Figure 4. PreNMDARs contribute significantly to burst-induced facilitation and spike transfer.
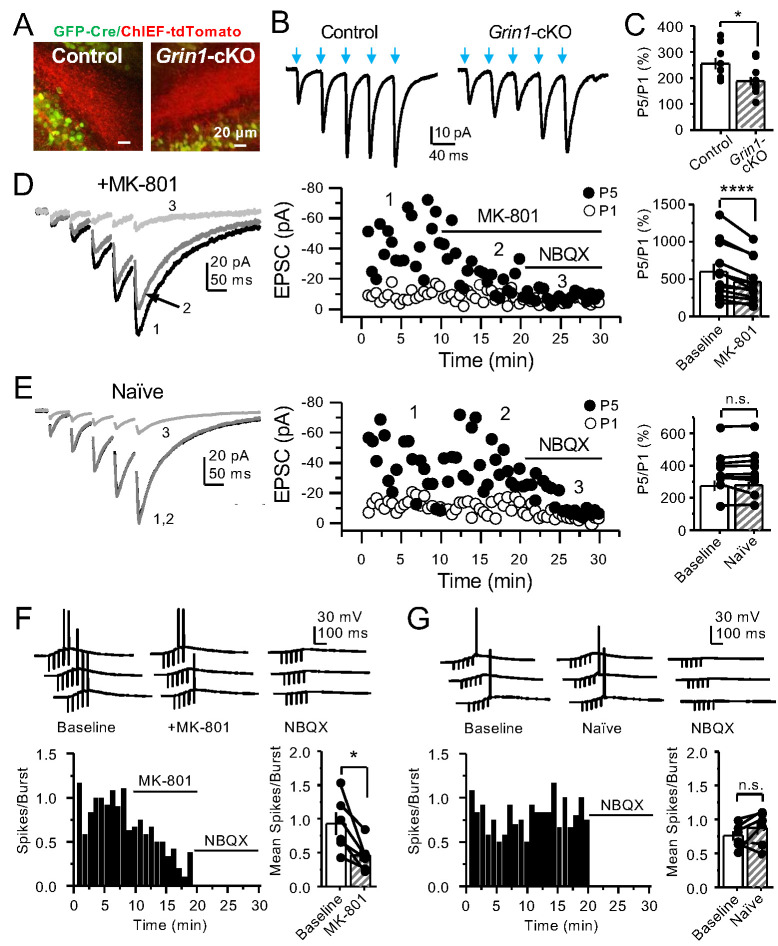
(A) Representative images showing expression of GFP-Cre (left) and ChIEF-tdTomato (right) in the DG of control and Grin1-cKO animals. (B) Representative AMPAR-EPSCs from control (left) and Grin1-cKO (right) CA3 pyramidal neurons recorded at Vh = −65 mV and evoked by optical burst-stimulation (5 pulses at 25 Hz) of stratum lucidum. Blue arrows indicate light stimulation. (C) Summary plot of burst-induced facilitation measured as P5/P1 ratio of optical responses; facilitation was significantly reduced in Grin1-cKO animals as compared to control (Grin1-cKO 187 ± 16%, n = 12 cells, nine animals; control 255 ± 22%, n = 9 cells, eight animals; Grin1-cKO vs control, p=0.0167, unpaired t-test). (D) Burst stimulation induced KAR-EPSCs were isolated and recorded as described in Figure 3, bath application of MK-801 (50 µM) significantly reduced facilitation (baseline 601 ± 107%, MK-801 464 ± 84%, n = 13 cells, 10 animals; baseline vs MK-801, p=0.00042, paired t-test). In (D) and (E) of this figure: representative traces (left), representative experiment (middle), and summary plot (right). (E) Burst-induced facilitation was stable in interleaved, naïve slices (baseline 369 ± 45%, naïve 367 ± 48%, n = 9 cells, nine animals; p=0.863, paired t-test). (F) Bath application of MK-801 (50 µM) reduced KAR-mediated action potentials induced by burst-stimulation (baseline 0.93 ± 0.17, MK-801 0.46 ± 0.09, n = 6 cells, five animals; p=0.036, Wilcoxon signed-rank test). In (F) and (G) of this figure: representative traces (top), representative experiment and summary plot (bottom). (G) Stable KAR-mediated action potentials in interleaved naïve slices (baseline 0.76 ± 0.07, naïve 0.88 ± 0.1, n = 6 cells, five animals; p=0.2084, Wilcoxon signed-rank test). NBQX (10 µM) was applied at the end of all experiments in (D–G). Data are presented as mean ± s.e.m. *p<0.05; ****p<0.001.
