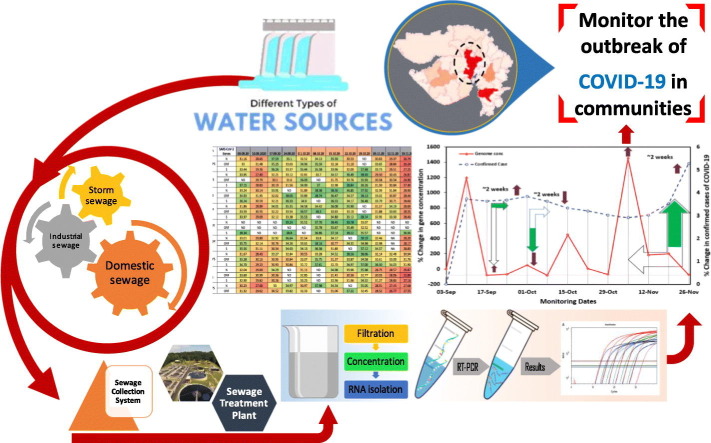
Abstract
Following the proven concept, capabilities, and limitations of detecting the RNA of Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) in wastewater, it is pertinent to understand the utility of wastewater surveillance data on various scale. In the present work, we put forward the first wastewater surveillance-based city zonation for effective COVID-19 pandemic preparedness. A three-month data of Surveillance of Wastewater for Early Epidemic Prediction (SWEEP) was generated for the world heritage city of Ahmedabad, Gujarat, India. In this expedition, 116 wastewater samples were analyzed to detect SARS-CoV-2 RNA, from September 3rd to November 26th, 2020. A total of 111 samples were detected with at least two out of three SARS-CoV-2 genes (N, ORF 1ab, and S). Monthly variation depicted a significant decline in all three gene copies in October compared to September 2020, followed by a sharp increment in November 2020. Correspondingly, the descending order of average effective gene concentration was: November (~10,729 copies/L) > September (~3047 copies/L) > October (~454 copies/L). Monthly variation of SARS-CoV-2 RNA in the wastewater samples may be ascribed to a decline of 20.48% in the total number of active cases in October 2020 and a rise of 1.82% in November 2020. Also, the monthly recovered new cases were found to be 16.61, 20.03, and 15.58% in September, October, and November 2020, respectively. The percentage change in the gene concentration was observed in the lead of 1–2 weeks with respect to the percentage change in the provisional figures of confirmed cases. SWEEP data-based city zonation was matched with the heat map of the overall COVID-19 infected population in Ahmedabad city, and month-wise effective gene concentration variations are shown on the map. The results expound on the potential of WBE surveillance of COVID-19 as a city zonation tool that can be meaningfully interpreted, predicted, and propagated for community preparedness through advanced identification of COVID-19 hotspots within a given city.
Keywords: Wastewater based epidemiology (WBE), COVID-19, SARS-CoV-2, Pandemic, Surveillance
Graphical abstract
1. Introduction
The contagious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the coronavirus pandemic, has infected 11 million people in India alone by February 22nd, 2021 (WHO, 2020). A large number of asymptomatic patients exerted never seen challenges over the actual estimation of disease spread based on clinical surveillance (Rimoldi et al., 2020; Medema et al., 2020). Earlier studies suggested that 18–45% of patients do not have signs of infection with COVID-19 but are capable of spreading the disease and pose an adverse impact on the actual containment of the disease (Lavezzo et al., 2020; Yang et al., 2020; Mizumoto and Chowell, 2020; Nishiura et al., 2020). Cheung et al. (2020) conducted a study on a total of 4243 COVID-19 patients and detected SARS-CoV-2 RNA in feces from a higher proportion of patients (48.1%) compared to the gastro-intestinal symptoms (17%). As up to 67% of infected people showed the presence of SARS-CoV-2 RNA in feces (Chan et al., 2020; Cheung et al., 2020; Parasa et al., 2020; Wong et al., 2020), alternative approaches such as wastewater-based epidemiology (WBE) surveillance has gained loads of recognition as a viable option that can provide early warning of the upcoming prevalence of the disease within a community (Hata et al., 2020; Kumar et al., 2021a, Kumar et al., 2021b). One of the advantages of WBE is that wastewater contains feces from a huge number of people. Therefore, it may require a far fewer number samples and less labor than clinical testing to know the presence of infected persons in the area. However, the sensitivity of WBE for SARS-CoV-2 detection is comparatively less than norovirus, presumably due to the low SARS-CoV-2 load in the patient's fecal matter and it's enveloped nature (Hata et al., 2020). Also, to evaluate WBE's potential as an early prediction tool for the COVID-19 pandemic, it is essential to explore the correlation between the SARS-CoV-2 genetic load in wastewater and the number of cases at the district level in each country.
Overall, following the proven concept and capabilities of detecting the RNA of SARS-CoV-2 in wastewater, several limitations and bottlenecks have been put forward towards its practical applicability (Zhu et al., 2021; Tran et al., 2020). At the same time, there is a extreme need for time-series data of SARS-CoV-2 RNA concentration in the wastewater that can be matched with the actual clinical survey data to confirm the utility and predictability of wastewater surveillance. This is also imperative for the adaptation of the Surveillance of Wastewater for Early Epidemic Prediction (SWEEP) on the policy level, which has been delayed for some reasons in the major parts of the globe (Tiwari et al., 2021). There has also been an active debate on varying levels of effectiveness of WBE based on the size of watersheds, catchment type, complexity of sewer systems, and population. Although the science, concepts, and knowledge pertaining to COVID-19 are still evolving and changing rapidly, it is pertinent to check that how effective SWEEP can be on the urban scale, that too if cases reported from the given city have been pretty high?. Under this scenario, the four major directions in the field of SWEEP may be summarised as i) substantiating the data to unravel the early warning capability of wastewater surveillance for COVID-19 through temporal studies on SARS-CoV-RNA detection; ii) need for the escalation of WBE monitoring in various parts of the globe to generate results from all the levels of COVID-19 situation; iii) developing a model that can use Ct-value obtained through SWEEP into the meaningful predictions for effective COVID-19 pandemic preparedness; and iv) collectively reach to the understanding of critical issues like removal, discharge, decay, dilution, and infectivity due to the presence of SARS-CoV-2 RNA in wastewaters (Prevost et al., 2015; Kumar et al., 2020b, Kumar et al., 2020c, Kumar et al., 2021a, Kumar et al., 2021c).
