Abstract
Objective
HCQ is an essential medication in SLE, proven to lengthen survival and reduce flares. Its use, however, is limited by its rare but severe ophthalmological complications. Here, we aimed to analyse factors associated with HCQ retinopathy including HCQ blood levels.
Methods
This case–control study compared SLE patients with and without HCQ retinopathy, defined by abnormal results for at least two of the following ophthalmological tests: automated visual fields, spectral-domain optical coherence tomography (SD-OCT), multifocal electroretinogram (mfERG) and fundus autofluorescence. We compared clinical and laboratory findings to assess risk factors for HCQ retinopathy.
Results
The study included 23 patients with confirmed retinopathy (cases) and 547 controls. In the univariate analysis, age (P < 0.001), height (P = 0.045), creatinine clearance (P < 0.001), haemoglobin concentration (P = 0.01), duration of HCQ intake, (P < 0.001), higher cumulative HCQ dose (P < 0.001) and geographical origin (West Indies and sub-Saharan Africa) (P = 0.007) were associated with the risk of retinopathy, while HCQ blood levels were not. In the multivariate analysis, only cumulative dose (P = 0.016), duration of intake (P = 0.039), creatinine clearance (P = 0.002) and geographical origin (P < 0.0001, odds ratio 8.7) remained significantly associated with retinopathy.
Conclusion
SLE patients on HCQ should be closely monitored for retinopathy, especially those from the West Indies or sub-Saharan Africa, or with renal insufficiency, longer HCQ intake or a high cumulative dose. Although reducing the daily dose of HCQ in patients with persistently high HCQ blood levels seems logical, these concentrations were not associated with retinopathy in this study with controls adherent to treatment.
Keywords: hydroxychloroquine, retinal toxicity, retinopathy, concentration, lupus
Rheumatology key messages
SLE patients on hydroxychloroquine should be closely monitored for retinopathy, especially those with risk factors.
Risk factors for hydroxychloroquine retinopathy were intake duration, cumulative dose, geographical origin and creatinine clearance.
Hydroxychloroquine levels were not associated with retinopathy in this study with controls adherent to treatment.
Introduction
Medical use of HCQ continues to rise. It has a well-established role in rheumatology, dermatology and infectious diseases, as well as an emerging role in oncology. It is recognized as an essential medication in SLE and is considered among the safest anti-rheumatic agents, with extremely rare serious side effects [1–4]. The most common adverse reactions are gastrointestinal and cutaneous, and the most severe ophthalmological and cardiac [2–4]. Despite its rarity, the clinical maculopathy known as bull’s eye lesion is a serious concern because there is little if any visual recovery after cessation of the drug and sometimes vision loss even progresses [5, 6].
Earlier literature on chloroquine or HCQ retinopathy recognized only clinical maculopathy (bull’s eye lesion or visual field loss), with incidence reported to range from 0% to 0.5% in large series [7–10]. The incidence is higher for retinopathy detected at a very early stage, by more sensitive screening techniques [11]. In a recent, large study of patients treated with HCQ for >5 years, according to pharmacy refill data, the prevalence of early toxicity, defined by characteristic damage on automated visual field (AVF) testing or spectral-domain optical coherence tomography (SD-OCT) before visible signs, reached 7.5% but varied with daily consumption [odds ratio (OR) 5.67, 95% CI: 4.14, 7.79 for >5.0 mg/kg], duration of use (OR 3.22, 95% CI: 2.20, 4.70 for >10 years), kidney disease (OR 2.08, 95% CI: 1.44, 3.01) and concurrent tamoxifen citrate therapy (OR 4.59, 95% CI: 2.05, 10.27) [12].
Measurements of HCQ blood levels are used increasingly often in SLE patients, in particular to monitor and improve adherence [13–16]. Low HCQ levels are a marker and predictor of SLE exacerbation, with a target blood level threshold of 1000 ng/ml [17]. The interindividual—but not the intraindividual—variability of HCQ levels is substantial; that is, levels in a given (adherent) patient vary only slightly, because of HCQ’s long elimination half-life [18]. Higher HCQ levels have been statistically associated with cutaneous hyperpigmentation lesions, but the association is not considered clinically meaningful as levels overlapped substantially between cases and controls [19]. Higher HCQ levels have also been associated with retinopathy in a small study [20] and in a recent large clinical retrospective study of 537 SLE patients [21]. To analyse the risk factors for retinal toxicity, we conducted a case–control study comparing 23 SLE patients with HCQ retinopathy and 547 SLE patients treated with HCQ but without retinopathy.
Patients and methods
This retrospective multicentre case–control study included subjects followed at the four participating centres (Cochin, Rothschild Foundation, Tenon, and internal medicine 2 Department at Pitié-Salpêtrière) with a diagnosis of SLE according to the ACR classification criteria [22] and treated with HCQ for at least 6 months.
Cases
Because the ophthalmological toxicity of chloroquine is greater than that of HCQ [6, 23] and since most SLE patients in France are treated with HCQ, we excluded patients treated with chloroquine for >3 months. To allow comparison with controls (see below), we also excluded patients with calculated creatinine clearance lower than 60 ml/min and those with liver failure.
