Abstract
Salamanders are recognized for their ability to regenerate a broad range of tissues. They have also have been used for hundreds of years for classical developmental biology studies because of their large accessible embryos. The range of tissues these animals can regenerate is fascinating, from full limbs to parts of the brain or heart, a potential that is missing in humans. Many promising research efforts are working to decipher the molecular blueprints shared across the organisms that naturally have the capacity to regenerate different tissues and organs. Salamanders are an excellent example of a vertebrate that can functionally regenerate a wide range of tissue types. In this review, we outline some of the significant insights that have been made that are aiding in understanding the cellular and molecular mechanisms of tissue regeneration in salamanders and discuss why salamanders are a worthy model in which to study regenerative biology and how this may benefit research fields like regenerative medicine to develop therapies for humans in the future.
1. Introduction
Tissue regeneration is widely distributed across both plant and animal phylogenies (Birnbaum & Sánchez Alvarado, 2008; Brockes & Kumar, 2008). The main goal of tissue regeneration is to restore the morphological and functional features of tissue after an injury. In contrast the majority of the mammals are experts at repairing wounds which results in scar tissue but have low regenerative capacity with the exception of tissues such as the liver, bone marrow, gut epithelium, that can recover after a moderate injury (Brockes & Kumar, 2008; Londono, Sun, Tuan, & Lozito, 2018). Among vertebrates, salamanders are one of the organisms that have the outstanding ability to regenerate different tissues and organs such as the limb, heart, spinal cord, and lens (Birnbaum & Sánchez Alvarado, 2008; Brockes & Kumar, 2008; Dinsmore & American Society of Zoologists, 2008; Tanaka, 2016; Tanaka & Ferretti, 2009). Different species of salamanders have been used to give some molecular and cellular insights into the mechanisms that promote a regenerative response (Joven, Elewa, & Simon, 2019) (Fig. 1).
Fig. 1.
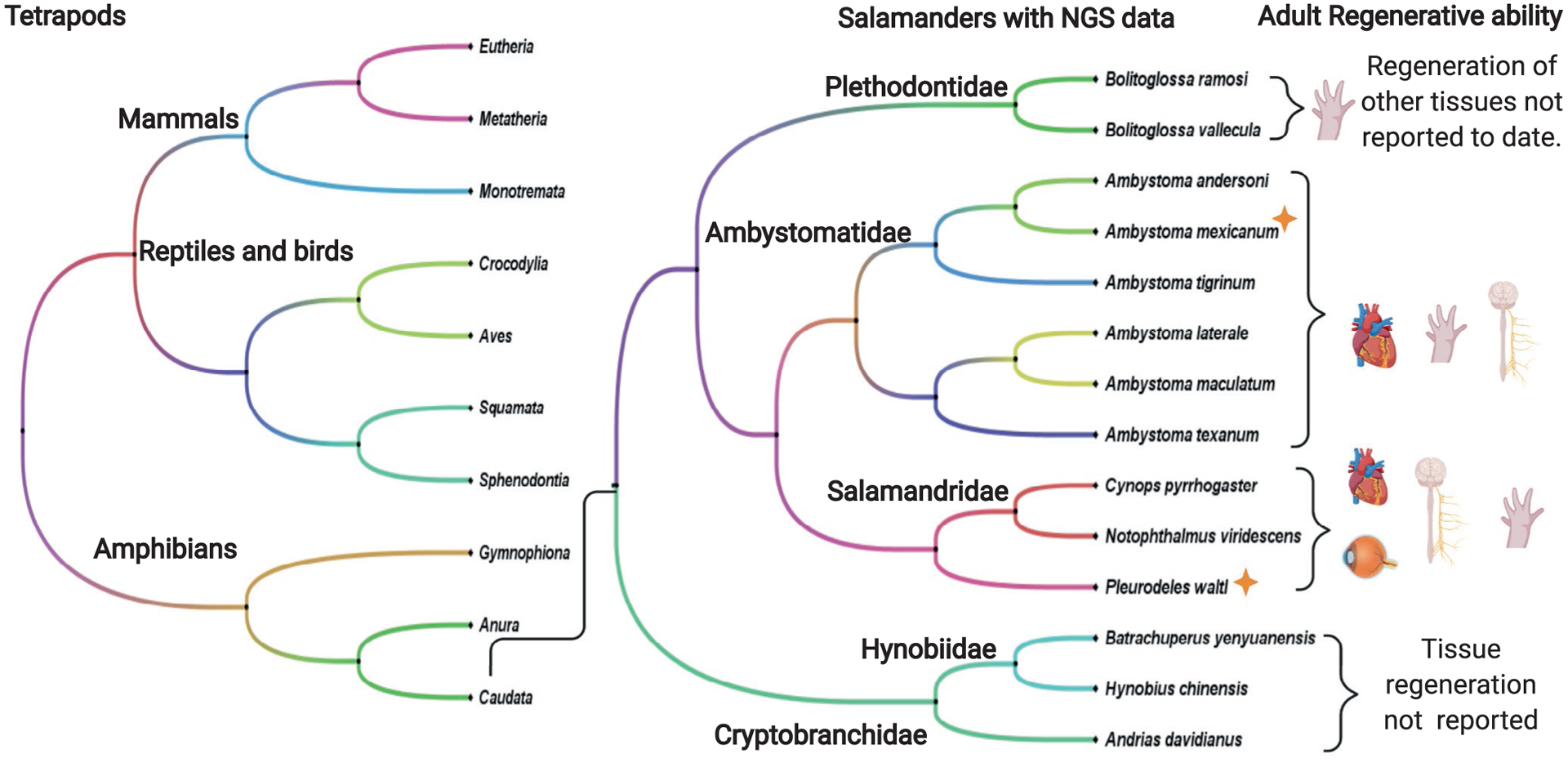
Salamanders species with Next-Generation Sequencing Data (NGS) and their regenerative capacities as adults. Salamanders are to date the only known tetrapod with the capacity to fully regenerate limbs. From the 10 salamanders families only species from 5 families have transcriptional profiling data from regenerating tissue available. A. mexicanum and P. waltl (orange star) are the only salamanders with sequenced genomes to date.
The most widely used salamander species in the developmental and regenerative biology fields are Ambystoma mexicanum (Mexican Axolotl) and Notophthalmus viridescens (Eastern Newt) (Joven et al., 2019) (Fig. 2A). However not all salamander species have the same regenerative ability, important insights into these divergent features have come from studying other species such as Pleurodeles waltl (Elewa et al., 2017), Cynops pyrrhogaster (Nakamura et al., 2014; Tsutsumi, Inoue, Yamada, & Agata, 2015), Bolitglossa ramose (Arenas Gómez, Gomez Molina, Zapata, & Delgado, 2017), Hynobius chinensis (Che, Sun, Wang, & Xu, 2014) and Andrias davidianus (Geng et al., 2015) (Fig. 1). Considering the differences in how highly related salamanders execute a regenerative program, it is clear that is will be important to understand more broadly the extent of diversity in the pro-regenerative programs found in salamanders, especially considering there are around 739 different species widely distributed around the world (AmphibiaWeb, 2019).
Fig. 2.
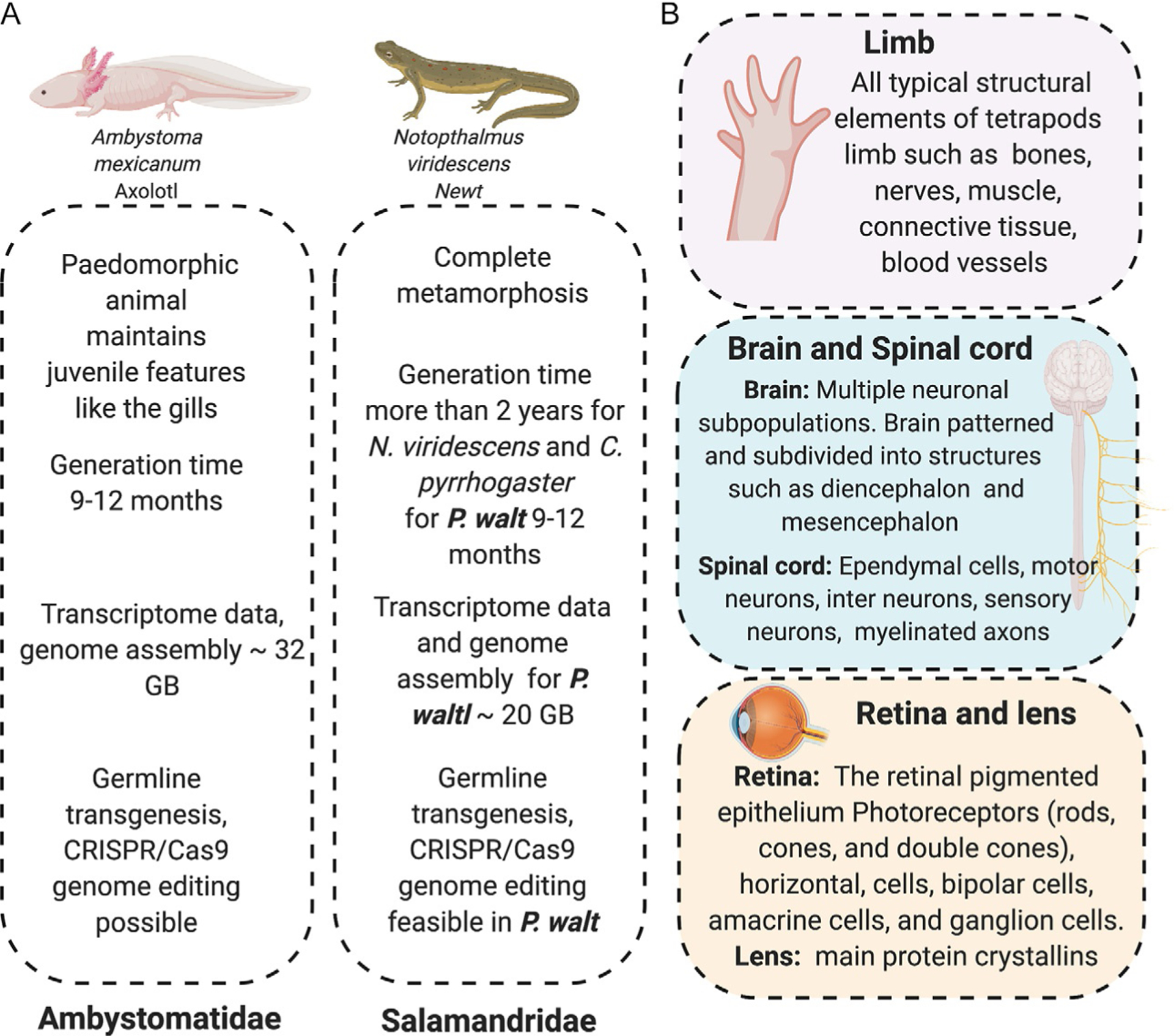
Main salamanders model used in field of regenerative biology. (A) A. mexicanum, N. viridescens and P. waltl are the main salamanders that have been established in the field to understand tissue regeneration. P. waltl has a genome assembled and the generation time is shorter than N. viridescens. (B) Main morphological features in the limb, spinal cord, brain, lens and retina that make salamanders comparable with human tissues.
To date researchers have made important discoveries regarding some cellular and molecular mechanisms that promote regenerative responses in salamanders. We now know that there is a nerve dependency to regeneration, remodeling of the extracellular matrix plays a crucial; possibly instructive role in directing cells and that the timing of the immune cells arriving to the injury site is important (Calve, Odelberg, & Simon, 2010; Campbell & Crews, 2008; Godwin, Pinto, & Rosenthal, 2013; Kumar, Godwin, Gates, Garza-Garcia, & Brockes, 2007; Tsai, Baselga-Garriga, & Melton, 2019). This knowledge may provide useful information to the field of regenerative medicine and tissue engineering in order to design novel therapies for diseases or to reduce scarring after wounding in humans (Brockes & Gates, 2014; Brockes & Kumar, 2005). Additionally these organisms may help us to understand complex diseases such as cancer, because the molecular and cellular landscape expressed during tissue regeneration is comparable with the environment during an oncogenic process (Brockes, 1998; Fior, 2014; Oviedo & Beane, 2009; Vieira, Wells, & McCusker, 2020). Finally, salamanders also could be useful organisms to understand the aging process (Vieira et al., 2020; Yun, 2015, 2018). It is known that most salamanders keep the regenerative potential throughout life; however, it is clear that younger animals regenerate faster and make fewer mistakes, suggesting that even in salamanders the aging process affects regenerative ability; however, old salamanders still regenerate much better than humans (Monaghan et al., 2014; Yun, 2015).
Here, we will review the knowledge that we have learnt from salamanders about tissue regeneration in a range of tissues and organs and how this response differs in humans. We will give an overview of the main molecular and cellular mechanisms known to be involved and how modern genetic tools have been aiding to elucidate this amazing biological process in order to translate it in the future to the field of regenerative medicine.
The urodele amphibians, commonly referred to as salamanders, have been studied for centuries. The 18th century was considered a golden era to study tissue regeneration in different research organisms, among them, Réaumur’s research on insect appendage regeneration, Tremblay’s work on Hydra regeneration, Bonnet’s work on worm regeneration and Lazzaro Spallanzani first documented salamander limb regeneration in 1768 (Dinsmore & American Society of Zoologists, 2008; Spallanzani, 1768). At that time the technology was not yet developed to enable researchers to decipher the molecular and cellular mechanisms of regeneration in salamanders. In the past 30 years, research on tissue regeneration in salamanders has started to reemerge thanks to the development of new tools that allow us to label and track cells in vivo and to explore molecular interactions in the genome; this is enabling researchers to decipher different cellular and molecular process that are key to promoting functional regeneration. In the next chapter, we will discuss some of the recent research on regeneration in salamanders such as limb, and neural tissue regeneration, these are tissues which have high homology to their mammalian counterparts (Fig. 2B).
2. Limb regeneration
Salamanders are to date the only known tetrapod able to regenerate limbs after an injury throughout their lives. Regenerative ability in salamanders appears to be an ancient trait, fossil records suggest that it has been conserved for approximately 300 million years. Using fossil records Fröbisch et al. identified that limb regeneration occured in Micromelerpeton, a distant relative of modern amphibians (Fröbisch, Bickelmann, & Witzmann, 2014). The salamander species, A. mexicanum and N. viridescens, have been the main salamanders used to study the molecular and cellular mechanism that promote limb regeneration (Brockes & Kumar, 2005, 2008; Haas & Whited, 2017; Joven et al., 2019; Simon & Tanaka, 2013; Tanaka, 2016; Tsonis & Fox, 2009). The main stages of limb regeneration after an amputation can be summarized as wound healing, blastema formation, blastema patterning and finally cell differentiation to replace all the lost cell types reviewed in (Stocum, 1979, 1991) (Fig. 3A).
Fig. 3.
