Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a potentially life-threatening hyperinflammatory syndrome that occurs after primary SARS-CoV-2 infection. The pathogenesis of MIS-C remains undefined, and whether specific inflammatory biomarker patterns can distinguish MIS-C from other hyperinflammatory syndromes, including Kawasaki disease and macrophage activation syndrome (MAS), is unknown. Therefore, we aimed to investigate whether inflammatory biomarkers could be used to distinguish between these conditions.
Methods
We studied a prospective cohort of patients with MIS-C and Kawasaki disease and an established cohort of patients with new-onset systemic juvenile idiopathic arthritis (JIA) and MAS associated with systemic JIA (JIA-MAS), diagnosed according to established guidelines. The study was done at Cincinnati Children's Hospital Medical Center (Cincinnati, OH, USA). Clinical and laboratory features as well as S100A8/A9, S100A12, interleukin (IL)-18, chemokine (C-X-C motif) ligand 9 (CXCL9), and IL-6 concentrations were assessed by ELISA and compared using parametric and non-parametric tests and receiver operating characteristic curve analysis.
Findings
Between April 30, 2019, and Dec 14, 2020, we enrolled 19 patients with MIS-C (median age 9·0 years [IQR 4·5–15·0]; eight [42%] girls and 11 [58%] boys) and nine patients with Kawasaki disease (median age 2·0 years [2·0–4·0]); seven [78%] girls and two [22%] boys). Patients with MIS-C and Kawasaki disease had similar S100 proteins and IL-18 concentrations but patients with MIS-C were distinguished by significantly higher median concentrations of the IFNγ-induced CXCL9 (1730 pg/mL [IQR 604–6300] vs 278 pg/mL [54–477]; p=0·038). Stratifying patients with MIS-C by CXCL9 concentrations (high vs low) revealed differential severity of clinical and laboratory presentation. Compared with patients with MIS-C and low CXCL9 concentrations, more patients with high CXCL9 concentrations had acute kidney injury (six [60%] of ten vs none [0%] of five), altered mental status (four [40%] of ten vs none [0%] of five), shock (nine [90%] of ten vs two [40%] of five), and myocardial dysfunction (five [50%] of ten vs one [20%] of five); these patients also had higher concentrations of systemic inflammatory markers and increased severity of cytopenia and coagulopathy. By contrast, patients with MIS-C and low CXCL9 concentrations resembled patients with Kawasaki disease, including the frequency of coronary involvement. Elevated concentrations of S100A8/A9, S100A12, and IL-18 were also useful in distinguishing systemic JIA from Kawasaki disease with high sensitivity and specificity.
Interpretation
Our findings show MIS-C is distinguishable from Kawasaki disease primarily by elevated CXCL9 concentrations. The stratification of patients with MIS-C by high or low CXCL9 concentrations provides support for MAS-like pathophysiology in patients with severe MIS-C, suggesting new approaches for diagnosis and management.
Funding
Cincinnati Children's Research Foundation, National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health, the Deutsche Forschungsgemeinschaft, and The Jellin Family Foundation.
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a severe post-infectious complication that can occur 3–6 weeks after a typically mild or asymptomatic SARS-CoV-2 infection. Initially described as Kawasaki-like disease or paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK, it became known as MIS-C in the USA. The condition was recognised in April, 2020, with initial descriptions highlighting overlapping features with Kawasaki disease, such as conjunctivitis, rash, mucositis, swelling of the hands and feet, and cardiac involvement.1, 2, 3, 4, 5, 6, 7 However, MIS-C also encompasses features uncommon in Kawasaki disease, such as marked hyperferritinaemia, cytopenia, coagulopathy, and greater organ dysfunction. These features are reminiscent of cytokine storm syndromes such as macrophage activation syndrome (MAS) or secondary haemophagocytic lymphohistiocytosis (HLH). As such, the management of MIS-C has largely been adapted from that of Kawasaki disease with addition of systemic steroids.2, 3, 8, 9
Research in context.
Evidence before this study
Multisystem inflammatory syndrome in children (MIS-C) has overlapping features with Kawasaki disease and macrophage activation syndrome (MAS), yet its pathophysiology remains unclear. We searched PubMed between April 27, 2020, and Sept 10, 2020, for studies published in English on MIS-C, including a combination of “MAS”, “Kawasaki disease”, “cytokine storm”, “CXCL9”, “interferon gamma”, and “IL-18”. Multiple observational studies have compared clinical and general laboratory findings between MIS-C and pre-COVID-19 Kawasaki disease and fewer between MAS. However, a comprehensive comparison of biomarkers between MIS-C, MAS, and Kawasaki disease was not found.
Added value of this study
Our study provides support that MIS-C and Kawasaki disease are characterised by similar S100 protein and interleukin-18 concentrations, but these two entities are distinguished by concentrations of the IFNγ-induced chemokine (C-X-C motif) ligand 9 (CXCL9). This finding is analogous to how systemic JIA with MAS is differentiated from systemic JIA without MAS. We provide evidence that MAS physiology might be involved in the pathogenesis of a severe subset of MIS-C, and that this severity of clinical presentation can be differentiated by CXCL9 concentrations. CXCL9 is a useful biomarker for distinguishing MIS-C from Kawasaki disease and can also be used to diagnose severity of MIS-C with high sensitivity and specificity.
Implications of all the available evidence
We suggest clinical biomarkers to distinguish hyperinflammatory diseases from each other and to determine clinical severity for MIS-C. Such biomarkers will be essential as the COVID-19 pandemic continues and seroconversion is likely to increase, and history of exposure and serology will become less reliable in diagnosing MIS-C. Furthermore, our findings suggest that CXCL9 could be used to monitor MIS-C clinically. MIS-C clinical trials are needed to help guide immunomodulator regimens and duration, particularly with medications that have clinically significant adverse effects and the potential for shortages given the high demand during the COVID-19 pandemic.
Kawasaki disease is a self-limited vasculitis of unknown cause but might be triggered by viral infections.10 Studies attempting to investigate whether Kawasaki disease and MIS-C could be the same disease by comparing immune cell compositions, cytokines, and autoantibodies, have found several differences in plasma protein profiles, T-cell subsets, and autoantibodies.9, 11
Because MIS-C has features resembling MAS, it is possible that MIS-C could represent a Kawasaki disease-like post-infectious process complicated by a cytokine storm similar to MAS. Although the pathogenesis of MAS is not fully understood, animal models and human studies have shown that IFNγ plays a central role in both familial HLH (also characterised by cytokine storm) and MAS.12, 13 Children with systemic juvenile idiopathic arthritis (JIA) complicated by MAS (systemic JIA-MAS) present with cytopenia, hyperferritinaemia, elevated systemic inflammatory markers, and greater organ dysfunction than typically seen in patients with active systemic JIA without MAS.12 Specifically, patients with MAS have significantly higher serum concentrations of IFNγ, the IFNγ-induced chemokine (C-X-C motif) ligand 9 (CXCL9), and interleukin (IL)-18 than do those with active systemic JIA without MAS.12, 14 One study that included a small number of patients reported that although CXCL9 and IL-18 were somewhat elevated in patients with MIS-C, concentrations were significantly lower than in patients with MAS or secondary HLH.3
S100A8/A9 and S100A12 are alarmin proteins produced by myeloid cells that amplify innate immune responses; excess production of these proteins can lead to apoptosis and organ damage.15 S100A8/A9 and S100A12 are sensitive diagnostic and disease activity biomarkers in various autoimmune and autoinflammatory diseases including systemic JIA and Kawasaki disease.16, 17, 18, 19 However, the degree of S100 protein elevation and the potential diagnostic role of these proteins in distinguishing systemic JIA and Kawasaki disease is unclear, and they have not been examined as biomarkers in MIS-C. Before the COVID-19 pandemic, we began a prospective study to establish the utility of inflammatory biomarkers including S100A8/A9, S100A12, and IL-18 to distinguish Kawasaki disease from rheumatic disease mimics including systemic JIA. With the emergence of COVID-19, our study was adapted to include patients with MIS-C and MAS, and to compare biomarkers patterns between MIS-C, Kawasaki disease, systemic JIA without MAS, and systemic JIA-MAS. Here, we aimed to establish whether patients with MIS-C display a distinct biomarker profile with key differences from these clinically similar hyperinflammatory states.
