Abstract
Background
The prevalence of SARS-CoV-2 infection among health care workers (HCWs) provides information about the spread of COVID-19 within health care facilities, and the risk groups.
Objectives
We aimed to describe the rate of SARS-CoV-2 seroprevalence and its determinants among HCWs.
Data sources
We used Web of Science, PubMed, Scopus, MEDLINE, EBSCOhost and Cochrane Library.
Study eligibility criteria
We included the reports of SARS-CoV-2 seroprevalence with a sample size of minimum 1000 HCWs.
Methods
The study was registered at the International Prospective Register of Systematic Reviews (PROSPERO, no. CRD42021230456). We used PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. The keywords were “COVID-19”, “SARS-CoV-2”, “Coronavirus”, “seroprevalence”, “health care workers” and “risk factors”.
Results
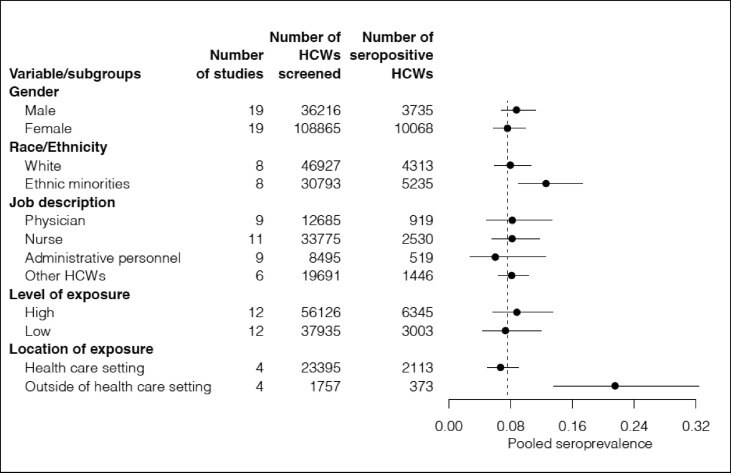
In total 4329 reports were retrieved, duplications were removed; after filtering according to the title and abstract, 25 studies were selected. Risk of bias was assessed in 25 studies; it was low in 13 studies, medium in four studies, and high in eight studies. In meta-analysis using the random effect model, the weighted average of seroprevalence was calculated as 8% (95% CI 6–10%). The pooled seroprevalence rates of the selected variables that have a rate above the average were male HCWs with 9% (95% CI 7–11%); HCWs from ethnic minorities with 13% (95% CI 9–17%); high exposure 9% (95% CI 6–13%); exposure to the virus outside the health care setting 22% (95% CI 14–32%).
Conclusions
Our analysis indicates a SARS-CoV-2 seroprevalence rate of 8% among studies that included >1000 HCWs for the year 2020, before vaccinations started. The most common risk factors associated with higher seroprevalence rate were ethnicity, male gender and having a higher number of household contacts. Working as a frontline HCW was inconsistent in its association with higher seroprevalence.
Keywords: Before vaccination, Covid-19, Healthcare workers, Risk factors, Seroprevalence
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) continues to spread worldwide since its first emergence in December 2019. Health care workers (HCWs) have been considered at high risk of contracting the virus and may also pose a significant risk of transmitting the virus to patients, colleagues and their social contacts [1]. According to the World Health Organization (WHO), one out of every seven patients (14%) is a HCW [2]. A study from the UK and the USA reported that frontline HCWs have a 3.4 times higher risk than people living in the community [3]. In a systematic review the hospitalization and mortality rate of HCWs were reported to be 15.1% and 1.5% respectively [4]. Inability to protect the safety of frontline HCWs poses a risk for collapse of the health care system as well as transmission of the virus from health care settings to the community [5]. The rate of SARS-CoV-2 seroprevalence among HCWs varies between and within countries and even between institutions due to the variations in infection control measures taken in health care settings, geographic areas, established health policies and procedures at national level along with individual behaviours of HCWs to adhere to these measures [6].
The prevalence of SARS-CoV-2 infection among HCWs provides valuable information to understand the spread of COVID-19 within health care facilities and to detect the risk groups for the infection [7]. In this study, we aimed to investigate the seroprevalence of SARS-CoV-2 among HCWs and related risk factors by including studies published in 2020 which were conducted before the unpredictable effects of highly spreading new variants appeared and vaccination programmes put in place in 2021.
Materials and methods
Study design, registration and search protocol
This is a systematic review and meta-analysis that has been registered at the International Prospective Register of Systematic Reviews (PROSPERO) with a registration number of CRD42020159198. We performed the review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (PRISMA checklist, Table S1) [8]. An independent librarian conducted the search to find articles published up to 31 December 2020 in PubMed, Web of Science, Scopus, MEDLINE, EBSCOhost and the Cochrane Library. Please see Table S2 for the search strategy, which included variations of the following terms “COVID-19, “SARS-CoV-2”, “Coronavirus”, “seroprevalence”, “health care workers” and “risk factors”. The librarian provided the list of articles after eliminating duplicate files. We have also searched Google for non-peer-reviewed publications and also the list of references of all relevant articles and previous reviews.