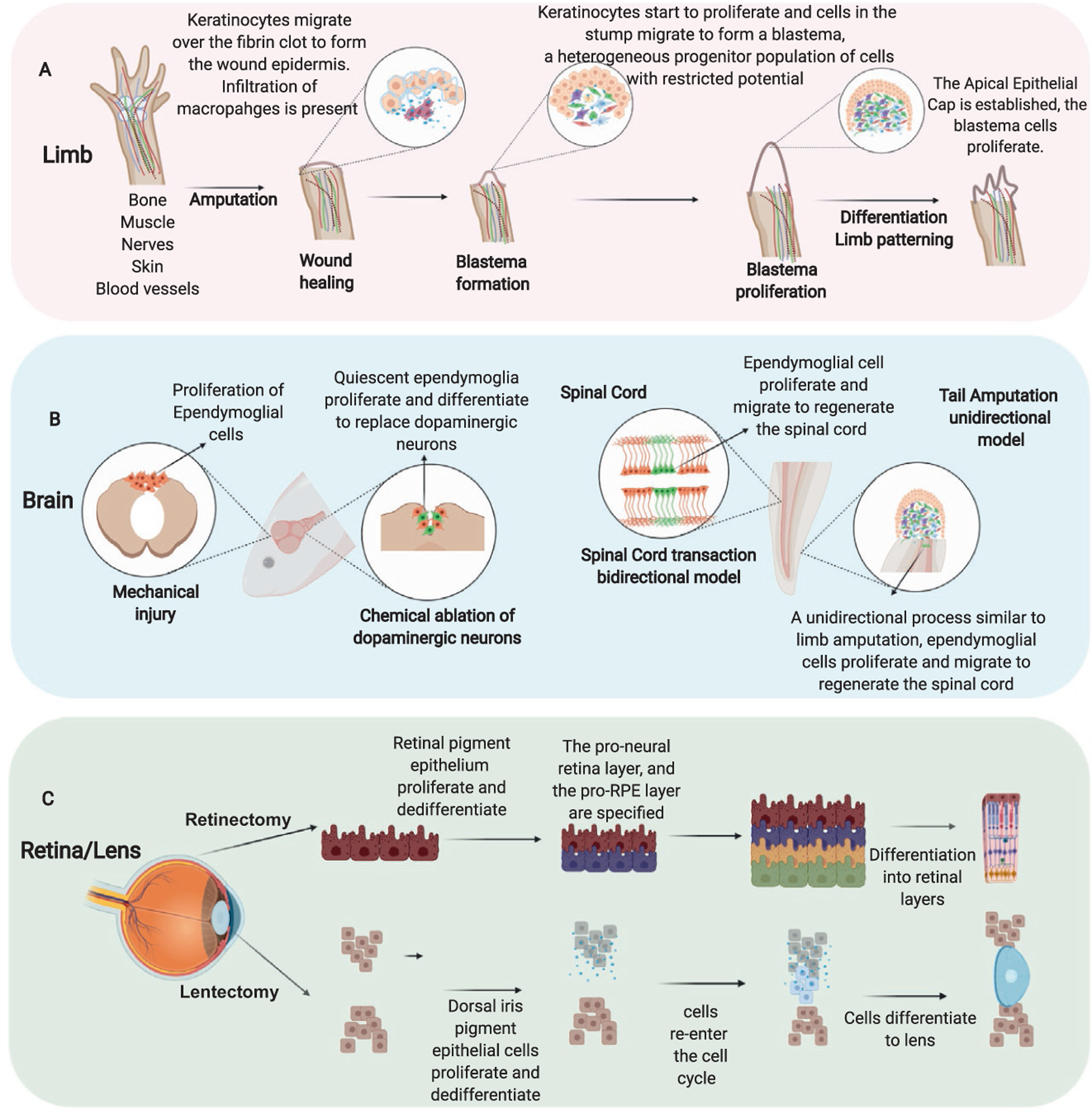
General process of tissue regeneration in salamanders. (A) Limb regeneration main stages, wound healing, blastema formation, blastema patterning and finally cell differentiation to replace all the lost cell types. (B) Brain and spinal cord regeneration is largely dependent on activation of ependymoglial cells. (C) Retina and lens regeneration is led by the transdifferentiation of the retinal pigmented epithelium (RPE) after a retinectomy and the dorsal iris pigment epithelial cells after a lentectomy.
After amputation, the wound healing phase is characterized by the formation of a fibrin clot and a fast migration of non-proliferative keratinocytes (Arenas Gómez, Sabin, & Echeverri, 2020; Chalkley, 1954; Murawala, Tanaka, & Currie, 2012; Thornton, 1954). Successful scar-free wound healing is necessary for regeneration to proceed. During the wound healing phase, cell migrate to close the wound and from the distal epithelium commonly referred to as the wound epithelium. Throughout the migration process the cells differentially regulate gene expression and potentially begin to express and/or secrete proteins that activate surrounding cells to form a blastema (Campbell & Crews, 2008). Older studies have shown that covering a wound with uninjured skins inhibits the regeneration process, this suggests that the changes in gene expression in response to injury are crucial for the scar free regenerative process to proceed (Tassava & Garling, 1979; Tassava & Loyd, 1977). The wound epithelium formed during regeneration is considered a specialized structure as when the keratinocytes start to migrate they start to produce their own ECM composed mainly of laminin, collagen type IV, collagen type XII, MMP3, and MMP9 (Campbell & Crews, 2008). During re-epithelization of the wound site other crucial processes are advancing in the stump tissue such as histolysis of remnant injured tissues like dermis, muscle, bond, and remodeling of ECM. Equally important, an immune response characterized by the increase of anti-inflammatory cytokines (e.g., IL-4, IL-10, IL-13), proinflammatory cytokines (e.g., TNF-α, IL-17) and macrophages chemotactic molecules (e.g., CCL4, CCL3, CXCL12) is activated by 1 day post-amputation (dpa) until 15 dpa (Godwin et al., 2013; Tsai et al., 2019).
Once the wound epithelium is formed the basal keratinocytes start to proliferate; they form an epithelium layer that is called the apical epithelial cap (AEC), which is thought to play a similar role to that of the apical ectodermal ridge during limb development (Chalkley, 1954; Iten & Bryant, 1973; Thornton, 1954). The AEC is also referred to in the literature as the regenerative epithelium (Campbell & Crews, 2008; Campbell et al., 2011; Christensen & Tassava, 2000; Satoh, Graham, Bryant, & Gardiner, 2008).
This structure, AEC or regenerative epithelium, is an important source of molecules that are thought to be essential in recruiting cells to the injury site to form a blastema; which is defined as a mound of proliferating cells that will eventually differentiate and replace the lost limb (Campbell et al., 2011; Satoh, Bryant, & Gardiner, 2012; Satoh, Graham, et al., 2008). The blastema is a pool of heterogeneous progenitor cells with mainly restricted potential that are going to be the source of the cells to re-establish the tissues that form the limb (Gardiner, Muneoka, & Bryant, 1986; Kragl et al., 2013; Muneoka, Fox, & Bryant, 1986; Nye, Cameron, Chernoff, & Stocum, 2003; Satoh, Bryant, & Gardiner, 2008). One of the big questions in the field is, what are the main cellular and molecular mechanism that contribute to the generation of the progenitor cells that form the blastema? One of the key components is the remodeling of the ECM, where spatio-temporal changes of Tenascin (TN), Fibronectin (FN), and Hyaluronic acid (HA) are essential during the early days to promote regeneration. In newts, a transitional matrix composed of TN and FN favors promotion of cell proliferation and an ECM rich in HA, TN, FN infiltrates the damaged basal membrane of the skeletal muscle to trigger dedifferentiation of muscle cells (Calve et al., 2010). Muscle dedifferentiation is a process which occurs in response to injury, causing a multi-nucleated muscle fiber to fragment and give rise to mono-nucleated cells that enter into the blastema (Hay, 1959, 1966). Muscle dedifferentiation is an important source of new cells to regenerate muscle during limb regeneration in newts. Interestingly the closely related axolotls use their resident +pax7 stem cells (satellite cells) to regenerate muscle in the context of limb regeneration. This difference was elegantly shown using the power of transgenic animals via a Cre-loxP genetic fate mapping of skeletal muscle during limb regeneration in A. mexicanum and N. viridescens (Sandoval-Guzman et al., 2014). It remains to be seen how widespread these two mechanisms of regenerating muscle are among other species of salamanders. Axolotls in this scenario appear more similar to mouse and humans which both can repair small pieces of muscle damage by activating their resident muscle stem cells, the Pax7 positive satellite cell (Lepper, Partridge, & Fan, 2011; Sambasivan et al., 2011). However, limb regeneration requires more than just muscle cells, so where do the other cells come from? The pool of progenitors cells in the blastema during limb regeneration express some but not all of the factors used to make induced pluripotent stem cells, c-myc, oct4, sox2 and klf4, commonly referred to as the Yamanaka factors (Takahashi & Yamanaka, 2006). Newts express three of the four Yamanaka factors used to induced pluripotent stem cells (iPSCs) (klf4, sox2, c-myc) (Maki et al., 2009) while axolotls limb blastema cells only express two of them (klf4 and c-myc), nevertheless further investigation is required to understand the dynamic of this factors in salamanders (Knapp et al., 2013). The cross-species conservation of the genetic factors necessary for forming pluripotent stem cells suggests that genetic factors necessary for regeneration are conserved but potentially not activated in mammals in response to injury. However, the exact role of these factors in regeneration has yet to be deciphered, the upregulation of these factors in newt might suggest they play a role in muscle dedifferentiation or that the cells in the newt blastema are pluripotent whilst the cells in the axolotl blastema, which only express klf4 and c-myc may be more restricted in their potential.
The blastema is a structure unique to many animals that regenerate, so where do all the cells come from to form a blastema? Early skin grafting work suggests that the dermal fibroblasts from the skin are the main cell contributor to the blastema cells (Muneoka et al., 1986). This work was further supported using grafting between transgenic GFP axolotls and non-transgenic animals, which also showed that many cells in the blastema come from the dermal fibroblasts in the skin (Kragl et al., 2009). This transgenic approach, of grafting embryonic tissue between transgenic and non-transgenic animals, also allowed the contribution of other cells types to the blastema to be mapped, suggesting that most cells remain lineage restricted during regeneration but that plasticity was observed in fibroblasts (Kragl et al., 2009). More recent work, using a Cre-loxP-Prrx1 reporter, that labels the fibroblasts of the connective tissue, combined with single-cell transcriptome (scRNA-seq) demonstrated the cell heterogeneity of the connective tissue that contributes to the limb blastema. Prrx1 is a homeobox gene expressed in the connective tissue precursors during limb development and regeneration. Interestingly, at an early stage point, the gene expression profile present in the dedifferentiated connective tissue cells differed for the limb development bud, whereas at 11 dpa the blastema cells have a similar genetic state as that of a limb bud (Gerber et al., 2018). This supports the idea that the early stages of forming a blastema requires a distinct molecular circuitry that is different from how limb development is executed.
Once the blastema is established these cells must then proliferate, work from several groups has shown that the interplay between the AEC and the nerve is crucial to stimulate the proliferation of the blastema cells. Initially the AEC had been proposed as the main source of mitogenic factors to induce proliferation in the blastema; however, other research has shown that there are factors released from the nerve that are essential for proliferation (Stocum, 2017). Among these factors is the expression of the anterior gradient protein (nAG) first described in N. viridesence. nAG is expressed in the first days (5–8 dpa) in the Schwann cells and at 10 dpa the expression switches to the AEC. The expression of nAG depends on the nerve, when the regenerative limb is denervated the expression of nAG is abolished (Grassme et al., 2016; Kumar et al., 2007). However, during limb development, nAG is expressed in the glands of the apical ectodermal ridge and is not nerve dependent (Kumar & Delgado, 2011). This is one interesting example of one of the different mechanisms between limb development and limb regeneration. When nAG starts to be expressed in the AEC the blastema cells express the cell surface protein PROD1 which is expressed in a proximal to distal gradient (da Silva, Gates, & Brockes, 2002). nAG was identified as the nerve dependent ligand for PROD1 (Kumar et al., 2007). To date PROD1 is thought to be a taxon-specific protein found in salamanders and its expression is a key to enable the interaction with nAG to activate blastema cell proliferation (Garza-Garcia, Driscoll, & Brockes, 2010). In newts PROD1 interacts with the EGFR by a GPI-anchor, which is a residue in the α-helical region of the protein crucial for the interaction and action of PROD1 in newts. Curiously, axolotls also have a PROD1 gene but it’s lacks a GPI anchor domain, however, it also interacts with the EGFR (Blassberg, Garza-Garcia, Janmohamed, Gates, & Brockes, 2011). It is known that PROD1 is expressed in different salamander families, however, how conserved this cell-signaling axis is in other species of salamanders is an open question (Garza-Garcia et al., 2010; Geng et al., 2015).
Several other factors involved in blastema cell proliferation in A. mexicanum have been reported such as FGF8, BMP (Satoh, Makanae, Nishimoto, & Mitogawa, 2016), FGF2 (Mullen, Bryant, Torok, Blumberg, & Gardiner, 1996), Neuregulin 1 (Farkas, Freitas, Bryant, Whited, & Monaghan, 2016). Neuregulin 1 (NRG1) is a nerve derived growth factor which can rescue proliferation in a denervated limb but intriguingly is also expressed by other cells types at different stages of the regenerative process. NRG1 and its receptors (ERBB2, ERBB3) are expressed by the basal keratinocytes of the AEC and by the mesenchymal cells of the blastema; however, the expression of NRG1 in the blastema cells alone does not suffice to trigger a mitotic response. The input of motor and sensory nerves are crucial to increase expression of NRG1 to induce the proliferation of the blastema cells (Farkas et al., 2016). This gives insight to the complexity of the crosstalk between the AEC, nerve, and blastema cells to trigger the correct amount of cell division to enable a new limb to be regenerated.