Methods
Study design and participants
We did a cohort study that prospectively enrolled children with suspected Kawasaki disease and MIS-C and a retrospective cohort of patients with new-onset systemic JIA and systemic JIA-MAS. All patients were enrolled from Cincinnati Children's Hospital Medical Center (Cincinnati, OH, USA), and all patients with Kawasaki disease except one were enrolled before the COVID-19 pandemic.
MIS-C was diagnosed by the treating physicians, and all patients satisfied classification criteria from the Centers of Disease Control and Prevention or WHO.8, 20 Complete and incomplete Kawasaki disease were diagnosed based on American Heart Association criteria.21 All patients with MIS-C and Kawasaki disease identified by an inpatient rheumatology consultation service were approached regarding study enrolment. Systemic JIA was diagnosed based on the Childhood Arthritis and Rheumatology Research Alliance provisional definition, which is a modified version of the diagnostic criteria of International League of Associations for Rheumatology.22, 23 The new-onset systemic JIA group included patients who had not initiated biologics at time of study enrolment and did not have MAS.23 All patients in the systemic JIA-MAS group had a history of systemic JIA and were independent of patients with new-onset systemic JIA without MAS. MAS was defined based on the 2016 MAS classification.23, 24
Written informed consent was obtained from patients or their legal guardians. The study was approved by the Institutional Review Board (Cincinnati Children's Hospital Medical Center IRB2018-2408).
Procedures
Clinical data were extracted from electronic records. Blood samples (collected in cell preparation tubes for serum, serum-separating tubes and lithium heparin tubes for plasma) were centrifuged at 1200 g for 10 min at room temperature to collect plasma or serum, and stored at −80°C until further analysis. S100 proteins, IL-18, CXCL9, and IL-6 are validated clinical tests done by the Cincinnati Children's Hospital Medical Center Diagnostic Immunology Laboratory (participates in the College of American Pathologists, Laboratory Accreditation Program and has Clinical Laboratory Improvement Amendments certification) as part of routine clinical laboratory tests or on a research basis using archived blood samples if available. S100A8/A9, IL-18, CXCL9, and IL-6 were measured using ELISA kits from R&D Systems (Minneapolis, MN, USA), and S100A12 was measured using ELISA kits from Medical and Biological Laboratories Co (Nagoya Aichi, Japan). The validated range of S100A8/A9 is 716–3004 ng/mL, S100A12 is 32–385 ng/mL, IL-18 is 89–540 pg/mL, CXLC9 is 121 pg/mL or less, and IL-6 is 5 pg/mL or less. A high concentration of CXCL9 was determined as greater than 739 pg/mL, and low concentration of CXCL9 as less than or equal to 739 pg/mL. High sensitivity troponin-I, brain natriuretic peptide (BNP), procalcitonin, N-terminal prohormone (NT-pro) BNP, D-dimer, and ferritin were tested by Cincinnati Children's Hospital Medical Center Clinical Laboratories as routine clinical laboratory tests or on a research basis using archived blood samples if available. Concentrations lower than the detection limit were seen for C-reactive protein in three patients in the systemic JIA-MAS group, and for troponin I in all patients with systemic JIA-MAS that had levels measured, as well as in one patient with Kawasaki disease and one patient with MIS-C. For statistical analysis, we replaced the values with the lowest concentrations the assay could detect, which for C-reactive protein was 0·4 mg/dL and for troponin I was 0·0025 ng/mL.
Statistical analysis
Percentages and counts were used to express categorical variables. The Shapiro-Wilk test was used to test for normality. Continuous variable comparisons among the several disease groups were analysed by one-way ANOVA, with Šidák correction for multiple comparisons and reported as mean (SEM) if data were parametric. If data were non-parametric, the Kruskal-Wallis test was used with Dunn's correction, reported as median (IQR). The MIS-C group was further stratified by initial CXCL9 concentrations and continuous variables were analysed using a non-paired Student t test if data were parametric or the Mann-Whitney test if data were non-parametric.
To assess the performance of concentrations of S100 proteins, IL-18, and CXCL9 in diagnoses, receiver operating characteristic (ROC) curve analysis was used; sensitivity and specificity were described at each biomarker value, with Wilson-Brown 95% CIs. The optimal cutoff points were chosen based on highest specificity when assessing the diagnostic performance of biomarkers in distinguishing systemic JIA from Kawasaki disease and MIS-C from Kawasaki disease. If there was more than one cutoff point with the same specificity; then the cutoff point with the highest sensitivity was selected. To distinguish a patient with MIS-C with and without MAS, an optimal cutoff point for CXCL9 was chosen based on highest sensitivity. If there were more than one cutoff with the same sensitivity, then the cutoff point with highest specificity was selected. All tests were two-sided, and p values less than 0·05 were considered to indicate a statistically significant difference. Statistical analyses and graphing were done using Prism, version 9.0.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing.
Results
Between April 30, 2019, and Dec 14, 2020, we enrolled 11 patients with systemic JIA (6 girls, 5 boys), nine with systemic JIA-MAS (4 girls, 5 boys), nine with Kawasaki disease (7 girls, 2 boys), and 19 with MIS-C (8 girls, 11 boys). Of the 19 patients with MIS-C, 16 (84%) had a documented history of COVID-19 exposure or evidence of current or previous SARS-CoV-2 infection (table 1 ). The remaining patients with MIS-C were diagnosed before wide availability of outpatient PCR and antigen testing but had potential for community exposure. As in previous reports, MIS-C most closely resembled Kawasaki disease from a clinical standpoint (eg, conjunctival injection, mucositis, and swelling of hands and feet), except for frequently reported abdominal pain in MIS-C (table 2 , appendix p 1). In addition, six (32%) of 19 patients with MIS-C had evidence of acute kidney injury at baseline. Conversely, no patients in the MIS-C group had active arthritis versus two (22%) of nine patients with Kawasaki disease, six (67%) of nine patients with systemic JIA-MAS, and six (55%) of 11 patients with systemic JIA.
Table 1.
