Abstract
Objectives
This study aims to examine the relationship between childhood socioeconomic position (SEP) and cognitive function in later life within nationally representative samples of older adults in the United States and England, investigate whether these effects are mediated by later-life SEP, and determine whether social mobility from childhood to adulthood affects cognitive function and decline.
Method
Using data from the Health and Retirement Study (HRS) and the English Longitudinal Survey of Ageing (ELSA), we examined the relationships between measures of SEP, cognitive performance and decline using individual growth curve models.
Results
High childhood SEP was associated with higher cognitive performance at baseline in both cohorts and did not affect the rate of decline. This benefit dissipated after adjusting for education and adult wealth in the United States. Respondents with low childhood SEP, above median education, and high adult SEP had better cognitive performance at baseline than respondents with a similar childhood background and less upward mobility in both countries.
Discussion
These findings emphasize the impact of childhood SEP on cognitive trajectories among older adults. Upward mobility may partially compensate for disadvantage early in life but does not protect against cognitive decline.
Keywords: Cognition, Cross-national comparison, Early origins of health, Education, Life course analysis
Prior research has demonstrated that socioeconomic status is strongly associated with inequalities in health. The association between educational attainment and other measures of socioeconomic position (SEP) and adult disease, including cognition, is firmly established. Adults with lower levels of education often perform poorer on neurological tests and have the highest risk of Alzheimer’s disease and dementia. Similarly, low SEP is related to cognitive impairment and dementia in old age (Clouston et al., 2015; Glymour and Manly, 2008; Holland and Rabbitt, 1991; Karlamangla et al., 2009; Katzman, 1993; Koster et al., 2005; Lyu, 2015; Marden et al., 2017; Zahodne et al., 2015; Zhang et al., 2016). It is usually theorized that education is protective against cognitive decline or may modify the expression of cognitive decline and dementia through either biologic or behavioral pathways (Katzman, 1993; Stern et al., 1994). Similarly, higher socioeconomic status in adulthood may confer health benefits through improved living and working conditions, access to health services, and differences in lifestyles and behavior.
However, the relationship often found between education and other adult SEP measures and cognition could also be a reflection of exposures early in life. Previous research suggests that parental education levels contribute to cognitive functioning in adulthood (Kaplan et al., 2001; Lyu and Burr, 2016; Rogers et al., 2009). But, the degree to which the effect of early life operates directly on later-life outcomes, including cognition, or operates indirectly, via later-life SEP is not well documented. It is important to the interpretation of the effects of life course SEP and application of potential intervention and policy to better understand the pathways through which SEP is linked to later-life health and cognition. If the effects of childhood SEP persist irrespective of educational attainment and later-life experiences, then this presents as an important time for intervention and support. However, if a poor SEP in childhood can be ameliorated by education and adult SEP, then resources could be focused on providing educational and occupational opportunities later in life.
Social epidemiologic research has established the importance of considering the timing and accumulation of advantage and disadvantage in both childhood and adulthood when investigating the effect of SEP on health outcomes and has proposed several explanations for the association between SEP and health over the life course that can be summarized in four life course frameworks including the early-life/sensitive period hypothesis, and the pathway, accumulation of risk, and social mobility models (Ben-Shlomo and Kuh, 2002; Kuh et al., 2003; Singh-Manoux et al., 2005; Walsemann et al., 2016). For example, according to the pathway model, parental education and childhood SEP may influence adult SEP through access to social and economic resources and adult SEP may in turn influence health and cognitive outcomes (i.e., childhood SEP operates indirectly through adult SEP). Whereas, according to the social mobility model, change in social mobility would affect SEP in later life, which in turn could modify health behaviors, access to health care, and opportunity to have a cognitively stimulating job. (Ben-Shlomo and Kuh, 2002; Kuh et al., 2003). It is important to note these are not competing theories and can be considered to operate jointly.
This type of life course approach has been applied to research on how socioeconomic exposures across different life stages influence cognition in middle and late age; however, the results have been somewhat varied. Some studies have shown that cognitive functioning in adulthood is independently affected by early-life circumstances, suggesting that childhood SEP has a lasting effect on cognition beyond its impact on educational attainment and later SEP, while others have found that childhood SEP has no direct effect on cognition but a substantial indirect effect mediated though education and adult SEP (Al Hazzouri et al., 2011; Barnes et al., 2012; Kaplan et al., 2001; Lyu and Burr, 2016; Singh-Manoux et al., 2005; Turrell et al., 2002; Zhang et al., 2008, 2016).
Researchers have also argued that a life course approach is important to understanding social variations in health because it implies a continuing relationship between SEP and health such that poor circumstances at one life stage can be moderated by better circumstances earlier or later in life (Graham, 2002). Life course research has demonstrated that disease in adulthood can be influenced by this type of socioeconomic mobility; however, very few studies have investigated the extent to which social mobility, both inter-generational and intra-generational, contributes to socioeconomic-related inequalities in cognitive performance and change (Hart et al., 1998; Power et al., 2005). Although it is difficult to disentangle the life course processes of cumulative exposure and social mobility, previous cross-sectional studies examining social mobility and cognition have found that the negative impact of disadvantaged SEP in childhood can be overcome by higher status later in life (Luo and Waite, 2005; Turrell et al., 2002). Of the existing studies investigating the effects of childhood and adulthood SEP on cognition, most have been limited to cross-sectional analyses and to date, looking at point in time cross-sectional cognitive performance and not cognitive change over time.
A cross-national comparison provides an opportunity to even better understand the mechanisms underlying the relationship between life course SEP and later-life cognition. Countries differ in their investment in public resources such as education, thereby making SEP attainment more or less difficult, which provides insight into the mechanisms that underlie the association between life course SEP and later-life health. Comparison between the United States and England is particularly interesting because while both countries have invested in public education systems, income inequality is higher in the United States than the United Kingdom, with the United Kingdom being a much more equal nation in the post-World War II years than it is currently (Lansley and Mack, 2013). In addition, while health care costs and out-of-pocket spending on health care is much higher in the United States, health insurance coverage is not universal under age 65 as it is in the United Kingdom, and more spending on average has not translated into better health status for all Americans (Banks et al., 2006, 2016). Poor health, disproportionately experienced by lower SES groups and specifically related to cardiovascular health, is associated with poor cognitive outcomes in later life such as vascular dementia. Differential access to health care across England and the United States could be one mechanism through which we would see differences in both health and cognitive outcomes.
Previous work investigated whether differential impacts of early-life conditions between England and the United States are related to differences in subsequent later-life health outcomes and found that poor health in later life has some origin in social and health inequalities in early life (Banks et al., 2011). However, this work did not examine comparative early-life effects on cognition nor did they focus on potential effects related to social mobility over the life course.
The contribution of childhood SEP to cognitive performance and rate of decline in later life, independent of both level of education and adult socioeconomic status, is not well characterized in an economically diverse population with several years of follow-up and has not been examined cross-nationally. In addition, to our knowledge, there are no studies investigating a cross-national comparison of the effects of social mobility on cognition and cognitive change.
We hypothesize that differences in social investment and opportunity for social mobility translate into differential cognitive performance and decline in later life across the two countries.
The primary goals of this study are to determine in both the United States and England (a) whether there is a direct effect of childhood SEP on cognitive performance and decline later in life or whether it is mediated entirely through education and measures of SEP in adulthood, (b) to determine whether upward or downward social mobility from childhood to adulthood affects cognitive function and decline, and (c) whether there are differences between the United States and England in these effects.