Eligibility criteria
We included antibody screening studies among HCWs with over 1000 participants. We excluded surveys, preprint reports, diagnostic or screening studies performed primarily by using polymerase chain reaction (PCR) for viral RNA, and studies that included only suspected or confirmed cases.
Study selection and data collection
There were two phases of the selection process. In the first phase, two reviewers (B.M. and İ.K.) independently screened the titles and abstracts of each study. Two other reviewers (Ş.K. and Ö.K.) conducted another screening to double check the first reviewers, which yielded consistent results. The studies which do not fulfil the eligibility criteria were excluded. Studies with promising titles but missing abstracts were included in the full text screening. Reference lists of included studies were searched to identify further studies. In the second phase, two reviewers (B.M. and İ.K.) read the full text of the articles that remained from the first phase and discrepancies in selection were resolved via a consensus discussion among the review team.
The data extracted from each article included authors, location, dates of starting and ending terms of screening, sample size, setting, study design, antibody tests, sensitivity and specificity of antibody tests and risk factors associated with seroprevalence of SARS-CoV-2 antibodies. The data required for the pooled analysis were extracted for the variables of gender, race/ethnicity, job description, level of exposure and location of exposure (in or outside of the hospital) to the virus. Race/ethnicity was dichotomized as white and ethnic minorities. Level of exposure was categorized as high for (1) frontline workers, (2) in high exposure conditions, (3) emergency department (ED), intensive care units (ICUs), infectious disease department and pulmonology departments.
Risk of bias assessment
In order to evaluate the systematic errors in each study one of the authors (İ.K.) assessed the risk of Bias (RoB) by using the Joanna Briggs Institute (JBI) critical appraisal tools for prevalence studies. This is a 9-point scale where a score of 8–9 indicates low risk of bias whereas a score of 5–7 indicates moderate and ≤4 indicates high risk of bias [9]. As we have included studies with over 1000 participants, the sample size item on the JBI scale was considered as fulfilled, but we have evaluated the item about response rate as low if it was less than 50% and unclear if it was not mentioned or we were unable to calculate according to the number of target population provided.
Data analysis
The primary outcome measure was the pooled seroprevalence rate obtained from the articles and pooled seroprevalence rate for selected risk factors. Heterogeneity assessment was done using the I 2 test. The estimation for the pooled seroprevalence for SARS-CoV-2 was carried out whenever appropriate with 95% CI. We performed meta-analysis on prevalence rates using both the fixed effect model and random effects model under logit transformation. Statistical analyses were done using meta package in R [10]. The outcome of this analysis was visualized using Forest plots.
Results
Study characteristics, SARS-CoV-2 seroprevalence and related factors
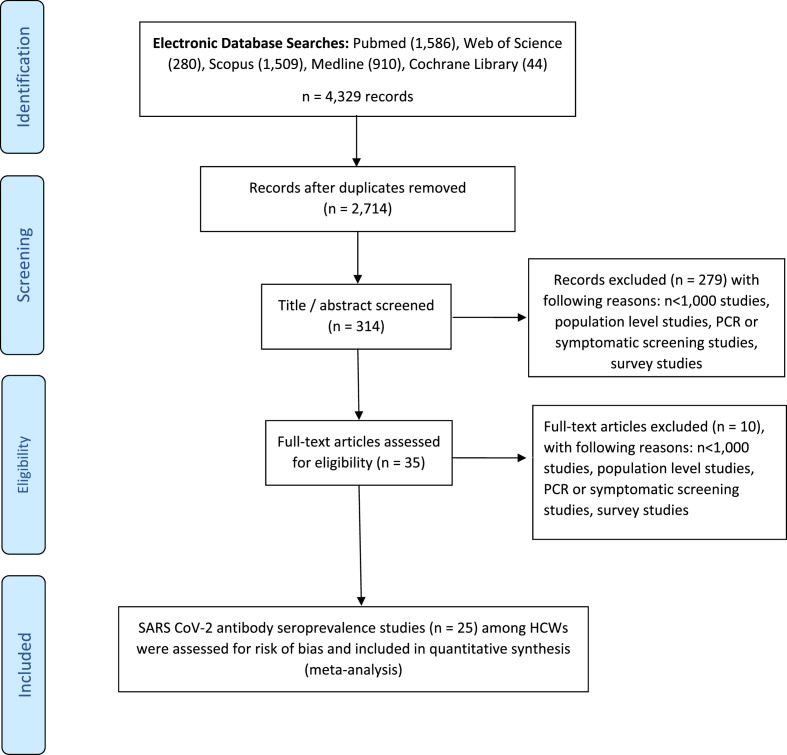
Our literature review yielded 4329 reports in total. After removal of duplications and initial screening according to the title and abstract we had 35 articles for full text review. Finally we had 25 studies that met the eligibility criteria (PRISMA flowchart, Fig. 1 ). According to the RoB assessment 13 studies showed low RoB, five medium RoB and seven high RoB (please see supplementary information 2).