Patient characteristics
| Systemic JIA (n=11) | Systemic JIA-MAS (n=9) | Kawasaki disease (n=9) | MIS-C (n=19) | ||
|---|---|---|---|---|---|
| Sex | |||||
| Girls | 6 (55%) | 4 (44%) | 7 (78%) | 8 (42%) | |
| Boys | 5 (45%) | 5 (56%) | 2 (22%) | 11 (58%) | |
| Age, years | 8·0 (3·0–10·0) | 4·0 (2·0–7·0) | 2·0 (2·0–4·0) | 9·0 (4·5–15·0) | |
| Race or ethnicity | |||||
| Hispanic | 0 | 0 | 3 (33%) | 3 (16%) | |
| White | 7 (64%) | 7 (78%) | 4 (44%) | 8 (42%) | |
| Black | 3 (27%) | 2 (22%) | 2 (22%) | 8 (42%) | |
| Asian | 1 (9%) | 0 | 0 | 0 | |
| Native American | 0 | 0 | 0 | 0 | |
| Pre-existing conditions | 6* (55%) | 9† (100%) | 2‡ (22%) | 8§ (42%) | |
| Duration of symptoms until admission, days | 21 (10–35) | 21 (21–28) | 5 (2–6) | 6 (4–8) | |
| Reported COVID-19 exposure | 1 (9%) | 0 | 1 (11%) | 9 (47%) | |
| Positive SARS-COV-2 serology | 1 (9%) | 0 | 0 | 13 (68%) | |
| Positive nasopharyngeal swab PCR | 0 | 0 | 0 | 6 (32%) | |
| Reported COVID-19 exposure or positive test including serology | 1¶ (9%) | 0 | 1‖ (11%) | 16 (84%) | |
| Met complete Kawasaki disease criteria | 1 (9%) | 0 | 6 (67%) | 5 (26%) | |
| Met incomplete Kawasaki disease criteria | 3 (27%) | 2 (22%) | 3 (33%) | 4 (21%) | |
| Met MAS criteria** | 0 | 9 (100%) | 0 | 8 (42%) | |
Data are n (%) or median (IQR). JIA=juvenile idiopathic arthritis. MAS=macrophage activation syndrome. MIS-C=multisystem inflammatory syndrome in children. Some patients had more than one pre-existing condition.
n=3 asthma, n=1 eczema, n=1 Hurler's syndrome, n=1 growth hormone deficiency.
All patients with MAS had a history of systemic JIA.
n=1 hypoplastic left heart syndrome, n=1 attention-deficit hyperactivity disorder.
n=4 obesity, n=1 type 2 diabetes, n=1 haemoglobin SC disease, n=1 subglottic stenosis, n=2 anxiety and depression, n=1 attention-deficit hyperactivity disorder, n=1 bipolar depression.
Patient with systemic JIA had positive COVID-19 antibodies and history of exposure at time of diagnosis, but clinical and laboratory assessment was consistent with systemic JIA.
Patient with Kawasaki disease with history of COVID-19 exposure but negative serology and PCR and according to their primary physicians (rheumatology, infectious disease, and general paediatric medicine), disease was typical for Kawasaki disease.
MAS criteria based on ferritin greater than 684 ng/mL and two other criteria (platelets ≤181 × 109 per L, aspartate aminotransferase >48 units per L, triglycerides ≥156 mg/dL, or fibrinogen ≤360 mg/dL).
Table 2.
Clinical manifestations, hospital admission details, and treatments
| Systemic JIA (n=11) | Systemic JIA-MAS (n=9) | Kawasaki disease (n=9) | MIS-C (n=19) |
Stratification of MIS-C group* |
|||
|---|---|---|---|---|---|---|---|
| Low CXCL9 (n=5) | High CXCL9 (n=10) | ||||||
| Fever | 11 (100%) | 9 (100%) | 9 (100%) | 19 (100%) | 5 (100%) | 10 (100%) | |
| Rash | 8 (73%) | 9 (100%) | 7 (78%) | 12 (63%) | 3 (60%) | 6 (60%) | |
| Conjunctival injection | 0 | 1 (11%) | 6 (67%) | 11 (58%) | 4 (80%) | 6 (60%) | |
| Mucositis | 1 (9%) | 1 (11%) | 7 (78%) | 7 (37%) | 1 (20%) | 6 (60%) | |
| Swelling of hands or feet | 2 (18%) | 0 | 5 (56%) | 7 (37%) | 1 (20%) | 4 (40%) | |
| Adenopathy | 4 (36%) | 4 (44%) | 6 (67%) | 8 (42%) | 3 (60%) | 2 (20%) | |
| Abdominal pain | 1 (9%) | 5 (56%) | 0 | 13 (68%) | 4 (80%) | 6 (60%) | |
| Diarrhoea | 1 (9%) | 1 (11%) | 3 (33%) | 10 (53%) | 3 (60%) | 5 (50%) | |
| Headache | 4 (36%) | 1 (11%) | 2 (22%) | 12 (63%) | 4 (80%) | 7 (70%) | |
| Respiratory symptoms | 3 (27%) | 2 (22%) | 3 (33%) | 10 (53%) | 4 (80%) | 4 (40%) | |
| Pharyngitis | 5 (45%) | 1 (11%) | 1 (11%) | 10 (53%) | 1 (20%) | 6 (60%) | |
| Arthritis | 6 (55%) | 6 (67%) | 2 (22%) | 0 | 0 | 0 | |
| Hepatomegaly | 1 (9%) | 3 (33%) | 1 (11%) | 2 (11%) | 0 | 2 (20%) | |
| Splenomegaly | 1 (9%) | 3 (33%) | 0 | 2 (11%) | 0 | 2 (20%) | |
| Acute kidney injury | 0 | 0 | 0 | 6 (32%) | 0 | 6 (60%) | |
| Shock† | 0 | 0 | 4 (44%) | 13 (68%) | 2 (40%) | 9 (90%) | |
| Requiring fluid bolus | 0 | 0 | 4 (44%) | 13 (68%) | 2 (40%) | 9 (90%) | |
| Requiring inotropes | 0 | 0 | 1 (11%) | 5 (26%) | 1 (20%) | 3 (30%) | |
| Coronary changes | 0 | 0 | 4 (44%) | 2 (11%) | 2 (40%) | 0 | |
| Left ventricular dysfunction, any | 0 | 0 | 3 (33%) | 7 (37%) | 1 (20%) | 5 (50%) | |
| Moderate or severe left ventricular dysfunction | |||||||
| Mild (45 to <55%) | 0 | 0 | 2 (22%) | 5 (26%) | 1 (20%) | 3 (30%) | |
| Moderate (40–45%) | 0 | 0 | 1 (11%) | 0 | 0 | 0 | |
| Moderate to severe (35–40%) | 0 | 0 | 0 | 1 (5%) | 0 | 1 (10%) | |
| Severe (<35%) | 0 | 0 | 0 | 1 (5%) | 0 | 1 (10%) | |
| Respiratory support | 2 (18%) | 0 | 3 (33%) | 11 (58%) | 3 (60%) | 6 (60%) | |
| Oxygen only | 2 (18%) | 0 | 2 (22%) | 10 (53%) | 3 (60%) | 5 (50%) | |
| High-flow nasal cannula | 0 | 0 | 1 (11%) | 3 (16%) | 0 | 3 (30%) | |
| CPAP or BiPAP | 0 | 0 | 0 | 1 (5%) | 0 | 1 (10%) | |
| Intubation ventilation | 0 | 0 | 0 | 1 (5%) | 0 | 1 (10%) | |
| Mental status changes | 0 | 0 | 0 | 4 (21%) | 0 | 4 (40%) | |
| Immunomodulatory treatments‡ | 2 (18%) | 9 (100%) | 9 (100%) | 17 (89%) | 5 (100%) | 10 (100%) | |
| Intravenous immunoglobulin | 1 (9%) | 0 | 9 (100%) | 17 (89%) | 5 (100%) | 10 (100%) | |
| Corticosteroids | 2 (18%) | 8 (89%) | 5 (56%) | 16 (84%) | 5 (100%) | 9 (90%) | |
| IL-1 inhibitor | 0 | 9 (100%) | 0 | 5 (26%) | 1 (20%) | 3 (30%) | |
| Infliximab | 0 | 0 | 2 (22%) | 0 | 0 | 0 | |
| IL-6 inhibitor | 0 | 1 (11%) | 0 | 0 | 0 | 0 | |
| Other drug treatments | 0 | 5 (56%)§ | 0 | 0 | 0 | 0 | |
| Intensive care unit | 0 | 2 (22%) | 3 (33%) | 12 (63%) | 3 (60%) | 6 (60%) | |
| Antibiotics | 4 (36%) | 1 (11%) | 7 (78%) | 14 (74%) | 4 (80%) | 7 (70%) | |
| Antiviral | 0 | 1 (11%) | 0 | 1 (5%) | 1 (20%) | 0 | |
Data are n (%). JIA=juvenile idiopathic arthritis. MAS=macrophage activation syndrome. MIS-C=multisystem inflammatory syndrome in children. CPAP=continuous positive airway pressure. BiPAP=bi-level positive airway pressure. IL=interleukin. CXCL9=chemokine (C-X-C motif) ligand 9.