Method
Study Population and Data
The samples for these analyses were drawn from the Health and Retirement Study (HRS) and the English Longitudinal Survey of Ageing (ELSA), both ongoing longitudinal studies of older adults. The HRS is a nationally representative, prospective panel study funded by the National Institutes of (NIA U01 AG009740). The study sample includes over 43,000 community-dwelling adults residing in the contiguous United States with oversamples of African-Americans and Hispanics. A new cohort of respondents is added to the sample every 6 years to adjust to maintain the steady state design and to account for aging and attrition. Interviews are conducted with sampled respondents and their spouse/partner every 2 years, including those who have entered nursing homes. Interviews are conducted by telephone and in person in both English and Spanish. Additional detail about the HRS is available elsewhere (Heeringa and Connor, 1995; Juster and Richard, 1995; Sonnega et al., 2014).
HRS respondents interviewed between 1998 and 2014, at least 50 years of age, who were not missing on baseline covariates, and had at least one interview wave with a valid cognitive test were eligible for inclusion in the analyses. The HRS did not begin asking questions about early childhood exposures until 1998, so respondents also had to have at least one interview subsequent to 1998. After restricting to those aged 50 years and older at interview and respondents to all three childhood questions (74%), our final HRS sample includes 23,229 individuals after excluding an additional 81 who were missing on one or more covariates, including immediate and delayed recall.
The ELSA is the English sister study to the HRS (Steptoe et al., 2013). ELSA is a multidisciplinary panel study of a cohort of men and women (aged 50 years and older). ELSA began in 2002 with an initial sample of 11,391 participants aged 50–100 years. ELSA includes follow-ups every 2 years.
There were 18,489 individuals who participated in one or more wave of ELSA. New cohorts were added in Wave 3, 4, and 6 (2006, 2008, and 2012). Questions about childhood exposures were administered in Wave 3, so our analytical sample is determined based on availability of these variables in Wave 3 (n = 6,342). In total, 6,008 individuals had no missing data on covariates, were aged 50 years and older, and had at least one observation with cognition data.
Assessment of Cognitive Function
Cognitive performance and decline are assessed using the episodic memory tasks at each wave. These tasks were selected because they have been shown to be sensitive measures of cognitive change (Small et al., 1999) and predictors of dementia (Crimmins et al., 2011). Respondents are asked to recall a list of 10 common nouns immediately after hearing them (immediate recall) and after approximately 5 min of additional test administration (delayed recall). Prior principal-components factor analyses showed that these recall tasks loaded on a single factor so scores on these tests were combined to create a composite score (Ofstedal et al., 2005).
Cognitively impaired respondents are less likely to participate in the study or complete the cognitive assessment (Rogers et al., 2009). In order to minimize the effects of item-level non-response among self-respondents, we used the imputed cognition data released by HRS (Fisher et al., 2017).
Each wave of ELSA includes an assessment of general cognitive function involving a battery of standard tests across the cognitive domains of memory, processing speed, and executive functioning (Zaninotto et al., 2018). Memory is measured using a word-learning test of 10 words. Both immediate and delayed recall (after 5 min) are assessed. Four separate versions of the word list are used, so that each participant would be presented a novel list at each time point.
Repeated administration of cognitive tests has been shown to result in practice effects such that there can be a boost in performance after the initial exposure to the test. To control for practice effects over time in these analyses, a binary variable was created to represent prior exposure to the cognitive test. Respondents were assigned a zero at their baseline wave of cognitive testing and a value of 1 at each subsequent test. The coefficient for this variable represents the average increase in test score between the baseline and first follow-up interview wave.
Measurement of SEP
Childhood SEP
SEP in childhood was examined using three items that were similarly assessed in HRS and ELSA: (a) father/parental unemployment in childhood, (b) experience of financial difficulty before age 16, and (c) father’s occupation (white collar vs not).
Parental unemployment was determined from a question about whether the father (in HRS) or parent (ELSA) was ever unemployed for several months or more before age 16.
Childhood financial adversity was defined as whether the family received help from relatives because of financial difficulties in HRS and in ELSA whether the respondent experienced extreme financial hardship before age 16.
Father’s occupation was coded on the basis of respondent reports of father’s or male carer’s primary occupation. Occupation was classified as white collar (professionals, managers, sales, clerical) or blue collar (operators, craftsmen, farmers, service industries) and unemployed/other. Primary occupation was assigned using the occupation code according to the 1980 and 2000 U.S. Census guidelines in the HRS. In ELSA, white-collar jobs included managerial or senior officials, running own business, professional or technical jobs, and administrative or clerical roles.
An index of childhood SEP was created by dichotomizing each of the childhood items (parent/father was ever unemployed [0] or not [1]; family ever experienced financial difficulty [0] or not [1]; and father/male career blue collar occupation [0] or white collar [1]) and summing them for a range of 0–3 with a higher score indicating higher SEP. This index was then dichotomized at the median to create two categories of childhood SEP (≤2 items and 3 items).
Measurement of education and adult SEP
SEP in adulthood was measured using respondent’s education and wealth at baseline. Education was measured in years with a maximum of 17. Years of education were imputed using the degree attained information where necessary. Years of education was then dichotomized to create two categories of education (≤12 years, 13+ years).
Household wealth at baseline was chosen as the primary measure of adult economic circumstances. Wealth was adjusted for inflation using the Consumer Price Index (CPI) calculated by the Bureau of Labor Statistics, and normalized to 2002 dollars/pounds. Wealth data for the HRS were drawn from the RAND HRS data files, version P (St. Clair et al., 2006). A dichotomized measure of adult SEP (≤ median baseline household wealth, >median baseline household wealth) was also created.
Life Course Socioeconomic Mobility
SEP mobility patterns
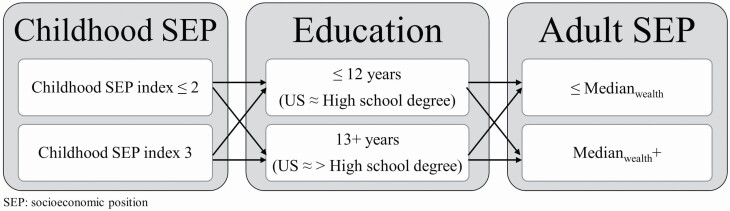
The availability of SEP at childhood, young adulthood, and older age enabled us to investigate of the influence of social mobility on cognitive function. Eight SEP mobility trajectories were created by cross-classifying the dichotomized childhood SEP variable with two-level education and wealth variables as represented in Figure 1 (e.g., “HHH” is equivalent to high childhood SEP index, high education, and high wealth). This measure reflected the respondent’s socioeconomic trajectory from childhood, through early adulthood, to mid- and later life.
Figure 1.
Socioeconomic trajectories using measurements of socioeconomic position at three periods in life resulting in eight possible trajectories (concept adapted from Hallqvist, 2004).
Covariates
Demographics
Using self-reported race/ethnicity questions, respondents were classified into white and non-white, including respondents of African descent, other racial groups, and respondents of Hispanic ethnicity. Sex was categorized as male/female using baseline responses to demographic questions. Married respondents and cohabiting partners were combined into one group and defined at baseline. Birth cohort was centered at 1930.