Since there are no consensual criteria for diagnosis of HCQ retinopathy, we chose a stringent definition requiring abnormalities confirmed by at least two of the following functional or objective structural tests: automated threshold central 10–2 VF, mfERG, SD-OCT and fundus autofluorescence. Other causes of retinopathy were excluded. The presence of bull’s eye maculopathy or evident fundus changes was not mandatory, but was reported if present. Cases were referred by clinical physicians of the four participating centres and confirmed by two ophthalmologists (S.S. and E.B.) who independently reviewed all tests. The three patients from Tenon Hospital have been previously reported (but without details of their eye involvement) [20].
Control group
We used the patients included in the PLUS study between 2007 and 2010 (ClinicalTrials.gov, number NCT00413361) as a control group. The PLUS study was a randomized, double-blind, placebo-controlled, multicentre trial to evaluate the impact of HCQ dose adaptation to its blood levels in SLE patients who had been treated with HCQ for at least 6 months [24]. Relevant exclusion criteria were known non-adherence, history of retinopathy, calculated creatinine clearance lower than 60 ml/min and liver failure. Patients underwent retinopathy screening at baseline and were monitored according to the recommendations. After contacting the investigators and reviewing the data of all patients in 2018, we further excluded from the controls the patients known to have developed HCQ retinopathy after their participation in the PLUS study (n = 26). Three of these patients had already been included among our cases since they are treated in our centres. The diagnosis of retinopathy in the other patients was based on reports by their investigators; because complete ophthalmological data were usually not available for independent review, those patients were not included in the cases.
Analysed data
We compared both groups for clinical and laboratory data including gender, geographical origin, age at diagnosis, SLE criteria, kidney disease (estimated by the Cockcroft–Gault equation), smoking status, other signs of HCQ toxicity (skin pigmentation lesions, aquagenic pruritus and cardiac toxicity), real and calculated ideal weight, height, laboratory findings, SLEDAI score and whole blood HCQ levels when available. These variables were all assessed at the control subjects’ inclusion in the PLUS study. One HCQ blood level measurement at inclusion was accordingly available for each control subject. Variables for the case patients were assessed at the last visit while receiving HCQ; the one exception was HCQ levels, for which we included all available measurements and then used the mean per patient. The HCQ levels were determined as previously described [17]. Ideal body weight was defined as 50 kg plus 2.3 kg per inch (2.54 cm) over 5 feet (152.4 cm) for men and 45.5 kg plus 2.3 kg per inch over 5 feet for women [9].
Ethics
French patients are informed on their copies of their medical records that their data may be used anonymously for medical research, by retrospective chart reviews. They can refuse at any moment by writing to the department mentioned on the records. Patients in the PLUS study provided written informed consent. A French ethics committee (Rothschild Ophthalmologic Foundation in Paris) approved this study in September 2018.
Statistical analysis
Conventional χ2 and Fisher’s exact tests were used to analyse qualitative differences, and the Mann–Whitney test was used to compare the means in large independent samples of similar variance. A P-value <0.05 was taken to indicate statistical significance. When several independent variables appeared statistically significant in the univariate analysis, a logistic regression test was performed for multivariate analysis (backward conditional) to rule out possible confounding variables. In this case, only those variables statistically significant in the multivariate analysis were considered significant in the study results. The OR was calculated to assess the risk of appearance of each variable.
Results
Cases with HCQ retinopathy
The total number of referred cases was 39. After careful review of all ophthalmological tests by our two reference ophthalmologists, the diagnosis of HCQ retinopathy was ruled out for eight patients, all considered to have another cause of retinopathy. Six additional patients were excluded because they had also been treated with chloroquine and two because they had kidney disease with clearance <60 ml/min. Finally, 23 SLE patients with HCQ retinopathy met our inclusion criteria.
The median age at SLE diagnosis was 30 [interquartile range (IQR): 20–42] years, and median age at discontinuation of HCQ treatment because of the retinopathy diagnosis 52 (41–59) years. The median cumulative dose was 2338 (1403–3268) g and the median duration of use 16.2 (9–21.5) years. Exposure was recorded for 381.5 patient-years. One patient had been treated for <5 years (4.59 years), six from 5–10 years and 16 for >10 years. One patient had a first-degree atrioventricular block, but none had confirmed HCQ cardiac toxicity. Only one patient (4.3%) had a cutaneous side effect of HCQ (skin pigmentation). Twelve patients (52%) had had regular HCQ blood level measurements, with a median of 7 (1–12) results available per patient. Table 1 details the characteristics of the cases.
Table 1.