15 of the 19 patients with MIS-C who had CXCL9 measured were further stratified based on CXCL9 concentrations less than or equal to or greater than 739 pg/mL.
Shock was defined as needing inotrope support or fluid resuscitation greater than 20 mL/kg.
Intravenous immunoglobulin, corticosteroids, anakinra, infliximab, or tocilizumab.
Cyclosporine (n=2) and emapulumab (n=3). Before admission, some patients with systemic JIA-MAS received tofacitinib and cyclophosphamide (n=1), etoposide (n=1), or tocilizumab (n=1).
Patients with MIS-C were more likely to present with shock relative to Kawasaki disease (13 [68%] of 19 vs four [44%] of nine), receive inotropic support (five [26%] of 19 vs one [11%] of nine), and require intensive care unit (ICU) care (12 [63%] of 19 vs three [33%] of nine). The rate of left ventricular dysfunction was 37% (seven of 19) in patients with MIS-C versus 33% (three of nine) in those with Kawasaki disease, but the degree of dysfunction was more severe in some patients with MIS-C (table 2). However, coronary dilation was less common in MIS-C (two [11%] of 19 vs four [44%] of nine). Requirement of respiratory support was also common in MIS-C (11 [58%] of 19), generally requiring only supplemental oxygen with one (5%) participant of 19 requiring intubation and ventilation.
Of the 19 patients with MIS-C, 17 (89%) received intravenous immunoglobulin, 16 (84%) received corticosteroids, and five (26%) required the IL-1 receptor antagonist anakinra. Two (11%) of 19 patients with MIS-C had self-resolved inflammation without receiving immunomodulatory therapy. All nine patients with Kawasaki disease received intravenous immunoglobulin, five (56%) received corticosteroids, and two (22%) received the TNF inhibitor infliximab (table 2). All patients with systemic JIA-MAS were on immunomodulators before their admission, and upon admission for MAS, treatment was escalated to include pulse corticosteroids, switch to or increased dose of anakinra, or to include cyclosporin or the IFNγ inhibitor emapalumab. For the systemic JIA group, treatment refers to pre-treatment given before diagnosis. Two (18%) of 11 patients with systemic JIA were pre-treated with intravenous immunoglobulin or corticosteroids or both, and upon systemic JIA diagnosis were started on oral corticosteroids and IL-1 or IL-6 inhibitors, but these are not mentioned in table 2 because for most patients, the diagnosis was made and treatment prescribed in the outpatient setting. Clinical manifestations stratified by sex for each hyperinflammatory disease and interpretation are shown in the appendix (pp 1–2), but no conclusions can be made regarding the influence of sex because of the small sample size.
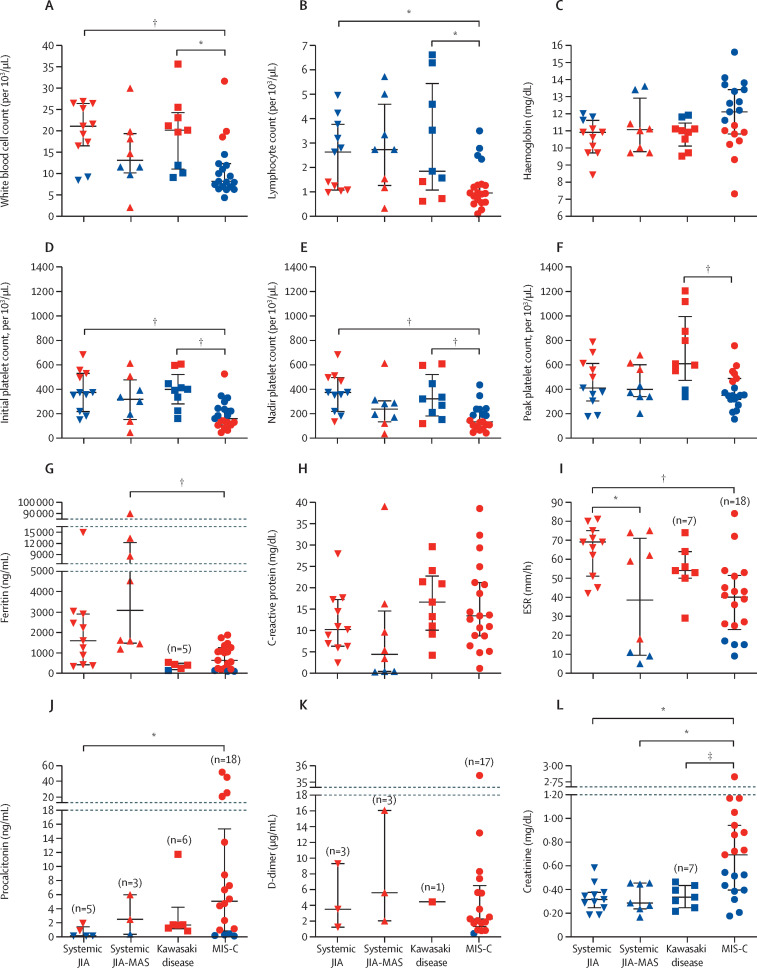
Patients with MIS-C typically presented with lymphopenia, thrombocytopenia, marked elevation of inflammatory markers, hyperferritinaemia, elevated cardiac biomarkers, and acute renal injury (figure 1 , appendix pp 3–4). Patients with MIS-C had lower white blood cell counts, with significantly more severe lymphopenia and thrombocytopenia compared with patients with Kawasaki disease, but less severe compared with patients with systemic JIA-MAS (albeit not significantly; figure 1). Peak platelet counts during hospital admission were within normal ranges for patients with MIS-C but were elevated and within thrombocytosis ranges in patients with Kawasaki disease (figure 1F).
Figure 1.
