Abstract
Background
A ‘catecholamine storm’ in a case of pheochromocytoma can lead to a transient left ventricular dysfunction similar to Takotsubo cardiomyopathy. A cardiogenic shock can thus develop, with high left ventricular end-diastolic pressure and a reduction in coronary perfusion pressure. This scenario can ultimately lead to a cardiac arrest, in which unloading the left ventricle with a peripheral left ventricular assist device (Impella®) could help in achieving the return of spontaneous circulation (ROSC).
Case summary
A patient affected by Takotsubo cardiomyopathy caused by a pheochromocytoma presented with cardiogenic shock that finally evolved into refractory cardiac arrest. Cardiopulmonary resuscitation was performed but ROSC was achieved only after Impella® placement.
Discussion
In the clinical scenario of Takotsubo cardiomyopathy due to pheochromocytoma, when cardiogenic shock develops treatment is difficult because exogenous catecholamines, required to maintain organ perfusion, could exacerbate hypertension and deteriorate the cardiomyopathy. Moreover, as the coronary perfusion pressure is critically reduced, refractory cardiac arrest could develop. Although veno-arterial extra-corporeal membrane oxygenation (va-ECMO) has been advocated as the treatment of choice for in-hospital refractory cardiac arrest, in the presence of left ventricular overload a device like Impella®, which carries fewer complications as compared to ECMO, could be effective in obtaining the ROSC by unloading the left ventricle.
Keywords: Cardiogenic shock, Cardiac arrest, Cardiac assist devices, Takotsubo cardiomyopathy, Pheochromocytoma, Case report
Learning points
Pheochromocytoma can induce Takotsubo cardiomyopathy and cardiogenic shock which can be difficult to treat pharmacologically because of a ‘catecholamine storm’.
Refractory cardiac arrest can develop in the setting of cardiogenic shock when coronary pulse pressure is critically reduced.
Impella® unloads the left ventricle and can help in achieving return of spontaneous circulation when cardiac arrest occurs in the setting of cardiogenic shock.
Introduction
Pheochromocytoma, a neuroendocrine tumour derived from enterochromaffin cells of the adrenal gland, may lead to a ‘catecholamine storm’ that could lead to a transient left ventricular dysfunction similar to Takotsubo cardiomyopathy.1 In this setting, the cardiogenic shock could develop with high left ventricular end-diastolic pressure (LVEDP) and a reduction of coronary perfusion pressure, ultimately leading to cardiac arrest. Based on the mechanism of cardiac arrest, unloading of the left ventricle could result in the achievement of a return of spontaneous circulation (ROSC).
Timeline
| Day 0 | Epigastric pain after consuming Levosulpiride, followed by haematemesis |
| Day 0, 1 h | Emergency Department: pH 6.8, PaO2/FiO2 150 mmHg requiring endotracheal intubation; electrocardiogram: sinus tachycardia, ST-segment elevation in anterior and lateral leads; transthoracic echocardiography: akinesia of all middle and apical segments of the left ventricle, left ventricular ejection fraction (LVEF) 20% |
| Day 0, 2 h | Thoracic and abdominal computed tomography scan showing a mass (5 cm) of the left adrenal gland (suggestive of a pheochromocytoma) |
| Day 0, 3 h | Coronary angiography: no evidence of relevant coronary artery disease; left ventricular angiography showing apical ballooning with severe reduction in LV function (LVEF 15%) |
| Day 0, 4 h | Cardiac arrest after Labetalol administration: ventricular fibrillation, CPR, DC-shock 6×, pulseless electrical activity; implantation of Impella CP®, high dose of epinephrine intravenous (i.v.) infusion |