In view of this, the objective of this study was to put forward the evidence of practical applicability of SWEEP for COVID-19 pandemic management by comparing the detected concentration of SARS-CoV-2 RNA in wastewater of various parts of the city with the COVID- 19 clinical cases. The idea is that clinical surveillance hardly classifies the city into precise zones where more tests or attention are required, while SWEEP-based information can help in zoning of the city and identifying the hotspots on a city scale. The detected concentrations of SARS-CoV-2 RNA in wastewater would reflect the true prevalence of COVID-19 infection in the sewer catchment, including clinically undiagnosed patients. On the other hand, the number of clinically reported cases covers only diagnosed patients and depends on the number of PCR diagnosis. We analyzed SARS-CoV-2 RNA in the wastewater samples (n = 116) from nine different locations, including wastewater pumping stations and sewage treatment plant (STP) in Ahmedabad, India, from September 3rd to November 26th, 2020 (thirteen weeks), with the following objectives: a) detection and quantification of SARS-CoV-2-RNA concentration in the influent wastewater samples of Ahmedabad to understand the temporal variation in the pandemic situation over three months, b) weekly resolution of the SARS-CoV-2 RNA data for three months in wastewater samples; and c) explicating the potential of WBE for COVID-19 surveillance as a potential tool for identifying hotspots and public health monitoring at the city level.
2. Material and methods
2.1. Study area
Ahmedabad is the seventh largest city in India and the second biggest trade centre in the western Indian region, with a population of 5.5 million (Census of India, 2011). It has a 1523 km long sewage network, assisted with 43 sewage pumping stations. The treatment capacity of the existing wastewater treatment plants in the city was 670 MLD in 2007, which is likely to be extended to 1075 MLD by 2021 (https://web.worldbank.org/archive/website01409/WEB/IMAGES/2010_1_1.PDF, AMC Report). There are 84 urban health centres present in different wards in Ahmedabad (Ahmedabad Municipal Corporation, 2021).
2.2. Sampling approach
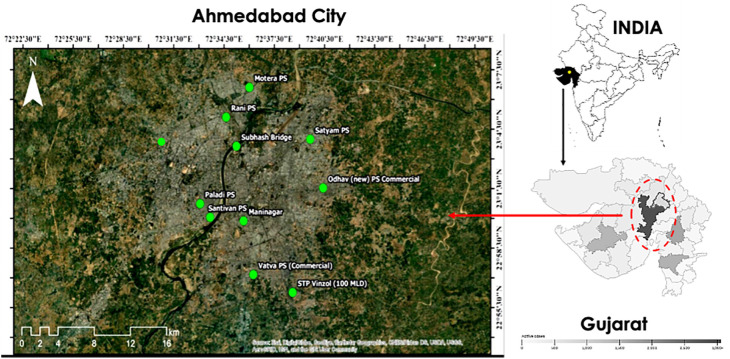
To achieve the objective; firstly, the entire city was divided based on urban & rural and north & south areas to the Sabarmati River- the major river that dissects the city; and identified 29 locations in association with Gujarat Pollution Control Board (GPCB) officials. We observed the data variations of 29 locations for the first four weeks. Thereafter, based on the significance of the variations within the data-set, we fixed thirteen locations to continue monitoring including nine different locations for the wastewater (eight wastewater pumping stations and a single sewage treatment plant) (Fig. 1 ); and four surface water locations (three lakes and one river sample). In the present study, we reported weekly data of wastewater samples collected from nine different locations for thirteen weeks during September to November 2020.
Fig. 1.
Geospatial position of sampling locations in Ahmedabad city.
A total of 116 samples were analyzed to detect SARS-CoV-2 RNA from nine different locations, comprising 103 samples from eight wastewater pumping stations and 13 samples from a single sewage treatment plant in Ahmedabad, India. All the samples were collected by grab hand sampling using 250 mL sterile bottles (Tarsons, PP Autoclavable, Wide Mouth Bottle, Cat No. 582240, India). Simultaneously, blanks in the same type of bottle were examined to know any contamination during the transport. The samples were kept cool in an ice-box until further process. The analysis was performed on the same day after bringing the samples to the laboratory. All the analyses were performed in Gujarat Biotechnology Research Centre (GBRC), a laboratory approved by the Indian Council of Medical Research (ICMR), New Delhi.
2.3. Detection and extraction of viral RNA from wastewater samples
2.3.1. Precipitation of viral particle
30 mL samples were centrifuged at 4000 ×g (Model: Sorvall ST 40R, Thermo Scientific) for 40 min in a 50 mL falcon tube followed by filtration of supernatant using 0.22-micron syringe filter (Mixed cellulose esters syringe filter, Himedia). After filtrating 25 mL of the supernatant, 2 g of PEG 9000 and 0.437 g of NaCl (17.5 g/L) were mixed in the filtrate, and this was incubated at 17 °C, 100 rpm overnight (Model: Incu-Shaker™ 10LR, Benchmark). Next day, the mixture was centrifuged at 14,000 ×g (Model: Kubota 6500, Kubota Corporation) for about 90 min. The supernatant was discarded after centrifugation, and the pellet was resuspended in 300 μL RNase-free water. The concentrated sample was kept in 1.5 mL eppendorf at −40 °C, and this was further used as a sample for RNA isolation.