Comparison of haematology and laboratory results
Data are median (IQR). All data are first sets of laboratory tests obtained during admission except for platelets in which (E) shows nadir and (F) shows peak concentrations recorded during admission. Red data points indicate concentrations outside the normal range for the indicated parameter. Participant numbers were as follows unless otherwise noted: n=11 systemic JIA, n=9 systemic JIA-MAS, n=9 Kawasaki disease, and n=19 MIS-C. Data analysed using one-way ANOVA if data were parametric or Kruskal-Wallis if non-parametric and post-hoc multiple comparison correction was made. JIA=juvenile idiopathic arthritis. MAS=macrophage activation syndrome. MIS-C=multisystem inflammatory syndrome in children. *p≤0·05. †p≤0·01. ‡p<0·1.
Acute inflammatory markers such as C-reactive protein, ESR, aspartate aminotransferase, and alanine aminotransferase were similar among patients with MIS-C, Kawasaki disease, and systemic JIA-MAS (figure 1H, 1I, appendix p 3). Although not significant, ferritin was higher in patients with MIS-C (median 635 ng/mL [IQR 202–1259]) than those with Kawasaki disease (395 ng/mL [182–484], p=1·0), but ferritin was significantly lower in patients with MIS-C than those with systemic JIA-MAS (3082 ng/mL [1477–12 266]; p=0·0028; figure 1G). Fibrinogen and lactate dehydrogenase concentrations were similar between patients with MIS-C and Kawasaki disease. Relative to patients with systemic JIA-MAS, those with MIS-C had significantly higher fibrinogen and lower lactate dehydrogenase concentrations, whereas triglyceride concentrations were not significantly different (appendix p 3). D-dimer and procalcitonin were elevated in almost all patients measured in the cohort.
Cardiac biomarkers were not routinely measured for patients with Kawasaki disease and systemic JIA-MAS during hospital admission but were measured in some archived blood samples. In patients with MIS-C, peak troponin I was elevated in 12 (67%) of 18 patients, and BNP was elevated in ten (71%) of 14; for patients with Kawasaki disease, peak troponin I was elevated in one (33%) of three and BNP was elevated in four (100%) of four; in patients with systemic JIA or systemic JIA-MAS, neither troponin I nor BNP were elevated in any patient (appendix p 4). NT-ProBNP was also elevated in patients with MIS-C (11 [85%] of 13), Kawasaki disease (three [100%] of three), systemic JIA (one [20%] of five), and systemic JIA-MAS (one [50%] of two).
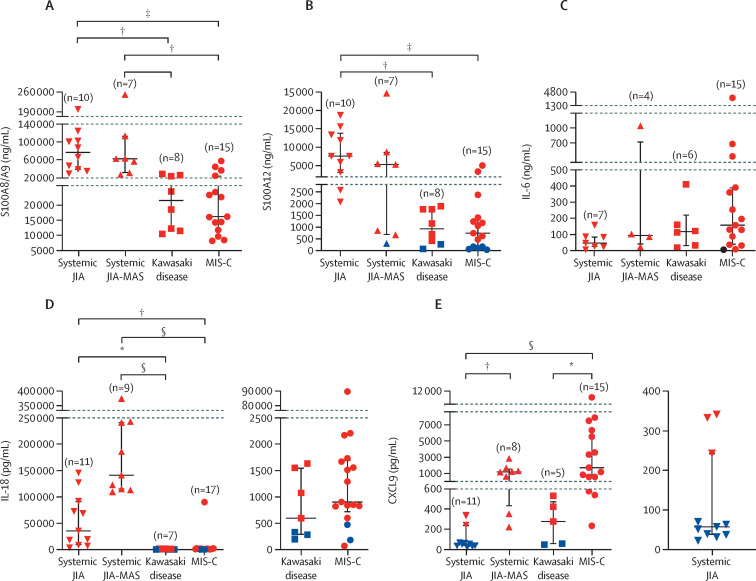
Median concentrations of S100A8/A9, S100A12, IL-18, CXCL9, and IL-6 were elevated in all groups, except for CXCL9 in the systemic JIA group (figure 2 ). Patients with systemic JIA and those with systemic JIA-MAS had similar markedly elevated S100A8/A9 and S100A12 concentrations, but patients with systemic JIA-MAS had significantly higher CXCL9 (1218 pg/mL [IQR 429–1616] vs 57 pg/mL [37–243]; p=0·0091) and higher concentrations of IL-18 (albeit not significant; figure 2). Additionally, in patients with systemic JIA and systemic JIA-MAS, concentrations of S100 proteins and IL-18 were markedly elevated compared with patients with Kawasaki disease or MIS-C. IL-6 concentrations were similar in all four groups (figure 2C).
Figure 2.
Comparison of S100A8/A9, S100A12, IL-18, CXCL9, and IL-6 concentrations
Data are median (IQR). All data are first sets of laboratory tests obtained during admission. Red data points indicate concentrations outside the normal range for the indicated parameter. Data analysed using one-way ANOVA if data were parametric or Kruskal-Wallis test if non-parametric and post-hoc multiple comparison correction was made. JIA=juvenile idiopathic arthritis. MAS=macrophage activation syndrome. MIS-C=multisystem inflammatory syndrome in children. IL=interleukin. CXCL=chemokine (C-X-C motif) ligand. *p≤0·05. †p≤0·01. ‡p≤0·001. §p≤0·0001.
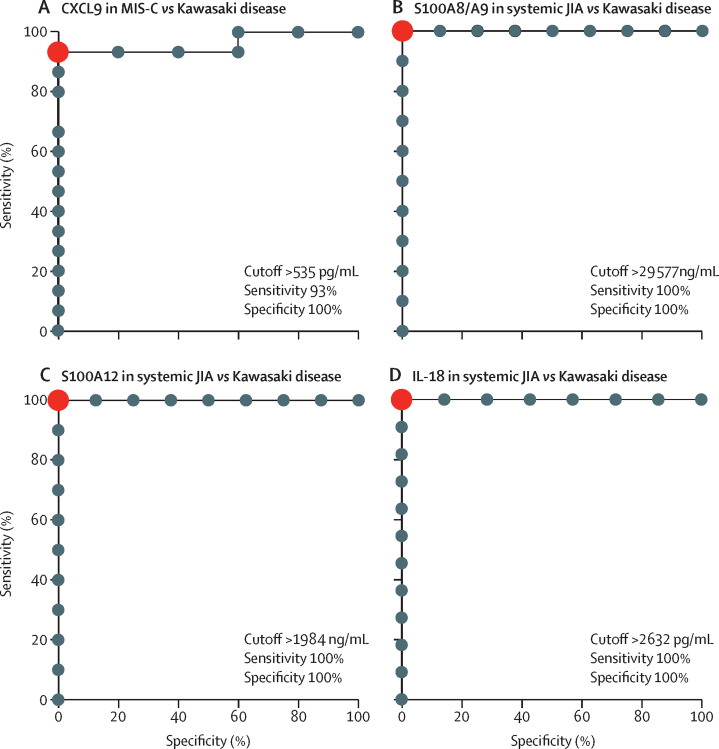
Patients with Kawasaki disease and MIS-C had similar biomarker profiles with respect to S100A8/A9, S100A12, and IL-18 (figure 2). However, patients with MIS-C had significantly higher concentrations of CXCL9 than did those with Kawasaki disease (1730 pg/mL [IQR 604–6300] vs 278 pg/mL [54–477]; p=0·038; figure 2E). The utility of CXCL9 concentration in differentiating MIS-C from Kawasaki disease was assessed by ROC curve analysis and performed well, with an area under the curve of 0·96 (p=0·0026). The optimal CXCL9 cutoff to differentiate MIS-C from Kawasaki disease was determined to be 535 pg/mL with a sensitivity of 93% and specificity of 100% (figure 3A ; appendix p 5).