| Day 1 | Tapering and end of epinephrine i.v. infusion |
| Day 2 | Introduction of Doxazosine, Metoprolol, and Amlodipine |
| Day 3 | Introduction of i.v. Urapidil due to resistant hypertension |
| Day 4 | Improved LV function (LVEF 45%), removal of Impella CP® |
| Day 33 | Pheochromocytoma confirmed by significantly increased urinary concentration of metanephrine and normetanephrine |
| Day 42 | Left sided laparoscopic adrenalectomy, uncomplicated |
| Day 46 | Patient’s discharge |
Case presentation
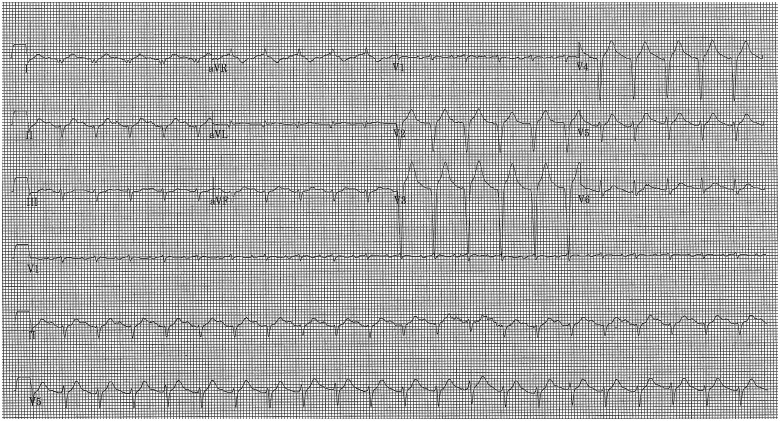
A 41-year-old man with no known previous significant medical history was seen in our Emergency Department (ED) with epigastric pain, haematemesis, and confusion arisen after having consumed Levosulpiride for a mild upper gastrointestinal discomfort. In the ED, the patient’s condition rapidly worsened with dyspnoea and oxygen desaturation, consistent with rales at physical examination. Moreover, despite a blood pressure as high as 163/127 mmHg the patient presented with signs of peripheral vasoconstriction. An arterial blood gas test showed respiratory acidosis (pH 6.8) and a PaO2/FiO2 ratio of 150 mmHg. In the view of this, the patient underwent endotracheal intubation and ventilation with elevated support pressure. An ECG showed sinus tachycardia (130 b.p.m.) with ST-segment elevation in anterior and lateral leads (Figure 1); a transthoracic echocardiography showed akinesia of all middle and apical segments of the left ventricle, with severe reduction of left ventricular ejection fraction (LVEF) (20%), moderate mitral regurgitation, and normal right ventricular function. Due to haematemesis at presentation, the patient underwent a thoracic and abdominal computed tomography scan, that did not demonstrate active pulmonary or gastric bleeding, but it demonstrated a mass in the left adrenal gland, suggestive of a pheochromocytoma.
Figure 1.
Electrocardiogram at admission.
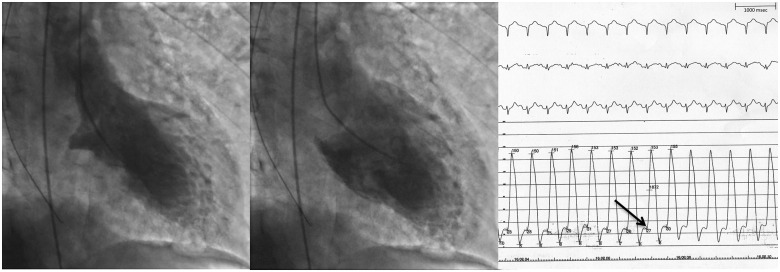
The patient underwent an emergency coronary angiography that showed normal coronary arteries (Video 1, right coronary artery angiography; Videos 2 and 3, left coronary artery angiography), and a left ventricular angiography which showed apical ballooning with severe reduction of LVEF due to akinesia of all middle and apical segments (Figure 2A,B, Supplementary material online, Video S1). Of note, LVEDP was very high (28 mmHg) (Figure 2C) and systolic pressure was the same at the level of both the left ventricular apex and the aorta (153 mmHg), thus excluding left ventricular outflow tract obstruction.
Figure 2.
(A) Left ventricular angiography, systole; (B) left ventricular angiography, diastole; (C) electrocardiogram (upper level) and left ventricular pressure recording (lower level), time scale on top right. Arrow: left ventricular end-diastolic pressure.
After careful evaluation of the clinical condition, a low dose of intravenous (i.v.) Labetalol (10 mg) was administered to the patient to treat the adrenergic crisis; however, the drug was administered while the patient was in the catheterization laboratory to provide immediate mechanical circulatory support if needed. Blood pressure showed a minor decline in the next 10 min, but showed a sudden and precipitous drop to 50/20 mmHg within the following 2 min and was refractory to i.v. Epinephrine. Subsequently, ventricular fibrillation (VF) occurred; chest compressions were commenced immediately along with six direct current (DC)-shocks being administered with a defibrillator in due time. In addition, intravenous bolus doses of Epinephrine and Amiodarone in keeping with advanced cardiac support protocol were administered. Eventually, wide QRS pulseless electrical activity (PEA) developed.