Statistical Methods
We performed descriptive statistics to compare the unweighted baseline characteristics between the two cohorts. We examined the effect of life course SEP on baseline and change in cognitive function using a series of mixed effect models with random effects without adjustment for sampling weights (Laird and Ware, 1982). The models were estimated using the full-information maximum likelihood estimation with an unstructured covariance matrix for the random effects and included all data available (HRS n = 23,229, D = 118,503; ELSA n = 6,008, D = 33,890). In these models, individuals are assumed to follow the mean path of the group except for random effects, which cause the initial level of cognitive performance to be higher or lower and the rate of change to be faster or slower, as described in more detail elsewhere (Wilson et al., 2002). This approach accommodates unbalanced data structures, both in terms of number of testing occasions and differences in intervals between assessments, and enables full use of data for all respondents with at least one valid cognitive score. Thus, all respondents contribute to the intercept term, whereas respondents with at least two valid cognitive tests contribute to the slope term. In addition, baseline level of cognition is explicitly modeled as a source of random variability and a possible correlate of how rapidly cognition changes over time.
Cognitive function was first modeled solely as function of age, allowing random effects for both the intercept and age-based change. For these analyses, we compared three progressively more complex models: linear, quadratic, and cubic polynomials on age. In addition, two-part linear spline models were estimated in an effort to best model the pattern of cognitive change with age. Fit was evaluated by comparing the log likelihood value and Bayesian information criterion (BIC) between models. For the sample as a whole, change in cognitive performance was found to be best described by a linear function.
We examined the main effect as well as the interaction of each childhood SEP variable with time (years since baseline) to test whether each was associated with cognitive function scores at baseline as well as the rate of change in cognitive function. All models were adjusted for baseline age, sex, race/ethnicity, marital/partner status, cohort, and cognitive test practice effect.
Similarly, to examine the effect of social mobility on cognitive performance and rate of change, we examined the interaction term between social mobility and time (years since baseline) after adjusting for baseline age, sex, race/ethnicity, marital status, cohort, and cognitive test practice effect. Finally, we performed a pooled analysis combining data from the HRS and ELSA to determine whether there were differences in cognitive performance, change, and the effect of social mobility by cohort. We used a two-side p value <.05 to determine statistical significance. All analyses were performed using STATA software, version 14.2 (Stata Corporation, College Station, TX).
Results
Table 1 presents the unweighted demographic characteristics of the HRS and ELSA samples. On average, HRS respondents were aged 63.2 years at baseline and women made up 56.4% of the sample. ELSA respondents were 61.4 years of age and 55.5% female. A higher percentage of HRS respondents had completed 13 or more years of education, 42.3% versus 39.6%. Over 78% of the HRS and 98% of the ELSA sample was non-Hispanic white. HRS respondents completed an average of 5.1 interview waves out of a possible 9 waves, and ELSA 5.6 out of 7 possible waves. The mean baseline memory score in HRS was 10.1 (SD 3.7) points out of a possible score of 20, whereas for ELSA, the mean score at baseline was 10.4 (SD 3.4).
Table 1.
Descriptive Statistics of Respondents 50+ Years in the Health and Retirement Study (HRS) and the English Longitudinal Study of Ageing (ELSA)a
| ELSA (n = 6,008) | HRS (n = 23,229) | |
|---|---|---|
| Mean age, years (SD) | 61.43 (9.37) | 63.2 (10.36) |
| Mean memory score at baseline (1–20) (SD) | 10.44 (3.55) | 10.11 (3.71) |
| Sex | ||
| Men | 44.51% | 43.62% |
| Women | 55.49% | 56.38% |
| Race/ethnicity | ||
| White | 98.15% | 78.32% |
| Non-white | 1.85% | 21.68% |
| Married or partnered | 76.05% | 69.62% |
| Childhood SEP | ||
| Parent employed, % | 92.78% | 79.62% |
| Parent had no financial difficulties, % | 97.14% | 88.21% |
| Father white-collar job, % | 35.97% | 54.41% |
| Overall SEP index, % in higher SEP group | 33.72% | 43.13% |
| Education ≥ 13 years, % | 39.58% | 42.30% |
| Median household incomeb, 2002 USD | $26,468 | $36,080 |
| Median household wealthb, 2002 USD | $31,475 | $106,700 |
| Mean number of waves tested (SD) | 5.64 (1.60) | 5.10 (2.90) |
| Known deceased at last contact | 8.70% | 33.30% |
| Ever required a proxy interview | 4.80% | 14.70% |
Notes: aEstimates are unweighted. Mean and SD are presented for continuous variables, except for household wealth and income, for which median is presented; percentages are presented for categorical variables.
bELSA income and wealth are converted from pounds to dollars.
We first estimated the amount of between- and within-person variance by estimating models that allowed random effects only for the intercept with no parameter for change over time (not shown). About half of the total variation in cognitive function was attributable to differences between persons; however, there was a non-ignorable amount of variation within persons. The linear model yielded both a significant time-constant intercept and age-dependent slope parameter representing the decrease in mean score with each year since baselines.
Effect of Childhood SEP
The results of the multivariate analysis of change in cognitive function are shown in Tables 2 and 3. The top portion of the table shows the coefficients from the random-effects regression models demonstrating the fixed effects of childhood SEP on the absolute level of cognitive function at baseline (baseline cognition) after the effects of the demographic control variables were considered. The effect of early-life conditions on cognitive function is presented in Model 2. Higher childhood SEP was associated with higher cognitive performance at baseline in both HRS and ELSA.
Table 2.
ELSA: Repeated Multivariate Analysisa of Baseline Performance and Change in Cognitive Function—Socioeconomic Position (SEP) Measures of Childhood and Adulthood
| Model 1: base model | Model 2: childhood SEP index | Model 3: education | Model 4: wealth | Model 5: all mobility variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE | ||||||
| Baseline cognition | |||||||||||||||
| Intercept | 18.54 | *** | 0.81 | 18.26 | *** | 0.79 | 17.00 | *** | 0.77 | 18.44 | *** | 0.79 | 17.04 | *** | 0.76 |
| Control variables | |||||||||||||||
| Age (years) | −0.17 | *** | 0.01 | −0.17 | *** | 0.01 | −0.16 | *** | 0.01 | −0.17 | *** | 0.01 | −0.16 | *** | 0.01 |
| Sex (women) | 0.70 | *** | 0.06 | 0.66 | *** | 0.06 | 0.09 | *** | 0.06 | 0.73 | *** | 0.06 | 0.88 | *** | 0.06 |
| Cohortb | −0.07 | 0.09 | −0.11 | 0.09 | −0.10 | 0.09 | −0.21 | * | 0.09 | −0.18 | * | 0.09 | |||
| Married/partnered | 0.37 | *** | 0.08 | 0.33 | *** | 0.08 | 0.28 | *** | 0.07 | 0.09 | 0.08 | 0.12 | 0.07 | ||
| Race/ethnicityc | 1.62 | *** | 0.23 | 1.77 | *** | 0.23 | 1.82 | *** | 0.22 | 1.59 | *** | 0.23 | 1.86 | *** | 0.22 |
| Practice effect | 0.56 | *** | 0.04 | 0.56 | *** | 0.04 | 0.56 | *** | 0.04 | 0.55 | *** | 0.04 | 0.55 | *** | 0.04 |
| Main effects | |||||||||||||||
| Childhood SEP index (3+) | 0.87 | *** | 0.07 | 0.56 | *** | 0.07 | |||||||||
| Education (>13 years) | 1.39 | *** | 0.07 | 1.21 | *** | 0.07 | |||||||||
| Wealth (>$31,475)d | 0.73 | *** | 0.08 | 0.32 | *** | 0.08 | |||||||||
| Rate of cognitive change | |||||||||||||||
| Years since baseline | −0.09 | *** | 0.00 | −0.10 | *** | 0.01 | −0.11 | *** | 0.01 | −0.13 | *** | 0.01 | −0.13 | *** | 0.01 |
| Childhood SEP index (3+) | 0.02 | * | 0.01 | 0.00 | 0.01 | ||||||||||
| Education (>13 years) | 0.03 | *** | 0.08 | 0.01 | 0.01 | ||||||||||
| Wealth (>$31,475)d | 0.07 | *** | 0.01 | 0.07 | *** | 0.01 | |||||||||
Notes: aModels fit with STATA 14.2 without sampling weights using n = 6,008 with D = 33,890.
