Abstract
PURPOSE
To determine the key optical coherence tomography (OCT) and OCT angiography (OCTA) parameters that correlate with visual field loss in optic disc drusen (ODD).
DESIGN
Retrospective cross-sectional study.
METHODS
SETTING
Single academic center.
PATIENT OR STUDY POPULATION
17 patients with ODD (29 eyes) and 35 age-matched controls (53 eyes)
INTERVENTION OR OBSERVATION PROCEDURES
Static perimetry, OCT and OCTA imaging of optic disc and macula.
MAIN OUTCOME MEASURES
static perimetry, OCT, and OCTA measurements.
RESULTS
We investigated the relationship between static perimetry and 14 OCT/OCTA measurements in patients with ODD vs. age-matched controls and found 5 key measurements that most correlated with visual field loss included: peripapillary retinal nerve fiber layer (RNFL), macular ganglion cell complex (GCC), peripapillary vessel area density (VAD), macular vessel diameter (VD) and flux. Hierarchical clustering of these 5 measurements vs. all clinical characteristics revealed 3 distinct clusters. ODD and control eyes with no visual field loss (mean deviation (MD) > −2.0 dB) had high RNFL, GCC, and low macular VD and flux. ODD eyes with mild visual field loss (MD −2.0 to −5.0 dB) had high RNFL, GCC, and increased macular VD and flux. ODD eyes with moderate/severe visual field loss (MD < −5.0 dB) had decreased RNFL, GCC, peripapillary VAD, and increased macular VD and flux.
CONCLUSIONS
OCT and OCTA provided objective measurements that can help predict visual field loss in ODD. Our data suggest that increased macular flow may be an early biomarker of visual field loss in ODD, while decreased peripapillary vessel density and RNFL thickness are late biomarkers of visual field loss in ODD.
Optic disc drusen (ODD) are semi-translucent multilobulated yellowish deposits at the optic disc.1 These deposits when on the surface can be seen on fundus color and autofluorescence photography and when calcified (even if buried) can be seen on orbital B-scan ultrasonography.2, 3 Pathological studies of ODD have shown that variable size deposits containing calcium phosphate are found in the optic nerve head.4, 5 These deposits are thought to arise from abnormal axonal metabolism and may form from extruded extracellular mitochondria.6
In children, ODD is often diagnosed during workup for suspected papilledema or visual field loss.7 In adults, ODD presents as visual field loss in a pattern consistent with optic neuropathy, such as nasal or altitudinal visual field loss.8 Visual field loss in ODD is thought to arise from compression of the unmyelinated retinal ganglion cell axons and surrounding blood vessels by the ODD.9 Vascular compression or altered autoregulation also lead to increased risk of nonarteritic ischemic optic neuropathy, central retinal artery occlusion, central retinal vein occlusion, and peripapillary choroidal neovascularization.7
Advances in noninvasive ophthalmic imaging have revolutionized the clinical diagnosis of ODD and may be the most easily obtained biomarker for predicting which ODD eyes will develop visual field loss. Optical coherence tomography (OCT) with enhanced depth imaging (EDI) can detect and quantify ODD and associated findings.10 OCT studies have shown that visual field loss in ODD is correlated with significantly thinned peripapillary retinal nerve fiber layer (RNFL) and macular ganglion cell complex (GCC).11, 12 Worse visual field is also associated with older age, presence of superficial ODD, and larger drusen volume.11, 12
Optical coherence tomography angiography (OCTA) is a promising new technology for visualizing macular and peripapillary microvasculature and quantification of changes in optic neuropathies, including vessel density, vessel length density, tortuosity, and flow.13 Case reports of focal microvascular attenuation corresponding to ODD were first published in 2017,14, 15 and 3 studies have focused on the changes of OCTA measurements compared to the controls as well as the correlation between OCTA changes and visual field loss.16–18 Despite these studies, we still do not know which OCT and OCTA measurements are the key parameters that can help predict visual field loss in ODD.
In this study, we performed structure-function analysis using paired static perimetry as well as macular and peripapillary OCT and OCTA measurements in ODD and age-matched controls in order to determine which parameters are most correlated with visual field loss. Identification of these key OCT and OCTA biomarkers can help diagnose the impact of ODD on retinal structures and potentially be used to predict the risk of vision loss in ODD.
METHODS
We performed a retrospective cross-sectional study of patients with ODD and who were evaluated at Byers Eye Institute at Stanford University Medical Center between January 2016 and April 2019. This study was approved by the Institutional Review Board of Stanford University and adhered to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
PARTICIPANTS AND CLINICAL EVALUATION
In total, 35 controls (53 eyes) and 17 patients (29 eyes) with ODD were enrolled. All subjects had comprehensive ophthalmic examination and comprehensive measurements, including best corrected visual acuity (BCVA) using the Snellen chart to calculate the logarithm of reciprocal decimal visual acuity (logMAR VA), refraction, intraocular pressure measurement, and ophthalmoscopy. Controls: We recruited age- and sex-matched controls of age ≥18 years. The control subjects had BCVA of equal or better than the logMAR VA 0.2 and normal optic nerves per fundus examination, no subjective visual field loss, and normal RNFL and GCC. ODD: All ODD subjects had comprehensive neuro-ophthalmic examination by one investigator (YJL) and confirmation by color and autofluorescence funduscopic photography and OCT according to the Optic Disc Drusen Studies Consortium.19 All ODD eyes had one or more hypo-reflective center with hyperreflective margin on EDI-OCT.19 ODD that is visible by ophthalmoscopy was classified as superficial ODD, while ODD that was only detected by B-scan ultrasound or OCT was classified as buried ODD.12, 20 Exclusion Criteria: We excluded all subjects with history of optic neuropathies other than ODD and those with ophthalmic, neurological, or systemic diseases that may impact measurements of peripapillary or macular OCT and OCTA. Subjects with unreliable visual field tests were excluded, which were defined as fixation loss > 20%, or false-positive or false-negative error rates > 20%. We also excluded ODD patients with vascular complications such as anterior ischemic optic neuropathy, central retinal artery or vein occlusion because they may affect visual function as well as OCT and OCTA measurements.