Comparison of 23 SLE patients with HCQ retinopathy and 547 controls
| HCQ retinopathy (n = 23) | Control(n = 547) | P a | Adjusted ORb (95% CI) | |
|---|---|---|---|---|
| Patients' characteristics | ||||
| Agec, median (IQR), years | 52 (41–59) | 37.5 (30–47) | <0.001 | 1.067 (1.03, 1.1) |
| Female, n (%) | 22 (96) | 501 (92) | 0.7 | 2 (0.26, 15.3) |
| Geographical origin, n (%) | ||||
| Sub-Saharan Africa and West Indies (Antilles) | 9 (39.1) | 90 (16.5) | 0.01 | 3.2 (1.37, 7.77) |
| Europe | 11 (47.8) | 322 (58.9) | ||
| North Africa | 0 (0) | 79 (14.4) | 0.06 | |
| Asia | 2 (8.7) | 46 (8.4) | 1 | |
| South America | 1 (4.3) | 0 (0) | ||
| Other | 0 (0) | 10 (1.9) | ||
| Tobacco smoker, n (%) | 5 (22) | 126 (23) | 0.88 | |
| Weight, median (IQR), kg | 59 (57–65) | 62 (55–72) | 0.32 | |
| BMI, median (IQR), kg/m2 | 23 (21–26) | 23 (21–26) | 0.9 | |
| Height, median (IQR), cm | 160 (156–167) | 164 (160–169) | 0.045 | 0.94 (0.88, 0.99) |
| Laboratory findings | ||||
| Creatinine clearance, median (IQR), ml/min | 73 (64–88) | 100 (80–121) | <0.001 | 0.95 (0.93, 0.97) |
| Hb concentration, median (IQR), g/dL | 12.3 (11.5–13.2) | 13.3 (12.4–14.1) | 0.01 | 0.66 (0.49, 0.89) |
| Leukocytes, median (IQR), G/L | 4.8 (3.2–7.2) | 6.1 (4.6–7.7) | 0.077 | |
| Lymphocytes, median (IQR), G/L | 1.34 (0.9–1.8) | 1.36 (0.9–1.8) | 0.8 | |
| Platelets, median (IQR), G/L | 226 (205–280) | 245 (209–291) | 0.28 | |
| SLE characteristics | ||||
| Age at SLE diagnosis, median (IQR), years | 30 (20–42) | 26 (21–36) | 0.63 | |
| Previous SLE renal involvement, n (%) | 9 (39) | 142 (26) | 0.16 | |
| Ever use of immunosuppressive drugs, n (%) | 12 (52) | 193 (36) | 0.098 | |
| Associated APS, n (%) | 3 (13) | 87 (16) | 0.78 | |
| SLEDAI, median (IQR) | 0 (0–4) | 2 (0–2) | 0.48 | |
| Associated side effects | ||||
| Aquagenic pruritus, n (%) | 0 (0) | 29 (5.3) | 0.62 | |
| HCQ skin pigmentation, n (%) | 1 (4.3) | 38 (7) | 1 | |
| Cardiac toxicity, n (%) | 0 (0) | NA | ||
| HCQ treatment | ||||
| Daily HCQ dose per real body weight, median (IQR), mg/kg/day | 6.78 (6–7.1) | 6.35 (5.3–7.3) | 0.48 | |
| Daily dose per ideal body weight, median (IQR), mg/kg/day | 7.26 (6.6–7.9) | 7 (6.4–7.6) | 0.21 | |
| Daily HCQ dose | ||||
| >6.5 mg/kg, n (%) | 13 (57) | 252 (46) | 0.32 | |
| >5 mg/kg, n (%) | 19 (83) | 428 (78) | 0.79 | |
| Duration of HCQ use, median (IQR), years | 16.2 (9–21.5) | 6.6 (2.7–11.4) | <0.001 | 1.13 (1.07, 1.18) |
| Cumulative HCQ dose, median (IQR), g | 2338 (1403–3268) | 884 (389–1551) | <0.001 | 1.001 (1.001, 1.001) |
| HCQ daily doses, median (IQR), mg/day | 400 (400–400) | 400 (400–400) | 0.88 | |
| HCQ blood measurement | ||||
| Blood HCQ level, median (IQR), ng/ml | 944 (746–1199) (n = 12) | 849 (623–1156) | 0.46 | |
| HCQ level >1000 ng/ml, n (%) | 6 (26) | 210 (38) | 0.23 | |
Values with statistical significance are shown in bold. aUnivariate P-values. bAdjusted OR for age, sex and all variables with P-values < 0.05 in the univariate analysis. cAge was calculated when HCQ was stopped for patients with retinopathy and at inclusion in the PLUS study for controls. APS: antiphospholipid syndrome; G/L: giga per litre; IQR: interquartile range; NA: not available; OR: odds ratio; SLE: systemic lupus erythematosus according to the ACR classification criteria [9].
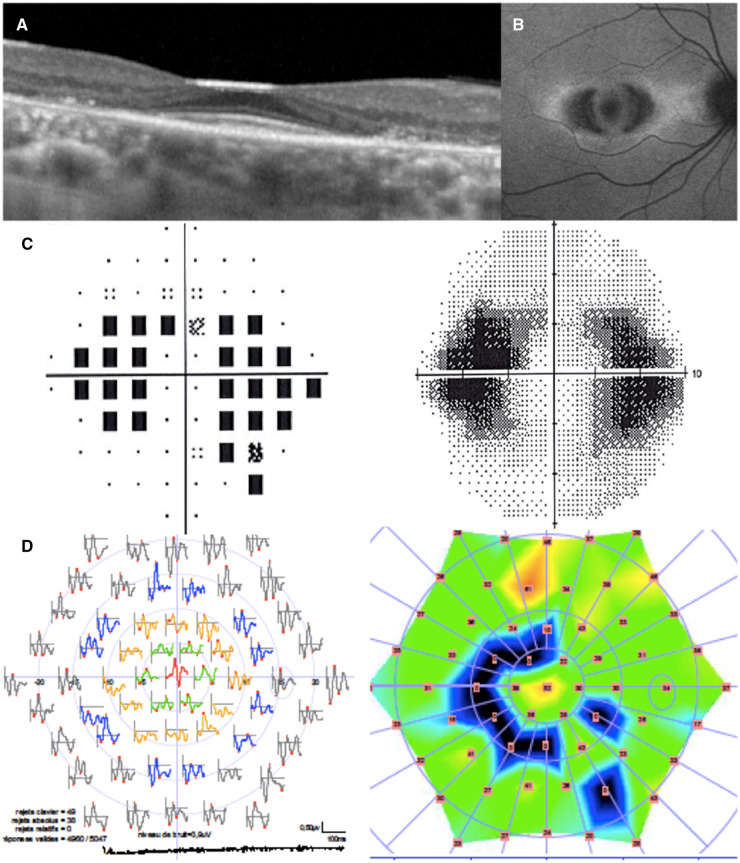
As required by our criteria for HCQ retinopathy, all patients had at least two pathological eye tests. No patient had a bull’s eye lesion, while 20 had typical automated visual field abnormalities (partial or full-ring scotomas, mainly involving the parafoveal region), 19 typical mf-ERG abnormalities (decreased parafoveal response amplitude), 16 typical abnormalities on the SD-OCT (parafoveal thinning of the outer retinal layers) and 11 typical fundus autofluorescence abnormalities (parafoveal autofluorescence changes) (Fig. 1). All patients but three had at least one abnormal functional and one abnormal structural test. The other three patients had functional tests that were both pathological and typical 6 months apart, but no abnormalities on structural tests.