bBirth cohort is centered at 1930.
cNon-Hispanic white.
dStandardized to 2002 pounds.
*p < .05. ***p < .001.
Table 3.
HRS: Repeated Multivariate Analysisa of Baseline Performance and Change in Cognitive Function—Socioeconomic Position (SEP) Measures of Childhood and Adulthood
| Model 1: base model | Model 2: childhood sep index | Model 3: education | Model 4: wealth | Model 5: all mobility variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE | ||||||
| Baseline cognition | |||||||||||||||
| Intercept | 22.86 | *** | 0.30 | 22.68 | *** | 0.30 | 22.53 | *** | 0.28 | 22.16 | *** | 0.29 | 22.07 | *** | 0.28 |
| Control variables | |||||||||||||||
| Age (years) | −0.23 | *** | 0.00 | −0.23 | *** | 0.00 | −0.23 | *** | 0.00 | −0.22 | *** | 0.00 | −0.22 | *** | 0.00 |
| Sex (women) | 1.07 | *** | 0.03 | 1.06 | *** | 0.03 | 1.14 | *** | 0.03 | 1.09 | *** | 0.03 | 1.14 | *** | 0.03 |
| Cohortb | −0.60 | *** | 0.03 | −0.58 | *** | 0.03 | −0.71 | *** | 0.03 | −0.51 | *** | 0.03 | −0.63 | *** | 0.03 |
| Married/partnered | 0.27 | *** | 0.03 | 0.27 | *** | 0.03 | 0.24 | *** | 0.03 | 0.12 | *** | 0.03 | 0.13 | *** | 0.03 |
| Race/ethnicityc | 1.61 | *** | 0.04 | 1.60 | *** | 0.04 | 1.43 | *** | 0.04 | 1.24 | *** | 0.04 | 1.19 | *** | 0.04 |
| Practice effect | 0.06 | ** | 0.02 | 0.06 | ** | 0.02 | 0.06 | ** | 0.02 | 0.05 | * | 0.02 | 0.05 | * | 0.02 |
| Main effects | |||||||||||||||
| Childhood SEP index | 0.20 | *** | 0.04 | −0.03 | 0.04 | ||||||||||
| Education (>13 years) | 1.56 | *** | 0.04 | 1.33 | *** | 0.04 | |||||||||
| Wealth (>$106,700)d | 1.30 | *** | 0.04 | 0.98 | *** | 0.04 | |||||||||
| Rate of cognitive change | |||||||||||||||
| Years since baseline | −0.16 | *** | 0.00 | −0.16 | *** | 0.00 | −0.17 | *** | 0.00 | −0.15 | *** | 0.00 | −0.16 | *** | 0.00 |
| Childhood SEP index | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||
| Education (>13 years) | 0.01 | 0.00 | 0.01 | *** | 0.00 | ||||||||||
| Wealth (> $106,700)d | −0.02 | *** | 0.00 | −0.23 | *** | 0.00 | |||||||||
Notes: aModels fit with STATA 14.2 without sampling weights using n = 23,229 with D = 118,503.
bBirth cohort is centered at 1930.
cNon-Hispanic white.
dStandardized to 2002 dollars.
*p < .05. **p < .01. ***p < .001.
The lower half of the table (rate of cognitive change) presents the coefficients from the longitudinal part of the model assessing whether childhood SEP is associated with change in cognitive function over time (represented as years since baseline). The unadjusted effect of childhood SEP on cognitive rate of change was significant for ELSA and nonsignificant in HRS. In ELSA, having a higher childhood SEP slowed decline by 0.02 points per year. These effects did not persist once adult education and adult SEP were included in the model.
Influence of Education and Adult SEP
Tables 2 and 3 also present the coefficients from the models that examined the effect of respondents’ education and adult SEP (represented as wealth) as independent predictors of baseline cognitive performance and change over time (Models 3 and 4) as well as whether these factors mediate the observed relationship between childhood SEP and cognitive function (Model 5). Adjusting for education and wealth attenuated the coefficient for the effect of childhood SEP on baseline cognition by approximately 35% in ELSA but the effect remained significant. In HRS, childhood SEP was no longer associated with baseline cognition once education and wealth were included in the model. Years of education was a significant predictor of baseline cognition and rate of change in HRS, with higher education associated with slower decline. In ELSA, higher education was associated with increased cognitive performance at baseline but not with decline over time.
Social Mobility and Cognitive Function
Table 4 shows the age- and demographic-adjusted mean effect on baseline cognition and age-related cognitive decline for the eight mobility trajectories from childhood to adulthood. The table shows a well-defined and graded pattern to results in both the United States and England with those in the stable low group, low SEP throughout childhood and adulthood, having the worst cognitive performance at baseline, while those in the stable high group having the highest scores. Respondents with low SEP in childhood who achieved an above median level of education and adult SEP (“Upward”) had better cognitive performance at baseline than respondents with a similar childhood background and less upward mobility. Respondents with low education, regardless of childhood SEP or adult wealth, had the lowest baseline cognitive scores in both cohort studies. It is clear that in both the United States and England, education is the dominant determinant of baseline cognitive performance.
Table 4.