Fig. 1.
Flowchart for selection of the study reports.
Table 1 shows the main characteristics of the studies, seroprevalence rates and risk factors investigated in each study. The start of data collection was March 2020 [11] and the end was August 2020 [12]. The mean duration of data collection was 45.8 days (min. 8 days [13] and max. 181 days [14]. The majority of the participants were female in each study with range 64–85%, except one in India with a rate of 34.6%. The range of the mean age was 37.0 and 49.4 years. Fourteen studies were performed in one centre, whereas 11 were multicentre studies. The lowest rate of seroprevalence was 1.1 %, reported in May from the USA [15]. The highest seroprevalence rate was reported to 35.4% in May from the USA [16]. Risk factors described in 25 studies are summarized in Table 2.
Table 1.
Review of the included studies
| Reference | Country | Females (%) |
Mean Age (years) |
Number of screened HCWs | Number of positive HCWs | Positivity rate | Response rate | Sampling start date (dd.mm.yyyy) |
Sampling end date (dd.mm.yyyy) |
|---|---|---|---|---|---|---|---|---|---|
| Moscola et al., 2020 | USA | 73.7 | 42.7 | 40 329 | 5523 | 13.7 | 65.1 | 20.04.2020 | 23.06.2020 |
| Iversen et al., 2020 | Denmark | 78.9 | 44.4 | 28 792 | 1163 | 4.0 | 96.3 | 15.04.2020 | 23.04.2020 |
| Martin et al., 2020 | UK | 79.8 | 44.0 | 10 662 | 1148 | 10.8 | 66.6 | 29.05.2020 | 13.07.2020 |
| Eyre et al., 2020 | UK | NR | NR | 9958 | 1069 | 10.7 | 73.0 | 23.04.2020 | 08.06.2020 |
| Dimeglio et al., 2020 | France | 80.4 | 40.0 | 8758 | 276 | 3.2 | 53.0 | 10.06.2020 | 10.07.2020 |
| Lidstrom et al., 2020 | Sweden | 77.0 | 44.0 | 8679 | 577 | 6.6 | NR | 27.05.2020 | 25.06.2020 |
| Plebani et al., 2020 | Italy | 71.6 | 43.2 | 8285 | 378 | 4.6 | NR | 22.02.2020 | 19.05.2020 |
| Jones et al., 2020 | UK | 77.8 | NR | 6858 | 638 | 9.3 | 56.0 | 01.01.2020 | 30.06.2020 |
| Calcagno et al., 2020 | Italy | 74.7 | 49.4 | 5444 | 377 | 6.9 | NR | 17.04.2020 | 20.05.2020 |
| Delmas et al., 2020 | France | 75.0 | 41.8 | 4600 | 527 | 11.5 | 91.8 | 14.05.2020 | 17.06.2020 |
| Xu et al., 2020 | China | 75.2 | 37.1 | 4384 | 81 | 1.8 | NR | 09.03.2020 | 10.04.2020 |
| Pere et al., 2020 | France | 74.8 | 39.6 | 3569 | 423 | 11.9 | NR | 02.05.2020 | 29.06.2020 |