During the blastema formation, another process is taking place the establishment of the axial patterns of the regenerative limb, which is necessary to restore the 3D limb structure (Brockes & Kumar, 2005; Bryant et al., 2017; Simon & Tanaka, 2013). One of the key molecules that helps to reestablish the positional hierarchy of the cells in a proximal to distal gradient is retinoic acid (RA) (Crawford & Stocum, 1988; Maden, 1982). Retinoic acid has been shown to change the molecular positional identity of cells in a blastema; a hand blastema exposed to specific level of retinoic reprograms and regenerates all upper arm elements and then the hand (Crawford & Stocum, 1988, Maden, 1982). The gene Prod1 was originally identified as a cell surface gene that is RA responsive (da Silva et al., 2002). Prod1 has also been identified to be expressed in a proximodistal pattern and when overexpressed in distal cells in a blastema changes their identity to proximal (Echeverri & Tanaka, 2005). Many Hox genes play important roles in patterning the blastema, the homeobox containing genes meis1 and meis2 have been identified as target genes of RA proximalizing activity during limb regeneration (Mercader, Tanaka, & Torres, 2005). Meis1 and meis2 have been shown in in vitro assays to regulate the axolotl PROD1 promoter (Shaikh, Gates, & Brockes, 2011). Furthermore, other homeobox genes such as hoxa9, hoxa13, hoxa11 are also expressed in a proximal to distal gradient in the limb blastema (Roensch, Tazaki, Chara, & Tanaka, 2013). It is well-established now that many genes that are used during limb development are re-used during limb regeneration, like genes that belong to the signaling pathways of WNT, BMP and Shh (Bryant, Endo, & Gardiner, 2002; Imokawa & Yoshizato, 1997; Knapp et al., 2013; Monaghan et al., 2012; Nacu, Gromberg, Oliveira, Drechsel, & Tanaka, 2016; Nacu & Tanaka, 2011; Satoh et al., 2016). Retinoic acid has been shown to play similar key roles in mammalian limb bud development, excess RA in limb development leads to defects in expression of shh and meis genes and subsequent mis-patterning of the limb, again illustrating the high degree of conservation of pathways between non-regenerative and regenerative animals (Dudley, Ros, & Tabin, 2002; Giguere, Ong, Evans, & Tabin, 1989; Logan, Simon, & Tabin, 1998). However, it is also becoming clear that the circuitry that activates and regulates these genes during regeneration is a unique combination and is not simply a recapitulation of limb development. Recent single cell approaches have given new insights into the molecular program and signatures of cells in the blastema, including intriguing data suggesting that connective tissue cells in the limb revert to a homogenous progenitor state in response to limb amputation (Gerber, Gerber, et al., 2018). Interestingly, a related paper using similar techniques suggests that the blastema contains a fibroblast-like progenitor cell (Leigh et al., 2018). Importantly both of these studies now show that at the molecular level the blastema essentially becomes an embryonic like limb bud, suggesting that regeneration re-uses the embryonic framework for building a limb. Most of the genes that have been identified to play crucial roles in blastema formation are present in humans; however, we do not form a blastema in response to injury. This lack of blastema appears to be one of the key missing aspects of response to injury in salamanders versus mammals and a key to regenerative therapies may lie in identifying how we direct cells toward blastema formation rather than to scar formation.
3. Regeneration of neural tissue
3.1. Spinal cord and brain regeneration
An area of immense interest for regenerative therapies is nervous system tissue. Millions of people around the world live with neurodegenerative disease and thousands more are diagnosed each year, with very limited therapies available. In contrast; salamanders can functionally regenerate complex networks of neural tissue, ranging from the spinal cord and brain to tissues in the eye (Freitas, Yandulskaya, & Monaghan, 2019; Diaz Quiroz & Echeverri, 2013; Joven et al., 2019; Tazaki, Tanaka, & Fei, 2017) (Fig. 3B–C). Nervous tissue regeneration is not limited to salamanders, there are many examples of vertebrates than can regenerate the nervous systems like lamprey, zebrafish and Xenopus (Davis, Troxel, Kohler, Grossmann, & McClellan, 1993; Edwards-Faret et al., 2017; Freed, de Medinaceli, & Wyatt, 1985; Ghosh & Hui, 2018; Jacyniak, McDonald, & Vickaryous, 2017). Xenopus is an interesting example as their extensive nervous system regeneration is limited to their pre-metamorphic state, loss of regenerative ability has to some extent been co-related to the development of a more complex immune systems and to lack of activation of neural progenitor cells among the amphibians (Gibbs, Chittur, & Szaro, 2011; Lee-Liu, Mendez-Olivos, Munoz, & Larrain, 2017).
Salamanders, like A. mexicanum and N. viridescens, are competent to regenerate the spinal cord through-out life (Butler & Ward, 1965, 1967; Clarke, Alexander, & Holder, 1988; Diaz Quiroz & Echeverri, 2013; Piatt, 1955; Tazaki et al., 2017). Different injury models have been used to understand the cellular and molecular profiles that drive this process. The tail amputation model which is unidirectional, is an injury model most comparable to limb amputation where the formation of a blastema is a key point in the process (Iten & Bryant, 1976). In contrast the spinal cord transection or ablation model is bidirectional, where cells from both sides of the injury side contribute to the regeneration of the ependymal tube and new neurons, but no clear blastema is formed; however, there is extensive cell death of different non-neural cell types around the injury site (Sabin, Santos-Ferreira, Essig, Rudasill, & Echeverri, 2015). This type of injury model is closer to spinal cord injuries that occur in humans; yet the outcomes are polar opposites.
Following a spinal cord injury (SCI) in salamander, the cells that line the central canal often referred to as glial cells, ependymal cells or ependymoglial cells respond to the injury signal by migrating and proliferating (Albors et al., 2015; Egar & Singer, 1972; O’ Hara, Ega, & Eag, 1992; O’Hara & Chernoff, 1994; Sabin et al., 2015). These cells have a characteristic oval shape, send long processes out to the plial surface and express both glial acidic fibrillar protein (GFAP), the classic glial cell marker and Sox2, the traditional neural stem cell marker (Fei et al., 2014; McHedlishvili, Epperlein, Telzerow, & Tanaka, 2007; McHedlishvili et al., 2012; O’Hara, Egar, & Chernoff, 1992). These cells act as neural stem cells after injury; they migrate and proliferate to first repair the lesion and then differentiate to replace lost glial cells and neurons. This provides the environment to allow the axon growth to ultimately regain sensory and motor function comparable to a pre-injury state. One major difference between salamander and mammals after SCI is that mammals form a glial scar, which is the main barrier to axonal regrowth (Bradbury & Burnside, 2019; Bradbury & McMahon, 2006; Fitch & Silver, 2008). Understanding the early signals that direct the cells that line the central canal toward a regenerative response versus formation of a glial scar may be key to unlocking regenerative potential, as previous work in mammals has shown that the severed axons have the potential to regrow if given the right environment (Adams & Gallo, 2018; Bradbury & Burnside, 2019).
A significant difference between humans and pro-regenerative vertebrates is the response of the glial cells to injury. Humans activate their glial cells to form a glial scar which prevents more injury from occurring but also reprograms the glial cells to reactive astrocytes that express many proteins that are inhibitory to axonal regrowth including vimentin, GFAP, chondroitin sulfate proteoglycans (CSPGs) reviewed in Adams and Gallo (2018), Dyck and Karimi-Abdolrezaee (2015), Fitch and Silver (2008), Silver (2016), Silver and Miller (2004), Tran, Warren, and Silver (2018). In contrast axolotl glial cells ramp up their cell division such that division happen faster, this process is dependent on the planar cell polarity pathway (Albors et al., 2015). Axolotls glial cells are activated to divide and migrate in a zone of 500μm adjacent to the injury site in both tail amputation and spinal cord ablation models (McHedlishvili et al., 2007; Sabin et al., 2015). Axolotl glial cells express GFAP, a traditional marker of glial cells that is transcriptionally upregulated after injury in humans and has become a hallmark of reactive gliosis; however, axolotl downregulate GFAP in response to injury. Recent work from Sabin et al. has identified a key highly conserved transcriptional complex that differs in its make up between axolotls and humans and is involved in the regulation of GFAP. The AP-1 transcription factor made up of the heterodimer c-Fos and c-Jun is activated after injury in human or mouse glial cells and binds to the promoter of GFAP activating its transcription (Gao et al., 2013). Axolotls also from an AP-1 transcription factor after injury but they form the complex via heterodimerization of c-Fos and JunB, which in contrast leads to the downregulation of GFAP. If c-Jun is activated in glial cells then genes involved in reactive gliosis are activated like CSPGs, vimentin and collagen and axon regeneration is inhibited (Sabin, Jiang, Gearhart, Stewart, & Echeverri, 2019). The formation of non-canonical AP-1 transcription factor may be a key step that prevents glial scar formation in axolotls and promotes a regenerative response. How conserved this molecular circuitry is for regulating the glial cell response to injury is known but it will be interesting in the future to examine it more broadly across several salamander species. As we move toward translating knowledge from salamanders to humans this molecular complex gives us a starting point of highly conserved genes to start to modulate in vitro in mouse or human cells. Today it is unknown if salamanders spinal cords have the same complexity of glial cells and astrocytes that humans have. It will be important to address this question by taking advantage of the advances in molecular technologies like single cell sequencing to identify cell signatures for all cells within an adult salamander spinal cord.
Thus far much of the more recent work in salamander spinal cord regeneration has focused on the role of the glial cells. A major question in the field is how is function restored. Earlier work in field has attempted to track the axonal regeneration using retrograde tracing. This works suggests that retrograde neurons are restored to their almost original number after complete transection (Clarke et al., 1988). How regrowing axons find their targets and how the functional circuits are restored is completely unknown. It is essential in the future to re-examine these aspects of regeneration with current technologies to understand how plasticity is there in making new connections and how is information relayed to the brain to reconnect circuits.
3.2. Brain regeneration
Salamanders can also functionally repair lesions to the brain. As in SCI, different injury models have been established to understand brain regeneration in salamanders. This includes, specific brain region extirpation (e.g., unilateral forebrain extirpation) and selective ablation of neuronal subtypes (Joven et al., 2019). When a brain extirpation is performed the GFAP positive ependymal glial cells are the cells that proliferate and differentiate to recover the neuronal diversity and form the new inter-neuronal connections that restore function but interestingly are not a faithful replication of the original (Amamoto et al., 2016; Maden, Manwell, & Ormerod, 2013; Urata, Yamashita, Inoue, & Agata, 2018). Axolotls can regenerate the full diversity of neurons that were present before injury, but although they regain function, they do not regenerate the same circuitry. Work in adult axolotl pallium has shown that while after mechanical injury new born neurons organise the same architecture of the brain they appear to fail to regenerate the long distance axonal tracts and exact circuitry that was present before injury (Amamoto et al., 2016). However, it is possible that the establishment of these long tract circuitry occurs over a much longer time period and longer observation points are necessary.
Work in the adult newt P. waltl using a model of excision of a quarter of the mesencephalon has given some interesting molecular insights into regeneration over the period of 1.6 years. This long-term observation of the brain regeneration process suggests there is an immediate response which is imperfect, followed by a longer regenerative response that leads to a better regenerative outcome. This data suggests that the rostral caudal region exhibits a self-organizing regenerative ability dependent upon the Pax7+ ependymoglia cells, while the isthmic region may represent a very early neurogenic niche (Urata et al., 2018).
Similarly, during the ablation of specific regions of the brain, the reactivation of quiescent resident GFAP+ ependymoglial cells are crucial for both axolotl and newt brain regeneration (Berg et al., 2010; Maden et al., 2013). This process is under the regulation of at least one neurotransmitter; dopamine. In newts dopamine appears to be essential to keep the ependymal glial cells in a quiescent state, ablation of dopamine neurons activates the ependymal glial cells to proliferate and undergo neurogenesis (Berg, Kirkham, Wang, Frisén, & Simon, 2011). Additionally, a Parkinson-like model has been developed in salamanders, where specific ablation of dopaminergic neurons was achieved using 6-hydroxydopamine. Thirty days post-ablation the neurons were regenerated, suggesting that the main cellular process involved was the reactivation of ependymoglial cells from the ventricular region that are GFAP and Sox2+ cells that mature into dopaminergic neurons to restore the affected area (Parish, Beljajeva, Arenas, & Simon, 2007). Salamanders are a promising model to understand the cellular and molecular mechanisms involved in the regeneration of the nervous system. This work illustrates the high conservation of cell identities and signaling molecules between salamanders and humans and strengthens the possibilities for developing novel therapeutic interventions for neurodegenerative diseases based on this research (Hedlund et al., 2016).
3.3. Lens and retina regeneration
In salamanders, the capacity to regenerate optic structures varies dramatically among different species. Newts such as N. viridescens, P. walt, and C. pyrrogaster retain the capacity to regenerate the lens and retina through-out their lives. However, salamanders like A. mexicanum lose this capacity 2 weeks post-hatching (Suetsugu-Maki et al., 2012) and different species of the family Plethodontidae are unable to regenerate lens as adults (Henry & Hamilton, 2018; Henry & Tsonis, 2010; Stone, 1967). This has led to newts being one of the main models that have been used in the field to understand this regenerative process. However, to be able to understand where in the evolution this trait was lost or potentially gained in certain salamanders then a comparative analysis across many salamander species is necessary to decipher the molecular circuitry that defines lens and retina regeneration.
The main cellular process used for lens and retina regeneration is transdifferentiation, where differentiated somatic cells dedifferentiate to convert into another cell type with a different developmental trajectory (Tsonis & Del Rio-Tsonis, 2004) (Fig. 3C). After a lentectomy, the dorsal iris pigment epithelial cells (PECs) start to proliferate and dedifferentiate, at the 4 day post-lentectomy (dpl) the cells re-enter the cell cycle and by 8 dpl begin to form a vesicle, at 15 dpl lens fiber start to be formed and lens regeneration is considered complete by 25–30 dpl (Eguchi, 1963; Tsonis & Del Rio-Tsonis, 2004; Tsonis, Madhavan, Tancous, & Del Rio-Tsonis, 2004). One of the initial signals after injury is the expression of thrombin from the dorsal iris, the ventral iris also has PECs but the lens never regenerates from this side, suggesting that the absence of thrombin expression in the ventral iris is part of the reason for lack of regeneration from this tissue (Imokawa, Simon, & Brockes, 2004). Interestingly, in vitro isolation of PECs from different organisms from dorsal and ventral iris including mammals, are capable of being induced to transdifferentiate in specific conditions to structures that resemble lentoids (Tsonis, 2006; Tsonis, Jang, Del Rio-Tsonis, & Eguchi, 2001). This suggests that elucidating the circuitry to induce the correct molecular environment that promotes lens regeneration is a key to understand this process.
Another important signal is FGF, when FGFR signaling is inhibited, lens regeneration is abolished. Like thrombin; FGF is also not expressed in the ventral iris, suggesting FGF signaling is crucial for the lens regeneration from the dorsal iris (Del Rio-Tsonis, Trombley, McMahon, & Tsonis, 1998; Hayashi, Mizuno, Ueda, Okamoto, & Kondoh, 2004; Rio-Tsonis, Jung, Chiu, & Tsonis, 1997). Using RNA-seq and comparing the genes expression profile between the dorsal and ventral iris after 4–8 dpl, Sousounis et al. identified a group of genes that are upregulated specifically in the dorsal iris. Among these genes are those related to cell cycle, cytoskeleton, transcriptional apparatus, and the immune system (Sousounis et al., 2013). The axolotl loses the ability to regenerate the lens as it matures, the timing correlates with the development of a more mature immune system and other hallmarks of aging. Transcriptional profiling studies on different ages of axolotl lens tissue reported that components of the immune system such as genes associated with macrophages, basophils and B-cells among others were upregulated mainly in the old larvae, which are unable to regenerate lens; however, there is no functional data to provide evidence that this loss is due to increased immune cell activity (Sousounis, Athippozhy, Voss, & Tsonis, 2014). In newts a model has been proposed where the leucocytes are attracted to the fibrin clot which is composed of thrombin and transmembrane protein tissue factor after a lentectomy and there the leucocytes could activate the expression of the FGF2 which induces the re-entry to the cell cycle of the PECs (Godwin, Liem, & Brockes, 2010). This suggests that the immune system has a positive role during lens regeneration in newts. It would be interesting to know if there is a difference in immune cell composition between the axolotls and newts in the context of lens regeneration. However, there may be other reasons to explain the lack of regeneration in axolotls, for example, 2 weeks after hatching axolotl larvae PECs start to express several regulators of cellular differentiation such notch and bmp, which may be implicated in the loss of the capacity to transdifferentiate of PECs. Additionally factors related to aging have been found to be upregulated in older axolotl larvae such as genes that restrict DNA synthesis and cell proliferation (Sousounis et al., 2014). In contrast aging does not effect lens regeneration in newts. In a long-term project using C. pyrrhogaster the lens was removed 18 times from the same animals for 16 years and by the time of the last tissue collection, the animals were at least 30 years old. The author compared the gene expression profile and the structural properties of the lens of young animals and old animals and no changes were observed (Eguchi et al., 2011). This suggests that age is not a limitation for lens regeneration in this species of newts but more studies are needed to understand the influence of aging-related genes in the context of lens regeneration in other salamanders. This is an emerging field of research in the study of tissue regeneration in salamanders and will discussed later in this chapter.
