Abstract
BACKGROUND:
The Department of Veterans Affairs cares for the largest population of patients with human immunodeficiency virus (HIV) of any healthcare system in the United States. Screening for anal dysplasia/cancer is recommended for all veterans with HIV. Exams are invasive, burdensome and resource intensive. We currently lack markers of disease to tailor screening.
OBJECTIVE:
To establish prevalence of advanced anal disease (high-grade dysplasia and anal cancer) and determine if CD4/CD8 ratio correlates with risk.
DESIGN:
Retrospective regional cohort study of veterans with HIV.
SETTING:
Eight medical centers between 2001–2019.
PATIENTS:
Patients with advanced disease were compared to patients with non-advanced anal pathology.
MAIN OUTCOME MEASURES:
Logistic regression modeling was utilized to estimate adjusted odds of disease as a function of CD4/CD8. Lowest (nadir) CD4/CD8 and nearest CD4/CD8 ratio in each cohort were evaluated.
RESULTS:
A total of 2,267 veterans were included. 15% had anal pathology (112 advanced disease (37 cancer, 75 high-grade), 222 non-advanced disease). Nadir and nearest ratio were lower in patients with advanced disease vs. non-advanced (0.24 vs. 0.45 (p<0.001) and 0.50 vs. 0.88 (p<0.001)), respectively. In adjusted models, one-unit increase in nadir or nearest ratio conferred decreased risk of advanced disease (OR 0.19(95%CI 0.07, 0.53,p<0.001)) and (OR 0.22(95%CI 0.12, 0.43,p<0.001)), respectively. Using a minimum sensitivity analysis, a cut-off nadir ratio of 0.42 or nearest ratio of 0.76 could be used to risk-stratify.
LIMITATIONS:
Retrospective analysis with a low screening rate.
CONCLUSIONS:
In a regional cohort of veterans with HIV, 15% were formally assessed for anal dysplasia. Advanced anal disease was present in 33% of those screened, 5% of the HIV+ population. A strong predictor of advanced disease in this cohort is the CD4/CD8 ratio which is a promising marker to stratify screening practices. Risk stratification using CD4/CD8 has potential to decrease burdensome invasive examinations for low-risk patients and intensify exams for those at high-risk. See Video Abstract at http://links.lww.com/DCR/Bxxx.
Keywords: Anal Cancer, Anal Dysplasia, Anal Intraepithelial Neoplasia (AIN), CD4/CD8 Ratio, Human Immunodeficiency Virus (HIV), Veterans
Abstract
ANTECEDENTES:
El Departamento de Asuntos de Veteranos atiende a la población más grande de pacientes con el virus de inmunodeficiencia humana (VIH) de cualquier sistema de salud en los Estados Unidos. Se recomienda la detección de displasia / cáncer anal para todos los veteranos con VIH. Los exámenes son invasivos, onerosos y requieren muchos recursos. Actualmente carecemos de marcadores de enfermedad para adaptar la detección.
OBJETIVO:
Establecer la prevalencia de enfermedad anal avanzada (displasia de alto grado y cáncer anal) y determinar si la relación CD4 / CD8 se correlaciona con el riesgo.
DISEÑO:
Estudio de cohorte regional retrospectivo de veteranos con VIH.
AJUSTE:
Ocho centros médicos entre 2001–2019.
PACIENTES:
Se comparó a pacientes con enfermedad avanzada con pacientes con patología anal no avanzada.
PRINCIPALES MEDIDAS DE RESULTADO:
Se utilizó un modelo de regresión logística para estimar las probabilidades ajustadas de enfermedad en función de CD4 / CD8. Se evaluó la relación CD4 / CD8 más baja (nadir) y la relación CD4 / CD8 más cercana en cada cohorte.
RESULTADOS:
Se incluyeron un total de 2267 veteranos. El 15% tenía patología anal (112 enfermedad avanzada (37 cáncer, 75 de alto grado), 222 enfermedad no avanzada). El nadir y el cociente más cercano fueron menores en los pacientes con enfermedad avanzada frente a los no avanzados (0,24 frente a 0,45 (p <0,001) y 0,50 frente a 0,88 (p <0,001)), respectivamente. En modelos ajustados, el aumento de una unidad en el nadir o el cociente más cercano confirió una disminución del riesgo de enfermedad avanzada (OR 0,19 (IC del 95%: 0,07, 0,53, p <0,001)) y (OR 0,22 (IC del 95%: 0,12, 0,43, p <0,001) )), respectivamente. Utilizando un análisis de sensibilidad mínima, se podría utilizar un cociente del nadir de corte de 0,42 o el cociente más cercano de 0,76 para estratificar el riesgo.
LIMITACIONES:
Análisis retrospectivo con una tasa de detección baja.
CONCLUSIONES:
En una cohorte regional de veteranos con VIH, el 15% fueron evaluados formalmente por displasia anal. La enfermedad anal avanzada estuvo presente en el 33% de los examinados, el 5% de la población VIH +. Un fuerte predictor de enfermedad avanzada en esta cohorte es la relación CD4 / CD8, que es un marcador prometedor para estratificar las prácticas de detección. La estratificación del riesgo usando CD4 / CD8 tiene el potencial de disminuir los exámenes invasivos onerosos para los pacientes de bajo riesgo e intensificar los exámenes para los de alto riesgo. Consulte Video Resumen en http://links.lww.com/DCR/Bxxx. (Traducción— Dr. Francisco M. Abarca-Rendon)
INTRODUCTION
People living with human immunodeficiency virus (HIV) (PLWH) are at increased risk for anal dysplasia and anal cancer development, with 40–80 times risk of anal cancer compared to patients without HIV infection.1,2 The Department of Veterans Affairs (VA) cares for the largest population of PLWH of any healthcare system in the United States.3 The VA recommends yearly anal cancer screening of HIV-infected veterans with anal cytology testing if resources and expertise are available.3 Most experts recommend rigorous follow-up with procedural examinations after dysplasia is detected, with standard or high-resolution anoscopy (HRA), biopsies, and excision/ablation of dysplastic lesions.4–6 Screening and surveillance procedures for anal dysplasia are invasive, leading to distress and decreased quality of life without evidence of improving cancer detection or survival.7,8
Although rates of anal dysplasia are known to be high in select groups of PLWH, there is a paucity of population data on the true national prevalence of anal dysplasia in PLWH.9 A pervasive issue in studying anal dysplasia is the inconsistent administrative coding to determine grade of disease. Rates of progression from dysplasia to invasive cancer are variably reported, ranging from 1%->8% over multiple years of follow-up.5,10,11 Considering the wide range of cancer development and the invasive nature of testing, patient risk stratification would be helpful to reduce unneeded burdensome examinations in those at decreased risk, while continuing to screen those at increased risk. Currently, no clinically useful marker is utilized to risk-stratify patients.3,6,12
CD4 and CD8 counts are immunologic markers routinely measured in HIV-positive patients to monitor response to HIV treatment. The CD4/CD8 ratio has been identified as a marker for risk of solid organ malignancies in PLWH, including cervical cancer, which is also a human papillomavirus (HPV)-associated malignancy.2,13–17 We have previously shown an association between low CD4/CD8 ratio and advanced anal disease (high-grade anal dysplasia (HGAIN) and anal cancer) within a single non-VA academic institution.18 The objective of this study was to determine prevalence of advanced anal disease in a larger sample of veterans living with HIV and determine if low CD4/CD8 ratio is associated with advanced disease.