| Self et al., 2020 | USA | 65.6 | 38.5 | 3248 | 194 | 6.0 | NR | 03.04.2020 | 19.06.2020 |
| De Carlo et al., 2020 | Italy | NR | 46.5 | 3242 | 62 | 1.9 | NR | 17.03.2020 | 18.05.2020 |
| Steensels et al., 2020 | Belgium | NR | NR | 3056 | 197 | 6.4 | 74.0 | 22.04.2020 | 30.04.2020 |
| Brant-Zawadzki et al., 2020 | USA | 72.0 | 42.6 | 2932 | 31 | 1.1 | NR | 01.05.2020 | 30.06.2020 |
| Racine-Brazostek et al., 2020 | USA | 64.5 | 37.0 | 2274 | 805 | 35.4 | NR | 17.04.2020 | 07.05.2020 |
| Rudberg et al., 2020 | Sweden | 85.0 | 44.0 | 2149 | 410 | 19.1 | 85.0 | 14.04.2020 | 08.05.2020 |
| Grant et al., 2020 | UK | NR | 40.3 | 2004 | 634 | 31.6 | 54.2 | 15.05.2020 | 05.06.2020 |
| Sydney et al., 2020 | USA | NR | NR | 1700 | 327 | 19.2 | NR | 28.04.2020 | 04.06.2020 |
| Jeremias et al., 2020 | USA | 70.2 | 42.8 | 1699 | 167 | 9.8 | NR | 01.03.2020 | 30.04.2020 |
| Psichogiou et al., 2020 | Greece | 69.7 | 46.4 | 1495 | 15 | 1.3 | 77.0 | 13.04.2020 | 14.05.2020 |
| Blairon et al., 2020 | Belgium | 72.4 | 43.9 | 1485 | 217 | 14.6 | 47.7 | 25.05.2020 | 19.06.2020 |
| Dimcheff et al., 2020 | USA | 64.0 | NR | 1476 | 72 | 4.9 | 51.0 | 08.06.2020 | 08.07.2020 |
| Goenka et al., 2020 | India | 34.6 | NR | 1122 | 134 | 11.9 | 25.0 | 12.07.2020 | 24.08.2020 |
Pooled analysis of SARS-CoV-2 seroprevalence and selected risk factors
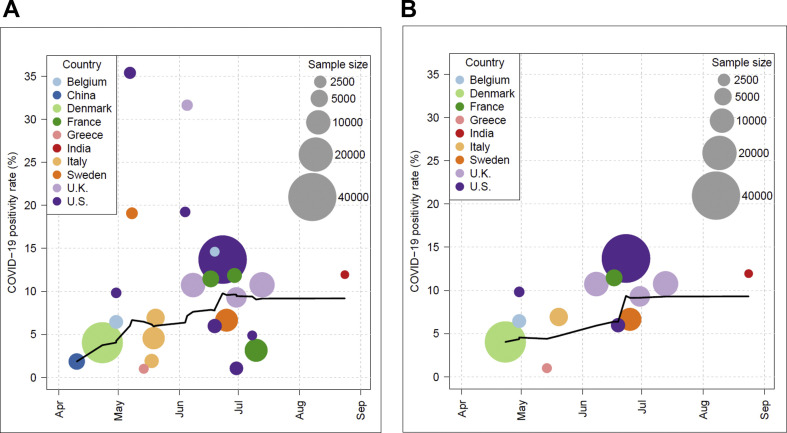
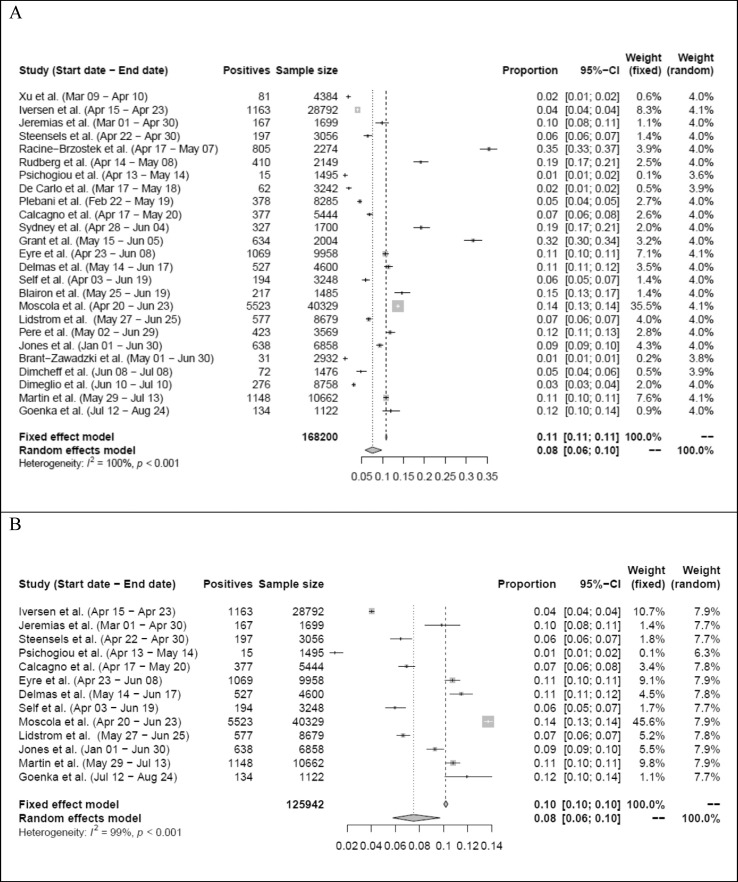
In total, data extracted for 168 200 HCWs were included. The lowest sample size was 1122 [12], the highest one was 40 329 [17] (Fig. 2 ). In meta-analysis using the random effects model, the weighted average of seroprevalence was calculated as 8% (95% CI 6–10%) with a heterogeneity of I 2 = 100% (p < 0.001) (Fig. 3, Fig. 4).
Fig. 2.
(A) The rates of SARS CoV-2 seropositivity among HCWs by antibody test among 25 studies. (B) The rates of SARS CoV-2 seropositivity among HCWs by antibody test after quality assessment among 13 studies.
Fig. 3.
(A) Forest plot of the severe acute respiratory syndrome coronavirus-2 seroprevalence rate among HCWs in 25 studies (see Fig. 2A). (B) Forest plot of the severe acute respiratory syndrome coronavirus-2 seroprevalence rate among HCWs after quality assessment, in 13 studies (see Fig. 2B).
Fig. 4.
Pooled analysis of SARS-CoV-2 seroprevalence rates among health care workers according to selected variables.