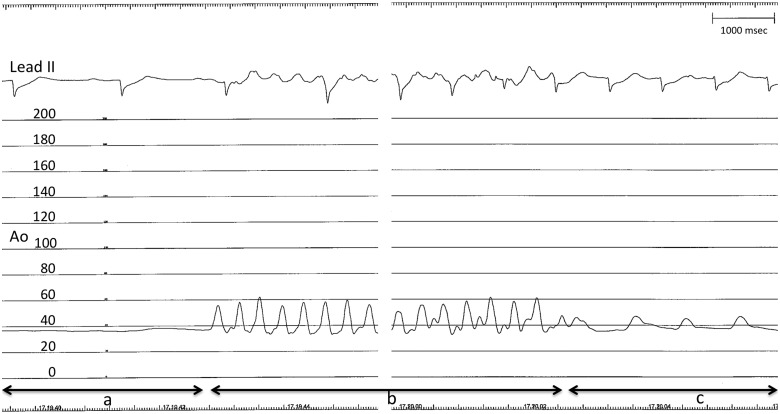
With almost uninterrupted chest compressions, a micro-axial percutaneous left ventricular assist device (pLVAD), Impella CP®, was implanted via the left femoral artery to unload the left ventricle and promote left ventricular output (Supplementary material online, Video S2: Impella® placement into the left ventricle, please note the absence of heart contraction). As the device was placed in the left ventricle, ROSC was achieved, as demonstrated by immediate pulse pressure restoration (Figure 3A–C; Supplementary material online, Video S3: please note the present albeit severely impaired heart contraction). In less than a minute, supply of circulatory support up to 3 L/min was established (low flow total time 27 min), with gradual improvement in blood pressure. The respiratory status also gradually improved, allowing reduction of support pressure. Post-ROSC care included temperature management with a target temperature of 36°C, maintained for 24 h.
Figure 3.
Electrocardiogram lead II (upper level) and aortic pressure recording (lower level, mmHg) during Impella® placement. Segment a: absence of pulse pressure while pushing the device into the left ventricle. Segment b: pulse pressure during cardiopulmonary resuscitation (CPR) (please note chest compression artefact on electrocardiogram tracing). Segment c: pulse pressure restoration after Impella® activation. Time scale on top right (14 s of CPR recording have been omitted).
Tapering of epinephrine was successfully accomplished 24 h later. Thereafter, treatment with Doxazosine, Metoprolol, Amlodipine, and ultimately Urapidil was required in view of hypertension.
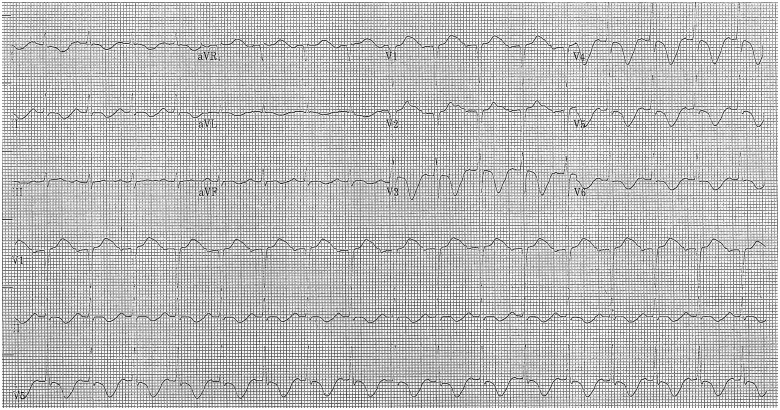
On the 4th day after admission, while the ECG showed deep negative T-waves in anterior and lateral leads and a long QT interval consistent with Takotsubo cardiomyopathy (Figure 4), an echocardiogram demonstrated an improvement of left ventricular function (LVEF 45%) and a reduction of mitral regurgitation. Thus, the Impella® device was removed.
Figure 4.
Electrocardiogram on Day 4 after admission.