Fig. 1.
Typical HCQ retinopathy on screening tests
(A) Spectral-domain optical coherence tomography; (B) fundus autofluorescence; (C) automated visual field; (D) multifocal electroretinogram.
Control group
In 2018, among the 573 patients initially included in the PLUS study, 26 (including three followed up in our centres) had been diagnosed with possible HCQ retinopathy since the end of the study and were thus excluded from the control group. The final number of SLE patients without HCQ retinopathy (controls) was thus 547. The median cumulative dose for the control patients was 884 (389–1551) g and their median duration of use 6.6 (2.7–11.4) years. Median age at inclusion was 37.5 (30–47) years and median age at last follow-up 46 (38–54) years. Table 1 details the characteristics of the control patients at inclusion in the PLUS Study.
Univariate comparison of groups
The characteristics of both groups are summarized in Table 1. They did not differ for sex or SLE characteristics, including for other SLE treatments (steroids and/or immunosuppressive drugs). Geographical origin differed significantly between the groups (P = 0.01) with more patients from sub-Saharan Africa and the West Indies among the cases than in the controls (respectively 39.1% vs 16.4%). Other patients were from Europe (48% of cases vs 59% of controls), North Africa (0% vs 14%), Asia (8.7% vs 8.4%), South America (4.3% vs 0%) or other (0% vs 1.8%).
The median duration of HCQ therapy was more than twice as long in the retinopathy group as among controls, and the median cumulative dose of HCQ more than twice as high (P < 0.001 for both). Patients were 14.5 years older at the diagnosis of retinopathy than at inclusion in the PLUS study (medians). Given the similarity of age at SLE diagnosis in both groups, this disparity probably reflects that duration of HCQ therapy (and/or age) is a risk factor for retinopathy.
Case patients had a shorter height, and mean BMI was very similar in both groups. Mean daily doses, regardless of definition (real or ideal body weight), did not differ significantly, nor did the percentage of patients prescribed high daily doses (>6.5 mg/kg).
Although we had excluded the two patients with kidney disease, defined by creatinine clearance <60 ml/min (to match the inclusion criteria in the PLUS study for the control group), the median creatinine clearance was quite significantly lower in the retinopathy group [73 (64–88) ml/min vs 100 (80–121) ml/min in the control group; P < 0.001]. Haemoglobin concentrations were also significantly lower among case patients, but leucocyte, lymphocyte and platelet counts did not differ significantly.
Daily doses of HCQ did not differ between groups; 91.3% of the cases and 92.3% of the controls were on HCQ at 400 mg/day. HCQ blood level measurements were available for 12 patients (52.2%) with HCQ retinopathy and, by definition, for all controls. The number of values in the cases ranged from 2 to 12 per patient. The median (IQR) HCQ blood level was 944 (746–1199) ng/ml in patients with HCQ retinopathy vs 849 (623–1156) ng/ml in controls (P = 0.46).
To compare the groups in more detail, a secondary analysis excluded control patients exposed to HCQ for <4 years; the control group then comprised 357 patients, with 23 retinopathy cases (Supplementary Table S1, available at Rheumatology online). The risk factors already identified remained significantly associated with HCQ retinopathy, except for height, which no longer differed between the groups.
Multivariate analysis
Finally, the multivariate analysis found that a higher cumulative dose (P = 0.012), longer use (P = 0.033), lower creatinine clearance (P = 0.001), and geographical origin from the West Indies or sub-Saharan Africa (P < 0.001, OR = 8.6) were associated with HCQ retinopathy (Table 2) whereas the associations with age, height and haemoglobin were not significant. In the secondary analysis restricted to the 357 controls exposed to HCQ for at least 4 years, these risk factors were still significantly associated with retinopathy (Supplementary Table S2, available at Rheumatology online).
Table 2.
Multivariate analysis of HCQ retinopathy risk factors (backward conditional)
| Risk factors | HCQ retinopathy (n = 23) | Control (n = 547) | P |
|---|---|---|---|
| Creatinine clearance, median (IQR), ml/min | 73 (64–88) | 100 (80–121) | 0.001 |
| Duration of HCQ use, median (IQR), years | 16.2 (9–21.5) | 6.6 (2.7–11.4) | 0.033 |
| Cumulative HCQ dose, median (IQR), g | 2338 (1403–3268) | 884 (389–1,551) | 0.012 |
| Geographical origin from Sub-Saharan Africa and West Indies, n (%) | 9 (39.1) | 90 (16.4) | <0.001 OR 8.6 |
Values with statistical significance are shown in bold. IQR: interquartile range.