MATERIALS AND METHODS
University of Wisconsin institutional review board approval (#2019-0276) and VA Research and Development Committee approval (MR-2019-0276) were obtained for this study
Study population
This is a retrospective regional study at the Veterans’ hospitals within Veterans Integrated Service Network (VISN) 12 (VA Great Lakes Healthcare System), one of the 19 geographic regions within the Veterans’ healthcare system in the United States, comprised of eight VA hospitals. The VA Corporate Data Warehouse (CDW), an electronic medical record database that houses national data on veterans was queried. A cohort of veterans with HIV cared for from 2001–2019 was identified using two validated diagnostic criteria (ICD-9 codes for HIV diagnosis and resultant immunologic laboratory markers (CD4 and CD8 markers)).19 Using natural language processing (NLP), we examined all anal pathology results (cytology and histopathology) and determined grade of disease, Appendix Table 1. All NLP results were validated with manual chart review. We also performed a chart review on all patients with an ICD9/10 diagnosis for anal dysplasia or anal cancer, Appendix Table 2. Two cohorts of patients were identified: 1) advanced anal disease (defined as history of high-grade anal intraepithelial neoplasia (HGAIN) and/or invasive anal cancer) and 2) negative for advanced anal disease (defined as anal pathology with a negative result, atypical squamous cells of undetermined significance (ASCUS), atypical squamous cells – cannot exclude high-grade (ASC-H) or low-grade anal intraepithelial neoplasia (LGAIN)).
Variables
Baseline patient characteristics included: age (at the time of anal pathology result), biological sex, race (defined as: White, Black, Pacific Islander, Native Indian/Alaska, and Other/unavailable), history of smoking, non-HPV sexually transmitted infections (STI), and clinical history of anal condyloma. We identified lowest (nadir) CD4/CD8 ratio and CD4/CD8 ratio nearest to anal pathology result. If a patient had multiple anal pathology results, the first date of the most advanced diagnosis (cancer > HGAIN > LGAIN > ASCUS & ASC-H > negative) was used to identify “nearest” time point. Mean CD4/CD8 ratios were determined and compared between the cohort of patients with advanced disease and those without advanced disease.
Statistical analysis
Demographic and clinical characteristics - including nadir CD4 counts, nadir CD4/CD8 ratio, CD4 counts nearest to anal pathology result and CD4/CD8 ratio nearest to anal pathology result - were compared between the two groups. Pearson’s chi square test was used for categorical variables and Student’s t-test for continuous variables.
Both unadjusted and adjusted logistic regression models were estimated to investigate whether CD4 counts or CD4/CD8 ratios were associated with the development of advanced disease. The covariates included in the adjusted models included age, sex, race, history of smoking, history of non-HPV STI, and history of condyloma.
Receiver Operating Characteristic (ROC) curves were estimated to determine the optimal threshold via the Youden index (J=sensitivity+specificity-1) with a minimum sensitivity analysis for using the CD4/CD8 ratio as a risk factor for stage of disease. Minimum sensitivity was set to 0.90. C-statistics for two models (first including only the covariates and second with the addition of the CD4/CD8 ratio) were calculated to assess whether the CD4/CD8 ratio variables contributed to the model. Statistical significance was defined at p<0.05. Analyses were performed using STATA SE 15.0.
RESULTS
From 2001–2019, 2,267 patients with HIV were cared for in the region. Of those, only 14.7% (N = 334) had anal pathology for review. Of the patients with anal pathology results, 33% (N = 112) had advanced anal disease (75 HGAIN, 37 anal cancer) and 66% (N = 222) had non-advanced anal disease (121 negative, 53 ASCUS, 3 ASC-H, 45 LGAIN) (Figure 1). Patients with advanced disease were older than those with non-advanced disease (56 v. 54 years old), more likely to have a non-HPV-related STI (72% v. 67%, p<0.001) and more likely to have a clinical history of condyloma (61% v. 29%, p<0.001). There was no difference in race, sex, or smoking history (Table 1).
Figure 1.