VISUAL FIELD EXAMINATION AND ANALYSIS
Automated static perimetry was performed using Humphrey Field Analyzer II 750 (SITA 24–2 programs, Swedish interactive threshold algorithm, Carl Zeiss Meditech, Inc, Dublin, CA). The mean deviation (MD) and pattern standard deviation (PSD) was automatically calculated by the Humphrey Field Analyzer. The criteria for normal visual field was defined as previous studies: a) Normal visual field (without visual field defect): mean deviation (MD) less than −2.0 dB; b) With visual field defect: MD more than −2.0 dB and must meet at least one of the three criteria: Glaucoma hemifield test (GHT) outside normal limits; PSD with probability less than 5%; a cluster of 3 or more adjacent points on pattern deviation plot with probability less than 5%, one of which must have a probability level of at least 1%.21, 22
SPECTRAL-DOMAIN OCT AND OCTA DATA ACQUISITION
OCT and OCTA images were acquired using Cirrus HD-OCT AngioPlex (Model 5000; Carl Zeiss Meditec In., Germany). This machine utilizes light of 840 nm wavelength. The OCT scanner has maximum A-scan speed of 68,000 scans/sec with optical axial resolution of 5 μm and scanning depth of 2 mm. We performed the Optic Disc Cube 200 × 200 acquiring 200 horizontal scan lines each composed of 200 A-scans and Macular Cube 512 × 128 scans acquiring 128 horizontal scan lines each composed of 512 A-scans. The thickness of the RNFL was automatically measured in a circle with 3.46 mm diameter centered on the optic disc. The thicknesses of the macular GCC was measured in an elliptical annulus (vertical inner and outer radius of 0.5 mm and 2.0 mm, horizontal inner and outer radius of 0.6 and 2.4 mm, respectively) around the fovea which consists of the combined thickness of the ganglion cell layer and inner plexiform layers. We also required a high resolution 5-line axial raster or radial scans through the optic disc, which helps to detect ODD.
For OCTA, we acquired 3 × 3 mm scans of the peripapillary and macular superficial capillary plexus. We automatically segmented en face OCTA images using optical microangiography (OMAG).23 The inner surface of superficial capillary plexus was defined by the internal limiting membrane (ILM). The outer surface of superficial capillary plexus was an approximation of inner plexiform layer (IPL), where IPL is estimated to be at 70% of the thickness between the ILM and the retinal pigment epithelium. All OCT and OCTA scans were performed by trained ophthalmic photographers. Only images with signal strength >7 were saved and used for analysis. Custom quantification software with an interactive interface was used to quantify vascular density and morphology in the peripapillary and macular OCTA images (MATLAB R2016a; MathWorks, Natick, MA) based on modification of previous algorithm, which measures 6 vessel parameters provided distinct and biologically relevant information about microvasculature perfusion in the peripapillary and macular regions.24–28 Briefly, the algorithm transforms the original OCTA image into a binary vessel map using a 3-way combined method consisting of global thresholding, Hessian filter, and adaptive threshold. We removed large vessels from the peripapillary and macular OCTA images. Vessel skeleton map was developed by linearizing vessel signals into single-pixel width. Vessel perimeter map was created by outlining all vessels identified in the binarized image.
To standardize region of interest, we used an annulus with an outer diameter of 2.75 mm and an inner diameter of 1.5 mm for the optic disc OCTA and an annulus with the same outer diameter and a smaller inner diameter of 1 mm for the macular OCTA. From the annulus, we calculated 6 measurements: (1) vessel area density (VAD) was the proportion of total sum area of white pixels (blood vessels) divided by the total area of all pixels in the binarized image; (2) vessel skeleton density (VSD) was the sum of white pixels divided by the sum of all pixels in the skeleton map; (3) vessel complexity index (VCI) was the square of the sum of pixels occupied by the vessel perimeter image divided by 4π times the sum of white pixels in the binarized image; (4) vessel perimeter index (VPI) was calculated using the vessel perimeter map as the ratio of the vessel perimeter to the total area of the OCTA image; (5) vessel diameter (VD) measured the averaged vessel caliber within the image; (6) flux measured the number of blood cells passing through a retinal vessel cross-sectional area per unit time.29 All OCTA data analysis was performed by one investigator to maximize consistency.
STATISTICAL ANALYSIS
The data were analyzed by SPSS version 23.0 for Mac (SPSS Inc., Chicago, IL, USA) and R (R Foundation for Statistical Computing, Vienna, Austria). Quantitative continuous variables were presented as mean ± standard error or median (95% confidence interval). A two-tailed Mann-Whitney u test was used to compare means of 2 groups of continuous variables. The frequencies of categorical variables were compared using chi-square test. Spearman correlation test was performed to determine the correlation between parameters. A value of P <0.05 was considered statistically significant. Principal component analysis, Spearman correlation matrix, and hierarchical clustering were performed using custom R scripts. In the hierarchical clustering, each measurement has been centered and scaled to have standard deviation one in order to see differences between eyes. The PSD values of visual field were multiplied by −1 because they were negatively correlated with some of the other measurements.
RESULTS
ODD EYES HAD SIGNIFICANTLY WORSE VISUAL FIELD AND PERIPAPILLARY OCT AND OCTA
To identify key determinants of vision function abnormalities in ODD, we conducted a cross-sectional study of 17 patients with ODD (29 eyes) and 35 controls (53 eyes). Representative ODD eyes with different severity of visual field loss, OCT, and OCTA images are shown in Figure 1 and 2. There were 12 (71%) patients with bilateral ODD and 5 (29%) with unilateral ODD. Nineteen (66%) eyes had superficial ODD, and 10 (34%) eyes had buried ODD. There were no significant differences between the groups with regard to age, sex. Both controls and ODD eyes had good LogMAR VA (P = .287). On static perimetry, the ODD eyes had significantly worse average MD compared with controls (controls: − 0.36 ± 0.15 dB; ODD: − 5.07 ± 1.15 dB, P < .0001). On OCT, ODD eyes had significantly decreased average peripapillary RNFL by 7.1 μm (P = .006) and no difference in average macular GCC. On OCTA, ODD eyes had significantly lower peripapillary VAD (controls: 0.45 ± 0.00; ODD: 0.41 ± 0.01, P = 0.011) but not macular VAD compared with controls (Table 1).