Salamanders can also regenerate the retina after extirpation of retina is performed. As in lens regeneration, transdifferentiation of the retinal pigment epithelium (RPE) is the source of cells to regrow the new retina. The RPE are quiescent cells and when an injury happens these cells are activated to transdifferentiate, start dividing again; many of the signaling molecules used during development like the Fgfs and Bmps play an important role in retina regeneration (Chiba, 2014). A second cell source has also been reported to contribute to the retina regeneration, these are the retinal progenitor cells present in the ciliary marginal zone (Chiba, 2014; Chiba et al., 2006; Grigoryan & Markitantova, 2016).
The RPE in humans and newt show close similarities in their intrinsic properties; however, in humans after a retinal injury the RPE has a different response whereby they lose their epithelial characteristics, migrate, and proliferate by transforming into mesenchymal cells (epithelial-mesenchymal transition, EMT) such as myofibroblasts, these cells are the ones that are going to contribute to retinal disorders. Interestingly when the human RPE undergoes the EMT these cells express c-myc, klf4, pax6 and mitf which make them behave as “stem cells,” however, they do not express sox2, which is a crucial transcription factor for neural specification in the retina (Salero et al., 2012). Islam et al. (2014) reported that the RPE in newts doesn’t contain retinal stem/progenitor cells. Instead after a retinal injury RPE cells re-enter the cell cycle (5–10days post-injury) and are reprogrammed via upregulation of reprogramming factors such as c-myc, klf4, pax6, sox2, and mitf to transform into multipotent cells termed retinal pigment epithelium stem cells. These cells have the potential to differentiate into two cell populations, the pro-neural retina layer, and the pro-RPE layer to rebuild the new retina (Islam et al., 2014). The exact cell reprogramming mechanisms used by salamander retinal cells is one of the big questions that remains in the field; interestingly they use only some of the factors that are needed to generate human iPSC. However, they appear to have all of the classical reprogramming genes like Klf4, Oct4, Myc and Sox2 and interestingly axolotl Pou2 can replace human Oct4 to reprogram somatic nuclei to pluripotent stem cells (Tapia et al., 2012). This suggests that there is a high degree of conservation among reprogramming circuitry but that there are different combinations of routes to the same endpoint. Nevertheless the biggest blackbox is still how to redirect cells toward the correct differentiation program to regenerate the correct tissue.
4. Cancer and regeneration: Similar pathways different outputs
Tumors have been described as wounds that never heal (Flier, Underhill, & Dvorak, 1986). However, wound healing and tumorigenesis share crucial mechanisms such as proliferation, migration, angiogenesis, and ECM remodeling (Flier et al., 1986). The formation of spontaneous tumors in salamander is very low (Brunst & Roque, 1969; Harshbarger, Chang, DeLanney, Rose, & Green, 1999; Khudoley & Ellselv, 1979; Shioda, Uchida, & Nakayama, 2011). Some studies have even suggested that amphibians are resistant to developing malignant neoplasms; however, tumors have been found in various salamanders (Okamoto, 1987, 1997; Rose & Rose, 1952; Rose et al., 1949; Tsonis & Eguchi, 1981, 1982). The similarities between a malignant tumor and a regenerating blastema has been recognized and pondered on by scientists for years. Both are mounds of proliferating cells, but one controls its environment in a very different manner to eventually regenerate specific tissue types while the other often invades the body destroying the tissues with devastating outcomes to human health (Brockes, 1998; Oviedo & Beane, 2009; Pearson & Alvarado, 2009; Sarig & Tzahor, 2017; Vieira et al., 2020).
The permissive microenvironment available for tissue regeneration in salamanders is very similar to the tumor environment, for example, the ECM composition, like fibronectin and tenascin which in a tumor are an important component for cell migration (Gopal et al., 2017; Sun, Londono, Hudnall, Tuan, & Lozito, 2018). Proteins of the ECM are not the only molecular commonalities, a database published recently summarizes the commonly expressed genes during tissue regeneration and cancer in mammals (Zhao, Rotgans, Wang, & Cummins, 2016). This represents an important source to develop studies that help further the understanding of the dynamics of these two-biological processes.
Lens regeneration represents a good model to test different conditions because regeneration only happens in the dorsal iris, the ventral iris doesn’t have this capacity (Okamoto, 1987, 1997). One study using different carcinogenic chemicals (e.g., nickel subsulfide) proved that when these chemicals are introduced in lentectomized newt eyes the dorsal iris proceeds normally with the len regeneration. However, in the ventral iris the formation of multiples lens was observed and the production of a melanoma-like ocular tumor were present only in the ventral iris. Surprisingly, when a high concentration of chemical was used the dorsal iris regeneration was stopped but didn’t show any formation of a tumor. Similarly, using the limb regeneration model, the blastema was exposed to chemical carcinogens, defects in regeneration and abnormalities in the limb patterning was observed but no uncontrolled growth similar to a tumor formation was seen (Tsonis & Eguchi, 1981, 1982). Other research has shown that if a renal tumor is grafted to the skin of a salamander forelimb, it starts to grown and generates a malignant mass, however, if a limb amputation is performed bissecting the tumor, the regeneration proceeds normally and the tumor heals, suggesting a reversion in the tumorigenic mechanism (Rose et al., 1949). Those observations raise different questions like, why are the dedifferentiated cells in a regeneration field more resistant to forming tumors? Or, is there specific molecular circuitry in the regeneration microenvironment that controls the gene network necesscary to induce regeneration rather than uncontrolled proliferation? One theory that has been proposed to understand this behavior in organisms that have the natural capacity to regenerate and avoid tumor formation is the expression pattern of tumor suppressor genes such as p53, retinoblastoma, Pten and Hippo; reviewed in Pearson and Alvarado (2009) and Pomerantz and Blau (2013). In non-regenerative organisms like mammals, the mutation in these genes is a known trigger of the tumorigenesis process (Wang, Wu, Rajasekaran, & Shin, 2018). However, these tumor suppressor genes in organisms like salamanders could be regulating the proliferation, dedifferentiation, and genomic stability of the cells implicated in the regeneration (Pomerantz & Blau, 2013). These genes are evolutionary conserved, found in different vertebrates and invertebrates organisms (Pearson & Alvarado, 2009). Nonetheless, in vertebrates like mammals the family of genes of tumor suppressors has diversified, for example, the family of p53 which include also p63 and p73, and in humans the tumor suppressor Arf; however, Arf is not found to date in any highly regenerative organisms like amphibians (Pomerantz & Blau, 2013). Understanding how the expansion of these tumor suppressor genes has occurred from salamanders to mammals, and how highly regenerative species use these gene families in a regeneration context may explain why the regeneration blastema is refractive to tumorigenesis at the molecular level. Elucidating this process could help to understand the genetic balance necessary to trigger a regenerative and not a tumorigenesis process in mammals.
5. Aging, cellular senescence and immune system: Their influence on tissue regeneration
Although humans have some limited capacity for regeneration, this capacity declines with age. As we age humans often accumulate more underlying health problems including high blood pressure, inflammation, high blood sugar levels, which all contribute negatively to our limited regenerative ability. A common question in the regeneration field is, do salamanders regenerate throughout life? The best example that they do, is the above discussion of lens regeneration where newts have been shown to perfectly regenerate the lens 18 times over a 30-year life span. The data on other types of regeneration in salamanders is much more limited. Most salamanders do regenerate throughout life, however, some studies on limb regeneration suggest that older animals make more patterning mistakes in the regeneration of limbs; the data on other forms of regeneration in old animals is lacking. More recently the role of cellular senescence, a process linked to aging has been shown to play an interesting novel role in the limb regeneration process in salamanders.
Cellular senescence is a stress response that stops proliferation, this process is triggered by different factors such as DNA damage, telomere shortening, oxidative stress (Yun, 2018), and is linked to age-related pathologies (e.g., fibrotic diseases). However, it has a role during physiological conditions such as tissue remodeling during embryogenesis and activation of the immune system (Muñoz-Espín & Serrano, 2014). Senescent cells express a senescence-associated secretory phenotype that allows them to secrete a variety of molecules such as growth factors, cytokines, chemokines and matrix remodeling proteins reviewed in (Kuilman & Peeper, 2009), that attract phagocytes to an injury site and have a role in the clearance of dead cells (Muñoz-Espín & Serrano, 2014). Cellular senescence has an important role during aging. In mammals senescent cells accumulate in adult organs as the organism age, for example, in skin, lung, liver, spleen, and kidney (McHugh & Gil, 2018). Senescence is induced in those adult tissues by different factors such as telomere shortening, metabolic dysfunction, and loss of proteostasis (López-Otín, Blasco, Partridge, Serrano, & Kroemer, 2013; McHugh & Gil, 2018). Also, senescence is related with the loss of mammals regenerative capacities, for example, during muscle regeneration in mouse their quiescent resident stem cells turn into senescent cells, which is driven-through epigenetic changes such as the repression of p16INK4a by the positive regulation of Bmi1, which is a component of the Polycomb Repressive Complex 1 (PRC1, Etienne, Liu, Skinner, Conboy, & Conboy, 2020). As mice age, although they have resident muscle stem cells their capacity for regeneration decreases as cellular senescence increases.
In the context of tissue regeneration, different model organisms have reported cellular senescence as a crucial step during regeneration such as fin regeneration in zebrafish (Da Silva-Álvarez et al., 2020) and limb regeneration in salamanders (Yun, Davaapil, & Brockes, 2015). Yun et al. (2015) reported a comparative assay to identify if the response to a stress input can trigger salamander cells to stop proliferating and identified that induced senescent cells display a molecular profile that is similar to that identified in mammal senescent cells (e.g., γH2AX foci, high levels of ROS), showing that the senescent state between mammals and salamanders is comparable. They interestingly reported that during limb regeneration at 7 dpa a high number of senescent cells where observed in the regenerating tissue, and that macrophages are essential to clear those cells from the regenerative microenvironment. They observed no increase in senescent cells in repeated amputations or older animals (3 years), suggesting that the axolotl has a unique mechanism for sensing and clearing senescent cells. In the future, it will be important to know if much older animals, 10 or 20 years old can clear senescent cells as efficiently or does accumulation and slower clearing of them correlate to mistakes in regeneration.
Besides tissue regeneration, cellular senescence is a conserved mechanism during organogenesis to allow tissue growth, remodeling, and patterning in different vertebrates (Muñoz-Espín et al., 2013; Storer et al., 2013) including amphibians. In axolotls, cellular senescence has been observed in development in different tissues including in the kidney (Davaapil, Brockes, & Yun, 2017; Villiard et al., 2017), olfactory epithelium of nerve fascicles and lateral organs (Villiard et al., 2017). Interestingly, in axolotls during limb development senescent cells are not present; they have only been found during limb regeneration, which is another example of how limb regeneration and limb development in salamanders could have molecular and cellular independent pathways (Yun et al., 2015).
The studies that have been done in cellular senescence in salamanders have shown how the transient expression of cellular senescence during tissue regeneration works as a positive input. What induces and controls the timing of this transient state and efficient clearing of these cells at the molecular level is unknown. Additionally, it is unknown if cellular senescence is essential for regeneration of other tissues or organs in salamanders.
5.1. The immune system and tissue regeneration
One of the currently discussed considerations to have successful regenerative therapies is the immunomodulation of the microenvironment of the affected tissue or organ (Pino, Westover, Johnston, Buf, & Humes, 2018), to have a balance between the host defense and the healing of the injury (Godwin, Pinto, & Rosenthal, 2017). A strict correlation between the complexity of the immune system and the capacity to trigger a regenerative response has been explored in different model organisms such as mammals (Porrello et al., 2011; Seifert et al., 2012), amphibians (Godwin, Debuque, Salimova, & Rosenthal, 2017; Godwin et al., 2013; King, Neff, & Mescher, 2012; Mescher, Neff, & King, 2013; Tsai et al., 2019; Tsai, Baselga-Garriga, & Melton, 2020) and fish (Lai et al., 2017; Tsarouchas et al., 2018). In mammals, it is well known that skin scar-free wound healing is possible in embryonic stages and the decline in this capacity is linked with the development of a more mature immune system as we age. Similarly, scar-free repair in mice is lost during the first days of birth, which correlates with the development of the immune system (Porrello et al., 2011). Also in Xenopus, young larval animals have high regenerative ability but when they undergo metamorphosis, during which time adaptative immune response develops, they largely lose their regenerative ability (King et al., 2012; Mescher et al., 2013).
In salamanders, the role of the immune system has mainly been explored in the context of limb and heart regeneration (Godwin et al., 2013; Tsai et al., 2019, 2020). One of the main cell populations that has been reported as crucial to promote tissue regeneration is macrophages, which secrete anti and pro-inflammatory molecules to immunomodulate the microenvironment of the injury site (Godwin, Kuraitis, & Rosenthal, 2014), which includes the novel role of clearing senescent cells during limb regeneration (Yun et al., 2015). In salamanders, drugs like Clodronate liposomes have been used to deplete the macrophage population during limb regeneration (Godwin, Debuque, et al., 2017). This approach has revealed a role for macrophages in blastema formation but they are not necessary for wound closure. These studies suggest that depletion of macrophages leads to a dys-regulation of the expression of genes such as collagens, MMPs, and collagen remodeling enzymes, which are crucial for the remodeling of the extracellular matrix and their disruption leads to the formation of a fibrotic tissue that is not favorable for tissue regeneration (Godwin et al., 2013). This is an example of how the crosstalk between the immune system and the ECM is an important modulator of tissue regeneration, this may be a feature of regeneration that is shared across the Metazoans that have outstanding regenerative capacities, reviewed in Arenas Gómez et al. (2020).
Some very interesting recent research has reported how early blastema cells upregulate Interleukin 8 (IL-8) expression, promoting the recruitment of monocytes and granulocytes (e.g., neutrophils) during wound healing by the interaction with the receptor CXCR-1/2. The knockdown of IL-8 or the inhibition of CXCR-1/2 leads to defects in the blastema formation (Tsai et al., 2019). Furthermore, the wound epidermis, the apical epidermal cap, and the blastema cells express cytokines like midkine that module the ECM, tissue histolysis and inflammatory microenvironment during early stages of limb regeneration (Tsai et al., 2020), which shows that the cells of the immune system are not the only ones that can secret cytokines to immunomodulate a regenerative microenvironment.