2.3.2. RNA isolation, and RT-PCR
RNA isolation from the pellet with the concentrated virus was performed using NucleoSpin® RNA Virus isolation kit (Macherey-Nagel GmbH & Co. KG, Germany). The samples were spiked with MS2 phage as an internal control prior to the RNA extraction, provided by TaqPathTM Covid-19 RT-PCR Kit. Some other specifics were, a) the nucleic acid was extracted by NucleoSpin® RNA Virus isolation kit, followed by the total RNA concentration estimation using Qubit 4 Fluorometer (Invitrogen), b) MS2 phage was taken as a molecular process inhibition control (MPC) for evaluating the efficiency of nucleic acid extraction and PCR inhibition (Haramoto et al., 2018). Briefly, steps were carried out as per the guidelines, provided with the product manual of Macherey-Nagel GmbH & Co. KG, and RNAs were detected using reverse transcription PCR (RT-PCR).
Applied Biosystems 7500 Fast Dx RT-PCR instrument (version 2.19 software) was used for SARS-CoV-2 gene detection. In the process, the probes anneal to three specific target sequences located between three unique forward and reverse primers for the N, ORF 1ab, and S genes. A template of 7 μL of extracted RNA was used in each reaction with TaqPath™ 1 Step Multiplex Master Mix (Thermofischer Scientific, USA). Total reaction mixture volume of 20 μL contained 10.50 μL Nuclease-free Water, 6.25 μL Master Mix, and 1.25 μL COVID-19 RT-PCR Assay Multiplex. Three controls were used, viz. positive control (TaqPath™ COVID 19 Control), one negative control (from extraction run spiked with MS2), and no template control (NTC). The RT-PCR contained 1 incubation step cycle of 25 °C & 2 min, 1 cycle of reverse transcription 53 °C & 10 min, 1 cycle of activation 95 °C & 2 min, and 40 cycles of amplification, including denaturation at 95 °C for 03 s and extension at 60 °C for 30 s. Finally, results were interpreted using Applied Biosystems Interpretive Software, and Ct values for three target genes i.e., ORF1ab, N Protein, and S Protein of SARS-CoV-2 along with MS2 used as an internal control.
2.3.3. Gene copy estimation, quality control and quality assurance
The samples were considered as positive if at least two out of the three primer probe sets showed amplification. The Ct-value of a given sample was then converted to gene copy numbers considering the equivalence of 500 copies of SARS-CoV-2 genes as 26 Ct-value (provided with the kit), and the same was extrapolated to derive approximate copies of each gene. To calculate the gene concentration in this semi-quantitative method, a calibration curve was prepared based on the well-established principle of 3.3 Ct change corresponding to a 10-fold gene concentration change. The gene concentration of SARS-CoV-2 present in a given sample was calculated by multiplying the RNA amount used as a template with the enrichment factor for each sample. In addition, we calculated the gene copy numbers based on the positive control provided with kit i.e., 104 copies/μL and the final concentration of 25 copies per reaction. It is worth noting that the primer efficiency of different genes slightly differ according to the primer sequence. However, based on hundreds of RT-PCR runs, it was observed that the positive control was robust enough to yield almost the same Ct values for all three genes, suggesting no discernible variation in primer efficiency. Relative to the Ct values of positive controls, copy numbers/ concentration of all three genes were calculated in test samples of different locations. The effective gene concentration was calculated by averaging the gene copies of all three genes in a particular sample. The effective gene concentration is considered as “zero” when RT-PCR results were positive for only one gene out of three in the wastewater sample. The limit of detection was set to 40 amplification cycle (Ct = 40) in the RT-PCR analysis.
Due to various constraints, analysis of duplicate samples was performed, considering that the samples were analyzed in the batch accompanied with negative and positive controls, and each sample was spiked with known concentrations of MS2. In the event of any variations (among duplicate and controls) of more than 10%, samples were re-analyzed.
2.4. Epdemiological information, data collection and interpretation
The data of affected people and their locations were obtained from the government mobile application ‘Arogya Setu’ which is published as 'Ahmedabad COVID-19 community vulnerability map' by SustainAbly and Accion Land Pvt. Ltd., and accessible at http://google.org/crisismap/a/gmail.com/amdcovid19. ‘Aarogya Setu’ mobile application was launched by the Ministry of Electronics and Information Technology of the Indian government for collecting data pertaining to tracing, syndromic mapping and self-assessment on COVID–19. This application reached more than 100 million installs in 40 days (Arogya setu, Wikipedia 2021). Other information was obtained from the Ahmedabad city portal accessed using link https://ahmedabadcity.gov.in/portal/web?requestType=ApplicationRH&actionVal=loadCoronaRelatedDtls&queryType=Select&screenId=114. Several other informations can be accessed using https://ahmedabadcity.gov.in/portal/jsp/Static_pages/water_project.jsp.
The percentage change showed for the confirmed and active cases were calculated using the formula:
Statistical Package for the Social Sciences (SPSS 21) has been used for hypothesis testing through Analysis of variance (ANOVA) and Duncan's Multiple Range Test (DMRT). The OriginPro 2019b data analysis software has been used to draw boxplots.