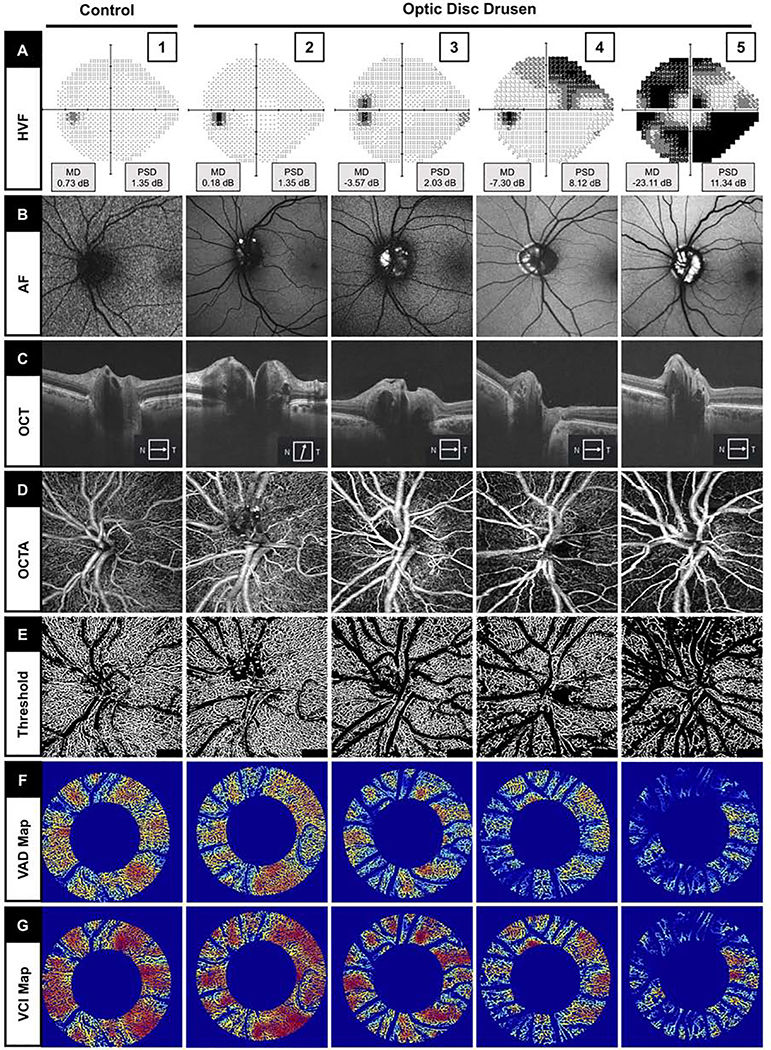
FIGURE 1.
Representative static perimetry and superficial peripapillary en face microvascular images of control (Column 1) and optic disc drusen (ODD) eyes with increasing severity of visual field loss (Column 2–5 from left to right). (A) Static perimetry using Humphrey visual field (HVF) with mean deviation (MD) and pattern standard deviation (PSD) measurements. (B) Fundus autofluorescence (AF) imaging showing ODD as hyperfluorescent areas. (C) OCT raster scan through the optic disc showing ODD as a signal-poor ovoid core with hyperreflective margin. (D) En face OCTA slab. (E) Threshold OCTA images of (D) after removing the large vessels. (F) Vessel area density (VAD) map with binary vessels after removing the large vessels. (G) Vessel complexity index (VCI) map after removing the large vessels.
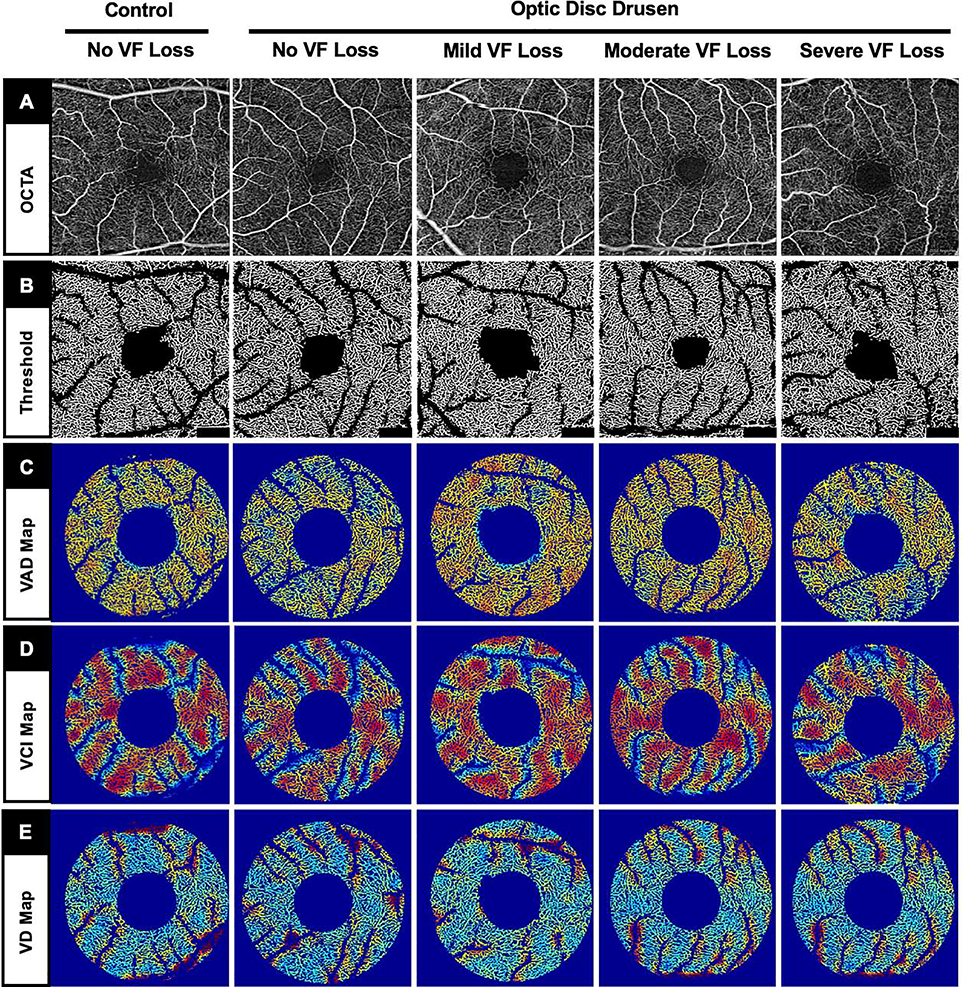
FIGURE 2.
Representative superficial macular optical coherence tomography angiography (OCTA) images (same eyes as Figure 1) showing no striking differences between control and optic disc drusen (ODD) eyes with different severities of visual field (VF) loss. (A) En face OCTA slab. (B) Threshold OCTA images. (C) Macular vessel area density (VAD) map with binary vessels after removing the large vessels. (D) Macular vessel complexity index (VCI) map after removing the large vessels. (E) Macular vessel diameter (VD) map after removing the large vessels.
TABLE 1.
