Abstract
Background
There is already extensive literature on the natural history of hypertensive heart disease (HHD) and aortic stenosis (AS). Once these patients develop severe left ventricular systolic dysfunction (LVSD) despite guideline-directed therapy for heart failure (HF), it is often thought to be end-stage from irreversible adverse remodelling. Our case series challenges this traditional paradigm. A more holistic model that factors in the interactions between the ventricle and vasculature is required. Based on a novel hypothetical concept of myocardial fatigue, we propose that occasionally LVSD is not an inherent myocardial or valvular disease but a consequence of an arterial afterload mismatch. By addressing this, the ventricle may recover and contract efficiently in unison with the arterial system.
Case summary
We present two cases of severe LVSD in a young lady with long-standing essential hypertension and a gentleman with stable severe AS. Both patients were already established on HF medications. After optimizing their blood pressure control, repeat echocardiography revealed normalization of left ventricular ejection fraction within 3 months, along with a demonstrable improvement in ventricular–arterial coupling and for AS, a reduction in valvular-arterial impedance.
Discussion
Just as Frank–Starling’s law was discovered by initially drawing analogies to skeletal muscle behaviour, it is biologically plausible that cardiac fatigue can occur in the setting of afterload mismatch. The chance of recovery rests upon early recognition before it transitions to irreversible myocardial damage. Only by testing new emerging theories of HF can we galvanize original research and find new avenues to understanding this complex syndrome.
Keywords: Case report, Myocardial fatigue, Heart failure, Afterload
Learning points
A proposed mechanism to hypertensive and valvular heart failure with reduced ejection fraction is not only to focus on the effect of the ventricle on the arterial circuit but also the effects of the vasculature on the ventricle; in that a markedly increased and protracted arterial resistance can impede myocardial performance.
Intrinsic contractile dysfunction of cardiomyocytes may only be temporary because of excessive opposing forces during systole before any irreversible damage to its contractile properties occurs. An exciting part of this thinking is that if this mechanism were true, left ventricular systolic function in these patients should recover fully if arterial resistance could be tackled effectively.
Introduction
This case series challenges the common notion that left ventricular systolic dysfunction (LVSD) defines the terminal phase in the natural history of hypertensive heart disease (HHD) or severe aortic stenosis (AS). The classical paradigm shared by both involves an initial adaptive left ventricular hypertrophy (LVH) to minimize wall stress, followed by maladaptive remodelling from progressive cardiomyocyte loss and fibrosis, before an eventual decline in contractility leading to heart failure with reduced ejection fraction (HFrEF).1,2 Alternatively proposed is the second-hit theory whereby an interval injury such as myocardial infarction accelerates this transition to HFrEF.3 However, the underlying problem may not always be a primary cardiac pathology but a mechanical inefficiency of an otherwise viable ventricle to effectively contract against an excessive afterload.4 This inverse relationship between afterload and myocardial performance has been a century-old concept but it remains unclear whether this phenomenon relates to the cardiomyocytes’ load-dependent shortening or intrinsic contractile properties.5 Not least, it is clinically difficult to distinguish. Consequently, when severe LVSD occurs, clinicians often treat the conspicuous problem e.g. implement guideline-directed medical therapies (GDMTs) for HFrEF or refer for aortic valve replacement (AVR) but may overlook the less conspicuous arterial afterload as a potential cause.