3. Results and discussions
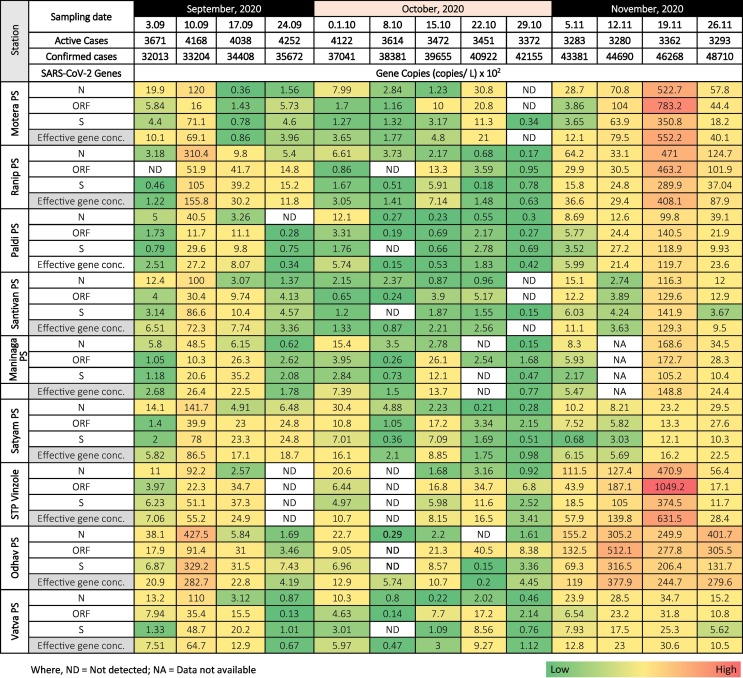
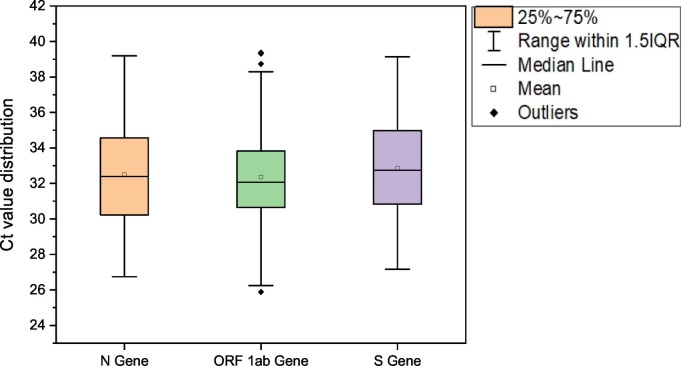
We detected and quantified variation in SARS-CoV-2 RNA from wastewater samples for three months (September and November) to understand the pandemic situation in Ahmedabad, Gujarat, India. Among the 116 samples analyzed in the study, 111 (95.7%) were found positive, comprising at least two positive RT-PCR results targeting SARS-CoV-2 ORF1ab, S gene, and N gene (Table 1 ). In addition to this, 109/116 (93.9%) samples showed positive RT-PCR results for each N, ORF 1ab, and S genes. The distribution analysis of Ct values for different genes using boxplot is represented in Fig. 2 . The average Ct values for N, ORF 1ab, and S genes were 32.50, 32.36, and 33.85, respectively. Likewise, the average Ct values of internal control (MS2 bacteriophage) was 28.2, and no SARS-CoV-2 genes were detected in the negative control samples.
Table 1.
Temporal variation in gene copies of the SARS-CoV-2 targeted genes and effective gene concentration at various locations in Ahmedabad city.
Fig. 2.
Distribution of Ct values of SARS-CoV-2 genes during the study period.
3.1. Monthly and weekly variations
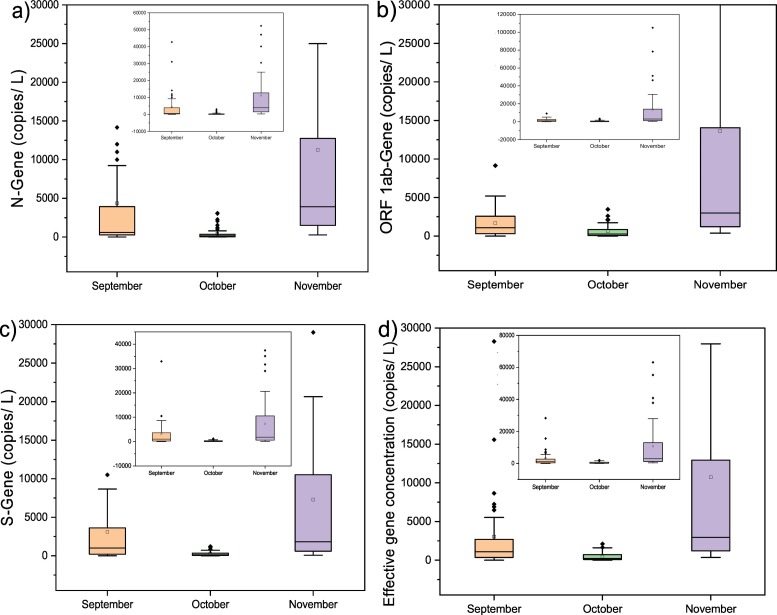
Monthly variation depicted a significant decline of 89.7, 63.7, and 90.9% in N, ORF-1ab, and S gene concentration (copies/L), respectively in October compared to September 2020, followed by a sharp increment in November 2020 i.e., ~25 folds in N gene, ~22 folds in ORF 1ab and ~26 folds in S gene. The PCR products for all three genes were maximum in wastewater samples of November, followed by September and October (Fig. 3 a–c). Likewise, the effective gene concentration of SARS-CoV-2 was maximum in the month of November (~10,729 copies/L), followed by September (~3047 copies/L), and October (454 copies/L) in line with a ~1.5-fold rise in the number of confirmed cases during the study period (3rd September 2020 and 26th November 2020) (Fig. 3d).
Fig. 3.
Distribution of SARS-CoV-2 gene copies on a temporal scale (monthly variation).
There had been a decline of 20.47% in active cases in October 2020 with respect to September, and a rise of 1.82% occurred in November 2020 compared to the preceding month i.e. October. While the increase of 1.82% in the active cases of November with respect to October was equivalent to a change of 59 cases (3234 cases on 1st November–3293 on 26th November); the same monthly change in the total new confirmed cases between October and November had been 14.1% due to addition of 6019 new cases to the tally of October on 26th November 2020. Also, a monthly decrease of 4.45% in recovered cases was noticed in November compared to October 2020. The monthly recovered new cases were 16.61, 20.03, and 15.58% in September, October, and November 2020, respectively. Apart from that, people's casual and reluctant attitude during the festive season in India (mid-October to mid-Nov) might be the reason for the piercing rise in COVID-19 cases.