Figure 3.
Diagnostic utility of inflammatory biomarkers for MIS-C, Kawasaki disease, and systemic JIA
(A) ROC curves for CXCL9 in differentiating MIS-C versus Kawasaki disease. ROC curves for S100A8/9 (B), S100A12 (C), and IL-18 (D) in differentiating systemic JIA from Kawasaki disease. Sensitivities and specificities for the optimal cutoff values are noted. Red dots represent optimal cutoff points. JIA=juvenile idiopathic arthritis. MAS=macrophage activation syndrome. MIS-C=multisystem inflammatory syndrome in children. ROC=receiver operating characteristic.
An original aim of this study was to examine biomarker profiles that could distinguish systemic JIA and Kawasaki disease. Relative to patients with Kawasaki disease, those with systemic JIA had significantly elevated S100A8/A9 (76 983 ng/mL [IQR 38 623–108 060] vs 16 504 [6704–25 595]; p=0·0040), S100A12 (7562 ng/mL [3008–13 912] vs 684 [153–1280]; p=0·0061), and IL-18 (35 288 pg/mL [8319–91 820] vs 598 pg/mL [285–1553]; p=0·0067; figure 2A–D). CXCL9 concentrations were similar between these groups (figure 2E). Using ROC analysis, we found that S100A8/A9, S100A12, and IL-18 concentrations performed well in distinguishing systemic JIA from Kawasaki disease, with area curves of 1 for all three biomarkers. The sensitivity and specificity were 100% for all biomarkers using a cutoff of 29 577 ng/mL for S100A8/A9, 1984 ng/mL for S100A12, and 2632 pg/mL for IL-18 (figure 3B–D, appendix pp 5–6).
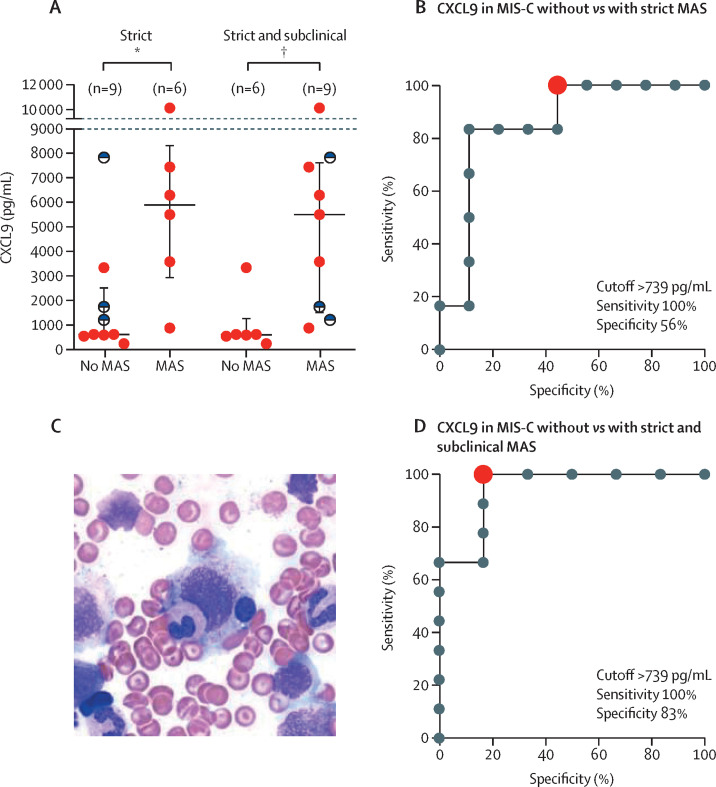
Given clinical and laboratory features of MIS-C that resemble MAS, the 2016 MAS classification criteria were applied to MIS-C. We found that eight (42%) of 19 patients with MIS-C fully satisfied MAS criteria (table 1).24 Patients with MIS-C that fulfilled MAS criteria had significantly higher CXCL9 concentrations (5905 pg/mL [IQR 2906–8354]) than did patients with MIS-C who did not satisfy MAS criteria (605 pg/mL [558–2534]; p=0·026; figure 4A ). ROC analysis showed that CXCL9 performed well in discriminating patients with MIS-C who met MAS criteria from those that did not, with an area under the curve of 0·85 (p=0·025), an optimal cutoff of 739 pg/mL, and a sensitivity of 100% and specificity of 56% (figure 4B, appendix p 6). One patient with MIS-C underwent bone-marrow biopsy as part of their diagnostic evaluation. This patient had a markedly elevated CXCL9 (7453 pg/mL) and satisfied MAS classification criteria. Biopsy results showed increased histiocytes and scattered haemophagocytosis (figure 4C).
Figure 4.
Differential CXCL9 concentrations in MIS-C with and without MAS
Data are median (IQR). (A) CXCL9 concentrations in patients with MIS-C that did or did not meet MAS classification criteria using only strict criteria (left) and including strict and subclinical MAS (right). CXCL9 concentrations of patients with subclinical MAS are shown with half-filled black circles. Data were analysed using the Mann-Whitney test. (B) ROC curves for CXCL9 differentiating MIS-C without and with MAS using strict 2016 MAS classification criteria. The red dot indicates the optimal cutoff point. (C) One patient with MIS-C underwent bone-marrow biopsy as part of their diagnostic evaluation and the smear shows haemophagocytosis with an ingested lymphocyte and a myeloid precursor in the process of being ingested by a histocyte. (D) ROC curve for CXCL9 differentiating MIS-C without and with strict plus subclinical MAS. The red dot indicates the optimal cutoff point. ROC=receiver operating characteristic. JIA=juvenile idiopathic arthritis. MAS=macrophage activation syndrome. MIS-C=multisystem inflammatory syndrome in children. *p≤0·05. †p≤0·01.
Additionally, four patients with MIS-C did not meet MAS criteria solely because their ferritin concentrations were less than the 684 ng/mL cutoff (mean 440 ng/mL). These four patients all had thrombocytopenia and at least two additional laboratory features in the MAS criteria. Three of them had CXCL9 measured, with a median concentration of 1730 pg/mL. If these patients were classified as subclinical MAS and moved to the MIS-C with MAS group, the optimal cutoff and sensitivity of CXCL9 was unchanged, but specificity increased to 83% (figure 4D, appendix p 6).25, 26
We further examined the 15 patients with MIS-C with initial CXCL9 concentrations less than (n=5) or greater than (n=10) the cutoff of 739 pg/mL, referred to as low and high CXCL9 groups. Both groups had the same median age and similar number of days of symptoms before admission (appendix p 7). No patients in the low CXCL9 group met strict 2016 MAS criteria compared with six (60%) of ten patients in the high group (appendix p 7). Additionally, more patients in the high CXCL9 group had organ dysfunction (acute kidney injury: six [60%] of ten vs none [0%] of five; shock: nine [90%] of ten vs two [40%] of five; left ventricular dysfunction: five [50%] of ten vs one [20%] of five; and altered mental status: four [40%] of ten vs none [0%] of five; table 2). The two patients with MIS-C in our cohort who had coronary changes were both in the low CXCL9 group. The proportion of patients who had respiratory support, ICU admission, and treatment were similar among the two MIS-C subgroups.