The pooled analysis of selected risk factors are presented in Table 3 (for the detailed Forest plots please see Figs S1–12). The pooled seroprevalence rates of the selected risk factors that have a rate over the average were male HCWs with 9% (95% CI 7–11%); non-white HCWs with 13% (95% CI 9–17%); frontline HCWs with 9% (95% CI 6–13%); exposure to the virus outside the health care setting 22% (95% CI 14–32%).
Discussion
Since the beginning of the pandemic HCWs were considered as the number one risk group for COVID-19, therefore our goal was to investigate seroprevalence rates among HCWs in scientific literature with over 1000 participants in order to ensure high level representation of the source population. The timeframe of our study was from the start of the pandemic until the vaccination programs began. At the first phase of the pandemic, the aim of the seroprevalence studies was to have an idea about the level of asymptomatic cases, risk factors and herd immunity in the population. Therefore, the seroprevalence studies among HCWs proved to be valuable to understand the risk and related factors as well. Towards the end of 2020, the vaccination programmes were in place, starting with HCWs. At the same time, there was another increasing concern regarding the new variants of the virus, all of which halted the efforts to detect antibodies among HCWs. Although vaccines have been stated to prevent the disease and severe illness, it is still under investigation whether vaccines will prevent acquiring and/or transmission of the infection [18,19].
Our analysis showed that the weighted average of the seroprevalence rate among HCWs in the selected studies was 8.0% (95% CI 6.0–10.0%) and the seropositivity rate reported in the articles ranged from 1.1% [15] to 35.4% [16]. However in a meta-analysis at the population level which excluded HCWs Rostami et al. reported that the SARS-CoV-2 seroprevalence rate was 3.38% (95% CI 3.05–3.72%) and in their report seroprevalence in the countries that we included in our analysis ranged from 0.36% in Greece to 15% in Sweden [20].
In our study, there were reports with a high seroprevalence rate (>20%) that required further explanation. One reason might be that two studies from the USA, in which the seroprevalence rates were reported as 35.4% [16] and 19% [21], representation of the African-American population, who already had higher rates of infection, in the general population was high, 48.4 % [16] and 27.2% [21] respectively. Additionally, the variance in seroprevalence rates reported in the studies was due to different commercial serological tests, statistical power or the studies and the timing of the study, corresponding to the waves in the epidemic curve of each country or region. For example, Racine-Brazostek et al. [16] conducted their study during the declining curve of the first wave in New York, USA, meaning that there were too many patients and too many unknowns about the prevention of COVID-19. Another study by Grant et al. [22] similarly reported a high level of seropositivity (31.6%) among HCWs and the authors suggested that it may be due to the spatial constraints in health care settings where exposures such as patient-to-HCWs or HCWs-to-HCWs might have played a role along with HCWs' awareness of and adherence to infection control measures. On the other hand, Brant-Zawadzki et al. [15] found the seroprevalence rate among HCWs in California, USA, to be 1.1%; however, the response rate in their research was below 50%. Xu et al. [23] reported a seroprevalence rate of 1.8% in Wuhan, China; however, their study was conducted after the first wave of the pandemic, when the daily number of cases in China was below 100 per day during their data collection period; the study is also subject to selection bias. Regarding the different conditions and the methods of data collection, it can be argued that the variance between studies is multifactorial.
Age
It has been well documented that older ages carry a higher risk for severe illness and death [24]. However, there were conflicting results in the studies we have included. Lidström et al. in Sweden [25], Iversen et al. in Denmark [13], Martin et al. [26], Eyre et al. [27] and Jones et al. [14] in the UK found that age was inversely associated with seropositivity. While their analysis depended on multivariate analysis, a similar statistically significant finding in univariate analysis by Goenka et al. [12] was diminished in the logistic regression model. Conversely, Xu et al. [23] in China found that seroprevalence significantly increased among HCWs over 65 years of age. Lastly, other studies [5,11,16,[27], [28], [29], [30], [31], [32], [33]] have found no significant association between seroprevalence rates and age. In a systematic review by Bandyopadhyay et al. [34] COVID-19 incidence rates at a global level were higher in older HCWs, especially in the 50–59 years age group. However, the incidence reports depend on PCR tests, which might represent active disease rather than past disease. Studies showing PCR screening results are out of our scope; however, it has been shown that symptomatic cases increase with age [35]. Detecting COVID-19 cases with PCR has the potential to yield results of relatively older patients. However, serological tests also convey past infections, which might have been asymptomatic or less symptomatic among younger populations. Hence, compared with PCR studies, seroprevalence studies can be expected to have a higher proportion of infected people from younger age groups. On the other hand, studies showing significant association between lower age and seropositivity might be a result of higher community transmission among younger adults and more active roles during patient care to protect older HCWs as well.
Gender
The majority of the studies included in our review have found no association between gender and seroprevalence rates. While Goenka et al. [12] found that the significant association between gender and seroprevalence rates diminished in multivariate analysis, males in the study by Iversen et al. [13] and females in the study by Self et al. [32] were significantly associated with higher seroprevalence rates. Our analysis shows that the pooled seroprevalence rates were 9% (95% CI 7–11%) for males and 8% (95% CI 6–10%) for females, which are very close to each other.