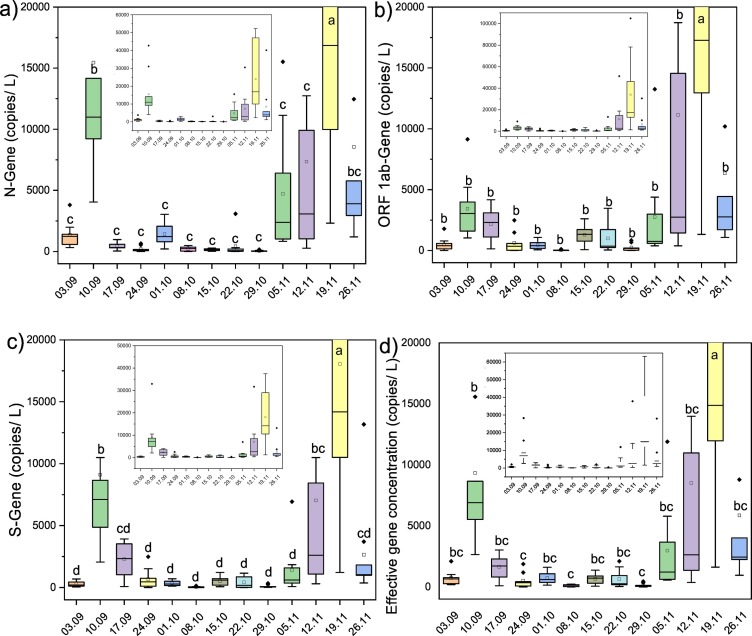
Weekly temporal variations in average SARS-CoV-2 gene copies were analyzed for SARS-CoV-2 RNA presence in samples collected from all the sampling locations in Ahmedabad and are displayed in Fig. 4a–d. One-way ANOVA and Duncan post hoc test (p < 0.05) were performed to see the significance level in gene copy variation among different sampling dates. The results showed significant differences in all three gene copies, i.e. N-gene (ANOVA, F = 7.49, p < 0.001), ORF-1ab genes (ANOVA, F = 5.94, p < 0.001), and S-gene (ANOVA, F = 8.25, p < 0.001) on the temporal scale (sampling dates). Similarly, differences were significant in the case of effective gene concentration (ANOVA, F = 7.12, p < 0.001).
Fig. 4.
Temporal variations in targeted gene copies of SARS-CoV-2, collected from different sampling points a.) N gene, b.) ORF 1ab gene, c.) S gene, and d) effective gene concentration.
The N-Gene concentration in wastewater samples collected on September 10th, 2020 was found to be significantly higher than other sampling dates, except November 26th, 2020, and lower than November 19th, 2020. The ORF 1ab gene copies/L in wastewater samples noticed maximum on November 19th, 2020 and were significantly higher than other sampling dates. Except for November 19th, 2020, the changes in ORF 1ab gene concentration were statistically insignificant among different sampling dates. Likewise, the highest S-Gene concentration was noticed on November 19th, 2020 (p < 0.05), followed by September 10th, 2020. The S gene copies/L in wastewater samples collected on September 10th, 2020 was significantly higher than other sampling dates except for November 12th, 2020. In addition to this, the alteration in S-Gene concentration was statistically insignificant among the remaining dates. Moreover, the SARS-CoV-2 effective gene concentration was found to be maximum and significantly higher on November 19th, 2020 than others. The effective gene concentration in wastewater sampled on September 10th, 2020 was significantly higher than the samples of September 24th, 2020 and October 8th & 29th 2020. All three gene copies (i.e. N, ORF1ab, and S genes) and effective gene concentration were detected maximum on November 19th, 2020, and values were significant (p < 0.05) as compared to other sampling dates. The exponential rise in virus gene concentration might be due to the decline in the decreasing trend (<−0.1%, November 12th, 2020) followed by the increase in the number of active cases (i.e. 2.5% which corresponded to the 82 new cases on November 19th, 2020), compared to the earlier sampling dates.
The primary implications of these temporal variations in monthly and weekly gene data can be explained in three ways: i) the evident effect of variations in new confirmed cases on gene copies; it is interesting to note that changes in the active cases is showing little correlation with the WBE statistics; ii) the monthly variations in gene data do not show much difference among the individual genes and effective gene concentrations; and iii) weekly variations highlight the differences among various genes, emphasizing the neccesity to normalize the data in the form of effective gene concentrations. Weekly data explicitly confirms that N genes are much more resistant among the three and ORF-1ab seems to be the least resistant gene. These two observations are clearly evident in data of 10th September and 5th November (Fig. 4) when the varitions/disagreements among the various genes are explicit. The further implications of these findings are related to the required number of sampling events and calculation of the effective gene concentration. It is evident here that weekly sampling should be enough to get a trend in a given Indian city. Also, COVID-19 wastewater surveillance based data must not be judged or evaluated based on a single particular gene of SARS-CoV-2 but its effective gene concertatio which includes multiple genes.