Additionally, multiple laboratory findings distinguished the lower and higher CXCL9 groups. Although there were no significant differences between S100 proteins, IL-6, or IL-18 concentrations (appendix p 8), more patients with MIS-C and high CXCL9 concentrations had increased concentrations of several markers of MAS physiology, including ferritin, D-dimer, aspartate aminotransferase, alanine aminotransferase, and triglycerides, and decreased platelet counts (appendix p 9). Furthermore, peak pro-BNP concentration was significantly increased in the high CXCL9 group (4229 pg/mL [IQR 954–9956] vs 202 [70–1247]; p=0·0007), as were troponin I concentrations (0·08 ng/mL [IQR 0·03–0·14) vs (0·03 [<0·01–0·59]), albeit this increase was not significant (p=0·19; appendix p 8).
In five patients with MIS-C, we followed CXCL9 concentrations over time and other inflammatory markers during their illness. CXCL9 concentrations consistently declined after treatment as the patient recovered (appendix p 10). For example, in one patient, CXCL9 concentrations progressively decreased whereas ferritin remained elevated despite clinical improvement. In some patients, CXCL9 concentrations plateaued or increased before recurrence or onset of new symptoms. In another patient, initial CXCL9 rapidly declined after initiation of treatment and clinical improvement. However, fever re-occurred on day 2 and continued on day 3 as CXCL9 concentrations plateaued. A further patient showed a slight increase in CXCL9 concentrations on day 8 of hospitalisation commensurate with the patient developing a new fever. After initiation of anakinra, the patient defervesced and CXCL9 declined the following day. These findings suggest that CXCL9 could be a sensitive marker of disease course in MIS-C.
Discussion
MIS-C overlaps clinically with other hyperinflammatory disorders including Kawasaki disease and MAS. However, the pathogenesis of MIS-C remains unclear, and a combination of inflammatory biomarkers to distinguish MIS-C from other conditions are similarly unknown. Here, we examined a prospective cohort of patients with MIS-C and Kawasaki disease with a high frequency of treatment failure, shock, myocardial dysfunction, and ICU care, along with an established cohort of patients with systemic JIA and systemic JIA-MAS. Our objective was to determine how S100 proteins, IL-18, and CXCL9 concentrations in patients with MIS-C compared to concentrations in patients with these clinically overlapping disorders. We found that patients with MIS-C showed a modest elevation in S100 proteins and IL-18, reflecting activation of innate immune effectors. This finding was in line with previous work showing elevated IL-18 concentrations in patients with MIS-C, and upregulation of S100A8/A9 and S100A12 genes in neutrophils and monocytes in six patients with MIS-C.27, 28, 29 Although MIS-C and Kawasaki disease had similar S100 and IL-18 elevations, these disease entities could be distinguished by markedly higher CXCL9 elevations in patients with MIS-C, comparable to those seen in patients with systemic JIA-MAS.
IFNγ and the IFNγ-induced chemokine CXCL9 are thought to play central roles in the pathogenesis of MAS and HLH.11, 12, 13, 30 Previous studies have suggested that IFNγ, CXCL9, CXCL10, or a combination thereof might be elevated in MIS-C.1, 12, 28, 29 In line with our findings, a 2021 report showed that a subset of patients with MIS-C showed significant upregulation of IFNγ and CXCL10 compared with patients with Kawasaki disease.29 This finding highlights a role for altered interferon responses and Th1-driven immunity, which are well described in MAS and in MIS-C pathogenesis, and that IFNγ activation could have a similarly key role in clinical and biochemical dysfunction in MIS-C.11, 12 It should be noted that although CXCL9 is a strong proxy for IFNγ, it can be difficult to discriminate the contribution of IFNγ versus type I IFN in stimulating CXCL9, and further study will be needed to address this question.
By contrast, Lee and colleagues found lower CXCL9 concentrations in patients with MIS-C than those with MAS associated with infection or systemic JIA, but they only examined concentrations in three patients with MIS-C.3 Other reports have found that MIS-C and Kawasaki disease have similar IFNγ cytokine profiles and that CXCL10 concentrations were higher in patients with Kawasaki disease than in those with MIS-C, but comparison to patients with MAS was not done in either of these reports.9, 29
By stratifying patients with MIS-C by high and low CXCL9 concentrations, our results revealed that the high CXCL9 group had greater multi-organ dysfunction, systemic inflammatory markers, and cytopenias. No patient in the low CXCL9 MIS-C group had acute kidney injury or altered mental status, and compared with the high CXCL9 group, they had a lower frequency of shock and myocardial dysfunction. The low CXCL9 MIS-C group more closely resembled the Kawasaki disease group including the frequency of coronary involvement. The CXCL9 cutoff of 739 pg/mL also provided a high likelihood ratio of 6·0, confirming moderate to large clinical importance. The stratification of patients with MIS-C by CXCL9 concentrations provided more support for a potential role for MAS in the pathophysiology of severe MIS-C, including the possibility that MIS-C could be considered as Kawasaki disease complicated by MAS. It is unclear whether these two subgroups of MIS-C (CXCL9 high and low) represent distinct phenotypes or are rather different ends of the same disease spectrum. This hypothesis aligns with observations from Esteve-Sole and colleagues, who define two subclasses of MIS-C: one more closely related to Kawasaki disease and the other defined by marked IFNγ overproduction.29 Carter and colleagues similarly proposed that pronounced increase of IFNγ, and less so IL-1β, in Kawasaki disease represents a distinction from MIS-C pathology.11 Our results describing the clinical spectrum of MIS-C are also similar to a large study that did a latent class analysis based on clinical characteristics and manifestation to identify three clinical spectrums in the patients with MIS-C reported to the Centers for Disease Control and Prevention.4 Class one in this study resembles our high CXCL9 patients with greater organ dysfunction, including myocardial dysfunction, while class 3 resembled our low CXCL9 cases with less severe organ dysfunction and similarities to pre-COVID-19 Kawasaki disease.
IL-18 and S100 protein concentrations were markedly different in patients with systemic JIA-MAS and MIS-C, but these distinctions might be more reflective of the autoinflammatory nature of the underlying systemic JIA in our patients with MAS. For example, S100 proteins have been found to distinguish systemic JIA from monogenic fevers or infections, although these studies did not include Kawasaki disease specifically.17, 18 A small 2021 study compared IL-18 in patients with MAS due to various rheumatic disorders, and found that although patients with Kawasaki disease and MAS had higher IL-18 than patients with Kawasaki disease without MAS, concentrations were similar to those in patients with MAS and lupus or juvenile dermatomyositis, but were markedly lower than in patients with systemic JIA-MAS.31 This finding is consistent with the hypothesis that IL-18 might be the main driver of MAS in systemic JIA but plays a smaller role in other rheumatological conditions or MIS-C. Other differences include that initial ferritin concentrations in patients with MIS-C did not exponentially increase to the levels seen in patients with MAS, and we observed that ferritin levels did not decline in parallel with the rate of clinical improvement in some patients with MIS-C.
The original purpose of our study was to compare S100 proteins and IL-18 concentrations as diagnostic biomarkers to distinguish patients with Kawasaki disease and new-onset systemic JIA. It is not uncommon that patients with systemic JIA are initially diagnosed with Kawasaki disease and given intravenous immunoglobulin with minimal or temporary improvement, are then discharged and subsequently re-admitted several times before the correct diagnosis is made. Our results show that S100 proteins and IL-18 can help differentiate systemic JIA from Kawasaki disease and improve diagnostic accuracy and prompt treatment, especially for patients with systemic JIA.
