Long-term vaccine-induced immunity is crucial for controlling the COVID-19 pandemic. Vaccination against COVID-19 is recommended for patients with rheumatic diseases, but a paucity of data are available regarding COVID-19 vaccines in patients with rheumatoid arthritis. Because patients receiving immunosuppressive treatment were excluded from the phase 3 clinical trials,1, 2 it is not clear whether disease-modifying anti-rheumatic drug (DMARD) treatment should be continued before and after vaccination. In addition, some published reports are limited to follow-up after a single vaccine dose.3, 4, 5
Here we report 53 consecutive patients with rheumatoid arthritis on DMARDs and 20 healthy controls (appendix p 1) who were eligible for vaccination according to the Swiss federal regulations and were enrolled in the RECOVER study, a non-randomised, prospective, observational trial. The RECOVER study was approved by the Ethical Committee of St Gallen, Switzerland, and written consent was obtained from all patients before inclusion. The vaccination itself was not part of the study. Nine patients received two doses of the mRNA-1273 vaccine (Moderna), all others received two doses of the BNT162b2 vaccine (Pfizer–BioNTech). Serum samples were collected at baseline, 3 weeks after the first vaccination, and 2 weeks after the second vaccination. Quantitative antibody testing was done using the Roche Elecsys Anti-SARS-CoV-2 spike subunit 1 (S1) assay that measures antibodies to SARS-CoV-2 spike protein 1 (range 0·4–2500 U/mL) and to SARS-CoV-2 nucleoprotein. This assay was used because it can distinguish between people who develop an anti-S1 response after vaccination or after natural infection, when typically antibodies to both S1 and nucleoprotein are generated. The threshold for this anti-SARS-CoV-2 S1 assay that might correspond to neutralisation of viral infectivity is still being discussed, but a cutoff level of 133 U/mL has been proposed.6 A lower cutoff level of >15 U/mL has been suggested,7 emphasising the need to establish formal cutoff levels of anti-SARS-CoV-2 antibody titres associated with protection against SARS-CoV-2 infection and severe disease.
Intervals between the first and second vaccine dose and the intervals between vaccination and serum sampling were comparable between patients and controls (appendix p 1). All patients with rheumatoid arthritis were receiving continuous therapy with conventional synthetic, biological, or targeted synthetic DMARDs. Methotrexate was used in 28 (53%) patients at a median dose of 15 mg per day (IQR 10–20). 17 (32%) of 53 patients were on low dose prednisone with a mean daily dose of 5 mg (SD 1·9 mg). None of the patients or controls reported symptoms suggestive of COVID-19 at baseline or during the observation period, and none had a positive SARS-CoV-2 antigen or RT-PCR test. Two patients had antibodies to nucleoprotein at baseline consistent with previously unnoticed COVID-19, and these patients were excluded from further analysis.
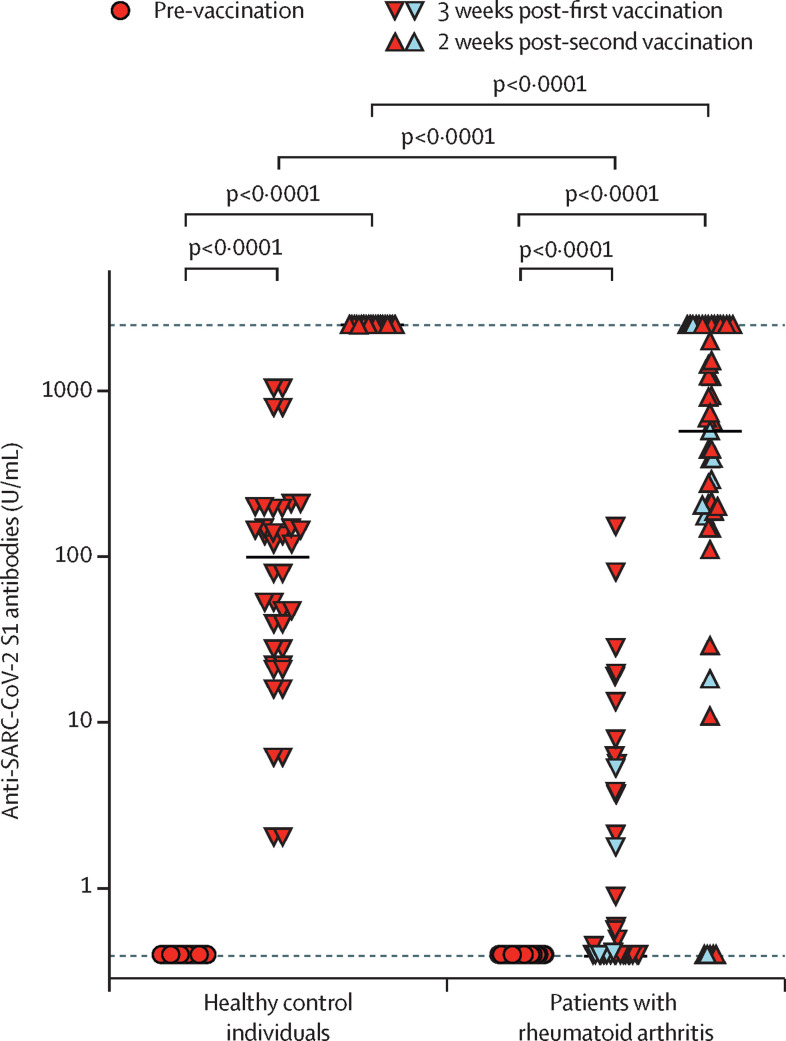
Vaccine-induced antibody titres to SARS-CoV-2 S1 protein were significantly lower in patients with rheumatoid arthritis 3 weeks after the first vaccination (median 0·4 U/mL, IQR 0·4–2·13) and 2 weeks after the second vaccination (657 U/mL, IQR 188–2500) than in the control group, which mainly consisted of health-care workers (3 weeks after first vaccination: 99·2 U/mL, IQR 24·8–172; 2 weeks after second vaccination: 2500 U/mL, IQR 2500–2500 (upper limit of detection of the assay was 2500 U/mL); p<0·0001; figure ). 18 (90%) of 20 of the controls reached a titre above 15 U/mL after the first vaccination, whereas only five (10%) of 51 of patients with rheumatoid arthritis did (p<0·001). Nine (45%) of 20 of the controls had a titre higher than 133 U/mL after their first vaccination, compared with only 1 (2%) of 51 patients with rheumatoid arthritis (p<0·001). Six (12%) of 51 patients with rheumatoid arthritis did not have titres of more than 15 U/mL after their second vaccine dose, four of these patients were using a JAK inhibitor in monotheray or combination therapy, one patient was on methotrexate and abatacept (appendix p 2). Nine (18%) of 51 patients had an anti-S1 titre of less than 133 U/mL after their second vaccine dose, whereas all control patients developed high anti-S1 titres after their second vaccine dose (ceiling at 2500 U/mL).