Many studies in limb regeneration to date have been performed in relatively young animals. It will be important in the future to also look at the interaction of the immune cells, the ECM and the role of cellular senescence in limb regeneration in aged animals, 3–5 years or older. Initial work from Yun et al. (Yun et al., 2015) suggests that senescent cells are cleared from the injury site by macrophages and may be necessary for regeneration. In the future, it will be important to understand the exact mechanism salamanders use to clear senescent cells, if this only evoked in a regeneration scenario or do old salamanders constantly use this system as they age to reduce senescent cells. This may suggest that salamanders have evolved an efficient way to remove senescent cells and compare to what is known to occur in aging and inflammatory disease progression in humans.
6. Modern tools for studying salamanders
For many years, the progression of tools in the salamander field was limited by the lack of a sequenced genome. One of the challenges with salamanders is that many have big genomes; in the case of A. mexicanum it has a size of ~32 GB, 10 times bigger than humans (Keinath et al., 2015). In the beginning, useful databases manually curated from contigs assembled from Expressed Sequences Tags (EST) collected mainly from A. mexicanum (Habermann et al., 2004) and A. tigrinum (Putta et al., 2004), were the main data available to perform molecular analysis. This helped with pushing forth the molecular analysis of regeneration and the next break through was techniques to transiently label cells in vivo and track their fate during regeneration (Echeverri & Tanaka, 2003). This was swiftly followed by the development of the first transgenic axolotls (Sobkow, Epperlein, Herklotz, Straube, & Tanaka, 2006) and newts (Casco-Robles et al., 2011; Ueda, Kondoh, & Mizuno, 2005), which opened up the possibility of using cell type specific promoters to image cells during regeneration (Casco-Robles et al., 2011; Hayashi et al., 2013; Joven & Simon, 2018; Khattak et al., 2014, 2013; Khattak & Tanaka, 2015; Sandoval-Guzman et al., 2014; Ueda et al., 2005).
In recent years major advances in computational abilities to handle big data sets has finally allowed the assembly of the huge axolotl genome, finally published in 2018 (Nowoshilow et al., 2018). This has been followed by publication of another genome project in axolotl (Smith et al., 2019) and the sequencing of the Pleurodeles genome (Elewa et al., 2017) and represents a new era in the research of salamanders.
The availability of salamander genomes has also aided in the use of Next Generation Sequencing (NGS) platforms such as RNA-Seq (Dwaraka, Smith, Woodcock, & Voss, 2019) or Single Cell Seq (Gerber, Gerber, et al., 2018; Leigh et al., 2018), which are powerful tools to facilitate the analysis of gene expression profiles during tissue regeneration at different time points, different experimental conditions and compare gene expression profile among different species of salamanders or other organisms.
This approach facilitates the identification of conserved molecular signatures during the time course of tissue regeneration. Transcriptomics, have also enabled the development of de novo reference transcriptome of salamanders that are endangered species such as Hynobius chinensis, Andrias davidianus, and Batrachuperus yenyuanensis, which are important to understand the evolution of gene families or specific traits in salamanders for the adaptation (Che et al., 2014; Huang, Ren, Xiong, Gao, & Sun, 2017; Li et al., 2015; Xiong, Lv, Huang, & Liu, 2019). The study of many different salamander species at the molecular levels enables the identification of conserved or divergent molecular circuitry for different types of regeneration (Arenas Gómez et al., 2017; Dwaraka & Voss, 2019). Ultimately sequenced genomes allow us to probe more deeply not just the pathways that are need to be activated or repressed to promote a regenerative response in salamanders but importantly allow us to dissect the possible conservation of coding sequences of genes between salamanders and humans and enables the comparison of enhancers and other regulatory regions that may be crucial to understand how different cells response to an injury signal.
The other area that having a sequenced genome is essential for is genome editing. The CRISPR/Cas system has changed the way knock-outs are carried out due to the efficiency of the system. Recent work has shown that this systems works very effectively in axolotl embryos for both gene knock outs and knockins (Fei et al., 2016, 2018, 2017, 2014; Flowers, Sanor, & Crews, 2017; Flowers, Timberlake, McLean, Monaghan, & Crews, 2014; Sanor, Flowers, & Crews, 2020).
In Newts, C. pyrrhogaster was one of the first in which transgenesis was established, however, it is not an ideal system as it takes a long time to reach sexual maturation (more than 3 years) (Hayashi et al., 2013; Ueda et al., 2005). Other species look more promising such as P. waltl, which have greater potential to regenerate lens throughout their lives, a reference genome is available and they have a shorter period to arrive at sexual maturation (9–12 months) (Joven et al., 2019). Both knockin and mutations are possible in Pleurodeles using CRISPR/cas based editing (Cai, Peng, Ren, & Wang, 2019; Elewa et al., 2017; Molla & Yang, 2019).
At the moment, A. mexicanum and P. waltl are the salamanders of choice in which to perform genome editing due to the range of established techniques and availability of genomic resources. In order to expand the toolkit available for studying development and regeneration in these animals more advances in knock-in strategies using CRISPR/Cas technology are needed, especially inducible systems which allow the function of a gene to be altered in a tissue and time specific manner. It is well known that salamanders have been a hard model to establish as a genetically tractable system, however, the recent advances in genome sequencing and genome editing make them a more accessible research organism to address molecular and cellular questions about development and regeneration.
7. Potential for translation from salamanders to humans
The question often asked about salamander regeneration studies is; how relevant is this to humans and can this knowledge ever be used to promote regeneration in humans?
Currently, the field of tissue engineering and regenerative medicine (TERM) have been creating different strategies to facilitate the replacement of damaged organs (e.g., artificial organs). These strategies include new cell-based therapies for diseases with limited treatments available (e.g., neurodegenerative diseases), the creation of 3D scaffolds mixed with cells that promote the local repair of damaged tissue (e.g., skin), building organs using bioreactors, and in the case of appendage amputations (e.g., limbs) the replacement with biomechanical structures; reviewed in (Baddour, Sousounis, & Tsonis, 2012; Binan, Ajji, De Crescenzo, & Jolicoeur, 2014; Bumbaširević et al., 2020; Han et al., 2020; Vig et al., 2017; Wang, 2019) (reviewed by Bumbaširević et al., 2020; Han et al., 2020; Kim, Lee, & Kim, 2013; Ravichandran, Liu, & Teoh, 2018; Vig et al., 2017; Wang, 2019). However, these strategies have limitations, especially with integrating the ex vivo structure to the in vivo system, some of those limitations include the re-vascularization, innervations and the reassembly of the mechanical properties of the tissue or organ (Dimmeler, Ding, Rando, & Trounson, 2014). However, insights into how to connect the vasculature and nervous systems between old and newly formed tissue may be gained by carefully studying how animals like salamanders integrate the old with the new.
Model organisms in tissue regeneration such as salamanders could help us to understand the molecular and cellular pathways needed for a successful regenerative response. It is clear that the modulation of the microenvironment where the regeneration takes place is a key element for successful tissue regeneration, including, the modulation of the extracellular matrix, the regulation of the immune system, the molecular factors required for innervation, and the cellular mechanisms activated in order to repopulate the tissue.
In this chapter, the regeneration of some of the tissues and organs in salamanders was included, these recent advances show the potential to translate this knowledge to develop new therapies in humans. For example; in the case of the eye, the conservation of the cell types and genetic pathways illustrate the potential for this knowledge to be used to design novel translational therapies (Barbosa-Sabanero et al., 2012; Carido et al., 2014; Chiba, 2014; Del Rio-Tsonis et al., 1998; Hayashi et al., 2004, 2013; Haynes, Gutierrez, Aycinena, Tsonis, & Del Rio-Tsonis, 2007; Islam et al., 2014; Tsonis & Del Rio-Tsonis, 2004; Zhu et al., 2013; Zhu, Schreiter, & Tanaka, 2016). Similarly, the research on spinal cord regeneration in salamanders has identified critical molecular pathways that are conserved in mammals but which axolotls specifically activate to promote regeneration instead of glial scar formation (Diaz Quiroz, Tsai, Coyle, Sehm, & Echeverri, 2014; Sabin et al., 2019, 2015), this knowledge of the axolotl spinal cord may help to inform potential cell based therapeutics in the future (Albors et al., 2015; Diaz Quiroz, Li, Aparicio, & Echeverri, 2016; Meinhardt et al., 2014). Similarly, the research done in brain regeneration using newts identified the role of dopamine in the modulation of the fate of the ependymoglial cells, a strategy that has been tested in mammals (Berg et al., 2010, 2011; Hedlund et al., 2016).
One of the biggest challenges in regenerative medicine may be the restoration of a full appendage like a leg, research on salamander limb regeneration is invaluable to this field. Salamanders effortlessly regenerate and integrate a new limb with the existing infrastructures and seamlessly reconnect the old and new vasculature and neural networks to regenerate a perfectly functioning limb. Research over several decades has given significant insights into how the salamanders do this, we now understand better where the cells come from to form a new limb and how much plasticity there is in given cell populations (Kragl et al., 2009; Muneoka & Bryant, 1984; Muneoka et al., 1986; Stocum, 1980, 1983, 1998, 2017). In the case of limbs, the skeletal system is very close to humans, however, in amphibians one of the differences is a group of bones calls the basale commune, which is an amalgamation in the base of digits I-II. Also, the polarity during digit development is different, in salamanders it is in a preaxial order (order of digit formation is IV-(V)-III-II-I) and in the other tetrapods it is postaxial (II-I-III-IV(-V)) (Fröbisch & Shubin, 2011). Despite this difference in development; the cellular and morphological composition is highly conserved cross-species, highlighting the possibilities for cross-species translational potential.
Researchers are making progress in understanding how cells know how much tissue to regenerate and how that new tissue is patterned to regenerate the correct structures, interestingly salamanders share many features with humans, like the use of Pax7 satellite cells to regenerate limb muscle and role of shh and Hox genes in patterning the limb, suggesting potential avenues for translating knowledge cross-species (Carlson, Komine, Bryant, & Gardiner, 2001; da Silva et al., 2002; Gardiner, Blumberg, Komine, & Bryant, 1995; Gerber et al., 2018; Imokawa & Yoshizato, 1997; Knapp et al., 2013; Mercader et al., 2005; Nacu et al., 2013, 2016; Roensch et al., 2013; Sandoval-Guzman et al., 2014; Sugiura, Wang, Barsacchi, Simon, & Tanaka, 2016).
8. Conclusion and perspectives
Salamanders represent a group of crucial vertebrates in which to understand the diversity of cellular and molecular mechanisms that have evolved to regenerate different tissue types. Understanding within one group of animals the multiple different pathways that can be deployed in different manners may help us understand why humans are very limited in their regenerative abilities. There are still many unknowns in the salamander regeneration field which are essential to decipher, for example, how do the injured cells know exactly how much tissue to regenerate? Despite many years of research, we still do not have a molecular answer to this question. Control of tissue size will be an essential element to understand both in terms of regenerating whole structures like limbs but also with regard to cell replacement in specific organs like the heart or brain. Also understanding how to control the connection of newly differentiated tissue to older tissue via vasculature and neuronal circuitry to restore functional tissue is essential for translational medicine to be possible. Salamander regeneration is a field with a long history; however, in terms of understanding how to translate the molecular knowledge of regenerating a limb to humans is still in its infancy.
References
- Adams KL, & Gallo V (2018). The diversity and disparity of the glial scar. Nature Neuroscience, 21(1), 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albors AR, Tazaki A, Rost F, Nowoshilow S, Chara O, & Tanaka EM (2015). Planar cell polarity-mediated induction of neural stem cell expansion during axolotl spinal cord regeneration. eLife, 4(NOVEMBER2015), 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amamoto R, Huerta VGL, Takahashi E, Dai G, Grant AK, Fu Z, et al. (2016). Adult axolotls can regenerate original neuronal diversity in response to brain injury. eLife, 5, e13998. 10.7554/eLife.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AmphibiaWeb. (2019). AmphibiaWeb:Information on amphibian biology and conservation.