Based on the interdependent circuit connecting left ventricle (LV) with aortic valve, root and arterial tree, several indices of this relationship have been established. In AS with systemic hypertension, LV faces a double afterload at the valve level from stenosis and arterial level from reduced compliance.6 This may explain why some patients with moderate AS are significantly symptomatic while some have low-flow severe AS despite preserved ejection fraction (EF).2 Valvular-arterial impedance (Zva) represents this global haemodynamic load with >3.5 mmHg/mL/m2 suggesting a significantly increased total afterload.6 In terms of systemic arterial stiffness, this is characterized by arterial elastance (Ea), which is a ratio of end-systolic pressure (ESP) to stroke volume (SV). A high elastance indicates a high ESP per SV change.7 Left ventricular elastance (Ees) is a reasonably load-independent index of contractility, which can be derived invasively from a family of pressure–volume loops at end-systole across different loads or from echocardiography using a single-beat technique.8,9 To achieve maximum cardiac efficiency, LV and systemic vasculature must work in unison, characterized by a ratio termed ventricular–arterial coupling (VAC). An Ea/Ees ratio of 0.7–1.0 indicates maximum stroke work while >1 indicates ineffective coupling.9,10 Brachial arterial blood pressure (BP) is a gross representation of these afterload parameters but is not without its limitations.9
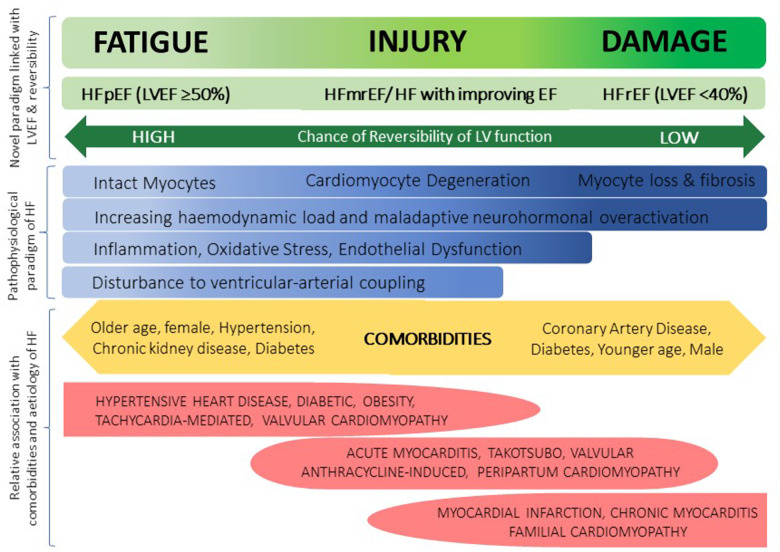
The current heart failure (HF) classification by LV ejection fraction (LVEF) does not capture these ventricular–arterial interactions or other pathophysiological models of HF; hence a novel perspective is demanded. Heart failure is a continuous spectrum as reflected by the intermediate phenotypes ‘HF with mid-range EF’ or ‘HF with improving EF’.10 A scattered plethora of mechanisms have been proposed including neurohormonal over-activation, inflammation and endothelial dysfunction but so far no unifying model exists.11 We propose a complementary framework of myocardial fatigue, injury, and damage discussed in detail elsewhere that reconciles LVEF classification with the physiological derangements across the HF spectrum (Figure 1).12 Just as Frank–Starling’s law emerged by originally drawing analogies from skeletal muscle behaviour, a similar analogy can be used to support the concept of myocardial fatigue in the face of arterial resistance. For example, by pushing against a boulder for a prolonged duration, muscle fatigue occurs and work diminishes over time; yet the muscle remains intact and recovers once the pathological load is removed. If myocardial fatigue persists, injury ensues followed by damage where the degree of damage predicts the chance of reversibility. The two following cases are cited in support of this hypothesis.
Figure 1.
Proposed spectral and descriptive model of myocardial fatigue, injury, and damage which reconciles with the left ventricular ejection fraction classification and pathophysiological and mechanical derangements underlying different aetiologies of heart failure and informs on the degree of potential myocardial recovery.