Baseline Cognitive Performance and Cognitive Change by Life Course Socioeconomic Mobility Groupsa
| Socioeconomic mobility group | Effects of social mobility group on baseline cognition and change | ||||||
|---|---|---|---|---|---|---|---|
| ELSA | HRS | ||||||
| Childhood SEP | Education | Wealth | Coefficient (CI) | n | Coefficient (CI) | n | |
| Baseline cognition | |||||||
| Stable low | Low | Low | Low | Ref. | Ref. | ||
| Downward | High | Low | Low | 0.819*** (0.561, 1.077) | 951 | −0.155* (−0.276, −0.035) | 3,154 |
| High | High | Low | 1.598*** (1.285, 1.911) | 441 | 1.672*** (1.517, 1.828) | 2,267 | |
| Varied | Low | High | Low | 1.255*** (0.983, 1.527) | 466 | 1.352*** (1.220, 1.484) | 2,983 |
| High | Low | High | 1.026*** (0.756, 1.297) | 848 | 1.030*** (0.897, 1.162) | 2,864 | |
| Upward | Low | Low | High | 0.230* (0.021, 0.438) | 411 | 1.106*** (0.988, 1.225) | 2,195 |
| Low | High | High | 1.740*** (1.522, 1.959) | 288 | 2.226*** (2.106, 2.347) | 1,378 | |
| Stable high | High | High | High | 1.979*** (1.760, 2.199) | 831 | 2.240*** (2.124, 2.355) | 3,390 |
| Decline per year | −0.133***(−0.149, −0.118) | 6,008 | −0.158***(−0.167, −0.149) | 23,229 | |||
| Stable low | Low | Low | Low | Ref. | 1,772 | Ref. | 4,998 |
| Downward | High | Low | Low | 0.012 (−0.019, 0.042) | 951 | 0.000 (−0.013, 0.013) | 3,154 |
| High | High | Low | 0.014 (−0.029, 0.050) | 441 | 0.004 (−0.013, 0.021) | 2,267 | |
| Varied | Low | High | Low | 0.021 (−0.011, 0.053) | 466 | 0.029*** (0.012, 0.045) | 2,983 |
| High | Low | High | 0.059*** (0.026, 0.091) | 848 | −0.020*** (−0.033, −0.006) | 2,864 | |
| Upward | Low | Low | High | 0.076*** (0.051, 0.100) | 411 | −0.022* (−0.034, −0.009) | 2,195 |
| Low | High | High | 0.073*** (0.048, 0.098) | 288 | −0.015* (−0.028, −0.002) | 1,378 | |
| Stable high | High | High | High | 0.091*** (0.065, 0.116) | 831 | −0.004 (−0.016, 0.001) | 3,390 |
Notes: SEP = socioeconomic position.
aModels fit with STATA 14.2 without sampling weights using ELSA: n = 6,008 with D = 33,890; HRS: n = 23,229 with D = 118,503. Adjusted for age, sex, birth cohort (centered at 1930), race/ethnicity, marital/partner status, and practice effect.
*p < .05. **p < .01. ***p < .001.
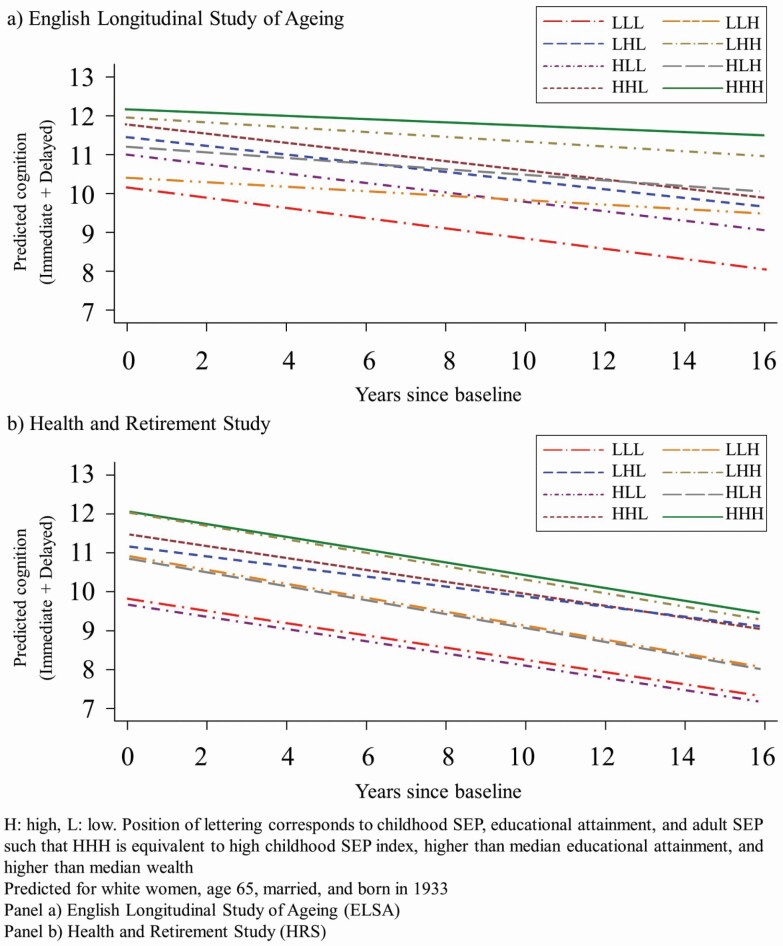
While the pattern in the coefficients for cognitive change is not as clear, all mobility groups in ELSA experienced slower cognitive decline with age relative to the stable low group, with the upwardly mobile and stable high groups experiencing the slowest decline. Whereas in the HRS, the upwardly mobile groups experienced faster decline comparatively.
Figure 2 shows the predicted trajectories of cognitive decline for the social mobility groups in HRS and ELSA.
Figure 2.
Predicted cognition (immediate + delayed) by life course social mobility group over 16 years.
The pooled cohort analyses (results not shown) showed that HRS had lower cognitive performance compared to ELSA and faster decline over time. In HRS, the mobility groups defined by above median wealth in adulthood experience faster rates of decline compared to the equivalent groups in ELSA. In addition, high childhood SEP confers less of a benefit in the United States relative to England.
We conducted sensitivity analyses to examine whether these effects and patterns held if we included only respondents aged 65 years and older and separately only those with two or more waves of observations with cognitive data. Results were not significantly different for any of the predictors of interest if we only included those with at least two waves of observation. Average cognitive decline was faster among those aged 65 years and older but the relationship between the predictors and cognitive outcomes was unchanged. In addition, we observed the same social mobility patterns (results not shown).
Discussion
Using longitudinal data from the HRS and ELSA for up to 16 years, we assessed the hypotheses that indicators of childhood SEP would be associated with cognitive performance and change in middle and older age and that social mobility from childhood to adulthood would be related to cognitive performance and change.
The first of these hypotheses was confirmed with an index of childhood SEP, including both parents’ employment and occupation, and childhood financial strain, found to be related to the absolute level of performance on memory-related cognitive tasks at baseline. However, childhood SEP was not a significant predictor of cognitive decline.
Respondents’ education and adult wealth mediated the effect of childhood SEP on cognitive performance in the U.S. cohort. This strong mediating relationship has also been found in studies on the childhood SEP on adult smoking and drinking, obesity, and cardiovascular disease (Lawlor et al., 2006). However, in the English cohort, childhood SEP had a lasting effect on baseline cognitive performance independent of the effects of education and SEP in adulthood. This provides evidence that early-life circumstances are important influences in shaping adult cognitive health. The significant direct effect of early childhood SEP on cognition in England suggests that in this context, early childhood could be considered a sensitive period that has effect on cognition later in life.
Our findings do not support the hypothesis that advantageous conditions in early life are associated with less rapid decline in cognitive function in older age once downstream factors are considered. The lack of association between early-life conditions and cognitive change has been found elsewhere in U.S.-based analyses using measures of memory, perceptual speed, and global cognitive function (Everson-Rose et al., 2003). There is a relative scarcity of studies of English aging cohorts that have investigated the associations between childhood socioeconomic status and later-life cognition. The small population of relevant studies evinces a predictive power of education and childhood SEP for estimations of cognitive ability and decline during old age. Higher educational attainment is associated with greater cognitive capacity in later life (Banks and Mazzonna, 2012; Gale et al., 2012; Lang et al., 2008). Offspring of fathers with lower status occupations have reduced scores and greater rates of decline on some cognitive tests administered in older age than do their peers with fathers of higher status occupations (Zaninotto et al., 2018). On the other hand, no association was found between educational attainment and incident dementia in old age (Cedar et al., 2018).