It has been shown that COVID-19 prevalence was higher among males for several reasons, including gender-based roles such as males being more likely to be employed in essential jobs, which increases their exposure to virus. At the same time, males had a higher tendency to engage in risky behaviours, including smoking [36]. Our findings from pooled analysis for gender data indicate a 1% higher seroprevalence for male HCWs. This slight difference is less likely to depend on gender roles, as the level of exposure to SARS-CoV-2 in health care settings might be a stronger determinant.
Race/ethnicity
Studies mainly from the USA and the UK have assessed the association between race/ethnicity and seropositivity among HCWs, and all [[14], [15], [16], [17],21,26,27,32] but one [11] found significantly increased rate among HCWs of African-American, Hispanic, Asian or indigenous populations. This finding is in line with previous studies indicating higher COVID-19 incidence among ethnic minority groups [6,37,38]. Our pooled analysis for seroprevalence according to race/ethnicity also confirms that white HCWs have lower levels of pooled seroprevalence (8%; 95% CI 6–11%) compared with HCWs from ethnic minorities (13%; 95% CI 9–17%).
The studies included in our review had limitations explaining the possible reasons of such a disparity in COVID-19 seroprevalence rate among HCWs based on race/ethnicity. It is well established that with few exceptions ethnic minorities are of lower socioeconomic status, and it is difficult to distinguish between the contributions of the two. Therefore, these findings indicate a deep inequality in exposure to SARS-CoV-2 against HCWs from ethnic minorities as a result of potential structural and social determinants of occupational health and safety conditions. Population-based studies could provide some explanations, such as crowded household conditions, cultural differences in social relation patterns, income and higher rates of comorbidities among ethnic minorities [37,39,40]. However, there is a need for further research in understanding the reasons of increased seropositivity among HCWs from ethnic minority populations, including access to PPE, adherence to infection control measures at clinical settings and their job descriptions.
Level and location of exposure to the virus
Our systematic review indicates that there has been a significantly increased seropositivity rate for HCWs in contact with patients either working in frontline service or a COVID-19 unit [5,22,25,26,28,41]. For example, Rudberg et al. [5] have shown that patient-related work increased the risk 2.3 times compared with other occupational groups. Moreover, even among the same occupational category, the ones with higher contact with patients had increased rates of seropositivity. It may be due to frequent contacts with the patients during the early stages of the disease, when they were more contagious. However, similar studies [11,17,30,33] did not report significant associations. While it is plausible that prolonged contact with patients, especially COVID-19 patients, increases the risk of transmission of the virus, some other studies have found no association between patient contact or work location, and the SARS-CoV-2 seropositivity rate indicates a contextual phenomenon [11,17,30,31,33].
Our pooled analysis results for level of exposure are in line with these findings. While HCWs with a high level of exposure to the virus have a higher pooled seropositivity rate (9% with 95% CI 6–13%) than average (8% with 95% CI 6–10%), the ones with a low level of exposure have a lower pooled seropositivity rate (7%; 95% CI 4–12%). As we have labelled high levels of exposure for the ones with increased patient contact, such a result indicates that the risk of contracting the disease lies with the group of HCWs who are spending more time at clinical encounters.
Another inquiry among the studies was the location of exposure. For example, Dimcheff et al. [30] and Steensels et al. [33] have shown no significant association between seropositivity rate and occupational factors, but there was only an increased seropositivity rate among the HCWs who had been exposed to a COVID-19 patients outside the health care system. Eyre et al. [27] reported household contact as the greatest risk factor for increased seropositivity among HCWs. Our pooled analysis for the seroprevalence rate based on location of exposure supports these findings. It is more likely that infection control measures and strict requirements to use PPE could have prevented patient-to-HCW as well as HCW-to-HCW transmission of SARS-CoV-2 in health care settings. However, it should not be underestimated that HCWs are at risk of getting infected in community settings, especially when the number of cases are on the increase [42].
Departments
Four studies from USA in our review have conveyed different results for the association of seropositivity and work place in health care setting. While Jeremias et al. [11], Self et al. [32] and Moscola et al. [17] have presented results showing low seropositivity rates in ICUs, there were no significant difference between departments. On the other hand, Sydney et al. [21] have found that seropositivity was significantly higher in EDs and significantly lower in ICUs. Similarly Eyre et al. [27] and Grant et al. [22] have found significantly lower rates of seropositivity in ICUs. The lower rates in ICUs might be the result of several factors, such as robust safety training for procedures in these units, availability of gowns and other superior PPE, strict adherence to preventive measures and well-ventilated wards [6,43]. Moreover, symptoms such as coughing or sneezing are limited in ICUs as patients use ventilators, and lastly aerosol-generating procedures might contain lower amounts of virus as patient admission to ICUs are at a later phase of the disease progression when viable virus secretion decreases [44].