Receiver operating characteristic curve analysis
Receiver operating characteristic curve analysis was used to determine the area under the curve of the cumulative HCQ dose associated with HCQ retinopathy (Supplementary Fig. S1, available at Rheumatology online). The optimal threshold was determined by the Youden index [sensitivity – (1 − specificity)]. The threshold value of 1450 g provided the best trade-off between sensitivity (73.9%) and specificity (72.2%).
Discussion
In this case–control study, we describe a series of 23 SLE patients with definite HCQ retinopathy and compare them with a large control group of SLE patients without retinopathy. The univariate analyses showed that mean age, duration of use, cumulative dose, height, calculated creatinine clearance and haemoglobin concentration were associated with HCQ retinopathy. In the multivariate analysis, however, the only risk factors that remained were duration of use, cumulative dose, creatinine clearance and geographical origin.
These 23 patients were managed in four large centres, and this small number confirms the rarity of HCQ retinopathy, especially when diagnosed according to stringent criteria. Indeed, the incidence of HCQ retinopathy depends on the screening tests used to diagnose it. A comparison of 10 studies using older screening methods and five studies using standard modern methods found that the reported rate of HCQ retinopathy has increased from 0.4–1.9% to 1.6–8% [25]. The main difficulty encountered in our study was the absence of a consensual definition for defining or diagnosing retinopathy, especially as more sensitive screening tests (mfERG, SD-OCT) have become available. The American Academy of Ophthalmology (AAO) recommendations for screening, revised in 2016, call for both AVF and SD-OCT for routine primary screening because they are widely available, with mfERG and fundus autofluorescence suggested as additional useful screening tests [26]. There is, however, no consensus on the number of tests necessary to define retinopathy. Studies have used many different definitions for HCQ retinopathy [7, 9, 27–31]. In the pivotal 2014 study by Melles and Marmor, for example, inclusion criteria only included a reliable central visual field examination or SD-OCT [12]. We chose to use stringent criteria that required that all patients have at least two pathological screening tests. This allowed us to exclude meaningless retinal changes that may not develop towards toxicity. In addition, and as recommended by Marmor et al., all the tests were performed by experienced staff in large clinical centres and interpreted by ophthalmologists with expertise in retinopathy to rule out causes other than HCQ [26].
Duration of use (>5 years) is a well-known risk factor. Consistent with this, all but one of our 23 patients with retinopathy had been treated with HCQ for >5 years and most (16/23) for >10 years. These findings are consistent with the revised guidelines, which suggest screening patients at baseline and after 5 years of treatment [26, 32] or sooner if they are at high risk, as was our patient with toxicity at 4.59 years. Her daily dose was 400 mg per day (6.25 mg/kg of real weight and 9.63 mg/kg of ideal weight), and her cumulative dose 670 g. The only risk factor for retinopathy in this patient was her short stature (149 cm) and high BMI (29 kg/m2), which meant that her daily dose was high (at least per ideal weight). This raises questions about the safety of a dosage based on actual weight in small patients, but the association of short stature and retinopathy was no longer significant in our secondary univariate analysis (Supplementary Table S1, available at Rheumatology online).
Daily dose in mg/kg is usually recognized as one of the main risk factors for HCQ retinopathy. A cutoff of 6.5 mg/kg was proposed three decades ago [33], chosen based on a very flawed study with substantial bias. The author compared patients with HCQ retinopathy with a control group of 900 patients with a daily HCQ <6.5 mg/kg/day among whom no retinopathy was found [34]. He concluded that 6.5 mg/kg/day ‘lies below the threshold at which retinal toxicity was found to develop’. Given the rarity of HCQ retinopathy, however, this result was to be expected. In 2011, the AAO recommended that this daily dose be calculated using ideal weight, given that overdosage is most likely to occur with individuals of short stature and especially as it was thought that HCQ is not retained in fat tissues; obese patients might thus be seriously overdosed if medicated on the basis of weight alone [5]. In 2014, a large study [12] showed that the use of real weight was more effective in preventing ophthalmological toxicity and proposed a cutoff of 5 mg/kg. The AAO followed that recommendation in revising its recommendations [12, 26]. Importantly (and often overlooked), this study used pharmacy data to calculate the doses patients actually used (i.e. refill data about the doses dispensed in pharmacies to the patient) rather than those prescribed by the physician [35]. In view of the rarity of 100% adherence, as we have previously stated [26, 35], this cutoff is probably similar to the previous one (based on prescription) or even less stringent. In any case, in our univariate analysis, the risk of retinopathy was not associated with the daily dose, whether assessed by real or ideal weight. Similarly, the proportions of patients with daily doses higher than either 5 mg/kg or 6.5 mg/kg did not differ significantly between the two groups.