3.2. SWEEP-based city zonation and identification of hot-spots
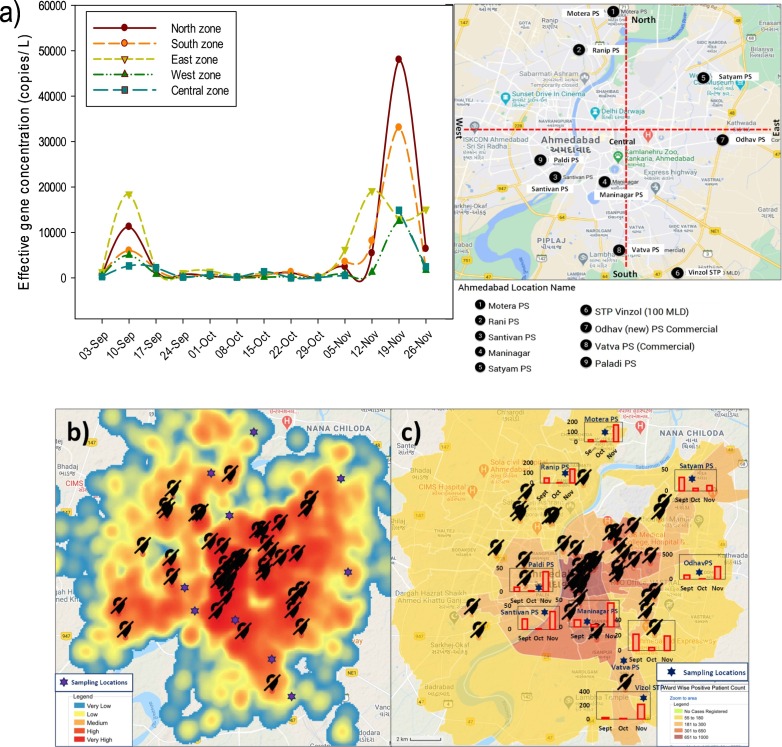
Depending on the SARS-CoV-2 effective gene concentration in wastewater samples based on analytical results, we identified highly susceptible areas for COVID-19 infection and its transmission among the community. Although we did not have explicit epidemiological data at the ward level/sampling locations; but variations were good enough to classify a city based on SARS-CoV-2 gene concentration in wastewater samples. The north (Motera and Ranip) and east (Odhav and Satyam) zones were highly affected areas with an average effective gene concentration of ~15,574 and ~13,397 copies/L, respectively, in November (Fig. 5a). Likewise, in September, wastewater samples collected from the east zone showed maximum effective gene concentration (~5734 copies/L), followed by the north zone (~3536 copies/L). Though areas present in north and east zones showed high virus genetic load, yet a sharp rise in SARS-CoV-2 RNA was noticed in all the zones in November 2020 (Fig. 5a). It has also been represented in a summarised format with a comparison to the affected population in the city (Fig. 5b & c).
Fig. 5.
a) Zone-wise Covid-19 pandemic status in Ahmedabad city; b) Heat map of the overall infected population in Ahmedabad City based on Aarogya-setu mobile application. Very low, low, medium, high and very high indicates no to up to 50, 51–180, 181–300, 301–650, and >651 registered positive covid-19 cases per ward. and c) Monthwise Effective gene concentration at the sampling locations (y-axis in bar diagrams represents SARS-CoV-2 effective gene concentration in copies ×102/L wastewater samples). Note: Positive patient count has been taken on ward basis not on the population-density.
It is imperative to note that Fig. 5b is a generalized status of the city as of 26th November 2020 pertaining to the COVID-19 total confirmed cases and Fig. 5c depicts three months change in SARS-CoV-2 effective gene concentration by bar diagram with existing positive cases of 26th November 2020 by colour coding. Although it would have been preferable to provide the heat maps of confirmed/ active cases distributions and effective gene concentrations during the entire study period to better evaluate the efficacy of WBE surveillance; yet, two observations are critical i.e. i) the monthly pattern of SARS-CoV-2 gene concentration at Satyam and Vinzol locations were diametrically opposite. It was found to be greater in case of Vinzol for the month of November when compared to Satyam, suggesting the capability of WBE to differentiate different parts of the city based on SARS- CoV-2 gene concentration; and ii) data of SARS-CoV-2 gene concentration and COVID-19 cases varied among the sample locations, and therefore seemed to be related to the size of the catchment and treatment plant, suggesting that analyzing month-wise variations alone would be not enough. Also, there is a need for the match between the epidemiological data and SARS-CoV-2 gene concentration in wastewater samples. Overall, despite several challenges in epidemiological and clinical data collection as well as sewage water collection and catchment delineation in India, the proper scrutiny and regular monitoring of wastewater could be useful for preparedness against adverse conditions as appeared in post-festive days in Ahmedabad.
The SWEEP technology offers a better picture of the pandemic situation at the sub-city or zone level, relying on the SARS-CoV-2 RNA concentration in wastewater samples of a particular area. SWEEP data can help to estimate the actual extent of the infection due to the SARS-CoV-2, as it covers both asymptomatic and presymptomatic patients, which may be underestimated by clinical surveillance. Therefore, SWEEP data-based zonation of the city can help to identify hot-spots to increase the preparedness in advance. On the other hand, clinical surveillance usually fails to classify the city into distinct zones as it is more dependent on the location of test centres rather than the COVID-19 patients, and owing to asymptomatic and presymptomatic patients. This is why several study could early detect the SARS-CoV-2 RNA in wastewater, before the first clinical report like Medema et al. (2020) in the Netherlands, La Rosa et al. (2020) in two different cities in Italy and Randazzo et al. (2020) in Spain. However, this is probably the first study where the SARS-CoV-2 RNA data has been compared with ward wise positive patient counts.