The Demographics and Clinical Characteristics of Controls and Optic Disc Drusen Patients (Eyes)
| Controls (n= 53 eyes) | ODD (n= 29 eyes) | P value | |
|---|---|---|---|
| Age a (y) | 43.3 ± 2.0 | 37.9 ± 4.3 | .208 |
| Sex a, n (%) | .558 | ||
| Female | 19 (54.3) | 11 (64.7) | |
| Male | 16 (45.7) | 6 (35.3) | |
| LogMAR | 0.01 ± 0.01 | 0.02 ± 0.01 | .287 |
| VF MD (dB) | −0.36 ± 0.15 b | −5.07 ± 1.15 c | <.0001* |
| VF PSD (dB) | 1.44 ± 0.07 b | 4.26 ± 0.71 c | .001* |
| OCT Average RNFL (μm) | 93.1 ± 1.2 | 86.0 ± 4.9 | .006* |
| OCT Average GCC (μm) | 81.8 ± 0.8 | 77.7 ± 2.1 | .244 |
| OCTA Optic disc VAD | 0.45 ± 0.00 | 0.41 ± 0.01 | .011* |
| OCTA Macula VAD | 0.41 ± 0.00 | 0.41 ± 0.01 | .182 |
GCC = ganglion cell complex; LogMAR= Logarithm of the minimum angle of resolution; ODD = optic disc drusen; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; PSD = pattern standard deviation; RNFL = retinal nerve fiber layer; VAD = vessel area density; VF = visual field.
calculated from 35 controls and 17 ODD subjects
calculated from 17 eyes
calculated from 24 eyes.
Data are expressed as mean ± standard error except sex. Values were compared by Mann-Whitney test.
P < .05
Sex was compared by Chi-square test.
CORRELATION OF VISUAL FIELD LOSS WITH OCT AND OCTA MEASUREMENTS
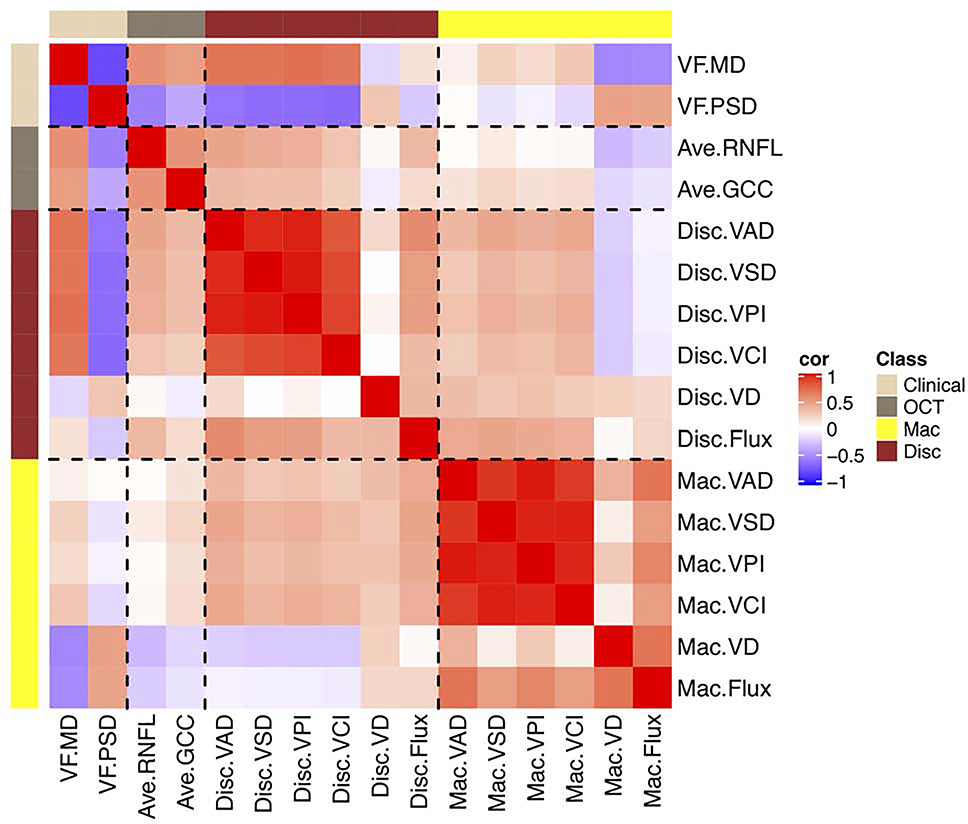
We performed Spearman correlation pairs analysis and defined r > 0.5 as strong correlation and r between 0.25–0.5 as moderate correlation (Figure 3). For both peripapillary and macular OCTA measurements, there was strong correlation among VAD, VSD, VPI and VCI (also see S1 and S2, Supplemental Material at AJO.com). Visual field loss, which is defined as decreased MD or increased PSD, was strongly correlated with decreased peripapillary VAD followed by thinning of OCT RNFL and then GCC. Interestingly, visual field loss was also strongly correlated with increased macular VD and flux. There was moderate correlation between OCT (RNFL and GCC) and most peripapillary OCTA measurements (disc VAD, VSD, VPI, VCI, and flux). There was no correlation between OCT (RNFL and GCC) and most macular OCTA measurements (macular VAD, VSD, VpI, VCI, and flux).
FIGURE 3.
Spearman correlation matrix heatmap showing visual field mean deviation was positively correlated with retinal nerve fiber layer (RNFL), ganglion cell complex (GCC), and peripapillary vessel area density (VAD) and negatively correlated with macular vessel diameter (VD) and flux. For both optic disc and macular OCTA measurements, there was strong correlation between VAD and vessel skeletal density (VSD), vessel perimetry index (VPI), and vessel complexity index (VCI). Abbreviations: Ave = average; cor = correlation; Disc = optic disc; GCC = ganglion cell complex; Mac = macular; MD = mean deviation; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; PSD = pattern standard deviation; RNFL = retinal nerve fiber layer; VAD = vessel area density; VCI = vessel complexity index; VD = vessel diameter; VF = visual field; VPI = vessel perimeter index; VSD = vessel skeleton density.
ODD EYES WITH VISUAL FIELD DEFECT HAD DECREASED OCT AND MOST PERIPAPILLARY OCTA MEASUREMENTS BUT INCREASED MACULAR VD AND FLUX
To examine which OCT and OCTA measurements that best correlate with visual field loss, we segregated the eyes into controls, ODD with no visual field defect (MD > − 2.0 dB) and ODD with visual field defect (MD < −2.0 dB) (Table 2). On OCT, the ODD with visual field defect had 24 μm thinner average RNFL compared with controls and 14 μm compared with ODD without visual field defect. ODD with visual field defect had 14 μm thinner average GCC compared with controls and 10 μm compared with ODD without visual field defect. On OCTA, ODD with visual field defect had relatively decreased peripapillary VAD, VSD, VPI, VCI and increased macular VD and flux compared with controls or ODD without visual field defect. Taken together, visual field loss in ODD eyes corresponded with a decrease in RNFL, GCC, and most peripapillary OCTA measurements and an increase in macular VD and flux.
TABLE 2.