Previous literature has described renal insufficiency as an important risk factor for HCQ retinopathy. Although our exclusion criteria prevent any definitive conclusion about the role of renal insufficiency as such, creatinine clearance was nonetheless significantly lower in cases than in controls, including in the multivariate analysis. Because creatinine clearance was calculated by the Cockcroft–Gault equation and our case patients were shorter, this difference might be overestimated and should be cautiously interpreted. Interestingly, haemoglobin concentrations were also significantly lower among cases in this univariate analysis. To our knowledge, no previous reports describe this association. It may be due to mild renal insufficiency, a hypothesis supported by the disappearance of this association in the multivariate analysis.
Our study is the first to describe geographical origin as a risk factor for retinopathy: patients with darker skin whose families came either from the West Indies (also called the Antilles) or sub-Saharan Africa were at significantly higher risk; the number of these subjects requires that these results be interpreted with caution. In the recent study of 23 cases of HCQ retinopathy, Petri et al. did not find any statistical difference between white and African American patients but other origins were not studied [21]. The retinal topography of CQ and HCQ toxicity has been shown to differ between ethnic groups (particularly between white and Asian patients). In Asian subjects the first signs of toxicity appear more pericentral with an extramacular pattern that can be missed by the usual 10-degree AVF [36]. The pathophysiology of these ethnic differences is currently unknown but may be due to distinct genetic predispositions to CQ and HCQ toxicity. We suggest adapting retinopathy screening to patients’ geographical original [closer monitoring for patients from the West Indies and sub-Saharan Africa and larger visual fields (30°) for Asian patients, as recommended by Giocanti-Aurégan et al. [36]].
HCQ can be measured by HPLC in whole blood. We have demonstrated that the blood level of HCQ is a marker and predictor of SLE flares [17, 24]. Its link with HCQ toxicity is less clear, however. We have shown an association between higher HCQ blood concentrations and skin pigmentation lesions in 24 patients, although its clinical meaning is unclear in view of the wide overlap: 1190 (465–2229) ng/ml in cases vs 841 (0–3316) ng/ml in controls, P = 0.008 [19]. One study has reported that high HCQ levels are associated with gastrointestinal but not ophthalmological side effects [37]. Data from a recent retrospective study of 537 SLE patients treated with HCQ have shown that HCQ blood levels are associated with retinopathy [21]. By contrast, we found no statistically significant difference between cases and controls, with a mean HCQ level of 973 (351) ng/ml and 914 (454) ng/ml, respectively (P = 0.46). We hypothesize that the discrepancies between these studies might be explained by treatment adherence. In our experience, and very logically, patients with confirmed HCQ retinopathy are usually among those who are adherent to treatment and they may thus have higher blood levels of HCQ than an unselected cohort of patients. The controls in our study, however, must be considered adherent to treatment since non-adherence was an exclusion criterion in the PLUS study. Their HCQ blood levels were thus probably higher than those of an unselected cohort (as in [21]), which may explain why we did not find a difference between cases and controls, unlike Petri et al. [21]. High cumulative levels of HCQ (>1500 ng/ml, for example) probably show that patients are particularly adherent to treatment and also possibly at higher risk of HCQ retinopathy. Since lower blood levels have proven to provide effective treatment, this might allow their daily dose of HCQ to be reduced to avoid unnecessary risk.
Finally, none of our case patients had aquagenic pruritus or significant cardiac toxicity, two other markers of HCQ toxicity, and only one had skin pigmentation, not different from the control group. This finding does not support a hypothesis that such pigmentation is a marker for patients at risk of ocular side effects. It is thus consistent with what we found when we studied 24 patients with HCQ skin pigmentation [19].
The limitations of our study come mainly from its retrospective nature. However, the rarity of HCQ retinopathy means that a prospective study is very unlikely to be performed. For example, Grierson et al. prospectively studied 758 patients for 10 years, without observing any retinopathy [8]. A further limitation is the absence of a consensual criterion for its diagnosis. To compensate for these weaknesses, we used a stringent criterion for diagnosing retinopathy, and two experienced ophthalmologists independently reviewed all screening tests. Another type of case–control study could have included only the PLUS patients and compared those with retinopathy at the end of the last follow-up with those without it. However, full eye examinations were not available everywhere for those with retinopathy, since PLUS was a nationwide study and since the screening was done locally. Although a detailed retinopathy monitoring was not available in 2018 for all patients of the control group, they all had an initial screening at inclusion and the monitoring is usually done annually in French centres and fully covered by the national health insurance fund. In addition, in our experience, we believe that many of the patients who reported retinopathy probably would not have met our stringent criteria for retinopathy or might have had a different cause. We nonetheless decided to exclude every suspected case from the control group to decrease the risk of false negatives (controls who might have developed retinopathy later). In any case, the similar inclusion and exclusion criteria ensure comparability between the groups. Finally, we were not able to assess the respective toxicity of chloroquine since we excluded the six patients who had been treated with chloroquine for >3 months. The high proportion of patients on chloroquine in light of its rare use for SLE treatment in France confirms that the ophthalmological toxicity of chloroquine is greater than that of HCQ [6, 23].
In conclusion, duration of use, cumulative dose, creatinine clearance and geographical origin (West Indies and sub-Saharan Africa) were associated with HCQ retinopathy, and patients with these risk factors probably need closer monitoring. Although reducing the daily dose of HCQ in patients with persistently high HCQ blood levels seems logical, these concentrations were not associated with retinopathy in this study with controls adherent to treatment.
Supplementary Material
Acknowledgements
To the patients, to the Association France Lupus (AFL) for a grant for the PLUS study, to the Clinical Research Unit of Pitié-Salpêtrière Hospital, which dealt with the methodological aspects, data management and monitoring, and to the sponsor of the PLUS study: Assistance Publique – Hôpitaux de Paris.