Timeline
| Time | Events |
|---|---|
| Patient 1 | |
| First admission with decompensated heart failure (HF) (Impaired LV) |
Grade 3 Hypertension. Cardiac magnetic resonance imaging showed severe left ventricular systolic dysfunction (LVSD) [left ventricular ejection fraction (LVEF) 14%]. On Spironolactone 12.5 mg once daily and maximal dose of Ramipril and Bisoprolol. Added furosemide. |
| Follow-up 3 months after discharge | Blood pressure (BP) 180/110. Severe LVSD on echocardiogram. Added hydralazine 25 mg thrice daily to control BP. |
| One month later in cardiac transplant clinic (improved LV) |
BP 126/76. Repeat echocardiogram: normalization of LVEF with good radial contractility seen. Discharged from cardiac transplant clinic. |
| Second decompensated HF 1 year later. |
BP 172/120. On above medications and bumetanide 3 mg twice daily. Increased hydralazine and spironolactone. |
| Follow-up in 1 month (Impaired LV) | Non-compliant with hydralazine TDS. Blood pressure uncontrolled. Echocardiogram: Severe LVSD, LVEF <35%. |
| Follow-up in 6 months (Improved LV) | Blood pressure 133/86 after addressing compliance with hydralazine. Echocardiogram: Non-dilated LV, mild LVSD. Left ventricular ejection fraction 47%. |
| Third decompensated HF one year later |
Blood pressure 180/130. NYHA III breathlessness. Echocardiogram- severe LVSD. Already on 6 months of Sacubitril/Valsartan 97/103 mg twice daily. |
| Follow-up in 1 year | Blood pressure 190/110. Still severe LVSD. Remains on Sacubitril/Valsartan 97/103 mg twice daily. |
| Patient 2 | |
| Initial diagnosis of aortic stenosis (AS) | 2012 echocardiogram showed mild degenerative AS and preserved LVEF. |
| Referral to HF clinic 6 years later |
Outpatient echocardiogram showed severe LVSD (LVEF 29%) and likely low-flow low-gradient severe AS. Already on maximal dose of bisoprolol and losartan. Seen by HF specialist who noted BP 156/111 in clinic. Started on hydralazine 25 mg twice daily. |
| Follow-up in 3 months |
Blood pressure 106/68. Exercise tolerance improved. Echocardiogram showing resolution of LVEF 60% and dobutamine stress echo confirmed severe AS. |
| Follow-up in 6 months |
Remained asymptomatic from severe AS. No changes to medication regimen. |
| Follow-up in 9 months (18 months since dobutamine stress echocardiography) | Developed exertional breathlessness. Echo showed LVEF 60%. Blood pressure controlled. Underwent successful transcatheter aortic valve implantation. |
Case presentation
Case 1: Fluctuating left ventricular systolic dysfunction in response to arterial blood pressure
A 33-year-old lady with a background of essential hypertension was admitted with New York Heart Association Class (NYHA) IV breathlessness attributed to de novo acute HF and severe LVSD. In the preceding 5 years, extensive investigations by nephrology and endocrinology specialists had excluded secondary causes including phaeochromocytoma, renovascular, and renal parenchymal disease. Body mass index was 33 kg/m2 but the STOP-Bang screen for obstructive sleep apnoea proved negative.
Echocardiogram revealed a mildly dilated LV with mild posterior LVH (septum 1.1 cm, posterior wall 1.5 cm, indexed LV mass 129 g/m2), LVEF 18% and indexed SV (SVi) 11mLs/m2. Using single-beat analysis for non-invasive estimations of Ea and Ees, net arterial load was found to be significantly raised (Ea 7.0 mmHg/mL; normal range 2.2 ± 0.8 mmHg/mL) in excess of a normal index of contractility (Ees 2.7 mmHg/mL; normal range 2.3 ± 1.0 mmHg/mL) giving a VAC of 2.6. This indicated considerable inefficiency of the ventricle working against an opposing afterload. Cardiac MRI confirmed HHD with LVEF 14% after excluding infarction, infiltration, myocarditis and coarctation of aorta. Coronary angiography demonstrated normal coronary arteries.
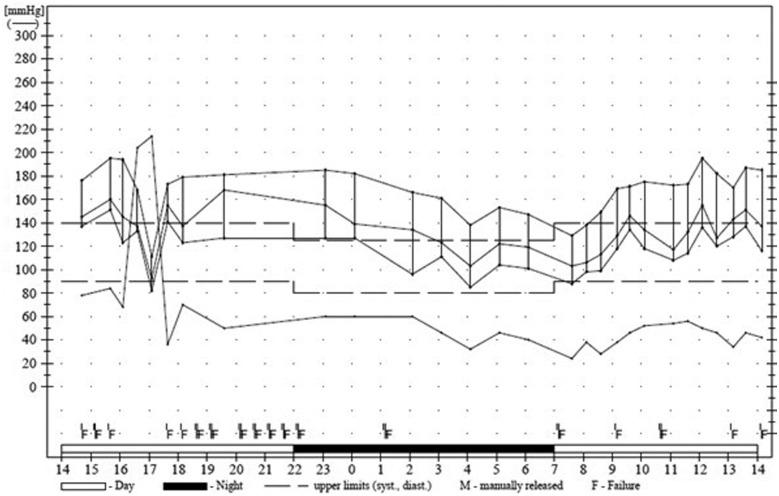
Before the diagnosis of HFrEF, she was already on spironolactone, maximum doses of bisoprolol and ramipril for the preceding 2 years. Despite this, she had grade 3 hypertension, confirmed on 24-h ambulatory monitor (Figure 2). With no evidence of interval injury, the HF team suspected a case of end-stage HHD prompting an urgent referral for cardiac transplant assessment. Hydralazine was concurrently added to optimize BP.