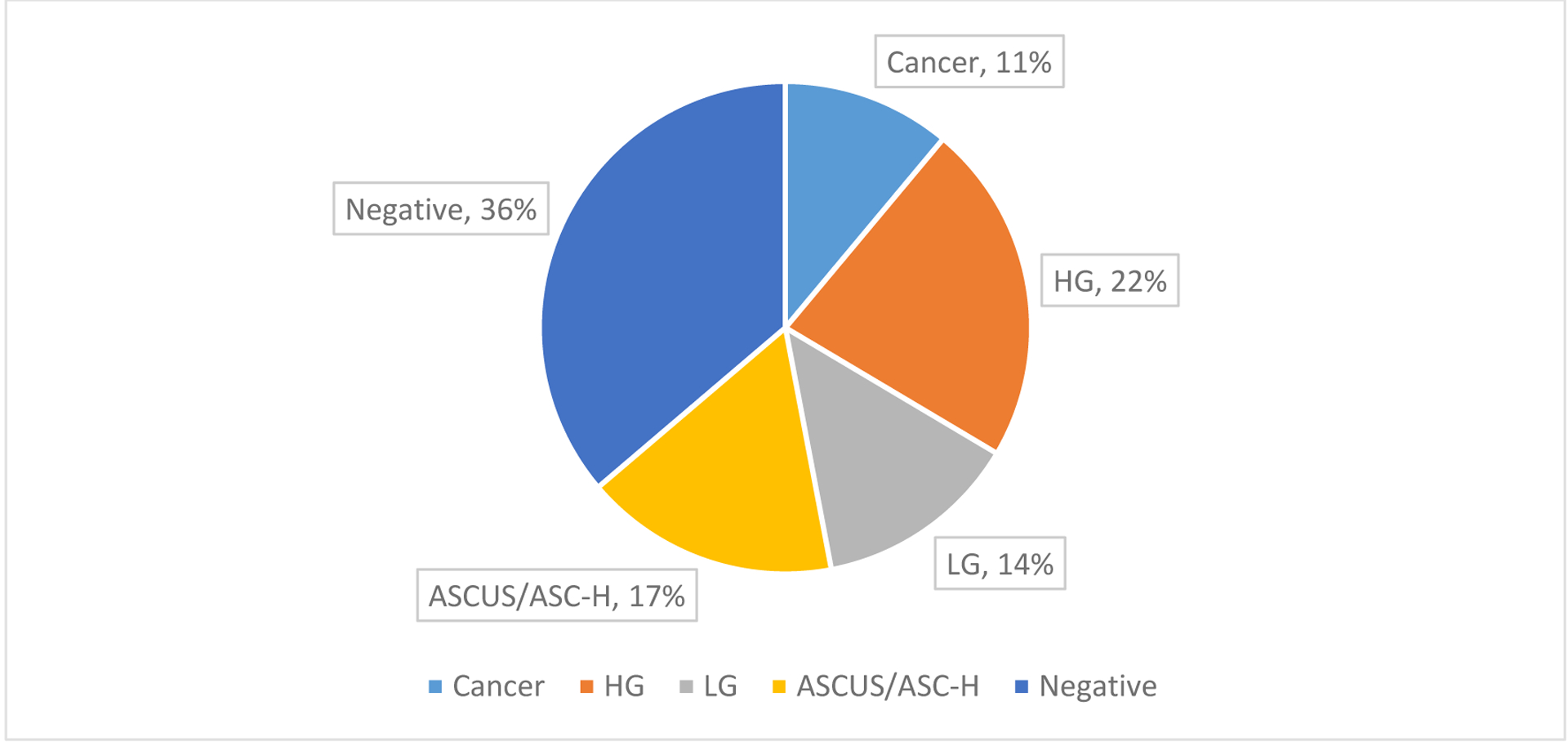
Breakdown by diagnosis of the 334 patients with anal pathology. 33% of patients had advanced anal disease (HG or cancer) and the remaining 66% had non-advanced anal disease (negative, ASCUS, ASC-H or LG). HG = high-grade, LG = low-grade, ASCUS = atypical squamous cells of undetermined significance, ASC-H = atypical squamous cells – cannot exclude high-grade.
Table 1.
Demographic characteristics of patients with HIV infection with 1. advanced anal disease (high-grade anal intraepithelial neoplasia (HGAIN) and/or invasive anal cancer) 2. non-advanced anal disease (negative, atypical squamous cells of undetermined significance (ASCUS), atypical squamous cells – cannot exclude high-grade (ASC-H) or low-grade anal intraepithelial neoplasia (LGAIN), and 3. those without anal pathology. SD = standard deviation, IQR = interquartile range, NA = not applicable, STI = sexually transmitted infection.
| Patient Characteristics | Advanced (n=112) | Non-advanced (n=222) | No screening (n=1933) | p-value | |
|---|---|---|---|---|---|
| Age | Mean age (SD) | 56.13 (10.20) | 53.63 (12.55) | 57.73 (11.95) | <0.001 |
| Median age (IQR) | 56 (12.5) | 56 (17) | 59 (15) | ||
| Race | White (%) | 46 (42.59) | 89 (40.64) | 695 (38.25) | 0.730 |
| Black (%) | 59 (54.63) | 120 (54.79) | 1056 (58.12) | ||
| Other/NA (%) | 3 (2.78) | 10 (4.57) | 66 (3.63) | ||
| Sex (female) (%) | 0 | 2 (0.90) | 48 (2.48) | 0.084 | |
| STI (%) | 81 (72.32) | 148 (66.67) | 1019 (52.72) | <0.001 | |
| Condyloma (%) | 68 (60.71) | 64 (28.83) | 110 (5.69) | <0.001 | |
| Smoking | Current smoker (%) | 63 (56.25) | 113 (50.90) | 959 (49.61) | 0.320 |
| Former user (%) | 16 (14.29) | 24 (10.81) | 213 (11.02) | ||
| Never used (%) | 33 (29.46) | 85 (38.29) | 761 (39.37) | ||
As shown in Figure 2, both nadir CD4 and nearest CD4 count were lower in patients with advanced disease compared to non-advanced (253 vs. 421 cells/mm3, p<0.001 and 444 vs. 659 cells/mm3, p<0.001). Nadir CD4/CD8 ratio and ratio nearest to time of anal pathology result were also lower in those with advanced disease (0.24 vs. 0.45, p<0.001 and 0.50 vs. 0.88, p<0.001).
Figure 2.