This review is subject to several limitations. As each study has reported varying levels of sensitivity and specificity levels for their test kits, it is hard to ensure standard measurement across studies. Therefore, seroprevalence reported in studies might be subject to overestimation as well as underestimation. Also, there were limited studies from developing countries. Lastly, the risk factors associated with seroprevalence rates were not standard. However, as a first step we have included studies with over 1000 participants to minimize bias and ensure generalizability of SARS-CoV-2 seroprevalence among HCWs. Second, we have presented the outcomes of our analysis according to RoB assessment to illustrate the variation in seroprevalence rates.
Conclusion
This study brings together studies with high sample size to ensure a representative sample of HCWs from the targeted health care settings. However, the unavailability of high-quality studies with low RoB especially from developing countries makes it difficult to understand the true level of seropositivity among HCWs and related factors. Nevertheless, our analysis indicates that SARS-CoV-2 seroprevalence rate was 8% (95% CI 6–10%) and seropositivity was higher among HCWs who have been exposed to the virus outside the health care setting, the ones with high level of exposure to the virus and HCWs from ethnic minorities. Decreasing the burden of COVID-19 among HCWs depends on ensuring high adherence to infection control measures, early detection of infection in the health care settings along with prevention of transmission of the virus to HCWs in the community. There is also a need for high-quality seroprevalence studies among HCWs in future phases of the pandemic especially from developing countries to understand the real burden of COVID-19 and assess the efficacy of the vaccines and effectiveness of the vaccination programmes.
Author contributions
İ.K. and B.M. contributed equally as first authors. All authors contributed to the development of the selection criteria, the risk of bias assessment strategy and data extraction criteria made by İ.K., B.M. and Ş.K. The manuscript was drafted by İ.K., Ş.K., B.M., Ö.K. and Ö.E. All authors developed the search strategy. M.G. and Ö.E. provided statistical expertise. Ö.E., J.R.A., N. Psh. and N. Pet. provided expertise on SARS CoV-2 seroprevalence. All authors read, provided feedback and approved the final manuscript.
Transparency declaration
Conflict of interest: None of the authors declared conflict of interest. Funding: There is no external funding.
Acknowledgement
We are thankful to Ertaç Nebioğlu from Koç University Health Sciences Library for his detailed literature search.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.05.036.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Adams J.G., Walls R.M. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020;323:1439–2140. doi: 10.1001/jama.2020.3972. [DOI] [PubMed] [Google Scholar]
- 2.WHO . 2020. Keep health workers safe to keep patients safe.https://www.who.int/news/item/17-09-2020-keep-health-workers-safe-to-keep-patients-safe-who [Google Scholar]
- 3.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.G., Ma M., et al. C.O.P.E. Consortium Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gholami M., Fawad I., Shadan S., Rowaiee R., Ghanem H., Hassan Khamis A., et al. COVID-19 and healthcare workers: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:335–346. doi: 10.1016/j.ijid.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudberg A.S., Havervall S., Manberg A., Jernbom Falk A., Aguilera K., Ng H., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivett L., Sridhar S., Sparkes D., Routledge M., Jones N.K., Forrest S., et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Santos W.M.D., Secoli S.R., Puschel V.A.A. The Joanna Briggs Institute approach for systematic reviews. Rev Lat Am Enfermagem. 2018;26 doi: 10.1590/1518-8345.2885.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzer G., Carpenter J.R., Rücker G. Springer International Publishing Switzerland; Cham, Switzerland: 2015. Meta-analysis with R. [Google Scholar]
- 11.Jeremias A., Nguyen J., Levine J., Pollack S., Engellenner W., Thakore A., et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020;180:1707–1709. doi: 10.1001/jamainternmed.2020.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goenka M., Afzalpurkar S., Goenka U., Das S.S., Mukherjee, Jajodia S., et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68:14–19. [PubMed] [Google Scholar]
- 13.Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J.H., Nielsen P.B., Pries-Heje M., et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones C.R., Hamilton F.W., Thompson A., Morris T.T., Moran E. SARS-CoV-2 IgG seroprevalence in healthcare workers and other staff at North Bristol NHS Trust: a sociodemographic analysis. J Infect. 2021;82:e24–e27. doi: 10.1016/j.jinf.2020.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brant-Zawadzki M., Fridman D., Robinson P.A., Zahn M., Chau C., German R., et al. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racine-Brzostek S.E., Yang H.S., Chadburn A., Orlander D., An A., Campion T.R., et al. COVID-19 viral and serology testing in New York City health care workers. Am J Clin Pathol. 2020;154:592–595. doi: 10.1093/ajcp/aqaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moscola J., Sembajwe G., Jarrett M., Farber B., Chang T., McGinn T., et al. For thee Northwell Health Consortium prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin E.J., Longo D.L. SARS-CoV-2 vaccination – an ounce (actually, much less) of prevention. N Engl J Med. 2020;383:2677–2678. doi: 10.1056/NEJMe2034717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipsitch M., Dean N.E. Understanding COVID-19 vaccine efficacy. Science. 2020;370:763–765. doi: 10.1126/science.abe5938. [DOI] [PubMed] [Google Scholar]