The remainder of the patient’s stay in the hospital was complicated by transient renal failure and sepsis. A diagnosis of pheochromocytoma was confirmed by elevated metanephrine (18 306 μg/24 h, normal value 64–302 μg/24 h) and normetanephrine (3626 μg/24 h, normal value 110–527 μg/24 h) urinary concentration. On the 42nd day after admission, the patient, who was neurologically intact, underwent an uncomplicated left-sided laparoscopic adrenalectomy (histological examination confirmed the diagnosis of pheochromocytoma), and was discharged from the hospital 4 days later. Normal left ventricular function (left ventricular end-diastolic volume 78 mL/m2, LVEF 55%) was demonstrated at the 1-month follow-up echocardiogram.
Discussion
Takotsubo cardiomyopathy is defined as a transient left ventricular dysfunction (hypokinesia, akinesia, or dyskinesia) presenting as apical ballooning or mid-ventricular, basal, or focal wall motion abnormalities.2 Although the precise pathophysiological mechanisms of Takotsubo cardiomyopathy are incompletely understood, there is considerable evidence that sympathetic stimulation is central to its pathogenesis, mainly related to an emotionally or physically triggering event.2 Pheochromocytoma, a nor/epinephrine-secreting tumour derived from enterochromaffin cells of the adrenal gland, may lead to a ‘catecholamine storm’,1 causing both severe hypertension and Takotsubo cardiomyopathy. About 5% of all Takotsubo cardiomyopathy patients develop cardiogenic shock, which carries a high mortality rate.3
In cardiogenic shock due to Takotsubo cardiomyopathy, beta-blockers are contraindicated unless left ventricular outflow tract obstruction is present.4 In this case, however, the initial scenario was consistent with pheochromocytoma-induced adrenergic crisis. Thus, as suggested in literature,5 a combined alpha- and beta-adrenoceptor blocker (Labetalol) was administered to the patient. The subsequent drop in the patient’s blood pressure might be connected to this drug, but it seems unlikely considering the low dose (10 mg) administered to the patient. However, based on the severely reduced LV function, we decided to administer the medication while the patient was in the catheterization laboratory, thereby being prepared to deliver prompt mechanical support, if needed.
In the clinical scenario of Takotsubo cardiomyopathy due to pheochromocytoma, when cardiogenic shock develops, treatment is difficult because exogenous catecholamines, required to maintain organ perfusion, could exacerbate the hypertension and worsen the cardiomyopathy which could result in a further deterioration in the patient’s condition. While the intra-aortic balloon pump is widely used in patients with cardiogenic shock, provided there is no left ventricular outflow tract obstruction,6 it could not be used in this case, because of the development of relapsing VF. Conversely, mechanical circulatory support with a pLVAD (Impella®) associated with veno-arterial extra-corporeal membrane oxygenation (va-ECMO) has been recently proposed to provide haemodynamic stabilization and sufficient organ perfusion.7 In addition, with respect to mechanical support, va-ECMO has been advocated as the treatment of choice for in-hospital refractory cardiac arrest.8 The benefit of using Impella® in refractory cardiac arrest has so far been described in a single case report comprising eight participants.9 However, in that paper, most patients were affected by acute myocardial infarction and percutaneous coronary intervention was performed either before or after device insertion, thus it is not clear whether the mechanical support alone allowed the ROSC.9
From a pathophysiological point of view, the current case clearly shows the role of coronary perfusion pressure in determining cardiac arrest; at the same time, it is also the most important determinant in achieving ROSC.10,11 Refractory VF, and ultimately PEA, developed in this patient as soon as the diastolic blood pressure dropped under the LVEDP (about 20 mmHg vs. 28 mmHg), thus critically reducing the coronary perfusion pressure and probably leading to diffuse subendocardial ischaemia.
The assumption of a negative coronary perfusion pressure as the trigger of cardiac arrest explains why implantation of an Impella CP® alone was chosen to unload the left ventricle in addition to guaranteeing organ perfusion. The va-ECMO, in fact, increases cardiac afterload establishing a reverse blood flow in the ascending aorta:12 which, in this case, would have further increased the LVEDP.
It may be noteworthy that as soon as the Impella® device was correctly placed into the left ventricle, ROSC was achieved, as demonstrated by invasive pressure monitoring which showed restoration of pulse pressure. This could be explained by the fact that unloading of the ventricle re-established myocardial perfusion as a result of achieving an adequate coronary perfusion pressure.