Visual Field Loss and OCT/OCTA Measurements from Controls and Optic Disc Drusen Eyes Categorized by Severity of Visual Field Defect
| Controls (n=17) | ODD without VF loss (n=9) | ODD with VF loss (n=15) | |
|---|---|---|---|
| VF | |||
| MD (dB) | −0.38 (−0.68 ∼ −0.04) | −1.03 (−1.32 ∼ −.51) | −6.35 (−10.83 ∼ −4.31) |
| PSD (dB) | 1.37 (1.29 ∼ 1.60) | 1.48 (1.30 ∼ 1.64) | 5.43 (4.02 ∼ 7.83) |
| OCT | |||
| Average RNFL (μm) | 96.0 (91.4 ∼ 99.2) | 86.0 (77.6 ∼ 91.5) | 72.0 (61.39 ∼ 89.54) |
| Average GCC (μm) | 82.0 (78.3 ∼ 85.6) | 78.0 (77.6 ∼ 86.4) | 68.0 (65.00 ∼ 78.20) |
| OCTA optic disc | |||
| VAD | 0.46 (0.45 ∼ 0.47) | 0.46 (0.42 ∼ 0.47) | 0.38 (0.34 ∼ 0.41) |
| VSD | 0.18 (0.18 ∼ 0.19) | 0.18 (0.17 ∼ 0.19) | 0.15 (0.13 ∼ 0.16) |
| VPI | 0.39 (0.37 ∼ 0.40) | 0.39 (0.36 ∼ 0.40) | 0.32 (0.29 ∼ 0.35) |
| VCI | 2206.1 (2129.0 ∼ 2292.8) | 2249.5 (2109.0 ∼ 2381.7) | 1946.3 (1706.6 ∼ 2033.9) |
| VD | 17.75 (17.58 ∼ 17.93) | 17.64 (17.37 ∼ 17.87) | 17.78 (17.53 ∼18.10) |
| Flux | 0.49 (0.44 ∼ 0.49) | 0.41 (0.39 ∼ 0.48) | 0.44 (0.41 ∼ 0.47) |
| OCTA macula | |||
| VAD | 0.42 (0.41 ∼ 0.43) | 0.40 (0.37 ∼ 0.42) | 0.42 (0.40 ∼ 0.44) |
| VSD | 0.17 (0.16 ∼ 0.17) | 0.16 (0.15 ∼ 0.16) | 0.17 (0.16 ∼ 0.17) |
| VPI | 0.37 (0.36 ∼ 0.38) | 0.35 (0.34 ∼ 0.37) | 0.37 (0.35 ∼ 0.38) |
| VCI | 2759.5 (2679.3 ∼ 2830.9) | 2691.0 (2543.6 ∼ 2740.7) | 2702.7 (2603.9 ∼ 2784.2) |
| VD | 17.34 (17.16 ∼ 17.71) | 17.43 (17.17 ∼ 17.73) | 17.89 (17.78 ∼ 18.02) |
| Flux | 0.30 (0.30 ∼ 0.32) | 0.30 (0.28 ∼0.34) | 0.35 (0.34 ∼0.37) |
GCC = ganglion cell complex; MD = mean deviation; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; ODD = optic disc drusen; PSD = pattern standard deviation; RNFL = retinal nerve fiber layer; VAD = vessel area density; VCI = vessel complexity index; VD = vessel diameter; VF = visual field; VPI = vessel perimeter index; VSD = vessel skeleton density.
Data are expressed as median (95% confidence interval).
PRINCIPAL COMPONENT ANALYSIS REVEALED 5 KEY MEASUREMENTS
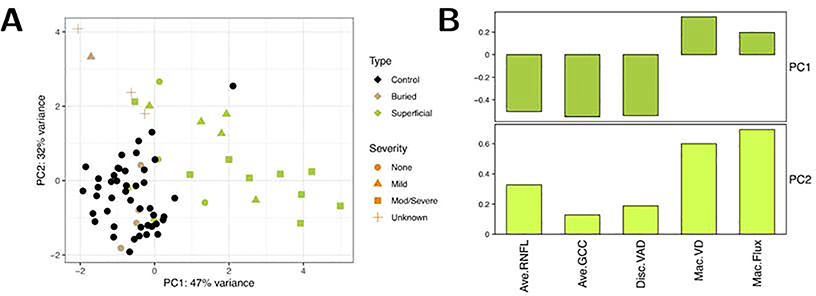
We performed principal component analysis (PCA) weighing the 5 key measurements: RNFL, GCC, peripapillary VAD, macular VD and macular flux (Figure 4). PC1 weighs RNFL, GCC and peripapillary VAD most heavily (and roughly equally), and PC2 weighs the two macular OCTA measures most heavily and also differentiates between the other three measurements. We found that the buried ODD eyes (tan color) mostly had no visual field loss (circles), while the superficial ODD eyes (green color) mostly had mild, moderate/severe visual field loss (triangle or square).
FIGURE 4.
Principal component analysis revealed that 5 key measurements retinal nerve fiber layer (RNFL), ganglion cell complex (GCC), peripapillary vessel area density (VAD), macular vessel density (VD), and macular flux were most informative for segregating eyes with no visual field loss (circle) from those with mild (triangle) or moderate/severe visual field loss (square). In addition, buried ODD eyes (tan color) mostly had no visual field loss (circle), while the superficial ODD eyes (green color) mostly had mild, moderate/severe visual field loss (triangle or square). Abbreviations: Ave = average; Disc = optic disc; GCC = ganglion cell complex; Mac = macular; mod/severe = moderate to severe; PC = principal component; RNFL = retinal nerve fiber layer; VAD = vessel area density; VD = vessel diameter.