PLUS Group: F. ACKERMANN, Z. AMOURA, B. ASLI, Leonardo ASTUDILLO, O. AUMAÎTRE, Cristina BELIZNA, Nadia BELMATOUG, Olivier BENVENISTE, Audrey BENYAMINE, Holly BEZANAHARY, B. BLANCHET, Patrick BLANCO, Olivier BLETRY, Bahram BODAGHI, Pierre BOURGEOIS, Benoît BRIHAYE, Emmanuel CHATELUS, J. COHEN-BITTAN, Richard DAMADE, Eric DAUGAS, Christian DE-GENNES, Jean-François DELFRAISSY, Céline DELLUC, Aurélien DELLUC, H. DESMURS-CLAVEL, Pierre DUHAUT, Alain DUPUY, Isabelle DURIEU, Hang-Korng EA, Olivier FAIN, Dominique FARGE, Christian FUNCK-BRENTANO, L. GALICIER, Frédérique GANDJBAKHCH, Justine GELLEN-DAUTREMER, Pascale GHILLANI-DALBIN, Bertrand GODEAU, Cécile GOUJARD, Catherine GRANDPEIX, Claire GRANGE, Lamiae GRIMALDI, Loïc GUILLEVIN, Eric HACHULLA, Jean-robert HARLE, Julien HAROCHE, Pierre HAUSFATER, J-S. HULOT, Jean JOUQUAN, Gilles KAPLANSKI, Homa KESHTMAND, J-E. KAHN, Mehdi KHELLAF, Olivier LAMBOTTE, David LAUNAY, D. LE THI HUONG, Philippe LECHAT, Hervé LEVESQUE, Olivier LIDOVE, F. LIOTE, Eric LIOZON, Kim LY, Matthieu MAHEVAS, Kubéraka MARIAMPILLAI, Xavier MARIETTE, Alexis MATHIAN, Karin MAZODIER, Marc MICHEL, Lucile MUSSET, Rokiya NGACK, Jacques NINET, Eric OKSENHENDLER, Jean-Luc PELLEGRIN, L. PERARD, Olivier PEYR, Anne-Marie PIETTE, Vincent POINDRON, J. POURRAT, Fabienne ROUX, David SAADOUN, K. SACRE, Sabrinel SAHALI, L. SAILLER, Bernadette SAINT-MARCOUX, Françoise SARROT-REYNAULD, J. SELLAM, Yoland SCHOINDRE, Damien SENE, Jacques SERRATRICE, Aude SERVAIS, Pascal SEVE, Jean SIBILIA, Claude SIMON, A. SMAIL, Christelle SORDET, J. STIRNEMANN, Benjamin TERRIER, Salim TRAD, Jean-François VIALLARD, Elisabeth VIDAL, Bertrand WECHSLER, Pierre-Jean WEILLER, N ZAHR. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. N.C.C. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. Study conception and design: Lenfant, Salah, Costedoat-Chalumeau. Acquisition of data: Lenfant, Salah, Leroux, Le Guern, Chasset, Francès, Morel, Chezel, Papo, Cacoub, Mouthon, Guettrot-Imbert, Cohen, Régent, Mauget-Faÿsse, Piette, Costedoat-Chalumeau. Analysis and interpretation of data: Lenfant, Salah, Bousquet, Jallouli, Costedoat-Chalumeau.
Funding: The PLUS study was supported by the 2005 French Programme Hospitalier de Recherche Clinique (PHRC) of Ministère de la santé, and the Direction de la Recherche Clinique et du Développement provided logistic and administrative support.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Costedoat-Chalumeau N, Amoura Z, Hulot J-S, Lechat P, Piette J-C.. Hydroxychloroquine in systemic lupus erythematosus. Lancet 2007;369:1257–8. [DOI] [PubMed] [Google Scholar]
- 2. Costedoat-Chalumeau N, Dunogué B, Morel N, Le Guern V, Guettrot-Imbert G.. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med 2014;43:e167–80. [DOI] [PubMed] [Google Scholar]
- 3. Durcan L, Petri M.. Immunomodulators in SLE: clinical evidence and immunologic actions. J Autoimmun 2016;74:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA.. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8. [DOI] [PubMed] [Google Scholar]
- 5. Marmor MF, Kellner U, Lai TYY, Lyons JS, Mieler WF.. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011;118:415–22. [DOI] [PubMed] [Google Scholar]
- 6. Costedoat-Chalumeau N, Dunogué B, Leroux G. et al. A critical review of the effects of hydroxychloroquine and chloroquine on the eye. Clinic Rev Allerg Immunol 2015;49:317–26. [DOI] [PubMed] [Google Scholar]
- 7. Mavrikakis I, Sfikakis PP, Mavrikakis E. et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology 2003;110:1321–6. [DOI] [PubMed] [Google Scholar]
- 8. Grierson DJ. Hydroxychloroquine and visual screening in a rheumatology outpatient clinic. Ann Rheum Dis 1997;56:188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolfe F, Marmor MF.. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res 2010;62:775–84. [DOI] [PubMed] [Google Scholar]
- 10. Levy GD, Munz SJ, Paschal J. et al. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum 1997;40:1482–6. [DOI] [PubMed] [Google Scholar]
- 11. Arndt C, Costantini M, Chiquet C. et al. Comparison between multifocal ERG and C-Scan SD-OCT (“en face” OCT) in patients with a suspicion of antimalarial retinal toxicity: preliminary results. Doc Ophthalmol 2018;136:97–111. [DOI] [PubMed] [Google Scholar]
- 12. Melles RB, Marmor MF.. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 2014;132:1453–60. [DOI] [PubMed] [Google Scholar]