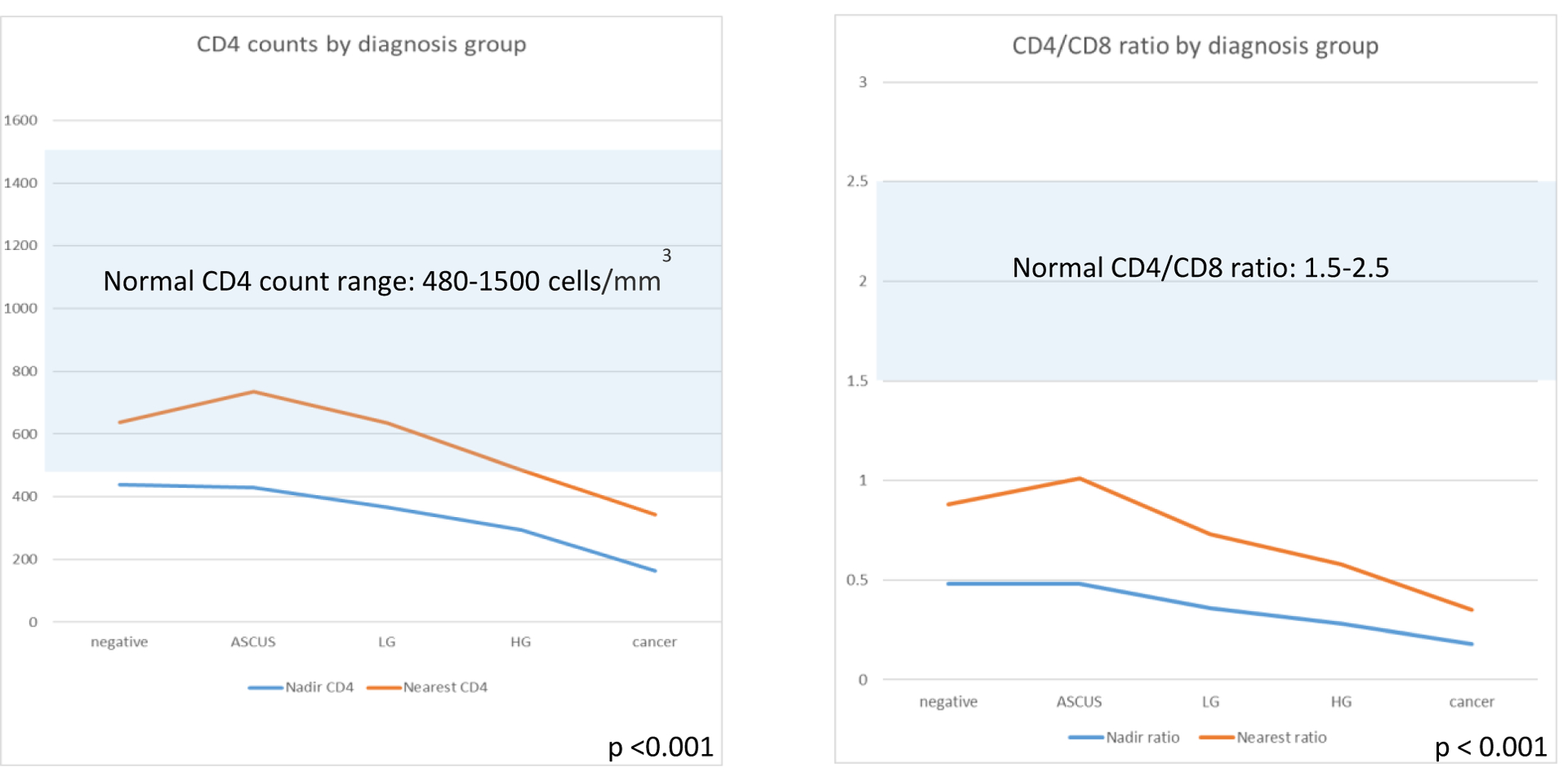
Immunologic markers by grade of anal disease. Both nadir and nearest CD4 count were lower with advanced anal disease, but often in the normal range. Nadir CD4/CD8 ratio and CD4/CD8 ratio nearest to time of anal pathology result were lower in those with advanced disease.
In adjusted analyses, a one-unit increase in the nadir ratio was associated with decreased odds of development of advanced anal disease (OR 0.19, 95% CI 0.07, 0.53, p = 0.001). Higher CD4/CD8 ratio nearest to anal pathology was also associated with decreased odds of advanced disease (OR 0.22, 95% CI 0.12, 0.43, p <0.001). Increase in nadir or nearest CD4 count by 100 units was predictive of decreased odds of advanced disease (OR 0.86 and 0.85 respectively, p<0.001), History of anal condyloma was associated with increased odds of advanced disease (OR 3.17, 95% CI 1.87, 5.36, p <0.001) (Figure 3).
Figure 3.
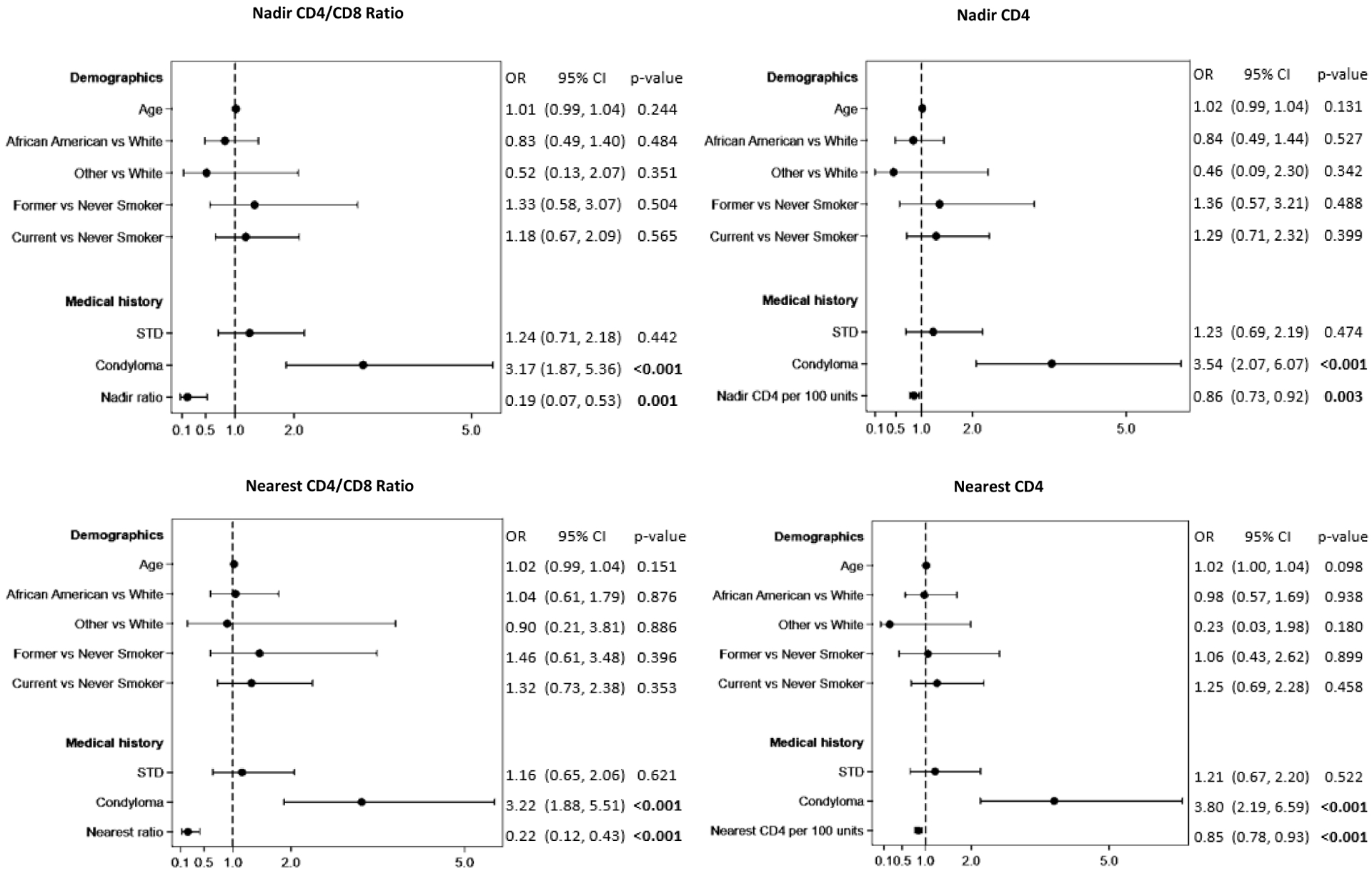
Multivariable logistic regression modeling for risk of advanced anal disease.