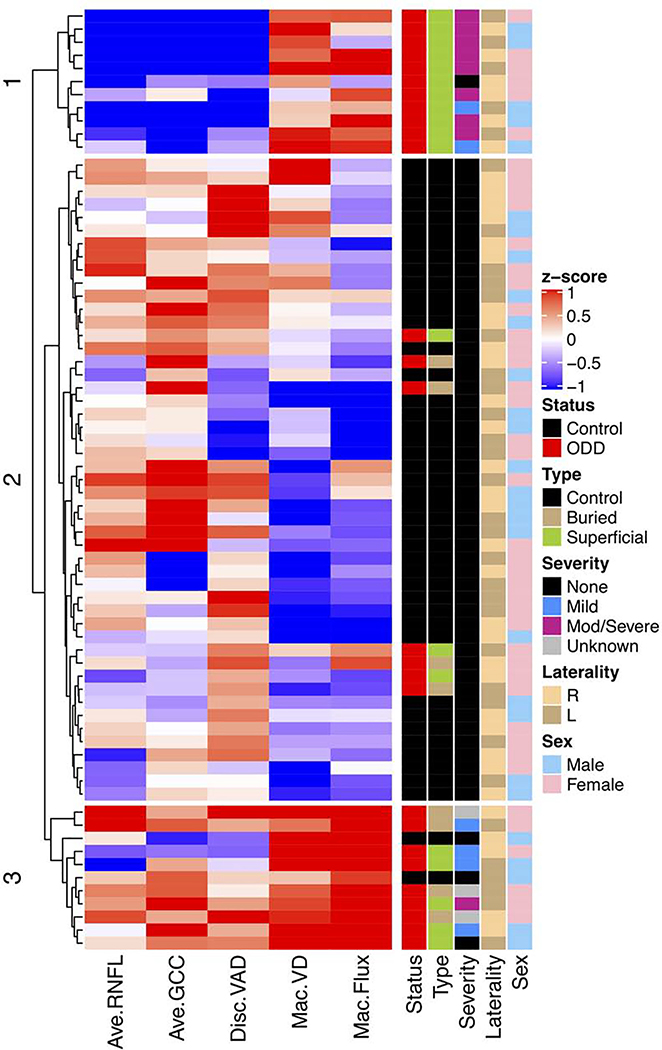
HIERARCHICAL CLUSTERING ANALYSIS OF OPTIC DISC AND MACULAR OCTA MEASUREMENTS SHOWED 3 GROUPS
We analyzed OCT and OCTA measurements objectively using hierarchical clustering of these 5 key measurements: RNFL, GCC, peripapillary VAD, macular VD and macular flux. All eyes were clustered into 3 distinct groups (Figure 5). The middle group contained most of the control eyes and 7 ODD eyes without visual field loss (MD > −2.0 dB). They had high RNFL, GCC and peripapillary VAD and relatively low macular VD and flux. In contrast, the bottom group contained mostly ODD eyes, including 5 with superficial ODD and 4 with buried ODD – the majority with normal visual field or mild visual field loss (MD −2.0 dB to −5.0 dB). This group had similarly high RNFL, GCC and peripapillary VAD, and much higher macular VD and flux compared with the middle group. The top group, which consisted of only superficial ODD eyes – most with moderate to severe visual field defect (MD < −5.0 dB), had low RNFL, GCC and peripapillary VAD but high macular VD and flux compared with the middle group. Comparing the top and bottom groups, which contained most of the ODD eyes with visual field loss, the main difference was that the top group had more severe visual field loss, thinning of RNFL and GCC, and decreased peripapillary VAD, while both had increased macular VD and flux.
FIGURE 5.
Hierarchical clustering of 5 key optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) measurements (horizontal dendrogram) and clinical characteristics (vertical dendrogram) showed that data clustered into 3 groups. Mild visual field defect is defined as mean deviation between −2.0 dB and −5.0 dB and moderate to severe visual field defect is defined as mean deviation worse than −5.0 dB. In the annotation to the right of the heatmap, the colors are defined by the legends on the far right. Abbreviations: Ave = average; Disc = optic disc; GCC = ganglion cell complex; Mac = macular; MD = mean deviation; mod/severe = moderate to severe; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; PSD = pattern standard deviation; RNFL = retinal nerve fiber layer; VAD = vessel area density; VCI = vessel complexity index; VD = vessel diameter; VF = visual field; VPI = vessel perimeter index; VSD = vessel skeleton density.
DISCUSSION
In vivo ophthalmic imaging has become an indispensable part of clinical assessment of patients with ODD and other optic neuropathies because they help diagnose disease and because changes in OCT and OCTA measurements serve as important biomarkers to help predict vision loss. A number of studies have examined the structure-function relationship of OCT in ODD,11, 12, 30 but only two studies have evaluated that of OCTA. 16, 18 To address this, we performed a structure-function study of ODD and found that 5 OCT and OCTA measurements of the superficial capillary plexus are particularly important in predicting visual field loss: peripapillary RNFL, macular GCC, peripapillary VAD, macular VD, and macular flux. Visual field loss was not only strongly correlated with changes in these 5 measurements, hierarchical clustering analysis identified patterns that help determine early and late changes that are associated with visual field loss in ODD. The eyes with mild visual field loss (MD −2.0 to −5.0 dB), which contained both buried and superficial ODD, was correlated with relatively normal RNFL, GCC, and peripapillary VAD and increased macular VD and flux. This may be a pattern consistent with early stage in optic neuropathy associated ODD, and timely therapeutic intervention to treat eyes with this pattern may be more likely to reverse or maintain vision. In contrast, the eyes with moderate/severe visual field loss (MD < −5.0 dB), which primarily contained eyes with only superficial ODD, was correlated with decreased RNFL, GCC, and peripapillary VAD and increased macular VD and flux. This may be a pattern consistent with late stage in optic neuropathy associated with ODD, and treatment may be more likely to maintain vision, given irreversible loss of retinal ganglion cells and axons.
In our study, the most important OCTA finding is that there was a relative increase in macular VD and flux of the superficial capillary plexus without changes in RNFL, GCC, and peripapillary VAD in ODD eyes with mild visual field loss. The flux we measured has a linear correlation with flow speed, especially after large vessel removal.29 This finding is compatible with a disease model in ODD where compensatory, autoregulatory changes in retinal microvasculature occurs prior to significant neurodegeneration. This increase in macular vessel diameter and flux has not previously been described in other optic neuropathies such as glaucoma, anterior ischemic optic neuropathy, and papilledema and may be unique to compressive, nonischemic optic neuropathy. Alternatively, this may be due to technical reasons since previous studies did not systematically remove large vessel contributions to OCTA measurements. A compensatory increase in macular capillary hemodynamic changes using OCTA has been described in pan-retinal photocoagulation (PRP) therapy in proliferative diabetic retinopathy. Following peripheral retinal PRP, there was an increase in macular capillary flow with no change in macular vessel density or vessel length density.31 Future OCTA studies of optic neuropathies should include both macular as well as peripapillary measurements in the same eyes, in order to validate this increase in macular vessel diameter and flux as the earliest imaging biomarkers for development of visual field loss.