- 20.Rostami A., Sepidarkish M., Leeflang M.M.G., Riahi S.M., Nourollahpour Shiadeh M., Esfandyari S., et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:331–340. doi: 10.1016/j.cmi.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sydney E.R., Kishore P., Laniado I., Rucker L.M., Bajaj K., Zinaman M.J. Antibody evidence of SARS-CoV-2 infection in healthcare workers in the Bronx. Infect Control Hosp Epidemiol. 2020;41:1348–1349. doi: 10.1017/ice.2020.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant J.J., Wilmore S.M.S., McCann N.S., Donnelly O., Lai R.W.L., Kinsella M.J., et al. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2021;42:212–214. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X., Sun J., Nie S., Li H., Kong Y., Liang M., et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 24.CDC . 2021. Older adults.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html [Google Scholar]
- 25.Lidström A.K., Sund F., Albinsson B., Lindback J., Westman G. Work at inpatient care units is associated with an increased risk of SARS-CoV-2 infection; a cross-sectional study of 8679 healthcare workers in Sweden. Ups J Med Sci. 2020;125:305–310. doi: 10.1080/03009734.2020.1793039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin C.A., Patel P., Goss C., Jenkins D.R., Price A., Barton L., et al. Demographic and occupational determinants of anti-SARS-CoV-2 IgG seropositivity in hospital staff. J Public Health (Oxf) 2020 doi: 10.1093/pubmed/fdaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyre D.W., Lumley S.F., O’Donnell D., Campbell M., Sims E., Lawson E., et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife. 2020;9 doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calcagno A., Ghisetti V., Emanuele T., Trunfio M., Faraoni S., Boglione L., et al. Risk for SARS-CoV-2 infection in healthcare workers, Turin, Italy. Emerg Infect Dis. 2021;27:303–305. doi: 10.3201/eid2701.203027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delmas C., Plu-Bureau G., Canoui E., Mouthon L., Meritet J.F. Clinical characteristics and persistence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) IgG antibodies in 4,607 French healthcare workers: comparison with European countries. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.1309. 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimcheff D.E., Schildhouse R.J., Hausman M.S., Vincent B.M., Markovitz E., Chensue S.W., et al. Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection among Veterans Affairs healthcare system employees suggests higher risk of infection when exposed to SARS-CoV-2 outside the work environment. Infect Control Hosp Epidemiol. 2020;42:392–398. doi: 10.1017/ice.2020.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Psichogiou M., Karabinis A., IPavlopoulou I.D., Basoulis D., Petsios K., Roussos S., et al. Antibodies against SARS-CoV-2 among health care workers in a country with low burden of COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Self W.H., Tenforde M.W., Stubblefield W.B., Feldstein L.R., Steingrub J.S., Shapiro N.I., et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network – 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steensels D., Oris E., Coninx L., Nuyens D., Delforge M.L., Vermeersch P., et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandyopadhyay S., Baticulon R.E., Kadhum M., Alser M., Ojuka D.K., Badereddin Y., et al. Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M., et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 36.Abate B.B., Kassie A.M., Kassaw M.W., Aragie T.G., Masresha S.A. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menachemi N., Yiannoutsos C.T., Dixon B.E., Duszynski T.J., Fadel W.F., Wools-Kaloustian K.K., et al. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample – Indiana, April 25–29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:960–964. doi: 10.15585/mmwr.mm6929e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin C.A., Jenkins D.R., Minhas J.S., Gray L.J., Tang J., Williams C., et al. Socio-demographic heterogeneity in the prevalence of COVID-19 during lockdown is associated with ethnicity and household size: results from an observational cohort study. EClinicalMedicine. 2020;25:100466. doi: 10.1016/j.eclinm.2020.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackey K., Ayers C.K., Kondo K.K., Saha S., Advani S.M., Young S., et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2021;174:362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razai M.S., Kankam H.K.N., Majeed A. Mitigating ethnic disparities in covid-19 and beyond. BMJ. 2021 doi: 10.1136/bmj.m4921. [DOI] [PubMed] [Google Scholar]
- 41.Blairon L., Mokrane S., Wilmet A., Dessilly G., Kabamba-Mukadi B., Beukinga I., et al. Large-scale, molecular and serological SARS-CoV-2 screening of healthcare workers in a 4-site public hospital in Belgium after COVID-19 outbreak. J Infect. 2021;82:159–198. doi: 10.1016/j.jinf.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belingheri M., Paladino M.E., Riva M.A. Beyond the assistance: additional exposure situations to COVID-19 for healthcare workers. J Hosp Infect. 2020;105:353. doi: 10.1016/j.jhin.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook T.M., Lennane S. Occupational COVID-19 risk for anaesthesia and intensive care staff – low-risk specialties in a high-risk setting. Anaesthesia. 2021;76:295–300. doi: 10.1111/anae.15358. [DOI] [PubMed] [Google Scholar]
- 44.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.