure. J Pediatr. 2007;151:506–12. [DOI] [PubMed] [Google Scholar]
- (65).Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Liz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet. 2006;367:1997–2004. [DOI] [PubMed] [Google Scholar]
- (66).van Rheenen P, de Moor L, Eschbach S, de Grooth H, Brabin B. Delayed cord clamping and haemoglobin levels in infancy: a randomised controlled trial in term babies. Trop Med Int Health. 2007;12:603–16. [DOI] [PubMed] [Google Scholar]
- (67).Emhamed MO, van Rheenen P, Brabin BJ. The early effects of delayed cord clamping in term infants born to Libyan mothers. Trop Doct. 2004;34:218–22. [DOI] [PubMed] [Google Scholar]
- (68).Gupta R, Ramji S. Effect of delayed cord clamping on iron stores in infants born to anemic mothers: a randomized controlled trial. Indian Pediatr. 2002;39:130–5. [PubMed] [Google Scholar]