We also found that more severe visual field loss in ODD was correlated with a decrease in peripapillary vessel density. Our finding is consistent with a recent publication of OCTA and ODD but not another.16, 18 Engelke et al. showed a strong negative correlation between peripapillary vessel density and pattern standard deviation.18 The difference with Cennamo et al. may be related to our inclusion of more advanced cases with older ages, worse visual field and more superficial ODD. This association of visual field loss with a decrease in peripapillary vessel density has also been described in OCTA studies of other optic neuropathies, including glaucoma and ischemic optic neuropathy. 18, 32, 33 The ODD eyes (with or without visual field loss) in our study exhibited an average of 9% decrease in superficial peripapillary vessel density compared with controls, which corresponded well with the 6–11% decrease in previous studies.16, 17 In ODD eyes with visual field defect, there was 17% decrease in vessel density in ODD eyes compared with controls. Although prior studies on ODD did not examine multiple OCTA parameters, we found that both peripapillary and macular VAD measurements were highly correlated with VSD, VCI, VPI, so commercial devices that only measure VAD still provides valuable data. The VCI and VPI measurements in our study likely reflect relative decrease in vascular branching in eyes with visual field loss relative to that of the perfused vasculature in healthy eyes, which has also been found in glaucoma.26, 28 An important part of retinal microvasculature analysis is removal of the contribution of the large vessels, so future analysis of VAD should include this detail. Overall, decrease in peripapillary VAD is an important biomarker in helping to predict visual field loss in ODD, but this likely occurs later in disease compared with the change in macular vessel diameter and flux.
Our data confirmed that OCTA is better correlated with visual field parameters than RNFL or GCC. Previous studies demonstrated the association between OCTA and visual field parameters were stronger than that between OCTA and OCT parameters in glaucoma patients.32, 34 Hata et al. found superficial peripapillary vessel density rather than RNFL was significantly decreased in anterior ischemic optic neuropathy patients with severe vision loss compared to mild to moderate vision loss.33 Liu et al. found peripapillary rather than parafoveal retinal vessel density was significantly decreased in chronic anterior ischemic optic neuropathy compared to the controls, and was significantly correlated with MD, RNFL and GCC.35 These are similar to our results. The discrepancy of vessel density and thickness changes in optic disc and macula may result from different corresponding microcirculation distribution, and the different macular scan size and depth between OCT and OCTA procedures as well.28 We also demonstrated RNFL was thinner in superficial ODD eyes than in controls and it was thicker in buried ODD eyes. Similar results were found in the study conducted by Gili P. et al., which demonstrated that RNFL in all quadrants except temporal region of superficial ODD was significant thinner than controls, while buried ODD did not show such a difference.21 The presence of superficial ODD was associated with some of the similar changes as that of visual field but may not correlate well with the severity of visual field defect, since eyes with superficial ODD exhibited a wide range of visual field loss. This may be explained by the previous study, which had demonstrated the worse visual field defects associated with superficial ODD is rather due to larger drusen volume than the more superficial anatomic location.12
There are several limitations in this study. Our study does not have longitudinal data points, which is critical for understanding of the evolution of ODD, and this should be addressed in a large, prospective study. We only observed superficial capillary plexus but couldn’t look deeper, although the deeper microvasculature also impacts visual field loss. Because of our sample size, there were relatively small number of eyes with buried ODD, so we are unable to compare the differences between those with superficial vs buried ODD. Using current analysis, we cannot distinguish changes in arterioles vs. venules vs. capillaries. This is important in ODD because a compressive lesion at the optic disc can cause reduced peripapillary arterioles at the same time as an increase in venules. Further exploration of these specific vascular changes will help us better understand the dynamic changes in vasculature in ODD and the role of the autoregulation.
In conclusion, our study showed that both OCT and OCTA measurements are important in structure-function analysis in patients with ODD. There was strong correlation between vision loss and an increase in macular vessel diameter and flux – an early biomarker – and a reduction in peripapillary vessel density, RNFL and GCC. Autoregulation may compensate for peripapillary effect of ODD on surrounding vasculature and structure by increasing overall retinal blood flow early in disease. As retinal ganglion cell axons are lost and more visual field loss develops, then a decrease of peripapillary vessel density occurs secondarily – a late biomarker of disease.
Supplementary Material
HIGHLIGHTS.
Optic disc drusen (ODD) is an optic neuropathy that is associated with visual field loss.
There are 5 OCT and OCTA measurements that most correlated with visual field loss.
Increased macular blood flow may be early biomarker of vision loss in ODD.
Decreased peripapillary vessel density may be late biomarker of vision loss in ODD.
ACKNOWLEDGMENTS/DISCLOSURE
Other Acknowledgments: We thank members of the Stanford Department of Ophthalmology for referral of study subjects, and we thank the photographers for performing ophthalmic imaging of these patients.
Funding: This work was supported by National Eye Institute (R01EY028753), Carl Zeiss Meditec, Inc. (Dublin, CA), the Research to Prevent Blindness, Inc., New York, NY, and unrestricted grant from Research Preventing Blindness grant and philanthropy.
Financial Disclosures: Dr. Wang has intellectual property owned by the Oregon Health and Science University and the University of Washington related to OCT angiography, which are licensed to commercial entities and related to the technology and analysis methods used in this manuscript. Dr. Wang also receives research support from Carl Zeiss Meditec, Inc and Moptim Inc. He is a consultant to Carl Zeiss Meditec, Inc. and Insight Photonic Solutions. Dr. Chu has intellectual property owned by the University of Washington related to OCT angiography, which are related to the technology and analysis methods described in this manuscript. The other authors have no disclosures. All authors attest that they meet the current ICMJE criteria for authorship.