Figure.
Anti-SARS-CoV-2 S1 serum antibodies from patients with rheumatoid arthritis
Patients with rheumatoid arthritis (n=51) and healthy control individuals (n=20) before vaccination and at the indicated time points following their first and second vaccination. Symbols show individual values, horizontal bars show medians, horizontal dotted lines show lower (0·4 U/mL) and upper (2500 U/mL) detection limits. Double red triangles show control individuals. Single red triangles show patients with rheumatoid arthritis. Blue triangles show patients with rheumatoid arthritis who were receiving Janus kinase inhibitors. Statistical analysis was done using the Mann–Whitney test for non-parametric data.
13 (81%) of 16 patients on csDMARDs, 17 (94%) of 18 patients on anti-cytokine directed biologics in monotherapy or combination therapy (appendix p 2), four (80%) of five patients on abatacept, and eight (67%) of 12 patients on JAK inhibitors in monotherapy or combination therapy developed a humoral immune response in titres that are in line with a neutralising capacity (ie, >15 U/mL) after their second vaccine dose. Anti-S1 titres did not differ between patients according to age, sex, disease duration, or the use of low-dose prednisone. These data suggest that the kinetics of the vaccine-induced humoral immune response differs between patients with rheumatoid arthritis who are taking DMARDs and healthy individuals. Significantly more patients with rheumatoid arthritis required the second vaccine dose to mount an immune response that is considered to correlate with neutralisation, according to the two cutoffs analysed.
In some countries, delayed administration of the second dose has been discussed to increase the number of people receiving at least one dose.8 Our data implies that successful vaccination in patients with rheumatoid arthritis on DMARDs might depend on the recommended schedule that includes two vaccinations within an interval of 3–6 weeks.
Limitations of our study include that the control group was not age-matched, as a result of regulatory recommendations regarding vaccination, a paucity of long-term data regarding the persistence of the humoral and cellular immune responses, and the use of numerical anti-S1 humoral response cutoffs as possible correlates of vaccine-induced protection.
Our study shows different kinetics of the humoral immune response to SARS-CoV-2 S1 protein, emphasising the importance of a second vaccination in patients with rheumatoid arthritis on DMARDs. Studies with large patient cohorts and longer duration are required to define the optimal vaccine strategy in patients with rheumatoid arthritis, to clarify whether DMARDs should be paused and whether monitoring anti-S1 concentrations is required to ensure vaccine-induced protection.
AR-R reports consulting fees from AbbVie, Gilead, Lilly, BMS, and Sanofi, honoraria from AbbVie, Pfizer, Sanofi, UCB, BMS, Lilly, Gilead, and Roche, payment for expert testimony from AbbVie and Gilead, support for travel or meeting attendance from Sanofi, Roche, and AbbVie, and compensation for participation on a Data Safety Monitoring Board from R-Pharm. KS reports support for travel or meeting attendance from AbbVie Deutschland. JvK reports consulting fees from AbbVie, BMS, Pfizer, and Sanofi, honoraria from Eli Lilly, and support for travel or meeting attendance from Pfizer. All other authors declare no competing interests. All authors have access to all the data in the study. AR-R and JvK accessed and verified all the data in the study. The RECOVER trial is registered by the Business Administration System for Ethics Committees (number2021-00156).
Supplementary Material
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220289. published online March 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220272. published online March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101544. published online April 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resman Rus K, Korva M, Knap N, Avšič Županc T, Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139 doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio-Acero R, Castelletti N, Fingerle V, et al. In search for the SARS-CoV-2 protection correlate: A head-to-head comparison of two quantitative S1 assays in a group of pre-characterized oligo-/asymptomatic patients. medRxiv. 2021 doi: 10.1007/s40121-021-00475-x. https://www.medrxiv.org/content/10.1101/2021.02.19.21252080v1 published online Feb 23. (preprint) [DOI] [PubMed] [Google Scholar]
- 8.Joint Committee on Vaccination and Immunisation Optimising the COVID-19 vaccination programme for maximum short-term impact. Jan 26, 2021. https://www.gov.uk/government/publications/prioritising-the-first-covid-19-vaccine-dose-jcvi-statement/optimising-the-covid-19-vaccination-programme-for-maximum-short-term-impact
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.