Although the specific mechanisms linking childhood SEP and cognition in older age are unclear, especially with regard to its effect on cognitive change, we would caution against interpreting these results as evidence that childhood SEP is unimportant in relation to adult cognitive functioning. While it is possible that the functional changes in the brain that lead to cognitive decline are unrelated to early-life socioeconomic factors, it is more likely that childhood SEP influences cognitive change through its effects on downstream circumstances and experiences in adulthood.
The results of the mobility analysis demonstrate that socioeconomic mobility over the life course has important consequences for cognitive health. Respondents in both the United States and England with disadvantaged circumstances in childhood who then achieved higher education or SEP in adulthood performed better on the cognitive tasks than those with similar childhood backgrounds who remained in the lower SEP groups at each life course stage. These results suggest that upward mobility can partially compensate for disadvantage early in life. However, downward socioeconomic mobility had similar, but negative, consequences for cognitive performance suggesting that the benefits of higher SEP in childhood can be diluted by lack of educational attainment and subsequent downward mobility over the life course. Our findings generally support the social mobility model, theorizing that early-life effects are modified by later-life circumstances, as has been found with other health outcomes (Ferraro and Shippee, 2009; Hallqvist et al., 2004; Pudrovska and Anikputa, 2014; Walsemann et al., 2016).
In both the U.S. and English cohorts, educational attainment was the most important predictor of high cognitive performance. In contrast, consistently high SEP across the life course and upward mobility were not consistently protective against cognitive decline. Respondents in the upwardly mobile groups experienced faster rates of decline in the United States than those in the stable low or downwardly mobile groups. However, in England, upward mobility and consistently high SEP across the life course decreased the rate of cognitive decline. Moreover, high childhood SEP was less beneficial in the United States relative to England. This result suggests that more proximate determinants of cognition, like education and later-life wealth, have significant effects on cognitive function in the United States. England historically has had programs to protect individuals from the economic consequences of poor health and has some sort of nationalized health care in place since 1911. It is possible that the steeper SES income/wealth gradient in the United States as well as the absence of health care programs throughout life erases much of the early childhood benefits on cognition by middle age.
The life course approach posits that lower SES and deprivation early in life may have an effect on later-life cognitive outcomes through a variety of mechanisms (Kuh and Ben-Shlomo, 2004; Kuh et al., 2003). Childhood deprivation may (a) cause biological changes in childhood that continue to have a lasting effect into adulthood such as decreased cortical thickness, less dendritic branching, and reduced neuronal communication, (b) have an effect by tracking of low SES into adulthood which could in turn result in poor adult heath, or (c) may be the first risk factor in a lifelong accumulation of risk (Guralnik et al., 2006). Given that the time from exposure to the measured outcome is 50 years or more, a number of childhood or adult social, behavioral, or biological factors may have contributed to patterns of adult cognitive functioning. However, it seems clear that cognition is sensitive to the environment and influences of early life, and especially education. Across both countries, upward mobility never quite erases disadvantage in early life but investment in education is the most influential determinant of cognitive performance.
The conclusions of this study are strengthened by a number of factors. The large size and representativeness of the HRS and ELSA samples enabled us to examine life course influences on cognitive performance and change in two countries. The nationally representative sample of these cohort studies provides greater representation than prior epidemiologic studies of cognitive change and dementia, and greater generalizability to populations that might not normally be represented in clinical-based samples. The longitudinal nature of these data, with an average of 5.5 waves and up to 16 years follow-up affords a unique opportunity to investigate trajectories of cognitive change. Additionally, the use of growth curve modeling takes advantage of the complex longitudinal nature of the data and allowed us to make full use of data for all subjects with at least one valid cognitive test.
Although causality between life course SEP and cognitive function in older age is implicit in the interpretation of these results, it is also possible that the association of SEP and cognition could be non-causal, arising because healthy individuals with better cognitive functioning are more likely to achieve higher education and social standing later in life. This type of selection effect theory suggests that individuals with better health or advantage early in life are more likely to achieve higher status and select into better occupations than individuals without these advantages (Osler et al., 2007). It is possible that selection in this manner could produce the pattern of results presented in this paper. Obtaining measures of early-life cognitive performance would help to disentangle the causal and reverse-causal explanations of these results and would allow for a better picture of the relationship between SEP and cognitive functioning across the life course. Moreover, collecting more specific or objective measures of SEP in childhood might also help to elucidate the social and biological mechanisms that link SEP at each life course stage with cognitive function in adulthood.
A limitation to this study is the use of retrospective reports on parental occupation and other early-life circumstances. Although, recall of childhood and family financial situation in childhood may be influenced by circumstances of adult health and socioeconomic status, it is less clear why respondent recall of parents’ education and occupation would be subject to systematic bias. Additionally, if a recall bias was present, it is unlikely that it would produce the pattern of results observed here. It is also possible that the effects of adverse social circumstances in early childhood may have manifested prior to enrollment in these studies. Specifically, disadvantaged persons may have died prior to being eligible for inclusion in the study at mid-life and thus may be underrepresented in this sample.
In addition, differences between the two studies in overall response rates and use of proxy respondents, whose data are not included in these analyses, could affect the comparison of cognitive function between groups. ELSA overall has a lower response rate than HRS and some of that sample selection could be due to cognitive status. Mode of interviewing could also affect results. ELSA near exclusively uses in-person interviews while HRS has both telephone and in-person interviewing. Lastly, it is important to note that there are far fewer non-white respondents in ELSA. We did not restrict our analyses to non-Hispanic whites in the HRS because we wanted to represent the full racial and ethnic diversity of the United States.
Despite these limitations, this research provides evidence that socioeconomic conditions early in life contribute to absolute levels of memory-based cognitive function in older age but are not protective of cognitive decline. While these results suggest a lasting influence of childhood SEP on adult cognitive performance, it appears that low cognitive ability is not an inevitable outcome of low SEP in early life. The social mobility findings suggest that improved SEP in later stages of life is associated with higher cognitive performance as an older adult.
These results have implications for the effects of residual confounding on potential links between indicators of SEP and cognition. We have shown that memory-based cognitive functioning is influenced by childhood SEP, education, and wealth. Thus, when studies aim to determine the association between an exposure and cognition or cognition and an outcome adjusted for SEP, they may underestimate confounding by life course socioeconomic factors if they only adjust for a single measure of SEP or measures from one stage of life.
The importance of this study lies in the measurement of SEP of over the life course and the implementation of analytical techniques that take advantage of the longitudinal design of the data and the cross-national comparison. Adopting a life course perspective underscores the need to think about adult health in a larger context and considers the positive and negative exposures that amass throughout a lifetime. However, a more thorough understanding of the pathways between childhood SEP and later-life cognitive performance and change will require better and more specific measures of the childhood socioeconomic environment. Consistent with much of the previous research on the effects of childhood SEP on cognition in older age, the results presented here suggest that, in addition to the impact on adult SEP, cognitive performance in adulthood may also have origins early in life and that upward social mobility may ameliorate the effects of childhood disadvantage. In both England and the United States, education is a significant predictor of cognitive performance; however, it may be even more essential for well-being later in life in a country like the United States that experiences high levels of income inequality.