When controlling for confounders using a minimum sensitivity analysis to capture at least 90% sensitivity, a cut-off nadir ratio of 0.42 (sensitivity 0.93, specificity 0.37) or nearest ratio of 0.76 (sensitivity 0.91, specificity 0.35) could be used to stratify risk in this population (Figure 4).
Figure 4.
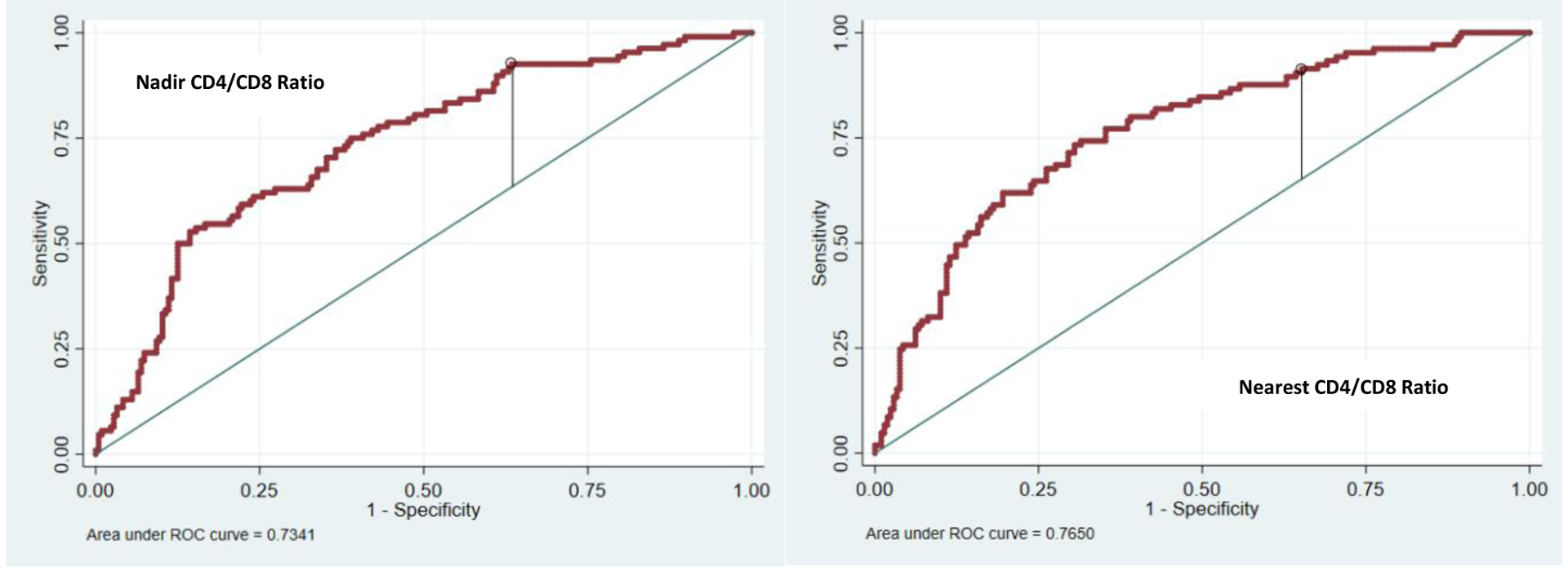
Optimal threshold for using nadir and nearest CD4/CD8 ratios clinically with a minimum sensitivity analysis. C-statistics=0.7074 with model including only the covariates; C-statistics=0.7341 with the addition of the nadir ratio and C-statistic =0.7650 with addition of nearest ratio.
DISCUSSION
With this regional study of veterans living with HIV, of which only 15% were screened or formally assessed for anal dysplasia, 33% were diagnosed with advanced anal disease (5% overall). When examining predictors of advanced anal disease, low CD4/CD8 ratios have a strong correlation and are a promising marker for patient risk stratification. In order to capture over 90% of PLWH that have advanced anal disease, a recent CD4/CD8 ratio below 0.8 or nadir ratio below 0.4 could be used.