Abbreviations
- (GCC)
ganglion cell complex
- (MD)
mean deviation
- (OCT)
optical coherence tomography
- (OCTA)
optical coherence tomography angiography
- (ODD)
optic disc drusen
- (PSD)
pattern standard deviation
- (RNFL)
retinal nerve fiber layer
- (VAD)
vessel area density
- (VCI)
vessel complexity index
- (VD)
vessel diameter
- (VPI)
vessel perimeter index
- (VSD)
vessel skeleton density
Footnotes
Supplemental Material available at AJO.com
This is a structure-function study using perimetry and optical coherence tomography and angiography in patients with optic disc drusen and age-matched controls. There were 5 key imaging measurements that most correlated with visual field loss. This data suggest that increased macular flow may be an early biomarker of visual field loss, while decreased peripapillary vessel density and retinal nerve fiber layer thickness are late biomarkers of visual field loss in optic disc drusen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Friedman AH, Gartner S, Modi SS. Drusen of the optic disc. A retrospective study in cadaver eyes. Br J Ophthalmol 1975;59(8):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustonen E, Nieminen H. Optic disc drusen--a photographic study. I. Autofluorescence pictures and fluorescein angiography. Acta Ophthalmol (Copenh) 1982;60(6):849–858. [DOI] [PubMed] [Google Scholar]
- 3.Boldt HC, Byrne SF, DiBernardo C. Echographic evaluation of optic disc drusen. J Clin Neuroophthalmol 1991; 11(2):85–91. [PubMed] [Google Scholar]
- 4.Kapur R, Pulido JS, Abraham JL, Sharma M, Buerk B, Edward DP. Histologic findings after surgical excision of optic nerve head drusen. Retina 2008;28(1): 143–146. [DOI] [PubMed] [Google Scholar]
- 5.Skougaard M, Heegaard S, Malmqvist L, Hamann S. Prevalence and histopathological signatures of optic disc drusen based on microscopy of 1713 enucleated eyes. Acta Ophthalmol 2020;98(2):195–200. [DOI] [PubMed] [Google Scholar]
- 6.Tso MO. Pathology and pathogenesis of drusen of the optic nervehead. Ophthalmology 1981;88(10): 1066–1080. [DOI] [PubMed] [Google Scholar]
- 7.Chang MY, Pineles SL. Optic disk drusen in children. Surv Ophthalmol 2016;61(6):745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee AG, Zimmerman MB. The rate of visual field loss in optic nerve head drusen. Am J Ophthalmol 2005; 139(6): 1062–1066. [DOI] [PubMed] [Google Scholar]
- 9.Hamann S, Malmqvist L, Costello F. Optic disc drusen: understanding an old problem from a new perspective. Acta Ophthalmol 2018;96(7):673–684. [DOI] [PubMed] [Google Scholar]
- 10.Merchant KY, Su D, Park SC, et al. Enhanced depth imaging optical coherence tomography of optic nerve head drusen. Ophthalmology 2013; 120(7): 1409–1414. [DOI] [PubMed] [Google Scholar]
- 11.Malmqvist L, Wegener M, Sander BA, Hamann S. Peripapillary Retinal Nerve Fiber Layer Thickness Corresponds to Drusen Location and Extent of Visual Field Defects in Superficial and Buried Optic Disc Drusen. J Neuroophthalmol 2016;36(1):41–45. [DOI] [PubMed] [Google Scholar]
- 12.Malmqvist L, Lindberg AW, Dahl VA, Jørgensen TM, Hamann S. Quantitatively measured anatomic location and volume of optic disc drusen: an enhanced depth imaging optical coherence tomography study. Invest Ophthalmol Vis Sci 2017;58(5):2491–2497. [DOI] [PubMed] [Google Scholar]
- 13.Spaide rF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res 2018;64:1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores-Reyes E, Hoskens K, Mansouri K. Optic nerve head drusen: imaging using optical coherence tomography angiography. J Glaucoma 2017;26(9):845–849. [DOI] [PubMed] [Google Scholar]
- 15.Gaier ED, Rizzo JF 3rd, Miller JB, Cestari DM. Focal capillary dropout associated with optic disc drusen using optical coherence tomographic angiography. J Neuroophthalmol 2017;37(4):405–410. [DOI] [PubMed] [Google Scholar]
- 16.Cennamo G, Tebaldi S, Amoroso F, Arvanitis D, Breve M, Cennamo G. Optical coherence tomography angiography in optic nerve drusen. Ophthalmic Res 2018;59(2):76–80. [DOI] [PubMed] [Google Scholar]
- 17.Abri Aghdam K, Ashraf Khorasani M, Soltan Sanjari M, et al. Optical coherence tomography angiography features of optic nerve head drusen and nonarteritic anterior ischemic optic neuropathy. Can J Ophthalmol 2019;54(4):495–500. [DOI] [PubMed] [Google Scholar]
- 18.Engelke H, Shajari M, Riedel J, Mohr N, Priglinger SG, Mackert MJ. OCT angiography in optic disc drusen: comparison with structural and functional parameters. Br J Ophthalmol 2019;0:1–5. [DOI] [PubMed] [Google Scholar]
- 19.Malmqvist L, Bursztyn L, Costello F, et al. The optic disc drusen studies consortium recommendations for diagnosis of optic disc drusen using optical coherence tomography. J Neuroophthalmol 2018;38(3):299–307. [DOI] [PubMed] [Google Scholar]
- 20.Roh S, Noecker RJ, Schuman JS, Hedges TR 3rd, Weiter JJ, Mattox C. Effect of optic nerve head drusen on nerve fiber layer thickness. Ophthalmology 1998; 105(5):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gili P, Flores-Rodriguez P, Martin-Rios MD, Carrasco Font C. Anatomical and functional impairment of the nerve fiber layer in patients with optic nerve head drusen. Graefes Arch Clin Exp Ophthalmol 2013;251(10):2421–2428. [DOI] [PubMed] [Google Scholar]
- 22.Keltner JL, Johnson CA, Cello KE, Wall M. Baseline visual field findings in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT). Invest Ophthalmol Vis Sci 2014;55(5):3200–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express 2007;15(7):4083–4097. [DOI] [PubMed] [Google Scholar]
- 24.Chu Z, Lin J, Gao C, et al. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J Biomed Opt 2016;21(6):66008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang R, Chu Z, Burkemper B, et al. Effect of scan size on glaucoma diagnostic performance using oct angiography en face images of the radial peripapillary capillaries. J Glaucoma 2019;28(5):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter GM, Sylvester B, Chu Z, et al. Peripapillary microvasculature in the retinal nerve fiber layer in glaucoma by optical coherence tomography angiography: focal structural and functional correlations and diagnostic performance. Clin Ophthalmol 2018;12:2285–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim AY, Rodger DC, Shahidzadeh A, et al. Quantifying retinal microvascular changes in uveitis using spectral-domain optical coherence tomography angiography. Am J Ophthalmol 2016; 171:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter GM, Chang R, Situ B, et al. Diagnostic performance of macular versus peripapillary vessel parameters by optical coherence tomography angiography for glaucoma. Transl Vis Sci Technol 2018;7(6):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi WJ, Qin W, Chen CL, et al. Characterizing relationship between optical microangiography signals and capillary flow using microfluidic channels. Biomedical Optics Express 2016;7(7):2709–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato T, Mrejen S, Spaide RF. Multimodal imaging of optic disc drusen. Am J Ophthalmol 2013; 156(2):275–282 e1. [DOI] [PubMed] [Google Scholar]
- 31.Fawzi AA, Fayed AE, Linsenmeier RA, Gao J, Yu F. Improved macular capillary flow on optical coherence tomography angiography after panretinal photocoagulation for proliferative diabetic retinopathy. Am J Ophthalmol 2019;206:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016;123(12):2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hata M, Oishi A, Muraoka Y, et al. Structural and functional analyses in nonarteritic anterior ischemic optic neuropathy: optical coherence tomography angiography study. J Neuroophthalmol 2017;37(2):140–148. [DOI] [PubMed] [Google Scholar]
- 34.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology 2017;124(5):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu CH, Kao LY, Sun MH, Wu WC, Chen HS. Retinal vessel density in optical coherence tomography angiography in optic atrophy after nonarteritic anterior ischemic optic neuropathy. J Ophthalmol 2017;2017:9632647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.