- Arenas Gómez CM, Gomez Molina A, Zapata JD, & Delgado JP (2017). Limb regeneration in a direct-developing terrestrial salamander, Bolitoglossa ramosi (Caudata: Plethodontidae). Regeneration, 4(4), 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas Gómez CM, Sabin KZ, & Echeverri K (2020). Wound healing across the animal kingdom: Crosstalk between the immune system and the extracellular matrix. Developmental Dynamics: An Official Publication of the American Association of the Anatomists, 249(7), 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour JA, Sousounis K, & Tsonis PA (2012). Organ repair and regeneration: An overview. Birth Defects Research. Part C, Embryo Today, 96(1), 1–29. [DOI] [PubMed] [Google Scholar]
- Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis PA, & Del Rio-Tsonis K (2012). Lens and retina regeneration: New perspectives from model organisms. The Biochemical Journal, 447(3), 321–334. [DOI] [PubMed] [Google Scholar]
- Berg DA, Kirkham M, Beljajeva A, Knapp D, Habermann B, Ryge J, et al. (2010). Efficient regeneration by activation of neurogenesis in homeostatically quiescent regions of the adult vertebrate brain. Development, 137(24), 4127–4134. [DOI] [PubMed] [Google Scholar]
- Berg DA, Kirkham M, Wang H, Frisén J, & Simon A (2011). Dopamine controls neurogenesis in the adult salamander midbrain in homeostasis and during regeneration of dopamine neurons. Cell Stem Cell, 8(4), 426–433. [DOI] [PubMed] [Google Scholar]
- Binan L, Ajji A, De Crescenzo G, & Jolicoeur M (2014). Approaches for neural tissue regeneration. Stem Cell Reviews and Reports, 10(1), 44–59. [DOI] [PubMed] [Google Scholar]
- Birnbaum KD, & Sánchez Alvarado A (2008). Slicing across kingdoms: Regeneration in plants and animals. Cell, 132(4), 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blassberg RA, Garza-Garcia A, Janmohamed A, Gates PB, & Brockes JP (2011). Functional convergence of signalling by GPI-anchored and anchorless forms of a salamander protein implicated in limb regeneration. Journal of Cell Science, 124(Pt. 1), 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, & Burnside ER (2019). Moving beyond the glial scar for spinal cord repair. Nature Communications, 10(1), 3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, & McMahon SB (2006). Spinal cord repair strategies: Why do they work? Nature Reviews. Neuroscience, 7(8), 644–653. [DOI] [PubMed] [Google Scholar]
- Brockes JP (1998). Regeneration and cancer. Biochimica et Biophysica Acta, Reviews on Cancer, 1377(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Brockes JP, & Gates PB (2014). Mechanisms underlying vertebrate limb regeneration: Lessons from the salamander. Biochemical Society Transactions, 42(3), 625–630. [DOI] [PubMed] [Google Scholar]
- Brockes JP, & Kumar A (2005). Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science (New York, N.Y.), 310(5756), 1919–1923. [DOI] [PubMed] [Google Scholar]
- Brockes JP, & Kumar A (2008). Comparative aspects of animal regeneration. Annual Review of Cell and Developmental Biology, 24, 525–549. [DOI] [PubMed] [Google Scholar]
- Brunst VV, & Roque AL (1969). A spontaneous teratoma in an axolotl (Siredon mexicanum). Cancer Research, 29(1), 223–229. [PubMed] [Google Scholar]
- Bryant SV, Endo T, & Gardiner DM (2002). Vertebrate limb regeneration and the origin of limb stem cells. International Journal of Developmental Biology, 46(7), 887–896. [PubMed] [Google Scholar]
- Bryant DM, Johnson K, Ditommaso T, Regev A, Haas BJ, & Whited JL (2017). A tissue-mapped axolotl De novo transcriptome enables identification of limb regeneration factors. Cell Reports, 18, 762–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumbaširević M, Lesic A, Palibrk T, Milovanovic D, Zoka M, Kravić-Stevović T, et al. (2020). The current state of bionic limbs from the surgeon’s viewpoint. EFORT Open Reviews, 5(2), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EG, & Ward MB (1965). Reconstitution of the spinal cord following ablation in urodele larvae. Journal of Experimental Zoology, 160(1), 47–65. [DOI] [PubMed] [Google Scholar]
- Butler EG, & Ward MB (1967). Reconstitution of the spinal cord after ablation in adult Triturus. Developmental Biology, 15(5), 464–486. [DOI] [PubMed] [Google Scholar]
- Cai H, Peng Z, Ren R, & Wang H (2019). Efficient gene disruption via base editing induced stop in newt Pleurodeles waltl. Genes, 10(11), 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calve S, Odelberg SJ, & Simon H-G (2010). A transitional extracellular matrix instructs cell behavior during muscle regeneration. Developmental Biology, 344(1), 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, & Crews C (2008). Wound epidermis formation and function in urodele amphibian limb regeneration. Cellular and Molecular Life Sciences, 65, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LJ, Suarez-Castillo EC, Ortiz-Zuazaga H, Knapp D, Tanaka EM, & Crews CM (2011). Gene expression profile of the regeneration epithelium during axolotl limb regeneration. Developmental Dynamics, 240(7), 1826–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carido M, Zhu Y, Postel K, Benkner B, Cimalla P, Karl MO, et al. (2014). Characterization of a mouse model with complete RPE loss and its use for RPE cell transplantation. Investigative Ophthalmology & Visual Science, 55(8), 5431–5444. [DOI] [PubMed] [Google Scholar]
- Carlson M, Komine Y, Bryant S, & Gardiner DM (2001). Expression of Hoxb 13 and Hoxc 10 in developing and regenerating axolotl limbs and tails. Developmental Biology, 229, 396–406. [DOI] [PubMed] [Google Scholar]
- Casco-Robles MM, Yamada S, Miura T, Nakamura K, Haynes T, Maki N, et al. (2011). Expressing exogenous genes in newts by transgenesis. Nature Protocols, 6(5), 600–608. [DOI] [PubMed] [Google Scholar]
- Chalkley DT (1954). A quantitative histological analysis of forelimb regeneration in triturus viridescens. Journal of Morphology, 94(1), 21–70. [Google Scholar]
- Che R, Sun Y, Wang R, & Xu T (2014). Transcriptomic analysis of endangered Chinese salamander: Identification of immune, sex and reproduction-related genes and genetic markers. PLoS One, 9(1), e87940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba C (2014). The retinal pigment epithelium: An important player of retinal disorders and regeneration. Experimental Eye Research, 123, 107–114. [DOI] [PubMed] [Google Scholar]
- Chiba C, Hoshino A, Nakamura K, Susaki K, Yamano Y, Kaneko Y, et al. (2006). Visual cycle protein RPE65 persists in new retinal cells during retinal regeneration of adult newt. The Journal of Comparative Neurology, 495(4), 391–407. [DOI] [PubMed] [Google Scholar]
- Christensen RN, & Tassava RA (2000). Apical epithelial cap morphology and fibronectin gene expression in regenerating axolotl limbs. Developmental Dynamics, 217(2), 216–224. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Alexander R, & Holder N (1988). Regeneration of descending axons in the spinal cord of the axolotl. Neuroscience Letters, 89(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Crawford K, & Stocum DL (1988). Retinoic acid coordinately proximalizes regenerate pattern and blastema differential affinity in axolotl limbs. Development (Cambridge, England), 102(4), 687–698. [DOI] [PubMed] [Google Scholar]
- da Silva SM, Gates PB, & Brockes JP (2002). The newt Ortholog of CD59 is implicated in Proximodistal identity during amphibian limb regeneration. Developmental Cell, 3(4), 547–555. [DOI] [PubMed] [Google Scholar]
- Da Silva-Álvarez S, Guerra-Varela J, Sobrido-Cameán D, Quelle A, Barreiro-Iglesias A, Sánchez L, et al. (2020). Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell, 19(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davaapil H, Brockes JP, & Yun MH (2017). Conserved and novel functions of programmed cellular senescence during vertebrate development. Development (Cambridge), 144(1), 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GR, Troxel MT, Kohler VJ, Grossmann ER, & McClellan AD (1993). Time course of locomotor recovery and functional regeneration in spinal transected lampreys: Kinematics and electromyography. Experimental Brain Research, 97, 83–95. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Trombley MT, McMahon G, & Tsonis PA (1998). Regulation of lens regeneration by fibroblast growth factor receptor 1. Developmental Dynamics: An Official Publication of the American Association of the Anatomists, 213(1), 140–146. [DOI] [PubMed] [Google Scholar]
- Diaz Quiroz JF, & Echeverri K (2013). Spinal cord regeneration: Where fish, frogs and salamanders lead the way, can we follow? The Biochemical Journal, 451(3), 353–364. [DOI] [PubMed] [Google Scholar]
- Diaz Quiroz J, Li Y, Aparicio C, & Echeverri K (2016). Development of a 3D matrix for modeling mammalian spinal cord injury in vitro. Neural Regeneration Research, 11(11), 1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Quiroz JF, Tsai E, Coyle M, Sehm T, & Echeverri K (2014). Precise control of mi R-125b levels is required to create a regeneration-permissive environment after spinal cord injury: A cross-species comparison between salamander and rat. Disease Models & Mechanisms, 7(6), 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Ding S, Rando TA, & Trounson A (2014). Translational strategies and challenges in regenerative medicine. Nature Medicine, 20(8), 814–821. [DOI] [PubMed] [Google Scholar]
- Dinsmore CE, & American Society of Zoologists. (2008). A history of regeneration research: Milestones in the evolution of a science. Cambridge University Press. [Google Scholar]
- Dudley AT, Ros MA, & Tabin CJ (2002). A re-examination of proximodistal patterning during vertebrate limb development. Nature, 418, 539–544. [DOI] [PubMed] [Google Scholar]
- Dwaraka VB, Smith JJ, Woodcock MR, & Voss SR (2019). Comparative transcriptomics of limb regeneration: Identification of conserved expression changes among three species of Ambystoma. Genomics, 111(6), 1216–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwaraka VB, & Voss SR (2019). Towards comparative analyses of salamander limb regeneration. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 10.1002/jez.b.22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck SM, & Karimi-Abdolrezaee S (2015). Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system. Experimental Neurology, 269, 169–187. [DOI] [PubMed] [Google Scholar]
- Echeverri K, & Tanaka EM (2003). Electroporation as a tool to study in vivo spinal cord regeneration. Developmental Dynamics, 226(2), 418–425. [DOI] [PubMed] [Google Scholar]
- Echeverri K, & Tanaka E (2005). Proximodistal patterning during limb regeneration. Developmental Biology, 279, 391–401. [DOI] [PubMed] [Google Scholar]
- Edwards-Faret G, Muñoz R, Méndez-Olivos EE, Lee-Liu D, Tapia VS, & Larraín J (2017). Spinal cord regeneration in Xenopus laevis. Nature Protocols, 12(2), 372–389. [DOI] [PubMed] [Google Scholar]
- Egar M, & Singer M (1972). The role of ependyma in spinal cord regeneration in the urodele, Triturus. Experimental Neurology, 37(2), 422–430. [DOI] [PubMed] [Google Scholar]
- Eguchi G (1963). Electon microscopic studies on lens regeneration I mechanism of depigmentation of the iris. Embryologia, 8(1), 45–62. [Google Scholar]
- Eguchi G, Eguchi Y, Nakamura K, Yadav MC, Millan JL, & Tsonis PA (2011). Regenerative capacity in newts is not altered by repeated regeneration and ageing. Nature Communications, 2, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewa A, Wang H, Talavera-López C, Joven A, Brito G, Kumar A, et al. (2017). Reading and editing the Pleurodeles waltl genome reveals novel features of tetrapod regeneration. Nature Communications, 8(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne J, Liu C, Skinner CM, Conboy MJ, & Conboy IM (2020). Skeletal muscle as an experimental model of choice to study tissue aging and rejuvenation. Skeletal Muscle, 10(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas JE, Freitas PD, Bryant DM, Whited JL, & Monaghan JR (2016). Neuregulin-1 signaling is essential for nerve-dependent axolotl limb regeneration. Development (Cambridge), 143(15), 2724–2731. [DOI] [PubMed] [Google Scholar]
- Fei J-F, Knapp D, Schuez M, Murawala P, Zou Y, Pal Singh S, et al. (2016). Tissue- and time-directed electroporation of CAS9 protein–gRNA complexes in vivo yields efficient multigene knockout for studying gene function in regeneration. npj Regenerative Medicine, 1(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei JF, Lou WP, Knapp D, Murawala P, Gerber T, Taniguchi Y, et al. (2018). Application and optimization of CRISPR-Cas 9-mediated genome engineering in axolotl (Ambystoma mexicanum). Nature Protocols, 13(12), 2908–2943. [DOI] [PubMed] [Google Scholar]
- Fei J-F, Schuez M, Knapp D, Taniguchi Y, Drechsel DN, & Tanaka EM (2017). Efficient gene knockin in axolotl and its use to test the role of satellite cells in limb regeneration. Proceedings of the National Academy of Sciences of the United States of America, 114(47), 12501–12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei JF, Schuez M, Tazaki A, Taniguchi Y, Roensch K, & Tanaka EM (2014). CRISPR-mediated genomic deletion of sox 2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration. Stem Cell Reports, 3(3), 444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fior J (2014). Salamander regeneration as a model for developing novel regenerative and anticancer therapies. Journal of Cancer, 5, 715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, & Silver J (2008). CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Experimental Neurology, 209(2), 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier JS, Underhill LH, & Dvorak HF (1986). Tumors: Wounds that do not heal. New England Journal of Medicine, 315(26), 1650–1659. [DOI] [PubMed] [Google Scholar]
- Flowers GP, Sanor LD, & Crews CM (2017). Lineage tracing of genome-edited alleles reveals high fidelity axolotl limb regeneration. eLife, 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers GP, Timberlake AT, McLean KC, Monaghan JR, & Crews CM (2014). Highly efficient targeted mutagenesis in axolotl using Cas9 RNA-guided nuclease. Development, 141(10), 2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed WJ, de Medinaceli L, & Wyatt RJ (1985). Promoting functional plasticity in the damaged nervous system. Science, 227(4694), 1544–1552. [DOI] [PubMed] [Google Scholar]
- Freitas PD, Yandulskaya AS, & Monaghan JR (2019). Spinal cord regeneration in amphibians: A historical perspective. Developmental Neurobiology, 79(5), 437–452. [DOI] [PubMed] [Google Scholar]
- Fröbisch NB, Bickelmann C, & Witzmann F (2014). Early evolution of limb regeneration in tetrapods: Evidence from a 300-million-year-old amphibian. Proceedings of the Royal Society B: Biological Sciences, 281(1794), 20141550. 10.1098/rspb.2014.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröbisch NB, & Shubin NH (2011). Salamander limb development: Integrating genes, morphology, and fossils. Developmental Dynamics, 240(5), 1087–1099. [DOI] [PubMed] [Google Scholar]
- Gao K, Wang CR, Jiang F, Wong AYK, Su N, Jiang JH, et al. (2013). Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia, 61(12), 2063–2077. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Blumberg B, Komine Y, & Bryant SV (1995). Regulation of HoxA expression in developing and regenerating axolotl limbs. Development, 121(6), 1731–1741. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Muneoka K, & Bryant SV (1986). The migration of dermal cells during blastema formation in axolotls. Developmental Biology, 118(2), 488–493. [DOI] [PubMed] [Google Scholar]
- Garza-Garcia AA, Driscoll PC, & Brockes JP (2010). Evidence for the local evolution of mechanisms underlying limb regeneration in salamanders. Integrative and Comparative Biology, 50(4), 528–535. [DOI] [PubMed] [Google Scholar]
- Geng J, Gates PB, Kumar A, Guenther S, Garza-Garcia A, Kuenne C, et al. (2015). Identification of the orphan gene prod 1 in basal and other salamander families. EvoDevo, 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Wei H, Shang H, Zhou M, Chen B, Zhang F, et al. (2015). Proteomic analysis of the skin of Chinese giant salamander (Andrias davidianus). Journal of Proteomics, 119, 196–208. [DOI] [PubMed] [Google Scholar]
- Gerber T, Gerber T, Murawala P, Knapp D, Masselink W, Schuez M, et al. (2018). Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science, 0681(September), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber T, Murawala P, Knapp D, Masselink W, Schuez M, Hermann S, et al. (2018). Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science (New York, N.Y.), 362(6413). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, & Hui SP (2018). Axonal regeneration in zebrafish spinal cord. Regeneration, 5(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs K, Chittur S, & Szaro B (2011). Metamorphosis and the regenerative capacity of spinal cord axons in Xenopus laevis. The European Journal of Neuroscience, 33(1), 9–25. [DOI] [PubMed] [Google Scholar]