One of the limitations of our study is the relatively small cohort of patients with systemic JIA-MAS, and that most patients were already on various immunomodulators at enrolment. Furthermore, our systemic JIA-MAS cohort had a low ICU admission and prevalence of multi-organ damage, such as liver and neurological dysfunction. These factors could also explain why CXCL9 concentrations in our MAS cohort were lower than those previously reported.3 Only one patient with MIS-C had bone-marrow aspiration to assess for HLH or underlying malignancy. Furthermore, the cutoff values were not verified in an external cohort, which limits the accuracy of the distinction between false positive and true positive results; this should be addressed in future studies. Finally, we cannot answer directly whether MIS-C is distinct from Kawasaki disease. However, our data provide further support to the idea that MAS might contribute to the pathogenesis of MIS-C and suggest that levels of MIS-C severity can be differentiated by assessing IFNγ activation via CXCL9.
In conclusion, distinguishing MIS-C from infection and other inflammatory conditions will continue to be a challenge as the COVID-19 pandemic continues and vaccination expands and seroconversion is likely to increase. Thus, it is essential that objective biomarker-based patterns are found to distinguish MIS-C from related conditions. Our findings support the use of inflammatory biomarkers, particularly CXCL9, to distinguish MIS-C from other hyperinflammatory diseases, and potentially identify and monitor patients with MIS-C with MAS features who could be prone to a more severe disease course.
Data sharing
All relevant anonymised patient-level data reported in this Article are available upon reasonable request to the corresponding author starting 3 months and ending 36 months after publication to researchers who provide a methodologically sound proposal.
Declaration of interests
AG has served as a consultant and received research support from Novartis, SOBI, NovImmune, and AB2Bio. GS reports consulting fees from Novartis and SOBI. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank Daniella Schocken and Laura Nedorezov, first year paediatric rheumatology fellows at Cincinnati Children's Hospital, who provided and cared for a substantial portion of the patients with MIS-C reported in this study. We thank Alyssa Rohde and Devin Tinker for identifying and consenting patients with Kawasaki disease. JRS's work was completed in partial fulfilment of the Master of Science degree in Clinical and Translational Research in the Division of Epidemiology, University of Cincinnati College of Medicine. This publication was supported by an Institutional Clinical and Translational Science Award (NIH/NCATS 1UL1TR001425). GS was supported by NIAMS/NIH K08-AR072075, AG by P30-AR070549, and JRS and GC by T32-AR069512. EV was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation, DFG/448863690). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Contributors
JRS, SL, AG, and GS contributed to the conception and design of the study. JRS, GC, YE, SDL, EB, TD, SD, and DG contributed to the acquisition of data. JRS, EV, and GC were responsible for data analysis and JRS, EV, GC, SDL, AG, and GS were responsible for data interpretation. JRS, EV, and GC wrote the original draft. All authors were involved in the reviewing and editing of the manuscript and approved the final version. JRS, EV, and GS verified the data, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Diorio C, Henrickson SE, Vella LA. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdoni L, Mazza A, Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee PY, Day-Lewis M, Henderson LA. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godfred-Cato S, Bryant B, Leung J. COVID-19-associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouletty M, Borocco C, Ouldali N. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein LR, Rose EB, Horwitz SM. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson LA, Canna SW, Friedman KG. American College of Rheumatology clinical guidance for pediatric patients with multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 and hyperinflammation in COVID-19. Version 2. Arthritis Rheumatol. 2021;73:e13–e29. doi: 10.1002/art.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Consiglio CR, Cotugno N, Sardh F. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968. doi: 10.1016/j.cell.2020.09.016. 81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouldali N, Pouletty M, Mariani P. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020;4:662–668. doi: 10.1016/S2352-4642(20)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter MJ, Fish M, Jennings A. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 12.Bracaglia C, de Graaf K, Pires Marafon D. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76:166–172. doi: 10.1136/annrheumdis-2015-209020. [DOI] [PubMed] [Google Scholar]
- 13.Locatelli F, Jordan MB, Allen C. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382:1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- 14.Lee PY, Schulert GS, Canna SW. Adenosine deaminase 2 as a biomarker of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2020;79:225–231. doi: 10.1136/annrheumdis-2019-216030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez LL, Garrie K, Turner MD. Role of S100 proteins in health and disease. Biochim Biophys Acta Mol Cell Res. 2020;1867 doi: 10.1016/j.bbamcr.2020.118677. [DOI] [PubMed] [Google Scholar]
- 16.Brown RA, Henderlight M, Do T. Neutrophils from children with systemic juvenile idiopathic arthritis exhibit persistent proinflammatory activation despite long-standing clinically inactive disease. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.02995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wittkowski H, Frosch M, Wulffraat N. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 2008;58:3924–3931. doi: 10.1002/art.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aljaberi N, Tronconi E, Schulert G. The use of S100 proteins testing in juvenile idiopathic arthritis and autoinflammatory diseases in a pediatric clinical setting: a retrospective analysis. Pediatr Rheumatol Online J. 2020;18:7. doi: 10.1186/s12969-020-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armaroli G, Verweyen E, Pretzer C. Monocyte-derived interleukin-1β as the driver of S100A12-induced sterile inflammatory activation of human coronary artery endothelial cells: implications for the pathogenesis of Kawasaki Disease. Arthritis Rheumatol. 2019;71:792–804. doi: 10.1002/art.40784. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) 2020. https://www.cdc.gov/mis-c/hcp/
- 21.McCrindle BW, Rowley AH, Newburger JW. Diagnosis, treatment, and long-term management of Kawasaki Disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 22.DeWitt EM, Kimura Y, Beukelman T. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care Res. 2012;64:1001–1010. doi: 10.1002/acr.21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petty RE, Southwood TR, Manners P. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 24.Ravelli A, Minoia F, Davì S. 2016 Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation collaborative initiative. Arthritis Rheumatol. 2016;68:566–576. doi: 10.1002/art.39332. [DOI] [PubMed] [Google Scholar]
- 25.Bleesing J, Prada A, Siegel DM. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 26.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34:1133–1138. [PubMed] [Google Scholar]
- 27.Ramaswamy A, Brodsky NN, Sumida TS. Post-infectious inflammatory disease in MIS-C features elevated cytotoxicity signatures and autoreactivity that correlates with severity. medRxiv. 2020 doi: 10.1101/2020.12.01.20241364. published online Dec 4. (preprint) [DOI] [Google Scholar]
- 28.Gruber CN, Patel RS, Trachtman R. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982. doi: 10.1016/j.cell.2020.09.034. 95.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteve-Sole A, Anton J, Pino-Ramírez RM. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric inflammatory multisystem syndrome and Kawasaki disease. J Clin Invest. 2021;131 doi: 10.1172/JCI144554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuta M, Shimizu M, Irabu H. Comparison of serum cytokine profiles in macrophage activation syndrome complicating different background rheumatic diseases in children. Rheumatology. 2021;60:231–238. doi: 10.1093/rheumatology/keaa299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant anonymised patient-level data reported in this Article are available upon reasonable request to the corresponding author starting 3 months and ending 36 months after publication to researchers who provide a methodologically sound proposal.