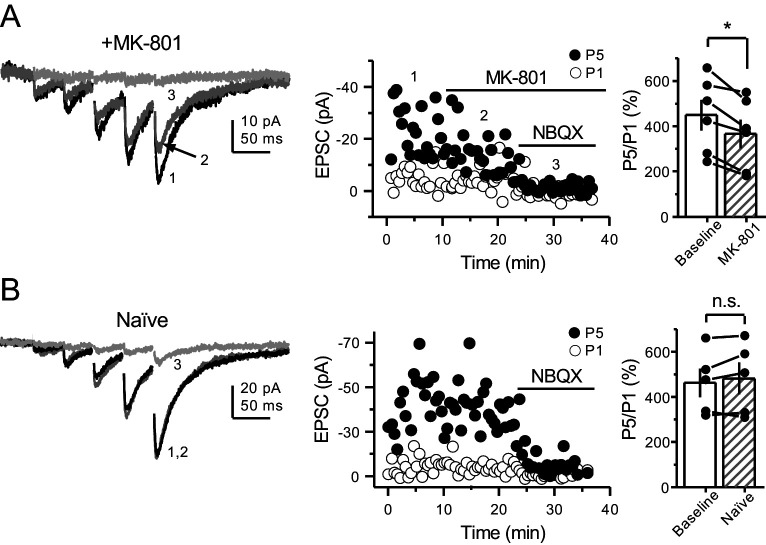
Figure 4—figure supplement 1. PreNMDARs contribute to burst-induced facilitation in more physiological conditions: 1.2 mM Mg+2, 1.2 mM Ca+2 and 35°C.
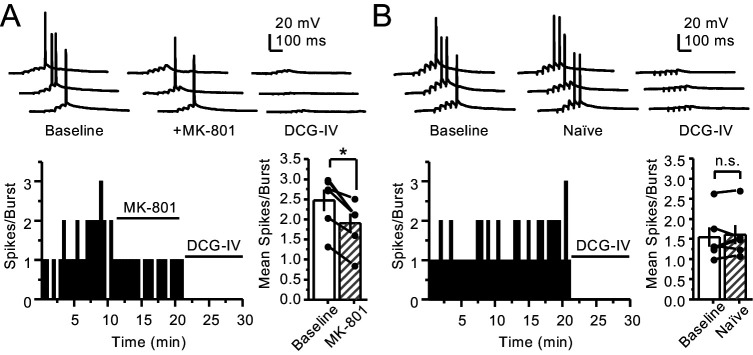
Figure 4—figure supplement 2. PreNMDARs contribute to action potential firing elicited by AMPAR-mediated transmission.
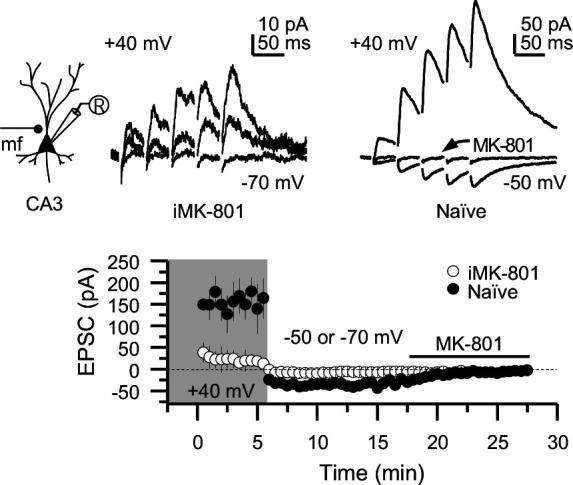
Figure 4—figure supplement 3. Intracellular MK-801 effectively blocked postsynaptic NMDARs elicited by burst stimulation (5 pulses at 25 Hz).