- Giguere V, Ong ES, Evans RM, & Tabin CJ (1989). Spatial and temporal expression of the retinoic acid receptor in the regenerating amphibian limb. Nature, 337(6207), 566–569 (published erratum appears in Nature 1989 Sep 7;341(6237):80). [DOI] [PubMed] [Google Scholar]
- Godwin JW, Debuque R, Salimova E, & Rosenthal NA (2017). Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen Med, 2, 22. 10.1038/s41536-017-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin J, Kuraitis D, & Rosenthal N (2014). Extracellular matrix considerations for scar-free repair and regeneration: Insights from regenerative diversity among vertebrates. The International Journal of Biochemistry & Cell Biology, 56, 47–55. [DOI] [PubMed] [Google Scholar]
- Godwin JW, Liem KF, & Brockes JP (2010). Tissue factor expression in newt iris coincides with thrombin activation and lens regeneration. Mechanisms of Development, 127(7–8), 321–328. [DOI] [PubMed] [Google Scholar]
- Godwin J, Pinto AR, & Rosenthal N.a. (2013). Macrophages are required for adult salamander limb regeneration. Proceedings of the National Academy of Sciences of the United States of America, 110(23), 9415–9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, & Rosenthal NA (2017). Chasing the recipe for a pro-regenerative immune system. Seminars in Cell & Developmental Biology, 61, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Veracini L, Grall D, Butori C, Schaub S, Audebert S, et al. (2017). Fibronectin-guided migration of carcinoma collectives. Nature Communications, 8(1), 14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassme KS, Garza-Garcia A, Delgado JP, Godwin JW, Kumar A, Gates PB, et al. (2016). Mechanism of action of secreted newt anterior gradient protein. PLoS One, 11(4), e0154176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan E, & Markitantova Y (2016). Cellular and molecular preconditions for retinal pigment epithelium (RPE) natural reprogramming during retinal regeneration in Urodela. Biomedicine, 4(4), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, & Whited JL (2017). Advances in decoding axolotl limb regeneration. Trends in Genetics, 33(8), 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann B, Bebin A, Herklotz S, Volkmer M, Eckelt K, Pehlke K, et al. (2004). An Ambystoma mexicanum EST sequencing project: Analysis of 17,352 expressed sequence tags from embryonic and regenerating blastema cDNA libraries. Genome Biology, 5(9), R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F, Wang J, Ding L, Hu Y, Li W, Yuan Z, et al. (2020). Tissue engineering and regenerative medicine: Achievements, future, and sustainability in Asia. Frontiers in Bioengineering and Biotechnology, 8, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshbarger JC, Chang SC, DeLanney LE, Rose FL, & Green DE (1999). Cutaneous mastocytomas in the neotenic caudate amphibians Ambystoma mexicanum (axolotl) and Ambystoma tigrinum (tiger salamander). Journal of Cancer Research and Clinical Oncology, 125(3–4), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED (1959). Electron microscopic observations of muscle dedifferentiation in regenerating Ambystoma limbs. Developmental Biology, 1, 555–585. [Google Scholar]
- Hay E (1966). Regeneration. New York: Holt, Rinehart, Wilson. [Google Scholar]
- Hayashi T, Mizuno N, Ueda Y, Okamoto M, & Kondoh H (2004). FGF2 triggers iris-derived lens regeneration in newt eye. Mechanisms of Development, 121(6), 519–526. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yokotani N, Tane S, Matsumoto A, Myouga A, Okamoto M, et al. (2013). Molecular genetic system for regenerative studies using newts. Development, Growth & Differentiation, 55(2), 229–236. [DOI] [PubMed] [Google Scholar]
- Haynes T, Gutierrez C, Aycinena JC, Tsonis PA, & Del Rio-Tsonis K (2007). BMP signaling mediates stem/progenitor cell-induced retina regeneration. Proceedings of the National Academy of Sciences of the United States of America, 104(51), 20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund E, Belnoue L, Theofilopoulos S, Salto C, Bye C, Parish C, et al. (2016). Dopamine receptor antagonists enhance proliferation and neurogenesis of midbrain Lmx1a-expressing progenitors. Scientific Reports, 6(1), 26448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, & Hamilton PW (2018). Diverse evolutionary origins and mechanisms of lens regeneration. Molecular Biology and Evolution, 35(7), 1563–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, & Tsonis PA (2010). Molecular and cellular aspects of amphibian lens regeneration. Progress in Retinal and Eye Research, 29(6), 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Ren HT, Xiong JL, Gao XC, & Sun XH (2017). Identification and characterization of known and novel microRNAs in three tissues of Chinese giant salamander base on deep sequencing approach. Genomics, 109(3–4), 258–264. [DOI] [PubMed] [Google Scholar]
- Imokawa Y, Simon A, & Brockes JP (2004). A critical role for thrombin in vertebrate lens regeneration. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 359(1445), 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa Y, & Yoshizato K (1997). Expression of sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limbbuds. Proceedings of the National Academy of Sciences, 94(17), 9159–9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Nakamura K, Casco-Robles MM, Kunahong A, Inami W, Toyama F, et al. (2014). The newt reprograms mature RPE cells into a unique multipotent state for retinal regeneration. Scientific Reports, 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iten LE, & Bryant SV (1973). Forelimb regeneration from different levels of amputation in the newt, Notophthalmus viridescens: Length, rate, and stages. Wilhelm Roux’ Archiv für Entwicklungsmechanik der Organismen, 173(4), 263–282. [DOI] [PubMed] [Google Scholar]
- Iten LE, & Bryant SV (1976). Regeneration from different levels along the tail of the newt, Notophthalmus viridescens. The Journal of Experimental Zoology, 196(3), 293–306. [DOI] [PubMed] [Google Scholar]
- Jacyniak K, McDonald RP, & Vickaryous MK (2017). Tail regeneration and other phenomena of wound healing and tissue restoration in lizards. Journal of Experimental Biology, 220(16), 2858–2869. [DOI] [PubMed] [Google Scholar]
- Joven A, Elewa A, & Simon A (2019). Model systems for regeneration: Salamanders. Development (Cambridge), 146(14), (dev167700-dev167700). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joven A, & Simon A (2018). Homeostatic and regenerative neurogenesis in salamanders. Progress in Neurobiology, 170(December 2017), 81–98. [DOI] [PubMed] [Google Scholar]
- Keinath MC, Timoshevskiy VA, Timoshevskaya NY, Tsonis PA, Voss SR, & Smith JJ (2015). Initial characterization of the large genome of the salamander Ambystoma mexicanum using shotgun and laser capture chromosome sequencing. Scientific Reports, 5, 16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak S, Murawala P, Andreas H, Kappert V, Schuez M, Sandoval-Guzman T, et al. (2014). Optimized axolotl (Ambystoma mexicanum) husbandry, breeding, metamorphosis, transgenesis and tamoxifen-mediated recombination. Nature Protocols, 9(3), 529–540. [DOI] [PubMed] [Google Scholar]
- Khattak S, Schuez M, Richter T, Knapp D, Haigo SL, Sandoval-Guzman T, et al. (2013). Germline transgenic methods for tracking cells and testing gene function during regeneration in the axolotl. Stem Cell Reports, 1(1), 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak S, & Tanaka EM (2015). Transgenesis in axolotl (Ambystoma mexicanum). Methods in Molecular Biology, 1290, 269–277. 10.1007/978-1-4939-2495-0_21. [DOI] [PubMed] [Google Scholar]
- Khudoley VV, & Ellselv VV (1979). Multiple melanomas in the axolotl Ambystoma mexlcanum 1. Journal of the National Cancer Institute, 63(I), 101–103. [PubMed] [Google Scholar]
- Kim SU, Lee HJ, & Kim YB (2013). Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology, 33(5), 491–504. 10.1111/neup.12020. [DOI] [PubMed] [Google Scholar]
- King MW, Neff AW, & Mescher AL (2012). The developing Xenopus limb as a model for studies on the balance between inflammation and regeneration. Anatomical record (Hoboken, N.J.: 2007), 295(10), 1552–1561. [DOI] [PubMed] [Google Scholar]
- Knapp D, Schulz H, Rascon CA, Volkmer M, Scholz J, Nacu E, et al. (2013). Comparative transcriptional profiling of the axolotl limb identifies a tripartite regeneration-specific gene program. PLoS One, 8(5), e61352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, et al. (2009). Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature, 460(7251), 60–65. [DOI] [PubMed] [Google Scholar]
- Kragl M, Roensch K, Nusslein I, Tazaki A, Taniguchi Y, Tarui H, et al. (2013). Muscle and connective tissue progenitor populations show distinct Twist1 and Twist3 expression profiles during axolotl limb regeneration. Developmental Biology, 373(1), 196–204. [DOI] [PubMed] [Google Scholar]
- Kuilman T, & Peeper DS (2009). Senescence-messaging secretome: SMS-ing cellular stress. Nature Reviews Cancer, 9(2), 81–94. [DOI] [PubMed] [Google Scholar]
- Kumar A, & Delgado JP (2011). The aneurogenic limb identifies developmental cell interactions underlying vertebrate limb regeneration. Proceedings of the National Academy of Sciences, 108(33), 13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, & Brockes JP (2007). Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science (New York, N.Y.), 318(5851), 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Marin-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, et al. (2017). Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. eLife, 6, e25605. 10.7554/eLife.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Liu D, Mendez-Olivos EE, Munoz R, & Larrain J (2017). The African clawed frog Xenopus laevis: A model organism to study regeneration of the central nervous system. Neuroscience Letters, 652, 82–93. [DOI] [PubMed] [Google Scholar]
- Leigh ND, Dunlap GS, Johnson K, Mariano R, Oshiro R, Wong AY, et al. (2018). Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution. Nature Communications, 9(1), 5153. 10.1038/s41467-018-07604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, & Fan C-M (2011). An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development (Cambridge, England), 138(17), 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang L, Lan Q, Yang H, Li Y, Liu X, et al. (2015). RNA-Seq analysis and gene discovery of Andrias davidianus using Illumina short read sequencing. PLoS One, 10(4), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M, Simon HG, & Tabin C (1998). Differential regulation of T-box and homeobox transcription factors suggests roles in controlling chick limb-type identity. Development, 125(15), 2825–2835. [DOI] [PubMed] [Google Scholar]
- Londono R, Sun AX, Tuan RS, & Lozito TP (2018). Tissue repair and Epimorphic regeneration: An overview. Current Pathobiology Reports, 6, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, & Kroemer G (2013). The hallmarks of aging. Cell, 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M (1982). Vitamin a and pattern formation in the regenerating limb. Nature, 295(5851), 672–675. [DOI] [PubMed] [Google Scholar]
- Maden M, Manwell LA, & Ormerod BK (2013). Proliferation zones in the axolotl brain and regeneration of the telencephalon. Neural Development, 8(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki N, Suetsugu-Maki R, Tarui H, Agata K, Del Rio-Tsonis K, & Tsonis PA (2009). Expression of stem cell pluripotency factors during regeneration in newts. Developmental Dynamics, 238(6), 1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHedlishvili L, Epperlein HH, Telzerow A, & Tanaka EM (2007). A clonal analysis of neural progenitors during axolotl spinal cord regeneration reveals evidence for both spatially restricted and multipotent progenitors. Development, 134, 2083–2093. [DOI] [PubMed] [Google Scholar]
- McHedlishvili L, Mazurov V, Grassme KS, Goehler K, Robl B, Tazaki A, et al. (2012). Reconstitution of the central and peripheral nervous system during salamander tail regeneration. Proceedings of the National Academy of Sciences of the United States of America, 109(34), E2258–E2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, & Gil J (2018). Senescence and aging: Causes, consequences, and therapeutic avenues. Journal of Cell Biology, 217(1), 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Brauninger M, et al. (2014). 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports, 3(6), 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader N, Tanaka EM, & Torres M (2005). Proximodistal identity during vertebrate limb regeneration is regulated by Meis homeodomain proteins. Development (Cambridge, England), 132(18), 4131–4142. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW, & King MW (2013). Changes in the inflammatory response to injury and its resolution during the loss of regenerative capacity in developing Xenopus limbs. PLoS One, 8(11), e80477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla KA, & Yang Y (2019). CRISPR/Cas-Mediated Base editing: Technical considerations and practical applications. Trends in Biotechnology, 37(10), 1121–1142. [DOI] [PubMed] [Google Scholar]
- Monaghan JR, Athippozhy A, Seifert AW, Putta S, Stromberg AJ, Maden M, et al. (2012). Gene expression patterns specific to the regenerating limb of the Mexican axolotl. Biology open, 1(10), 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan JR, Stier AC, Michonneau F, Smith MD, Pasch B, Maden M, et al. (2014). Experimentally induced metamorphosis in axolotls reduces regenerative rate and fidelity. Regeneration, 1(1), 2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen LM, Bryant SV, Torok MA, Blumberg B, & Gardiner DM (1996). Nerve dependency of regeneration: The role of distal-less and FGF signaling in amphibian limb regeneration. Development, 122(11), 3487–3497. [DOI] [PubMed] [Google Scholar]
- Muneoka K, & Bryant SV (1984). Cellular contribution to supernumerary limbs resulting from the interaction between developing and regenerating tissues in the axolotl. Developmental Biology, 105(1), 179–187. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Fox WF, & Bryant SV (1986). Cellular contribution from dermis and cartilage to the regenerating limb blastema in axolotls. Developmental Biology, 116(1), 256–260. [DOI] [PubMed] [Google Scholar]
- Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, et al. (2013). Programmed cell senescence during mammalian embryonic development. Cell, 155(5), 1104. [DOI] [PubMed] [Google Scholar]
- Muñoz-Espín D, & Serrano M (2014). Cellular senescence: From physiology to pathology. Nature Reviews Molecular Cell Biology, 15(7), 482–496. [DOI] [PubMed] [Google Scholar]
- Murawala P, Tanaka EM, & Currie JD (2012). Regeneration: The ultimate example of wound healing. Seminars in Cell & Developmental Biology, 23(9), 954–962. [DOI] [PubMed] [Google Scholar]
- Nacu E, Glausch M, Le HQ, Damanik FF, Schuez M, Knapp D, et al. (2013). Connective tissue cells, but not muscle cells, are involved in establishing the proximo-distal outcome of limb regeneration in the axolotl. Development, 140(3), 513–518. [DOI] [PubMed] [Google Scholar]
- Nacu E, Gromberg E, Oliveira CR, Drechsel D, & Tanaka EM (2016). FGF8 and SHH substitute for anterior-posterior tissue interactions to induce limb regeneration. Nature, 533(7603), 407–410. [DOI] [PubMed] [Google Scholar]
- Nacu E, & Tanaka EM (2011). Limb regeneration: A new development? Annual Review of Cell and Developmental Biology, 27, 409–440. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Islam MR, Takayanagi M, Yasumuro H, Inami W, Kunahong A, et al. (2014). A transcriptome for the study of early processes of retinal regeneration in the adult newt, Cynops pyrrhogaster. PLoS One, 9(10), e109831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowoshilow S, Schloissnig S, Fei J-F, Dahl A, Pang AWC, Pippel M, et al. (2018). The axolotl genome and the evolution of key tissue formation regulators. Nature, 554(7690), 50–55. [DOI] [PubMed] [Google Scholar]
- Nye HL, Cameron JA, Chernoff EA, & Stocum DL (2003). Regeneration of the urodele limb: A review. Developmental Dynamics, 226(2), 280–294. [DOI] [PubMed] [Google Scholar]
- O’ Hara C, Ega MW, & Eag C (1992). Reorganization of the ependyma during axolotl spinal cord regeneration: Changes in interfilament and fibronectin expression. Developmental Dynamics, 193, 103–115. [DOI] [PubMed] [Google Scholar]