Figure 2.
Twenty-four-hour ambulatory blood pressure monitor showing average blood pressure 180/110.
At the transplant clinic, BP improved to 126/76 and echocardiogram revealed normalization of LV systolic function associated with better exercise tolerance. She was therefore discharged from the transplant service. As presented in the timeline, subsequent BP escalations were associated with profound deteriorations in clinical and LV systolic function. When she became more compliant with the evening hydralazine doses, a reasonable BP 133/86 was achieved and echocardiogram showed a mildly impaired LV systolic function, LVEF 48%. Calculated Ea was 2.9 mmHg/mL, Ees 3.0 mmHg/mL with VAC ratio 0.97 indicating a closely matched elastance for greater ventricular efficiency.
Unfortunately, on recent follow-ups, she reported NYHA III breathlessness and was found again to have uncontrolled hypertension with severe LVSD. Despite 6 months of Sacubitril/Valsartan 97/103 mg BD, LV remained poor in the face of uncontrolled hypertension.
Case 2: Resolved left ventricular systolic dysfunction with blood pressure control in severe aortic stenosis
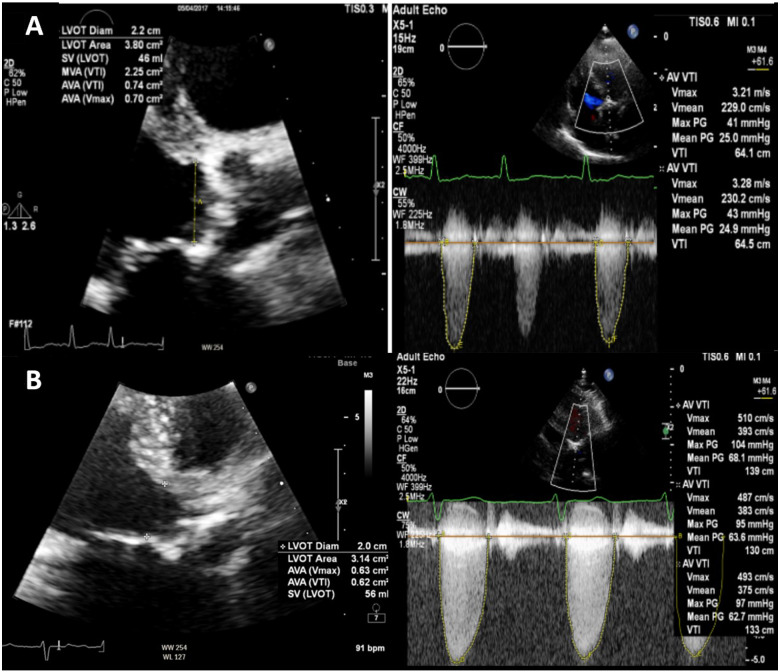
An 86-year-old gentleman was referred to the HF clinic with NYHA III breathlessness. He was known to have AS on a background of hypertension, diabetes, and coronary artery bypass grafting in 1998. Prior to referral, he was established on maximal doses of bisoprolol and losartan, and furosemide. N-terminal prohormone of brain natriuretic peptide was 1719 pmol/L. Compared with his baseline echocardiogram, repeat scan revealed new severe LVSD, LVEF 29%, SVi 23 mL/m2 with mild concentric LVH and low-flow low-gradient severe AS (mean gradient 25 mmHg, peak gradient 41 mmHg, aortic valve area 0.7 dimensionless valve index 0.2).(Figure 3A). There was no evidence of aortic coarctation. On review, BP was 156/111 and heart rate 86 b.p.m. To optimize BP control, hydralazine 25 mg twice daily was initiated.
Figure 3.
Transthoracic echocardiogram showing appearances of severe aortic stenosis in parasternal long-axis view and corresponding aortic valve Doppler’s at time of severe left ventricular systolic dysfunction (A row) and normal left ventricular systolic function during dobutamine stress echocardiography (B row).