Funding
This work was supported by the National Institute on Aging (NIA R01 AG055654, NIA R01 AG055406, NIA R03 AG048806).
This paper was published as part of a supplement with funding from the National Institute on Aging (R01AG030153).
Acknowledgement
This analysis uses data from the HRS Cross-Wave Imputation of Cognitive Functioning Measures 1992-2014 (V6) (https://hrs.isr.umich.edu), the RAND HRS Longitudinal File 2014 (V1) (https://www.rand.org/) and the Harmonized English Longitudinal Study on Ageing (Harmonized ELSA) (https://g2aging.org/). The development of the Harmonized HRS and Harmonized ESLA was funded by the National Institute on Aging (R01 AG030153, RC2 AG036619, 1R03AG043052). For more information, please refer to www.g2aging.org.
Conflict of Interest
None declared.
References
- Al Hazzouri, A., Haan, M. N., Kalbfleisch, J. D., Galea, S., Lisabeth, L. D., & Aiello, A. E. (2011). Life-course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: Results from the Sacramento Area Latino Study on Aging. American Journal of Epidemiology, 173(10), 1148–1158. doi: 10.1093/aje/kwq483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, J., Keynes, S., & Smith, J. P. (2016). Health, disability and mortality differences at older ages between the US and England. Fiscal Studies, 37(3–4), 345–369. doi: 10.1111/j.1475-5890.2016.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, J., Marmot, M., Oldfield, Z., & Smith, J. P. (2006). Disease and disadvantage in the United States and in England. Journal of American Medical Association, 295(17), 2037–2045. doi: 10.1001/jama.295.17.2037 [DOI] [PubMed] [Google Scholar]
- Banks, J., & Mazzonna, F. (2012). The effect of education on old age cognitive abilities: Evidence from a regression discontinuity design. Economic Journal (London, England), 122(560), 418–448. doi: 10.1111/j.1468-0297.2012.02499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, J., Oldfield, Z., & Smith JP. (2011). Childhood health and differences in late life health outcomes between England and the United States. NBER Working Paper Series, Working Paper 17096. Retrieved from http://www.nber.org/papers/w17096 [Google Scholar]
- Barnes, L. L., Wilson, R. S., Everson-Rose, S. A., Hayward, M. D., Evans, D. A., & Mendes de Leon, C. F. (2012). Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology, 79(24), 2321–2327. doi: 10.1212/WNL.0b013e318278b607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo, Y., & Kuh, D. (2002). A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology, 31(2), 285–293. doi: 10.1093/ije/31.2.285 [DOI] [PubMed] [Google Scholar]
- Cadar, D., Lassale, C., Davies, H., Llewellyn, D. J., Batty, G. D., & Steptoe, A. (2018). Individual and area-based socioeconomic factors associated with dementia incidence in England: Evidence from a 12-year follow-up in the English longitudinal study of ageing. Jama Psychiatry, 75(7), 723–732. doi: 10.1001/jamapsychiatry.2018.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston, S. A., Glymour, M., & Terrera, G. M. (2015). Educational inequalities in aging-related declines in fluid cognition and the onset of cognitive pathology. Alzheimer’s & Dementia (Amsterdam, Netherlands), 1(3), 303–310. doi: 10.1016/j.dadm.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Kim, J. K., Langa, K. M., & Weir, D. R. (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl. 1), i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose, S. A., Mendes de Leon, C. F., Bienias, J. L., Wilson, R. S., & Evans, D. A. (2003). Early life conditions and cognitive functioning in later life. American Journal of Epidemiology, 158(11), 1083–1089. doi: 10.1093/aje/kwg263 [DOI] [PubMed] [Google Scholar]
- Ferraro, K. F., & Shippee, T. P. (2009). Aging and cumulative inequality: How does inequality get under the skin? The Gerontologist, 49(3), 333–343. doi: 10.1093/geront/gnp034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, G. G., Hassan, H., Faul, J. D., Rodgers, W. L., & Weir, D. (2017). Health and Retirement Study: Imputation of cognitive functioning measures, 1992 – 2014. Retrieved from https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/COGIMP9216_dd_0.pdf [Google Scholar]
- Gale, C. R., Allerhand, M., & Deary, I. J.; HALCyon Study Team . (2012). Is there a bidirectional relationship between depressive symptoms and cognitive ability in older people? A prospective study using the English Longitudinal Study of Ageing. Psychological Medicine, 42(10), 2057–2069. doi: 10.1017/S0033291712000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour, M. M., & Manly, J. J. (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18(3), 223–254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Graham, H. (2002). Building an inter-disciplinary science of health inequalities: The example of lifecourse research. Social Science & Medicine (1982), 55(11), 2005–2016. doi: 10.1016/s0277-9536(01)00343-4 [DOI] [PubMed] [Google Scholar]
- Guralnik, J. M., Butterworth, S., Wadsworth, M. E., & Kuh, D. (2006). Childhood socioeconomic status predicts physical functioning a half century later. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 61(7), 694–701. doi: 10.1093/gerona/61.7.694 [DOI] [PubMed] [Google Scholar]
- Hallqvist, J., Lynch, J., Bartley, M., Lang, T., & Blane, D. (2004). Can we disentangle life course processes of accumulation, critical period and social mobility? An analysis of disadvantaged socio-economic positions and myocardial infarction in the Stockholm Heart Epidemiology Program. Social Science & Medicine (1982), 58(8), 1555–1562. doi: 10.1016/S0277-9536(03)00344-7 [DOI] [PubMed] [Google Scholar]
- Hart, C. L., Smith, G. D., & Blane, D. (1998). Social mobility and 21 year mortality in a cohort of Scottish men. Social Science & Medicine (1982), 47(8), 1121–1130. doi: 10.1016/s0277-9536(98)00061-6 [DOI] [PubMed] [Google Scholar]
- Heeringa, S., & Connor, J. (1995). Technical description of the Health and Retirement Study sample design. HRS Documentation Report DR-002. University of Michigan. [Google Scholar]
- Holland, C., & Rabbitt, P. (1991). The course and causes of cognitive change with advancing age. Review of Clinical Gerontology, 1, 181–196. doi: 10.1017/S0959259800002598 [DOI] [Google Scholar]
- Juster, F., & Richard, S. (1995). An overview of the Health and Retirement Study. Journal of Human Resources, 30(suppl.), S7–S56. doi: 10.2307/146277 [DOI] [Google Scholar]
- Kaplan, G. A., Turrell, G., Lynch, J. W., Everson, S. A., Helkala, E. L., & Salonen, J. T. (2001). Childhood socioeconomic position and cognitive function in adulthood. International Journal of Epidemiology, 30(2), 256–263. doi: 10.1093/ije/30.2.256 [DOI] [PubMed] [Google Scholar]
- Karlamangla, A. S., Miller-Martinez, D., Aneshensel, C. S., Seeman, T. E., Wight, R. G., & Chodosh, J. (2009). Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. American Journal of Epidemiology, 170(3), 331–342. doi: 10.1093/aje/kwp154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman, R. (1993). Education and the prevalence of dementia and Alzheimer’s disease. Neurology, 43(1), 13–20. doi: 10.1212/wnl.43.1_part_1.13 [DOI] [PubMed] [Google Scholar]