3.3. Early warning potential of WBE
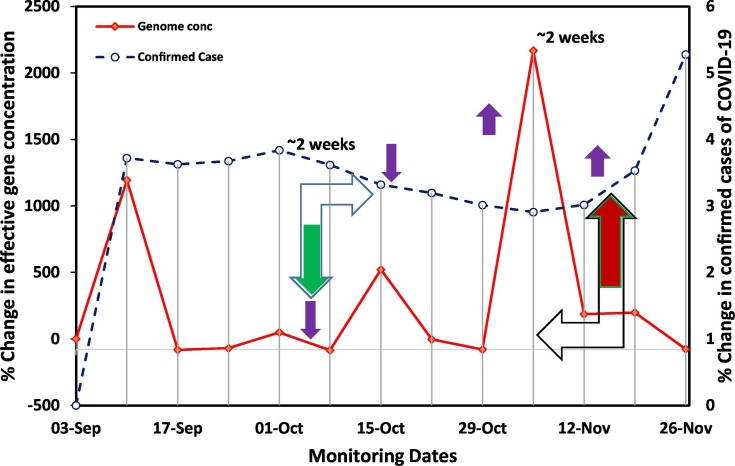
In this view, the present research work followed our first proof concept study, where we detected SARS-CoV-2 genetic material in wastewater and proposed its wide applicability for COVID-19 surveillance in the community (Kumar et al., 2020a). The linear regression between changes in SARS-CoV-2 effective gene concentration and the number of confirmed cases showed a positive correlation (Fig. S1) but was not statistically significant (p = 0.135, R = 0.438). There was no linear relationship between the SARS-CoV-2 gene concentration and epidemiological data. Therefore, we showed the relationship between percentage changes in effective gene concentration and confirmed cases that can be used as a pre-alarming tool, which gives a lead of ~2 weeks for the upcoming scenario (Fig. 6 ). Examining the potential of WBE for COVID-19 surveillance as a potential tool showed that the percentage change in effective gene concentration level on a particular date was in conjunction with the confirmed cases registered 1–2 weeks later on the temporal scale by the regulatory authority based on clinical tests (Fig. 6). For example, on October, 8th, 2020, a sharp decline of ~86% was noticed in the percentage change in the average effective gene concentration which was followed by ~0.4% decline in the percentage change in confirmed COVID-19 cases on October, 22nd, 2020. Likewise, on November 5th, 2020, a steep hike of >22-folds in the percentage change in the average effective gene concentration was noticed compared to the earlier sampling date, which was followed by ~0.6% and 2.37% increment in the percentage change in confirmed COVID-19 cases on November 19th and November, 26th, 2020, respectively. In the contrary, more than >1000% and ~500% increase were noticed in percentage change in SARS-CoV-2 effective gene concentration in wastewater in early September and mid-October, respectively. However, no notable increase was observed in the number of confirmed cases 1–2 weeks later. Still, this technique displayed positive prediction in most of the events during the study period. Therefore, we can predict the severity of the pandemic situation 1–2 weeks prior to the official reports by the regulatory body based on clinical tests.
Fig. 6.
Potential and evidence of wastewater based epidemiology surveillance of Covid-19 pandemic as an early warning tool.
The results unravel the potential of WBE surveillance of COVID-19 as an early warning tool displayed by the adequate presence of SARS-CoV-2 genetic material in wastewater samples though limited cases were documented and based on the immediate future trends. These findings were in agreement with those of Ahmed et al. (2020b), who noticed a longitudinal decline in the presence of SARS-CoV-2 RNA with the tapering of the first epidemic wave; however, there was no concrete relationship between virus RNA and daily cases numbers.
3.4. Limitations and perspectives
Epidemiological data accessible for this study has been weak as the clear catchment delineation and exact population being catered by each treatment or pumping stations is not precise. We just matched RNA data with secondary sourced information on COVID-19 vulnerability maps. Although it may still be considered as a good beginning, yet it emphasizes the need for collaborations among the different governmental organizations. Nevertheless, we explicitly put forward an example of the effectiveness of SWEEP for the early warning of COVID-19, and emphasize the continuous long-term monitoring with the following future objectives: i) monitoring the COVID-19 curve in the post-vaccination period through quantifying the genetic material of SARS-CoV-2 in the wastewaters of a given city (Ahmedabad); ii) understanding the association of antibiotic resistance with COVID-19 prevalence (Kumar et al., 2021b); iii) developing an online portal for the weekly update of gene concentration with accessibility provided to the public and policymakers; iii) estimating the potential risk of SARS-CoV-2 in natural water bodies through various water activities using a quantitative microbial risk assessment (QMRA) framework; iv) generating longer time-series data to further check the robustness of early warning capability of the techniques and its possible benefits; and v) developing predictive models for connecting the missing points in SWEEP generated database, meaningful interpretations, and supporting other surveillance protocols. SWEEP can be considered for developing advisory in the context of rapid-testing, the number of testing, community clearance, hotspot identification, vaccine need identification zones, as well as making a recommendation on staying at home and implementing curfews.
In this first phase, we have explicitly shown the capability of WBE as an early warning and city zonation tool, however in a country like India, where sewer systems are not complete and treatment systems are not well-managed, it is important to have long-term monitoring for a year at least so that precious meaningful data for the developing countries can be obtained. Furthermore, a practical guide and pandemic management tools can be developed by integrating the virtues of information technology with the early warning capability of wastewater surveillance. Confidence may be generated among the commons as well as to the government agencies like Ahmedabad Municipal Corporation (AMC) for incorporating WBE into regular monitoring program for the management of the current/future COVID-19 like epidemic/pandemic outbreak.
4. Conclusion
A temporal variation of SARS-CoV-2 RNA presence in wastewater was studied for a period of three months in Ahmedabad, India. A total 111 samples (95.7%) of the total 116 samples tested in the study were found to be positive, with at least two positive RT-PCR results targeting SARS-CoV-2 ORF1ab, S gene, and N gene assays. Monthly variation depicted a significant decline in all three gene targets in October compared to September 2020, followed by a sharp increment in November 2020. Correspondingly, the descending order of average effective gene concentration was November (~10,729 copies/L) > September (~3046 copies/L) > October (~454 copies/L). This finding was further supported by the relation between the percentage change in effective gene concentration level and confirmed cases, which followed a similar trend on the temporal scale with a ~1 to 2 weeks' time distance. The results unveiled the untapped potential of WBE surveillance of COVID-19 as an early warning tool for its practical use in city zonation based on SWEEP data to know about actual scenarios and future predictions. This approach may also help the authorities to identify the hotspots within a city and tuning effective management interventions. Further research should be focused on the quantification of correlation of SWEEP results with clinical surveillance data and development of a predictive model that can translate SWEEP data for easy propagation to the policymakers and common public to enhance the preparedness and management of pandemics.