Coronary angiography showed patent grafts without de novo lesions and no signs of tachyarrhythmia were found on Holter monitoring, excluding a second-hit injury. Dobutamine stress echocardiography (DSE) was arranged to assess for contractile reserve before potential transcatheter aortic valve implantation (TAVI). He returned a BP diary over 3 months, which showed significantly better control and reported improved exercise tolerance.
On the day of DSE, BP was 106/68. Resting echocardiogram (before dobutamine infusion) showed resolution of LVSD with an LVEF 60% and SVi 52.9mLs/m2. Dobutamine stress echocardiography excluded regional wall motion abnormalities on peak stress and recovery, and reaffirmed severe AS (mean gradient 68 mmHg, peak 104 mmHg, aortic valve area 0.7 cm2) (Figure 3B). Using steady data from echocardiography, VAC normalized to 0.99 (Ea 1.28 mmHg/mL over Ees 1.29 mmHg/mL) from an initial inefficient VAC 1.62 (Ea 3.42 mmHg/mL over Ees 2.11 mmHg/mL). Global haemodynamic load, characterized by ZVa, was calculated by dividing the estimated LV systolic pressure (systolic arterial pressure + mean transvalvular pressure) by SVi. At the time of severe LVSD, ZVa was significantly raised at 6.18 mmHg/mL/m2 indicating a high global impedance opposing ventricular ejection, absorbing most of the mechanical energy developed by the LV.6 On BP control, this improved to 3.3 mmHg/mL/m2. Accordingly, as symptoms and LV function improved, AVR was not required until 18 months later when he developed exertional breathlessness. Blood pressure remained well-controlled and echocardiogram showed LVEF 60%. He finally underwent a successful TAVI.
Discussion
This case series provides a unique perspective on the mechanisms behind reversible HFrEF and reminds us that in HF, the mechanical pump should be examined mutually with the arterial vasculature rather than in isolation. As demonstrated in both cases, a systolic BP 150 may not appear very high but can be detrimental to the LV. Consequently, modest BP rises can lead to exaggerated LV ESP which in turn increases myocardial oxygen consumption and energy costs that do not translate to adequate mechanical work, which corresponds with the description of muscle fatigue.7
By controlling BP through principles of ZVa, VAC, and excluding interval myocardial damage, we suggest that LV systolic function can be restored (independent of neurohormonal therapies) without having to resort to potentially harmful and premature valve replacement or cardiac transplant. Ventricular–arterial coupling is a central determinant of net cardiovascular performance and the European Society of Cardiology HF Association (HFA) consensus document on VAC has outlined strategies on restoring this.9 It recommends a common therapeutic intervention for both AS and hypertension-related HFrEF in using anti-hypertensive drugs that predominantly reduce arterial wall stiffness and central afterload, namely angiotensin-converting enzyme inhibitors, angiotensin-receptor blocker, and nitrates over beta-blockers. Importantly, it points out that VAC can potentially worsen post-TAVI if co-existing hypertension is not controlled since the acute rise in SV immediately after TAVI can disproportionately increase aortic BP and in turn, impose excessive afterload on the LV. However, the guidance does not mention hydralazine, which is a potent direct arterial vasodilator that benefitted both cases, which did not appear to respond to GDMTs including Sacubitril/Valsartan.
The two cases are on either extreme of age and gender. Such differences exhibit both similarities and variances on the LV’s response to load and likelihood of developing HF, not least we know that old age and female sex are independent risk factors of HFpEF.13 Ventricular–arterial coupling is similar based on age or gender at rest but during exercise, a steeper rise in Ea was observed in hypertensive women compared with age-matched hypertensive men.4 Another study found younger HFpEF patients to have similar VAC to stable hypertensive elderly at rest but with exercise, higher VAC was seen in the younger group.13 Furthermore, these studies have suggested that elderly healthy individuals with adequate contractile reserve develop age-related arterial stiffness (i.e. increased Ea) but have preserved VAC ratio due to a proportionate Ees rise. Such observations provide some insight into why the young lady with long-standing hypertension developed HFrEF earlier than the elderly man with hypertension who developed HFrEF much later only when additional load was imposed from progressive AS. Both cases have various rates of acceleration to myocardial fatigue and the chances of myocardial recovery thus depend on early recognition.