PLWH are known to be at increased risk for anal dysplasia due to a combination of associated behavioral risk factors such as anoreceptive intercourse and immunocompromised state.2 The incidence of anal dysplasia in PLWH has been variably reported, ranging from as low as 14% in average-risk populations (mainly HIV positive women) to as high as 81% in HIV-positive men who have anoreceptive intercourse.20–24 Due to the high prevalence and improved outcomes when anal cancer is detected at earlier stages, aggressive screening and surveillance protocols have been proposed.6,25,26 Prevalence data, however, is almost exclusively derived from single institution studies in highly selected, at-risk patients. This is the first study, to our knowledge, that has been able to determine grade of dysplasia using a large US dataset. With this study, we examined a regional cohort of >2,200 veterans with HIV in which the screening and/or disease detection rate was 15%. Of those that were screened, one third were diagnosed with advanced anal disease (5% of the overall cohort). This rate is in contrast to highly selected and screened groups of PLWH who have reported up to 52% prevalence of advanced anal disease.23 Although we are unable to determine how many patients who were not screened have asymptomatic advanced anal disease, our study demonstrates a lower prevalence compared to other published literature, thus illustrating the value of objective markers to tailor screening practices.
With modern antiretroviral therapy, PLWH usually normalize their CD4 counts quickly. However, compensatory rises in CD8 that occur at time of infection often do not normalize on therapy, resulting in a low CD4/CD8 ratio (normal range 1.5–2.5).27,28 Low CD4/CD8 ratios (<0.5) have been reported as risk factors in development and increased severity of other solid-organ malignancies in PLWH, including cervical cancer.13–15 In anal cancer specifically, lower CD4/CD8 ratios have been noted in a Swiss cohort of patients with anal cancer compared to those without when examining ratios many years prior to cancer diagnosis.29 We have previously examined CD4/CD8 ratios in patients at a single non-VA hospital and found a strong correlation between lower ratios and advanced anal disease development, especially with low nadir ratios.18 In the current study, we confirmed the consistent association of CD4/CD8 ratio with risk of advanced anal disease in a larger geographic area with a higher number of anal cancer cases. In this study there is evidence that a ratio recovery to >0.8 is associated with less risk, which is helpful as sometimes the true nadir of a patient on therapy for many years is unknown. We also found that lower CD4 counts correlate with advanced anal disease, however, the values are often in the normal range making the CD4 count alone less useful than the CD4/CD8 ratio as a clinical marker.
When examining a cut-off CD4/CD8 ratio that could be used clinically to stratify risk we determined patients with nadir ratios <0.4 and ratios <0.8 nearest to time of anal pathology result were at the highest risk for advanced anal disease with an >90% sensitivity. Both nadir and nearest ratio add value to other clinically relevant risk predictors as evidenced by the increased C-statistic. Cut-off values closely resemble those identified in our prior work in a single non-VA academic institution (<0.5 for nadir and <1.0 for nearest).18 The consistency in findings in these two different settings suggests the importance of the ratio in anal cancer risk for both veteran and non-veteran patients with HIV infection, despite older age of veteran populations. PLWH with higher ratios may be able to forgo or lessen invasive, burdensome screening examinations while those with low ratios should be examined more closely. Ratio cutoffs in our study were >90% sensitive in predicting advanced anal disease and could be added to discussion of individualizing/altering yearly anal screening. For example, a patient may choose to increase intervals between invasive exams (less frequent than yearly) in the context of risk tolerance.
Our study has limitations. It is a retrospective analysis and we are limited to the data available in the medical record. This study is only of veterans who have their HIV care within the VA system, and it is unknown if veteran data can be extrapolated to the general population, particularly considering the older average age of patients followed in a VA HIV clinic compared to community clinics. In addition, it is possible that anal dysplasia care is not consistent across all VAs. If anal dysplasia diagnoses were made outside of VA and not coded by the VA clinician, these may have been missed on chart review. In addition, we were not able to capture laboratory values drawn outside of VA. As anal dysplasia screening rates in this VA cohort are only 15%, we do not know the number of veterans that have asymptomatic advanced anal disease or reason screening was or was not performed. We collected data from 2001–2019 and routine assessment of anal pathology was only recommended in the VA in 2009.3 During this time the life expectancy as viewed by both providers and PLWH improved dramatically which likely factored into why anal disease was formally assessed in only a minority of patients. Finally, we used both cytology and histopathology results to determine grade of disease despite known limitations in sensitivity and specificity of cytopathology.30
CONCLUSION
In conclusion, about 15% of veterans within a regional population of veterans with HIV infection were formally assessed for anal dysplasia. Of these, 33% have advanced anal disease, 5% of the total cohort. A strong predictor of advanced anal disease in this cohort of veterans is a low CD4/CD8 ratio (especially nadir ratios below 0.4 and nearest ratios <0.8). The CD4/CD8 ratio is a promising marker to help stratify who to screen and individualize surveillance intervals.
ACKNOWLEDGMENTS
The authors thank Dr. Bret Hanlon for assistance in study design and data analysis.
Funding/Support: This work is supported in part by the UW Cancer Center Support Grant (P30CA014520). Dr. Corrine Voils was supported by a Research Career Scientist award from the Department of Veterans Affairs Health Services Research and Development Service (RCS 14-443).
Appendix
Appendix Table 1.
Natural Language Processing terminology utilized to examine all anal cytology and histopathology results.