- Koster, A., Penninx, B. W., Bosma, H., Kempen, G. I., Newman, A. B., Rubin, S. M., Satterfield, S., Atkinson, H. H., Ayonayon, H. N., Rosano, C., Yaffe, K., Harris, T. B., Rooks, R. N., Van Eijk, J. T., & Kritchevsky, S. B. (2005). Socioeconomic differences in cognitive decline and the role of biomedical factors. Annals of Epidemiology, 15(8), 564–571. doi: 10.1016/j.annepidem.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Kuh, D., & Ben-Shlomo, Y. (2004). A life course approach to chronic disease epidemiology. Oxford University Press. [PubMed] [Google Scholar]
- Kuh, D., Ben-Shlomo, Y., Lynch, J., Hallqvist, J., & Power, C. (2003). Life course epidemiology. Journal of Epidemiology and Community Health, 57(10), 778–783. doi: 10.1136/jech.57.10.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, N. M., & Ware, J. H. (1982). Random-effects models for longitudinal data. Biometrics, 38(4), 963–974. doi: 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- Lang, I. A., Llewellyn, D. J., Langa, K. M., Wallace, R. B., Huppert, F. A., & Melzer, D. (2008). Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: Analyses from the English Longitudinal Study of Ageing. Journal of the American Geriatrics Society, 56(2), 191–198. doi: 10.1111/j.1532-5415.2007.01557.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley, S., & Mack, J. (2013). A more unequal country? Retrieved from http://www.poverty.ac.uk/editorial/more-unequal-country
- Lawlor, D. A., Sterne, J. A., Tynelius, P., Davey Smith, G., & Rasmussen, F. (2006). Association of childhood socioeconomic position with cause-specific mortality in a prospective record linkage study of 1,839,384 individuals. American Journal of Epidemiology, 164(9), 907–915. doi: 10.1093/aje/kwj319 [DOI] [PubMed] [Google Scholar]
- Luo, Y., & Waite, L. J. (2005). The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60(2), S93–S101. doi: 10.1093/geronb/60.2.s93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, J. (2015). Gender differences in the association between childhood socioeconomic status and cognitive function in later life. Journal of Geriatrics, 2015. doi: 10.1155/2015/896876 [DOI] [Google Scholar]
- Lyu, J., & Burr, J. A. (2016). Socioeconomic status across the life course and cognitive function among older adults: An examination of the latency, pathways, and accumulation hypotheses. Journal of Aging and Health, 28(1), 40–67. doi: 10.1177/0898264315585504 [DOI] [PubMed] [Google Scholar]
- Marden, J. R., Tchetgen Tchetgen, E. J., Kawachi, I., & Glymour, M. M. (2017). Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: Early and late predictors of dementia risk. American Journal of Epidemiology, 186(7), 805–814. doi: 10.1093/aje/kwx155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofstedal, M., Fisher, G., & Herzog, A. (2005). Documentation of cognitive functioning measures in the health and retirement study. HRS Documentation Report DR-006. University of Michigan. [Google Scholar]
- Osler, M., McGue, M., & Christensen, K. (2007). Socioeconomic position and twins’ health: A life-course analysis of 1266 pairs of middle-aged Danish twins. International Journal of Epidemiology, 36(1), 77–83. doi: 10.1093/ije/dyl266 [DOI] [PubMed] [Google Scholar]
- Power, C., Graham, H., Due, P., Hallqvist, J., Joung, I., Kuh, D., & Lynch, J. (2005). The contribution of childhood and adult socioeconomic position to adult obesity and smoking behaviour: An international comparison. International Journal of Epidemiology, 34(2), 335–344. doi: 10.1093/ije/dyh394 [DOI] [PubMed] [Google Scholar]
- Pudrovska, T., & Anikputa, B. (2014). Early-life socioeconomic status and mortality in later life: An integration of four life-course mechanisms. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69(3), 451–460. doi: 10.1093/geronb/gbt122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, M. A., Plassman, B. L., Kabeto, M., Fisher, G. G., McArdle, J. J., Llewellyn, D. J., Potter, G. G., & Langa, K. M. (2009). Parental education and late-life dementia in the United States. Journal of Geriatric Psychiatry and Neurology, 22(1), 71–80. doi: 10.1177/0891988708328220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux, A., Richards, M., & Marmot, M. (2005). Socioeconomic position across the lifecourse: How does it relate to cognitive function in mid-life? Annals of Epidemiology, 15(8), 572–578. doi: 10.1016/j.annepidem.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Small, S. A., Stern, Y., Tang, M., & Mayeux, R. (1999). Selective decline in memory function among healthy elderly. Neurology, 52(7), 1392–1396. doi: 10.1212/wnl.52.7.1392 [DOI] [PubMed] [Google Scholar]
- Sonnega, A., Faul, J. D., Ofstedal, M. B., Langa, K. M., Phillips, J. W., & Weir, D. R. (2014). Cohort profile: The Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Clair, P., Blake, D., Bugliari, D., Chien, S., Hayden, O., Hurd, M.,…Zissimopoulos, J. (2006). RAND HRS data documentation, version F. In Labor & population program. RAND Center for the Study of Aging. [Google Scholar]
- Steptoe, A., Breeze, E., Banks, J., & Nazroo, J. (2013). Cohort profile: The English Longitudinal Study of Ageing. International Journal of Epidemiology, 42(6), 1640–1648. doi: 10.1093/ije/dys168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Gurland, B., Tatemichi, T. K., Tang, M. X., Wilder, D., & Mayeux, R. (1994). Influence of education and occupation on the incidence of Alzheimer’s disease. Journal of American Medical Association, 271(13), 1004–1010. doi: 10.1001/jama.1994.03510370056032 [DOI] [PubMed] [Google Scholar]
- Turrell, G., Lynch, J. W., Kaplan, G. A., Everson, S. A., Helkala, E. L., Kauhanen, J., & Salonen, J. T. (2002). Socioeconomic position across the lifecourse and cognitive function in late middle age. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 57(1), S43–S51. doi: 10.1093/geronb/57.1.s43 [DOI] [PubMed] [Google Scholar]
- Walsemann, K. M., Goosby, B. J., & Farr, D. (2016). Life course SES and cardiovascular risk: Heterogeneity across race/ethnicity and gender. Social Science & Medicine (1982), 152, 147–155. doi: 10.1016/j.socscimed.2016.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R. S., Beckett, L. A., Barnes, L. L., Schneider, J. A., Bach, J., Evans, D. A., & Bennett, D. A. (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17(2), 179–193. [PubMed] [Google Scholar]
- Zahodne, L. B., Stern, Y., & Manly, J. J. (2015). Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology, 29(4), 649–657. doi: 10.1037/neu0000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto, P., Batty, G. D., Allerhand, M., & Deary, I. J. (2018). Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. Journal of Epidemiology and Community Health, 72(8), 685–694. doi: 10.1136/jech-2017-210116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Gu, D., & Hayward, M. D. (2008). Early life influences on cognitive impairment among oldest old Chinese. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63(1), S25–S33. doi: 10.1093/geronb/63.1.s25 [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Hayward, M. D., & Yu, Y. L. (2016). Life course pathways to racial disparities in cognitive impairment among older Americans. Journal of Health and Social Behavior, 57(2), 184–199. doi: 10.1177/0022146516645925 [DOI] [PMC free article] [PubMed] [Google Scholar]