Still, it remains a challenge to promptly recognize and predict the possibility of reversibility in individual cases of severe LVSD. A cohort study of HHD patients with LVSD found that BP control was an independent predictor of LVEF normalization over a long median follow-up of 41 months and found that these patients tended to be younger with a shorter history of hypertension.14 One case report of HHD with fully reversible severe LVSD after 2 months of optimizing BP suggested that this was a global variant of hypertensive-induced Takotsubo cardiomyopathy—perhaps acute afterload acted as the stressor. The authors acknowledged that current literature on this is limited.15 In AS, LV systolic function can begin to deteriorate even when AS is moderate in the absence of coronary artery disease or a second-hit injury.2 Clearly, the prevailing concept that severe LVSD in AS is primarily a consequence of the valve is not entirely true. Aortic stenosis is not simply a valve but systemic disease with myocardial and vascular components that exert a global haemodynamic demand on the LV as illustrated in Case 2. Perhaps this diffuse effect on a strained and fatigued ventricle may be seen on cardiac imaging. Cardiac magnetic resonance T1 mapping quantifies diffuse myocardial tissue injury and can help to differentiate focal fibrosis which corresponds to myocyte loss from diffuse fibrosis which suggests potential myocardial recovery. This is supported by a study that found after a year post-AVR, focal fibrosis persisted while diffuse fibrosis regressed with slight recovery in LV function.16
These observations support the biologically plausible framework of myocardial fatigue, which arises when the LV struggles to pump into a high-pressure vascular circuit. It lies at the beginning of a spectral transition to myocardial injury followed by irreversible damage (Figure 1). The recognition of chronically elevated troponin has led to a recognition of myocardial injury and reduced contractile reserve in hypertensive HFpEF patients from a small prospective study,17 which fits well with our proposed paradigm. In fact, the idea of myocardial fatigue was first mentioned 50 years ago in an animal experiment that demonstrated gradual reduction in LV contractions after intermittent clamping of the ascending aorta over time.18 However, due to technological limitations, it was unable to establish whether this was a problem with intrinsic myocardial contractility, load-dependent myocyte shortening or external influences of the neurohormonal system.
The limitation of using brachial BP is a crude way to characterize the complex components of arterial afterload and its responses on the LV. It would have been ideal to obtain an invasive pressure–volume analysis before and after intervention to accurately document the load-dependent effects on cardiac function under physiological and pathological conditions. This would also elucidate other factors that influence end-systolic wall stress (ESWS) such as LV geometry (based on the Law of LaPlace) and systemic vascular resistance (SVR). Just as VAC and ZVa can be estimated non-invasively, well-validated methods to non-invasively calculate ESWS19 and SVR,20 which correlate well with invasive measures, are applied to both cases (Table 1). Both parameters are seen to reduce significantly after better BP control but interesting differences are seen. Case 1 after intervention had normalized VAC ratio but even though SVR had reduced, it remained high which may partially explain why LV systolic function did not completely normalize. In contrast, Case 2 had normalized SVR when normotension was achieved but with the persistent severe AS, a high ESWS is still present. It is clear from this case series that non-invasive measurements, albeit not as accurate as invasive references, can still provide useful clinical haemodynamic data.
Table 1.
Changes in end-systolic wall stress and systemic vascular resistance in Cases 1 and 2 before and after blood pressure control
| Cases | ESWS (kdynes/cm2)a | SVR (dynes/sec/cm5)b |
|---|---|---|
| Case 1 (severe LVSD + HTN) | 206 | 5435 |
| Case 1 (mild LVSD + better BP control) | 133 | 2833 |
| Case 2 (severe LVSD + HTN + severe AS) | 260 | 2581 |
| Case 2 (normal LVSF + normotensive + severe AS) | 195 | 1111 |
AS, aortic stenosis; BP, blood pressure; ESWS, end-systolic wall stress; HTN, hypertension; LVSD, left ventricular systolic dysfunction; LVSF, left ventricular systolic function; SVR, systemic vascular resistance.
End-systolic left ventricular wall stress calculated non-invasively as (0.334 × Systolic BP + mean pressure gradient) × (LV end-systolic diameter)/posterior wall thickness [1 + posterior wall thickness/LV end-systolic diameter].
Systemic vascular resistance calculated non-invasively as mean arterial pressure—RA pressure (based on IVC collapsibility)/cardiac output multiplied by 80 (Porter 2015) with normal ranges between 800 and 1500 dynes/s/cm5.