- 13. Costedoat-Chalumeau N, Amoura Z, Hulot J-S. et al. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Ann Rheum Dis 2007;66:821–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costedoat-Chalumeau N, Pouchot J, Guettrot-Imbert G. et al. Adherence to treatment in systemic lupus erythematosus patients. Best Pract Res Clin Rheumatol 2013;27:329–40. [DOI] [PubMed] [Google Scholar]
- 15. Durcan L, Clarke WA, Magder LS, Petri M.. Hydroxychloroquine blood levels in systemic lupus erythematosus: clarifying dosing controversies and improving adherence. J Rheumatol 2015;42:2092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costedoat‐Chalumeau N, Houssiau F, Izmirly P. et al. A prospective international study on adherence to treatment in 305 patients with flaring SLE: assessment by drug levels and self-administered questionnaires. Clin Pharmacol Ther 2018;103:1074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costedoat-Chalumeau N, Amoura Z, Hulot J-S. et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54:3284–90. [DOI] [PubMed] [Google Scholar]
- 18. Jallouli M, Galicier L, Zahr N. et al. Determinants of hydroxychloroquine blood concentration variations in systemic lupus erythematosus. Arthritis Rheumatol 2015;67:2176–84. [DOI] [PubMed] [Google Scholar]
- 19. Jallouli M, Francès C, Piette J-C. et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol 2013;149:935–40. [DOI] [PubMed] [Google Scholar]
- 20. Tétu P, Hamelin A, Lebrun-Vignes B. et al. Prevalence of hydroxychloroquine-induced side-effects in dermatology patients: a retrospective survey of 102 patients. Ann Dermatol Venereol 2018;145:395–404. [DOI] [PubMed] [Google Scholar]
- 21. Petri M, Elkhalifa M, Li J, Magder LS, Goldman DW.. Hydroxychloroquine blood levels predict hydroxychloroquine retinopathy. Arthritis Rheumatol 2020;72:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 23. Easterbrook M. An ophthalmological view on the efficacy and safety of chloroquine versus hydroxychloroquine. J Rheumatol 1999;26:1866–8. [PubMed] [Google Scholar]
- 24. Costedoat-Chalumeau N, Galicier L, Aumaître O. et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis 2013;72:1786–92. [DOI] [PubMed] [Google Scholar]
- 25. Jorge A, Ung C, Young LH, Melles RB, Choi HK.. Hydroxychloroquine retinopathy – implications of research advances for rheumatology care. Nat Rev Rheumatol 2018;14:693–703. [DOI] [PubMed] [Google Scholar]
- 26. Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF.. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision). Ophthalmology 2016;123:1386–94. [DOI] [PubMed] [Google Scholar]
- 27. Bergholz R, Schroeter J, Rüther K.. Evaluation of risk factors for retinal damage due to chloroquine and hydroxychloroquine. Br J Ophthalmol 2010;94:1637–42. [DOI] [PubMed] [Google Scholar]
- 28. Yam JCS, Kwok A.. Ocular toxicity of hydroxychloroquine. Hong Kong Med J 2006;12:294–304. [PubMed] [Google Scholar]
- 29. Lyons JS, Severns ML.. Using multifocal ERG ring ratios to detect and follow Plaquenil retinal toxicity: a review. Doc Ophthalmol 2009;118:29–36. [DOI] [PubMed] [Google Scholar]
- 30. Michaelides M, Stover NB, Francis PJ, Weleber RG.. Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol 2011;129:30–9. [DOI] [PubMed] [Google Scholar]
- 31. Payne JF, Hubbard GB, Aaberg TM, Yan J.. Clinical characteristics of hydroxychloroquine retinopathy. Br J Ophthalmol 2011;95:245–50. [DOI] [PubMed] [Google Scholar]
- 32. Couturier A, Giocanti-Aurégan A, Dupas B. et al. [Update on recommendations for screening for hydroxychloroquine retinopathy]. J Fr Ophtalmol 2017;40:793–800. [DOI] [PubMed] [Google Scholar]
- 33. Bernstein HN. Ocular safety of hydroxychloroquine. Ann Ophthalmol 1991;23:292–6. [PubMed] [Google Scholar]
- 34. Mackenzie AH. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med 1983;75:40–5. [DOI] [PubMed] [Google Scholar]
- 35. Costedoat-Chalumeau N, Isenberg D, Petri M.. Letter in response to the 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus by Fanouriakis et al. Ann Rheum Dis 2019, doi: 10.1136/annrheumdis-2019-215573. [DOI] [PubMed] [Google Scholar]
- 36. Giocanti-Aurégan A, Couturier A, Girmens J-F. et al. [Variability of chloroquine and hydroxychloroquine retinopathy among various ethnicities]. J Fr Ophtalmol 2018;41:363–7. [DOI] [PubMed] [Google Scholar]
- 37. Munster T, Gibbs JP, Shen D. et al. Hydroxychloroquine concentration–response relationships in patients with rheumatoid arthritis. Arthritis Rheum 2002;46:1460–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.