| Disease Grade | NLP Search Term |
|---|---|
| Negative | NEGATIVE FOR INTRAEPITHELIAL |
| NEGATIVE FOR SQUAMOUS INTRAEPITHELIAL LESION | |
| NEGATIVE FOR MALIGNANCY OR INTRAEPITHELIAL LESION | |
| NEGATIVE FOR CONDYLOMA OR MALIGNANCY | |
| NEGATIVE FOR DYSPLASIA | |
| NO EVIDENCE OF INTRAEPITHELIAL LESION | |
| NO EVIDENCE OF DYSPLASIA | |
| NO DEFINITIVE EVIDENCE OF INTRAEPITHELIAL LESION OR MALIGNANCY | |
| NO DEFINITIVE EVIDENCE OF DYSPLASIA | |
| NEGATIVE FOR CHRONIC CHANGES, DYSPLASIA | |
| NO INTRA-EPITHELIAL OR INVASIVE NEOPLASM | |
| NAGATIVE FOR INTRAEPITHELIAL LESION OR MALIGNANCY | |
| Atypical Squamous Cells of Undetermined Significance (ASCUS) | ASCUS |
| ASC-US | |
| ATYPICAL SQUAMOUS CELLS OF UNDETERMINED SIGNIFICANCE | |
| ATYPICAL SQUAMOUS CELLS OF UNDETERINED SIGNIFICANCE | |
| ATYPICAL SQUAMOUS CELLS OF UNDETEREMINED SIGNIFICANCE | |
| Atypical Squamous Cells – Cannot Exclude High-Grade (ASC-H) | ASC-H |
| Low-grade anal intraepithelial neoplasia (LGAIN) | LSIL |
| LGSIL | |
| LOW GRADE | |
| LOW-GRADE | |
| ACIN-I) | |
| ACIN-I | |
| ACIN I) | |
| ACIN I | |
| AIN-I) | |
| AIN-I | |
| AIN I) | |
| AIN I | |
| MILD DYSPLASIA | |
| AIN-1 | |
| High-grade anal intraepithelial neoplasia (HGAIN) | CARCINOMA IN SITU |
| CARCINOMA IN-SITU | |
| IN-SITU SQUAMOUS CARCINOMA | |
| MODERATE DYSPLASIA | |
| SEVERE DYSPLASIA | |
| MODERATE SQUAMOUS DYSPLASIA | |
| SEVERE SQUAMOUS DYSPLASIA | |
| AIN-2 | |
| AIN 2 | |
| AIN2 | |
| AIN 1–2 | |
| ACIN-II | |
| ACIN II | |
| CIN II | |
| CIN-II | |
| AIN II | |
| AIN-II | |
| AIN II | |
| AIN-3 | |
| AIN 3 | |
| AIN3 | |
| (AIN) II | |
| ANAL INTRAEPITHELIAL NEOPLASIA-3 | |
| ANAL INTRAEPITHELIAL NEOPLASIA-II | |
| ANAL INTRAEPITHELIAL NEOPLASIA II | |
| ANAL INTRAEPITHELIAL NEOPLASIA 3 | |
| ANAL INTRAEPITHELIAL NEOPLASIA 2 | |
| HIGH GRADE SQUAMOUS INTRAEPITHELIAL LESION | |
| HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION | |
| HSIL Exclude: CANNOT EXCLUDE HSIL CANNOT RULE OUT HSIL |
|
| H.S.I.L | |
| HGSIL Exclude: CANNOT EXCLUDE HGSIL CANNOT RULE OUT HGSIL |
|
| HIGH GRADE Exclude: NO EVIDENCE OF HIGH GRADE NEGATIVE FOR HIGH GRADE NOT EXCLUDE HIGH GRADE NOT EXCLUDE A HIGH GRADE HIGH GRADE CAN NOT HIGH GRADE CANNOT |
|
| HIGH-GRADE Exclude: NO EVIDENCE OF HIGH-GRADE NEGATIVE FOR HIGH-GRADE NOT EXCLUDE HIGH-GRADE NOT EXCLUDE A HIGH-GRADE HIGH-GRADE CAN NOT HIGH-GRADE CANNOT |
|
| Anal Cancer | INVASIVE SQUAMOUS CELL CARCINOMA |
| METASTATIC SQUAMOUS CELL CARCINOMA | |
| INVASIVE MODERATELY DIFFERENCIATED SQUAMOUS CELL CARCINOMA | |
| INVASIVE MODERATELY DIFFERENTIATED SQUAMOUS CELL CARCINOMA | |
| POORLY DIFFERENTIATED SQUAMOUS CELL CARCINOMA | |
| DIFFERENTIATED SQUAMOUS CELL Also contains: CARCINOMA |
|
| MODERATELY DIFFERENTIATED SQUAMOUS CELL CARCINOMA | |
| POORLY DIFFERENTIATED CARCINOMA | |
| SQUAMOUS CELL CARCINOMA INVASIVE Also contains: DIFFERENTIATED |
|
| Unsatisfactory | UNSATISFACTORY FOR EVALUATION |
| UNSATISFACTORY FOR INTERPRETATION |
Appendix Table 2.
Diagnosis codes for anal dysplasia or anal cancer (utilized in selecting study population).
| DIAGNOSIS DESCRIPTION | ICD-9 code | ICD-10 code |
|---|---|---|
| Anal dysplasia | 569.44 | K62.82 |
| Carcinoma in situ of the anus and anal canal | 230.5, 230.6 | D01.3 |
| Anal cancer precursor lesion | 569.44 | K62.82 |
| Malignant neoplasm of the anal canal | 154.2 | C21.1 |
| Malignant neoplasm of the anus, unspecified/Anal cancer | 154.3 | C21.0 |
| Malignant neoplasm of cloacogenic zone/Overlap sites rectum anus and anal canal | 154.8 | C21.2, C21.8 |
| Abnormal pap smear anus | 796.70 | R85.619 |
| Abnormal pap smear anus with ASCUS | 796.71 | R85.610 |
| Abnormal pap smear anus ASCUS cannot rule out high grade | 796.72 | R85.611 |
| Abnormal pap smear anus with Low grade intraepithelial lesion (LGSIL) | 796.73 | R85.612 |
| Abnormal pap smear anus with High grade intraepithelial lesion (HGSIL) | 796.74 | R85.613 |
| Anal HPV positive | 796.75, 796.79 | R85.81, R85.82 |
| Anal malignancy on pap smear | 796.76 | R85.614 |
Footnotes
Presentation: This work was presented at the American Society of Colon and Rectal Surgeons Annual Scientific Meeting, Quickshot Oral Presentation (#QS348) August, 2020
REFERENCES
- 1.Dandapani SV, Eaton M, Thomas CR Jr, Pagnini PG. HIV- positive anal cancer: an update for the clinician. J Gastrointest Oncol. 2010;1:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000;342:792–800. [DOI] [PubMed] [Google Scholar]
- 3.Primary Care of Veterans with HIV. 2019. https://www.hiv.va.gov/pdf/pcm-manual.pdf. Accessed August 10, 2020.