Conclusion
This case series reminds us that in HF, the LV should be examined mutually with the vascular circuit rather than in isolation, applying well-established notions of VAC, ZVa, and other components of afterload such as SVR and wall stress. Based on this biomechanical model, it proposes a novel concept of myocardial fatigue within both cases. We hope this case series galvanizes the research community to revisit the hypothesis of myocardial fatigue and attest this with modern state-of-the-art tools such as force-length work-loop models on human cardiomyocytes to add another piece to the puzzle of HF syndrome.
Lead author biography

Dr Patrick Tran is a cardiology specialist registrar with an interest in heart failure and cardiovascular physiology. He is currently studying a PhD into the biomechanical and load-bearing behaviour of human cardiomyocytes using a cardiac contractility model.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Sorrentino MJ. The evolution from hypertension to heart failure. Heart Fail Clin 2019;15:447–453. [DOI] [PubMed] [Google Scholar]
- 2. Carabello BA. Aortic stenosis: from pressure overload to heart failure. Heart Fail Clin 2006;2:435–442. [DOI] [PubMed] [Google Scholar]
- 3. Rame JE, Ramilo M, Spencer N, Blewett C, Mehta SK, Dries DL. et al. Development of a depressed left ventricular ejection fraction in patients with left ventricular hypertrophy and a normal ejection fraction. Am J Cardiol 2004;93:234–237. [DOI] [PubMed] [Google Scholar]
- 4. Shim CY, Hong GR, Ha JW.. Ventricular stiffness and ventricular-arterial coupling in heart failure: what is it, how to assess, and why? Heart Fail Clin 2019;15:267–274. [DOI] [PubMed] [Google Scholar]
- 5. Sonnenblick E, Downing S.. Afterload as a primary determinat of ventricular performance. Am J Physiol 1963;204:604–610. [DOI] [PubMed] [Google Scholar]
- 6. Levy F, Luc Monin J, Rusinaru D, Petit-Eisenmann H, Lelguen C, Chauvel C. et al. Valvuloarterial impedance does not improve risk stratification in low-ejection fraction, low-gradient aortic stenosis: results from a multicentre study. Eur J Echocardiogr 2011;12:358–363. [DOI] [PubMed] [Google Scholar]
- 7. Chantler PD, Lakatta EG, Najjar SS.. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol 2008;105:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA. et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 2001;38:2028–2034. [DOI] [PubMed] [Google Scholar]
- 9. Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA. et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail 2019;21:402–424. [DOI] [PubMed] [Google Scholar]
- 10. Srivastava PK, Hsu JJ, Ziaeian B, Fonarow GC.. Heart failure with mid-range ejection fraction. Curr Heart Fail Rep 2020;17:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mann DL, Bristow MR.. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 2005;111:2837–2849. [DOI] [PubMed] [Google Scholar]
- 12. Banerjee P. Heart failure: a story of damage, fatigue and injury? Open Heart 2017;4:e000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phan TT, Shivu GN, Abozguia K, Sanderson JE, Frenneaux M.. The pathophysiology of heart failure with preserved ejection fraction: from molecular mechanisms to exercise haemodynamics. Int J Cardiol 2012;158:337–343. [DOI] [PubMed] [Google Scholar]
- 14. Anguita Sánchez M, Rodríguez Esteban M, Ojeda Pineda S, Ruiz Ortiz M, Romo Peña E, Mesa Rubio D. et al. [Clinical outcome and reversibility of systolic dysfunction in patients with dilated cardiomyopathy due to hypertension and chronic heart failure]. Rev Esp Cardiol 2004;57:834–841. [PubMed] [Google Scholar]
- 15. Chockalingam A, Kumar SA.. Reversible cardiac dysfunction in long-standing hypertension may be global variant of stress cardiomyopathy. BMJ Case Rep 2018;2018:bcr2018225044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN. et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol 2018;71:860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P. et al. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bakli NM, Meerson FZ, Pogosian LA. et al. The effect of nucleosides, strophanthin and combinations of these factors on the development of the process of fatigue in the myocardium. Biull Eksp Bio Med 1964;57:27–31. [PubMed] [Google Scholar]
- 19. Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, Augeul L. et al. Influence of afterload on left ventricular radial and longitudinal systolic functions: a two-dimensional strain imaging study. Eur J Echocardiogr 2009;10:914–921. [DOI] [PubMed] [Google Scholar]
- 20. Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ. et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 2015;28:40–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.