- O’Hara CM, & Chernoff EA (1994). Growth factor modulation of injury-reactive ependymal cell proliferation and migration. Tissue & Cell, 26(4), 599–611. [DOI] [PubMed] [Google Scholar]
- O’Hara C, Egar M, & Chernoff E (1992). Reorganization of the ependyma during axolotl spinal cord regeneration: Changes in intermediate filament and fibronectin expression. Developmental Dynamics, 193(2), 103–115. [DOI] [PubMed] [Google Scholar]
- Okamoto M (1987). Induction of ocular tumor by nickel subsulfide in the japanese common newt, cynops pyrrh ogaster. Cancer Research, 47(19), 5092–5140. [PubMed] [Google Scholar]
- Okamoto M (1997). Simultaneous demonstration of lens regeneration from dorsal iris and tumour production from ventral iris in the same newt eye after carcinogen administration. Differentiation, 61(5), 285–292. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, & Beane WS (2009). Regeneration: The origin of cancer or a possible cure? Seminars in Cell & Developmental Biology, 20, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CL, Beljajeva A, Arenas E, & Simon A (2007). Midbrain dopaminergic neurogenesis and behavioural recovery in a salamander lesion-induced regeneration model. Development, 134(15), 2881–2887. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, & Alvarado AS (2009). Regeneration, stem cells, and the evolution of tumor suppression. Spring, LXXIII, 565–572. [DOI] [PubMed] [Google Scholar]
- Piatt J (1955). Regeneration of the spinal cord in the salamander. Journal of Experimental Zoology, 129(1), 177–207. [Google Scholar]
- Pino CJ, Westover AJ, Johnston KA, Buf DA, & Humes HD (2018). Regenerative medicine and immunomodulatory therapy: Insights from the kidney, heart, brain, and lung. Kidney International Reports, 3(December 2017), 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz JH, & Blau HM (2013). Tumor suppressors: Enhancers or suppressors of regeneration? Development (Cambridge), 140(12), 2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, James A, Olson EN, et al. (2011). Transient regenerative potential of the neonatal mouse heart. Science, 331(6020), 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putta S, Smith J, Walker J, Rondet M, Weisrock D, Monaghan J, et al. (2004). From biomedicine to natural history research: EST resources for ambystomatid salamanders. BMC Genomics, 5(1), 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran A, Liu Y, & Teoh S-H (2018). Review: Bioreactor design towards generation of relevant engineered tissues: Focus on clinical translation. Journal of Tissue Engineering and Regenerative Medicine, 12(1), e7–e22. https://pubmed.ncbi.nlm.nih.gov/28374578/. [DOI] [PubMed] [Google Scholar]
- Rio-Tsonis KD, Jung JC, Chiu I-M, & Tsonis PA (1997). Conservation of fibroblast growth factor function in lensregeneration. Proceedings of the National Academy of Sciences, 94(25), 13701–13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roensch K, Tazaki A, Chara O, & Tanaka EM (2013). Progressive specification rather than intercalation of segments during limb regeneration. Science, 342(6164), 1375–1379. [DOI] [PubMed] [Google Scholar]
- Rose M, & Rose F (1952). Tumor agent transformations in amphibia. Cancer Research, 12(1), 1–12. [PubMed] [Google Scholar]
- Rose SM, Wallingford HM, Moore CR, Price D, Bard P, & Mountcastle VB (1949). Transformation of renal tumors of frogs to Normal tissues in regenerating limbs of salamanders. Science, 109(2835), 435–444. [PubMed] [Google Scholar]
- Sabin K, Jiang P, Gearhart M, Stewart R, & Echeverri K (2019). AP-1cFos/Jun B/mi R-200a regulate the pro-regenerative glial cell response during axolotl spinal cord regeneration. Communications Biology, 2(1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin K, Santos-Ferreira T, Essig J, Rudasill S, & Echeverri K (2015). Dynamic membrane depolarization is an early regulator of ependymoglial cell response to spinal cord injury in axolotl. Developmental Biology, 408(1), 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salero E, Blenkinsop TA, Corneo B, Harris A, Rabin D, Stern JH, et al. (2012). Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell, 10(1), 88–95. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, et al. (2011). Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development (Cambridge, England), 138(17), 3647–3656. [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzman T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, et al. (2014). Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell, 14(2), 174–187. [DOI] [PubMed] [Google Scholar]
- Sanor LD, Flowers GP, & Crews CM (2020). Multiplex CRISPR/Cas screen in regenerating haploid limbs of chimeric axolotls. eLife, 9, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarig R, & Tzahor E (2017). The cancer paradigms of mammalian regeneration: Can mammals regenerate as amphibians? Carcinogenesis, 38(4), 359–366. [DOI] [PubMed] [Google Scholar]
- Satoh A, Bryant SV, & Gardiner DM (2008). Regulation of dermal fibroblast dedifferentiation and redifferentiation during wound healing and limb regeneration in the axolotl. Development, Growth & Differentiation, 50(9), 743–754. [DOI] [PubMed] [Google Scholar]
- Satoh A, Bryant SV, & Gardiner DM (2012). Nerve signaling regulates basal keratinocyte proliferation in the blastema apical epithelial cap in the axolotl (Ambystoma mexicanum). Developmental Biology, 366(2), 374–381. [DOI] [PubMed] [Google Scholar]
- Satoh A, Graham GM, Bryant SV, & Gardiner DM (2008). Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum). Developmental Biology, 319(2), 321–335. [DOI] [PubMed] [Google Scholar]
- Satoh A, Makanae A, Nishimoto Y, & Mitogawa K (2016). FGF and BMP derived from dorsal root ganglia regulate blastema induction in limb regeneration in Ambystoma mexicanum. Developmental Biology, 417(1), 114–125. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, & Maden M (2012). Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature, 489(7417), 561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh N, Gates PB, & Brockes JP (2011). The Meis homeoprotein regulates the axolotl prod 1 promoter during limb regeneration. Gene, 484(1–2), 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda C, Uchida K, & Nakayama H (2011). Pathological features of olfactory neuro-blastoma in an axolotl (Ambystoma mexicanum). The Journal of Veterinary Medical Science, 73(8), 1109–1111. [DOI] [PubMed] [Google Scholar]
- Silver J (2016). The glial scar is more than just astrocytes. Experimental Neurology, 286, 147–149. [DOI] [PubMed] [Google Scholar]
- Silver J, & Miller JH (2004). Regeneration beyond the glial scar. Nature Reviews. Neuroscience, 5(2), 146–156. [DOI] [PubMed] [Google Scholar]
- Simon A, & Tanaka EM (2013). Limb regeneration. Wiley Interdisciplinary Reviews: Developmental Biology, 2(2), 291–300. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Timoshevskaya N, Timoshevskiy VA, Keinath MC, Hardy D, & Voss SR (2019). A chromosome-scale assembly of the axolotl genome. Genome Research, 29(2), 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkow L, Epperlein HH, Herklotz S, Straube WL, & Tanaka EM (2006). A germline GFP transgenic axolotl and its use to track cell fate: Dual origin of the fin mesenchyme during development and the fate of blood cells during regeneration. Developmental Biology, 290(2), 386–397. [DOI] [PubMed] [Google Scholar]
- Sousounis K, Athippozhy AT, Voss SR, & Tsonis P.a. (2014). Plasticity for axolotl lens regeneration is associated with age-related changes in gene expression. Regeneration, 1(3), 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousounis K, Looso M, Maki N, Ivester CJ, Braun T, & Tsonis PA (2013). Transcriptome analysis of newt lens regeneration reveals distinct gradients in gene expression patterns. PLoS One, 8(4), e61445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallanzani L (1768). Prodromo sa un Opera da Imprimersi sopra le Riproduzioni animali. Modena, 7. [Google Scholar]
- Stocum DL (1979). Stages of forelimb regeneration in Ambystoma maculatum. The Journal of Experimental Zoology, 209(3), 395–416. [DOI] [PubMed] [Google Scholar]
- Stocum DL (1980). Intercalary regeneration of symmetrical thighs in the axolotl, Ambystoma mexicanum. Developmental Biology, 79(2), 276–295. [DOI] [PubMed] [Google Scholar]
- Stocum DL (1983). Amphibian limb regeneration: Distal transformation. Progress in Clinical and Biological Research, 110(Pt A), 467–476. [PubMed] [Google Scholar]
- Stocum DL (1991). Limb regeneration: A call to arms (and legs). Cell, 67(1), 5–8. [DOI] [PubMed] [Google Scholar]
- Stocum DL (1998). Regenerative biology and engineering: Strategies for tissue restoration [see comments]. Wound Repair and Regeneration, 6(4), 276–290. [DOI] [PubMed] [Google Scholar]
- Stocum DL (2017). Mechanisms of urodele limb regeneration. Regeneration, 4(October), 159–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS (1967). An investigation recording all salamanders which can and cannot regenerate a lens from the dorsal iris. Journal of Experimental Zoology, 164(1), 87–103. [DOI] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, et al. (2013). Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell, 155(5), 1119. [DOI] [PubMed] [Google Scholar]
- Suetsugu-Maki R, Maki N, Nakamura K, Sumanas S, Zhu J, Del Rio-Tsonis K, et al. (2012). Lens regeneration in axolotl: New evidence of developmental plasticity. BMC Biology, 10(1), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Wang H, Barsacchi R, Simon A, & Tanaka EM (2016). MARCKS-like protein is an initiating molecule in axolotl appendage regeneration. Nature, 531(7593), 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AX, Londono R, Hudnall ML, Tuan RS, & Lozito TP (2018). Differences in neural stem cell identity and differentiation capacity drive divergent regenerative outcomes in lizards and salamanders. Proceedings of the National Academy of Sciences of the United States of America, 115(35), E8256–E8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, & Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676. [DOI] [PubMed] [Google Scholar]
- Tanaka EM (2016). The molecular and cellular choreography of appendage regeneration. Cell, 165(7), 1598–1608. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, & Ferretti P (2009). Considering the evolution of regeneration in the central nervous system. Nature Reviews. Neuroscience, 10(10), 713–723. [DOI] [PubMed] [Google Scholar]
- Tapia N, Reinhardt P, Duemmler A, Wu G, Arauzo-Bravo MJ, Esch D, et al. (2012). Reprogramming to pluripotency is an ancient trait of vertebrate Oct4 and Pou2 proteins. Nature Communications, 3, 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassava RA, & Garling DJ (1979). Regenerative responses in larval axolotl limbs with skin grafts over the amputation surface. The Journal of Experimental Zoology, 208(1), 97–110. [DOI] [PubMed] [Google Scholar]
- Tassava RA, & Loyd RM (1977). Injury requirement for initiation of regeneration of newt limbs which have whole skin grafts. Nature, 268(5615), 49–50. [DOI] [PubMed] [Google Scholar]
- Tazaki A, Tanaka EM, & Fei JF (2017). Salamander spinal cord regeneration: The ultimate positive control in vertebrate spinal cord regeneration. Developmental Biology, 432(1), 63–71. [DOI] [PubMed] [Google Scholar]
- Thornton CS (1954). The relation of epidermal innervation to limb regeneration in Amblystoma larvae. Journal of Experimental Zoology, 127(3), 577–601. [Google Scholar]
- Tran AP, Warren PM, & Silver J (2018). The biology of regeneration failure and success after spinal cord injury. Physiological Reviews, 98(2), 881–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SL, Baselga-Garriga C, & Melton DA (2019). Blastemal progenitors modulate immune signaling during early limb regeneration. Development, 146(1) (dev169128-dev169128). [DOI] [PubMed] [Google Scholar]
- Tsai SL, Baselga-Garriga C, & Melton DA (2020). Midkine is a dual regulator of wound epidermis development and inflammation during the initiation of limb regeneration. eLife, 9, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarouchas TM, Wehner D, Cavone L, Munir T, Keatinge M, Lambertus M, et al. (2018). Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nature Communications, 9(1), 4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis P.a. (2006). How to build and rebuild a lens. Journal of Anatomy, 209(4), 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis PA, & Del Rio-Tsonis K (2004). Lens and retina regeneration: Transdifferentiation, stem cells and clinical applications. Experimental Eye Research, 78(2), 161–172. [DOI] [PubMed] [Google Scholar]
- Tsonis P, & Eguchi G (1981). Carcinogens on regeneration. Differentiation, 20(1–3), 52–60. [DOI] [PubMed] [Google Scholar]
- Tsonis P, & Eguchi G (1982). Abnormal limb regeneration without tumor production in adult newts directed by carcinogens, 20-Methylcholanthrene and benzo (α) pyrene: Amphibia/limb regeneration/carcinogen/teratogenesis. Development, Growth & Differentiation, 24(2), 183–190. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, & Fox TP (2009). Regeneration according to Spallanzani. Developmental Dynamics, 238(9), 2357–2363. [DOI] [PubMed] [Google Scholar]
- Tsonis P, Jang W, Del Rio-Tsonis K, & Eguchi G (2001). A unique aged human retinal pigmented epithelial cell line useful for studying lens differentiation in vitro. International Journal of Developmental Biology, 45(5–6), 753–758. [PubMed] [Google Scholar]
- Tsonis PA, Madhavan M, Tancous EE, & Del Rio-Tsonis K (2004). A newt’s eye view of lens regeneration. The International Journal of Developmental Biology, 48(8–9), 975–980. [DOI] [PubMed] [Google Scholar]
- Tsutsumi R, Inoue T, Yamada S, & Agata K (2015). Reintegration of the regenerated and the remaining tissues during joint regeneration in the newt\n Cynops pyrrhogaster. Regeneration, 2(1), 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kondoh H, & Mizuno N (2005). Generation of transgenic newt Cynops pyrrhogaster for regeneration study. Genesis (New York, N.Y.: 2000), 41(2), 87–98. [DOI] [PubMed] [Google Scholar]
- Urata Y, Yamashita W, Inoue T, & Agata K (2018). Spatio-temporal neural stem cell behavior leads to both perfect and imperfect structural brain regeneration in adult newts. Biology Open, 7(6). 10.1242/bio.033142.bio033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira WA, Wells KM, & McCusker CD (2020). Advancements to the axolotl model for regeneration and aging. Gerontology, 66(3), 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, et al. (2017). Advances in skin regeneration using tissue engineering. International Journal of Molecular Sciences, 18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiard É, Denis JF, Hashemi FS, Igelmann S, Ferbeyre G, & Roy S (2017). Senescence gives insights into the morphogenetic evolution of anamniotes. Biology Open, 6(6), 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X (2019). Bioartificial organ manufacturing technologies. Cell Transplantation, 28(1), 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-H, Wu C-F, Rajasekaran N, & Shin YK (2018). Loss of tumor suppressor gene function in human cancer: An overview. Cellular Physiology and Biochemistry, 51(6), 2647–2693. [DOI] [PubMed] [Google Scholar]
- Xiong J, Lv Y, Huang Y, & Liu Q (2019). The first transcriptome assembly of Yenyuan stream salamander (Batrachuperus yenyuanensis) provides novel insights into its molecular evolution. International Journal of Molecular Sciences, 20(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MH (2015). Changes in regenerative capacity through lifespan. International Journal of Molecular Sciences, 16(10), 25392–25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MH (2018). Cellular senescence in tissue repair: Every cloud has a silver lining. International Journal of Developmental Biology, 62(6–8), 591–604. [DOI] [PubMed] [Google Scholar]
- Yun MH, Davaapil H, & Brockes JP (2015). Recurrent turnover of senescent cells during regeneration of a complex structure. eLife, 4, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Rotgans B, Wang T, & Cummins SF (2016). REGene: A literature-based knowledgebase of animal regeneration that bridge tissue regeneration and cancer. Scientific Reports, 6(February), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Carido M, Meinhardt A, Kurth T, Karl MO, Ader M, et al. (2013). Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS One, 8(1), e54552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Schreiter S, & Tanaka EM (2016). Accelerated three-dimensional Neuroepithelium formation from human embryonic stem cells and its use for quantitative differentiation to human retinal pigment epithelium. Methods in Molecular Biology, 1307, 345–355. [DOI] [PubMed] [Google Scholar]