Additionally, unloading of the ventricle, together with pharmacological reduction of blood pressure, resulted in a rapid recovery of ventricular function, leading to an LVEF of 45% in 3 days and consequently weaning off mechanical support in <4 days.
When comparing va-ECMO and Impella®, it should be noted that Impella® is associated with a lower risk of bleeding than va-ECMO, since it requires a smaller arterial access point (14 Fr vs. 15–17 Fr) and a lower anticoagulation regimen, and it does not require a large-bore venous access.13 Moreover, the time taken to correctly position Impella® is shorter than the same for ECMO; and in this case, the device was implanted minimizing chest compression interruption to <30 s overall i.e. while crossing the aortic valve with the guiding wire and, later, while pushing the device into the left ventricle.
Conclusion
Takotsubo cardiomyopathy could be caused by excessive production of endogenous catecholamines by a pheochromocytoma. In such a setting, if cardiogenic shock develops, mechanical support with a micro-axial pLVAD could significantly improve the patient’s outcome. Moreover, Impella® could be effective in supporting cardiopulmonary resuscitation whenever left ventricular overload and loss of coronary perfusion pressure play a relevant role as causes of cardiac arrest.
Lead author biography

Filippo Zilio graduated in Medicine and Surgery at the University of Padua in 2009, and he obtained a Postgraduate Diploma in Cardiology at the same University in 2015. Since 2016, he works in the Cardiology Department of Santa Chiara Hospital, Trento, as clinical and interventional cardiologist. He is author or co-author of articles on various cardiology topics published in national and international journals. He is co-chairperson of the Young Group of Italian Association of Hospital Cardiologists (Area Giovani ANMCO) for the period 2019–21.
Supplementary Material
Acknowledgements
The authors would like to thank the patient for agreeing to have information linked to the case published anonymously.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagege A, Amar L.. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart 2013;99:1438–1444. [DOI] [PubMed] [Google Scholar]
- 2. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ. et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E.. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–1529. [DOI] [PubMed] [Google Scholar]
- 4. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ. et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic workup, outcome, and management. Eur Heart J 2018;39:20472047–20472062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazza A, Armigliato M, Marzola MC, Schiavon L, Montemurro D, Vescovo G. et al. Anti-hypertensive treatment in pheochromocytoma and paraganglioma: current management and therapeutic features. Endocrine 2014;45:469–478. [DOI] [PubMed] [Google Scholar]
- 6. Sangen H, Imori Y, Tara S, Yamamoto T, Takano H, Shimizu W.. Haemodynamic deterioration due to intra-aortic balloon counterpulsation in takotsubo cardiomyopathy. Eur Heart J 2018;39:2118. [DOI] [PubMed] [Google Scholar]
- 7. Mierke J, Loehn T, Linke A, Ibrahim K.. Reverse takotsubo cardiomyopathy - life-threatening symptom of an incidental pheochromocytoma: a case report. Eur Heart J Case Rep 2019;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooks SC, Anderson ML, Bruder E, Daya MR, Gaffney A, Otto CW. et al. Part 6: Alternative techniques and ancillary devices for cardiopulmonary resuscitation. 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S436–S443. [DOI] [PubMed] [Google Scholar]
- 9. Vase H, Christensen S, Christiansen A, Therkelsen CJ, Christiansen EH, Eiskjær H. et al. The Impella CP device for acute mechanical circulatory support in refractory cardiac arrest. Resuscitation 2017;112:70–74. [DOI] [PubMed] [Google Scholar]
- 10. Sutton RM, Friess SH, Maltese MR, Naim MY, Bratinov G, Weiland TR. et al. Hemodynamic-directed cardiopulmonary resuscitation during in-hospital cardiac arrest. Resuscitation 2014;85:983–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA.. Myocardial perfusion pressure: a predictor of 24-h survival during prolonged cardiac arrest in dogs. Resuscitation 1988;16:241–250. [DOI] [PubMed] [Google Scholar]
- 12. Werdan K, Gielen S, Ebelt H, Hochman JS.. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014;35:156–167. [DOI] [PubMed] [Google Scholar]
- 13. Kar B, Basra SS, Shah NR, Loyalka P.. Percutaneous circulatory support in cardiogenic shock: interventional bridge to recovery. Circulation 2012;125:1809–1817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.