CRediT authorship contribution statement
Manish Kumar: Conceptualization, Data curation, Supervision, Validation, Writing- Original draft preparation; Response.
Vaibhav Srivastava: Writing - Review & Editing, Visualization; Validation, Response.
Anil V Shah: Sampling and analyses, Illustrations.
Madhvi Joshi: Writing - Review & Editing, Validation;
Shyamnaryan Dave: Supervision and Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This work is funded by UNICEF, Gujarat and UKIERI. We also acknowledge the help received from Dr. Arbind K Patel, Mr. Alok Thakur, and other GBRC staffs who contributed towards sample and data analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.148367.
Appendix A. Supplementary data
Supporting dataset
References
- Aarogya Setu 2021. https://en.wikipedia.org/wiki/Aarogya_Setu accessed 20 March 2021.
- Ahmed W., Tscharke B., Bertsch P.M., Bibby K., Bivins A., Choi P., Clarke L., Dwyer J., Edson J., Nguyen T.M.H., O’Brien J.W. SARS-CoV-2 RNA monitoring in wastewater as an early warning system for COVID-19 transmission in the community: a temporal case study. Sci. Total Environ. 2020:144216. doi: 10.1016/j.scitotenv.2020.144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmedabad Municipal Corporation, 2021
- Census of India, 2011
- Chan P.K., Lui G., Hachim A., Ko R.L., Boon S.S., Li T., Kavian N., Luk F., Chen Z., Yau E.M., Chan K.H. Serologic responses in healthy adult with SARS-CoV-2 reinfection, Hong Kong, August 2020. Emerg. Infect. Dis. 2020;26(12):3076. doi: 10.3201/eid2612.203833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W., Tam A.R., Yip C.C. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Hata A., Hara-Yamamura H., Meuchi Y., Imai S., Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2020:143578. doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Thakur A.K., Mazumder P., Kuroda K., Mohapatra S., Rinklebe J., Ramanathan A.L., Cetecioglu Z., Jain S., Tyagi V.K., Gikas P. Frontier review on the propensity and repercussion of SARS-CoV-2 migration to aquatic environment. J. Hazard. Mater. Lett. 2020;1:100001. doi: 10.1016/j.hazl.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Kuroda K., Patel A.K., Patel N., Bhattacharya P., Joshi M., Joshi C.G. Decay of SARS-CoV-2 RNA along the wastewater treatment outfitted with Upflow Anaerobic Sludge Blanket (UASB) system evaluated through two sample concentration techniques. Sci. Total Environ. 2020;754:142329. doi: 10.1016/j.scitotenv.2020.142329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Alamin M., Kuroda K., Dhangar K., Hata A., Yamaguchi H., Honda R. Potential discharge, attenuation and exposure risk of SARS-CoV-2 in natural water bodies receiving treated wastewater. npj Clean Water. 2021;4(1):1–11. doi: 10.1038/s41545-021-00098-2. [DOI] [Google Scholar]
- Kumar M., Dhangar K., Thakur A.K., Ram B., Chaminda T., Sharma P., Kumar A., Raval N., Srivastava V., Rinklebe J., Kuroda K. Antidrug resistance in the Indian ambient waters of Ahmedabad during the COVID-19 pandemic. J. Hazard. Mater. 2021:126125. doi: 10.1016/j.jhazmat.2021.126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Joshi M., Kuroda K., Bhattacharya P., Barcello D. First comparison of conventional activated sludge versus root-zone treatment for SARS-CoV-2 RNA removal from wastewaters: statistical and temporal significance. Chem. Eng. J. 2021;425 doi: 10.1016/j.cej.2021.130635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C., Rossi L., Manganelli R., Loregian A., Navarin N., Abate D. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo. Nature. 2020;584(7821):425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Enviro.n Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Chowell G. Estimating risk for death from coronavirus disease, China, January–February 2020. Emerg. Infect. Dis. 2020;26(6):1251. doi: 10.3201/eid2606.200233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasa S., Desai M., Thoguluva Chandrasekar V., Patel H.K., Kennedy K.F., Roesch T., Spadaccini M., Colombo M., Gabbiadini R., Artifon E., Repici A., Sharma P. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA network open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost B., Lucas F.S., Goncalves A., Richard F., Moulin L., Wurtzer S. Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ. Int. 2015;79:42–50. doi: 10.1016/j.envint.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Moja L., Gismondo M.R., Salerno F. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S.B., Gahlot P., Tyagi V.K., Zhang L., Zhou Y., Kazmi A.A., Kumar M. Surveillance of Wastewater for Early Epidemic Prediction (SWEEP): environmental and health security perspectives in the post COVID-19 Anthropocene. Environ. Res. 2021 doi: 10.1016/j.envres.2021.110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H.N., Le G.T., Nguyen D.T., Juang R.S., Rinklebe J., Bhatnagar A., Lima E.C., Iqbal H.M.N., Sarmah A.K., Chao H.P. SARS-CoV-2 coronavirus in water and wastewater: a critical review about presence and concern. Environ. Res. 2020 doi: 10.1016/j.envres.2020.110265. https://web.worldbank.org/archive/website01409/WEB/IMAGES/2010_1_1.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M.C., Huang J., Lai C., Ng R., Chan F.K., Chan P.K. Detection of SARS-CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J. Inf. Secur. 2020 doi: 10.1016/j.jinf.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Novel coronavirus (2019-nCoV) situation report – 1. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation432-reports
- Yang R., Gui X., Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw. Open. 2020;3(5):e2010182. doi: 10.1001/jamanetworkopen.2020.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Oishi W., Maruo C., Saito M., Chen R., Kitajima M., Sano D. Early warning of COVID-19 via wastewater-based epidemiology: potential and bottlenecks. Sci. Total Environ. 2021;145124 doi: 10.1016/j.scitotenv.2021.145124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting dataset