- 4.Crawshaw BP, Russ AJ, Stein SL, et al. High-resolution anoscopy or expectant management for anal intraepithelial neoplasia for the prevention of anal cancer: is there really a difference? Dis Colon Rectum. 2015;58:53–59. [DOI] [PubMed] [Google Scholar]
- 5.Goldstone SE, Johnstone AA, Moshier EL. Long-term outcome of ablation of anal high-grade squamous intraepithelial lesions: recurrence and incidence of cancer. Dis Colon Rectum. 2014;57:316–323. [DOI] [PubMed] [Google Scholar]
- 6.Stewart DB, Gaertner WB, Glasgow SC, Herzig DO, Feingold D, Steele SR; Prepared on Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for Anal Squamous Cell Cancers (Revised 2018). Dis Colon Rectum. 2018;61:755–774. [DOI] [PubMed] [Google Scholar]
- 7.Cajas-Monson LC, Ramamoorthy SL, Cosman BC. Expectant management of high-grade anal dysplasia in people with HIV: long-term Data. Dis Colon Rectum. 2018;61:1357–1363. [DOI] [PubMed] [Google Scholar]
- 8.Palefsky JM. Screening to prevent anal cancer: current thinking and future directions. Cancer Cytopathol. 2015;123:509–510. [DOI] [PubMed] [Google Scholar]
- 9.Tomassi MJ, Abbas MA, Klaristenfeld DD. Expectant management surveillance for patients at risk for invasive squamous cell carcinoma of the anus: a large US healthcare system experience. Int J Colorectal Dis. 2019;34:47–54. [DOI] [PubMed] [Google Scholar]
- 10.Fazendin EA, Crean AJ, Fazendin JM, et al. Condyloma acuminatum, anal intraepithelial neoplasia, and anal cancer in the setting of HIV: do we really understand the risk? Dis Colon Rectum. 2017;60:1078–1082. [DOI] [PubMed] [Google Scholar]
- 11.Lee GC, Kunitake H, Milch H, et al. What is the risk of anal carcinoma in patients with anal intraepithelial neoplasia III? Dis Colon Rectum. 2018;61:1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: A review. World J Gastrointest Surg. 2016;8:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das D, Sarkar B, Mukhopadhyay S, Banerjee C, Biswas Mondal S. An altered ratio of CD4+ And CD8+ T lymphocytes in cervical cancer tissues and peripheral blood – a prognostic clue? Asian Pac J Cancer Prev. 2018;19:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hema MN, Ferry T, Dupon M, et al. ; ANRS CO 8 (APROCO/COPILOTE) study group. Low CD4/CD8 ratio is associated with non AIDS-defining cancers in patients on antiretroviral therapy: ANRS CO8 (Aproco/Copilote) prospective cohort study. PLoS One. 2016;11:e0161594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigel K, Wisnivesky J, Crothers K, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV. 2017;4:e67–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tancini G, Barni S, Rescaldani R, Fiorelli G, Vivani S, Lissoni P. Analysis of T helper and suppressor lymphocyte subsets in relation to the clinical stage of solid neoplasms. Oncology. 1990;47:381–384. [DOI] [PubMed] [Google Scholar]
- 17.Tao CJ, Chen YY, Jiang F, et al. A prognostic model combining CD4/CD8 ratio and N stage predicts the risk of distant metastasis for patients with nasopharyngeal carcinoma treated by intensity modulated radiotherapy. Oncotarget. 2016;7:46653–46661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geltzeiler CB, Xu Y, Carchman E, et al. CD4/CD8 ratio as a novel marker for increased risk of high-grade anal dysplasia and anal cancer in HIV+ patients: a retrospective cohort study. Dis Colon Rectum. 2020;63:1585–1592. [DOI] [PubMed] [Google Scholar]
- 19.Kramer JR, Hartman C, White DL, et al. Validation of HIV-infected cohort identification using automated clinical data in the Department of Veterans Affairs. HIV Med. 2019;20:567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaves EB, Folgierini H, Capp E, von Eye Corleta H. Prevalence of abnormal anal cytology in women infected with HIV. J Med Virol. 2012;84:1335–1339. [DOI] [PubMed] [Google Scholar]
- 21.Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006;43:223–233. [DOI] [PubMed] [Google Scholar]
- 22.Goldstone SE, Winkler B, Ufford LJ, Alt E, Palefsky JM. High prevalence of anal squamous intraepithelial lesions and squamous-cell carcinoma in men who have sex with men as seen in a surgical practice. Dis Colon Rectum. 2001;44:690–698. [DOI] [PubMed] [Google Scholar]
- 23.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. [DOI] [PubMed] [Google Scholar]
- 24.Rosa-Cunha I, Degennaro VA, Hartmann R, et al. Description of a pilot anal pap smear screening program among individuals attending a Veteran’s Affairs HIV clinic. AIDS Patient Care STDS. 2011;25:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin-Hong PV, Palefsky JM. Natural history and clinical management of anal human papillomavirus disease in men and women infected with human immunodeficiency virus. Clin Infect Dis. 2002;35:1127–1134. [DOI] [PubMed] [Google Scholar]
- 26.Morency EG, Harbert T, Fatima N, Samolcyzk J, Maniar KP, Nayar R. Anal cytology: institutional statistics, correlation with histology, and development of multidisciplinary screening program with review of the current literature. Arch Pathol Lab Med. 2019;143:23–29. [DOI] [PubMed] [Google Scholar]
- 27.McBride JA, Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017;13:e1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutoh Y, Nishijima T, Inaba Y, et al. Incomplete recovery of CD4 cell count, CD4 percentage, and CD4/CD8 ratio in patients with human immunodeficiency virus infection and suppressed viremia during long-term antiretroviral therapy. Clin Infect Dis. 2018;67:927–933. [DOI] [PubMed] [Google Scholar]
- 29.Bertisch B, Franceschi S, Lise M, et al. ; Swiss HIV Cohort Study Investigators. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178:877–884. [DOI] [PubMed] [Google Scholar]
- 30.Gonçalves JCN, Macedo ACL, Madeira K, et al. Accuracy of anal cytology for diagnostic of precursor lesions of anal cancer: systematic review and meta-analysis. Dis Colon Rectum. 2019;62:112–120. [DOI] [PubMed] [Google Scholar]


