Abstract
Peripheral nerve injuries of the upper extremity can result from a wide array of etiologies, with the two most common being compression neuropathy and traumatic injuries. These types of injuries are common and can be psychologically, functionally, and financially devastating to the patient. A detailed preoperative evaluation is imperative for appropriate management. Traumatic injuries can typically be treated with local burial techniques, targeted muscle reinnervation, and regenerative peripheral nerve interfaces. Median nerve compression is frequently managed with complete release of the antebrachial fascia/transverse carpal ligament and/or use of flap coverage such as the hypothenar fat pad flap and local muscle flaps. Ulnar nerve compression is commonly managed via submuscular transposition, subcutaneous transposition, neurolysis, and nerve wrapping. In this review, we discuss the preoperative evaluation, surgical techniques, and advantages and disadvantages of each treatment modality for patients with compressive and traumatic upper extremity nerve injuries.
Keywords: upper extremity nerve injury, compression neuropathy, revision peripheral nerve surgery
Peripheral nerve pathology of the upper extremity can take on many forms, with compression neuropathy and traumatic injuries being two major etiologies. Peripheral compression neuropathies tend to be more common, with carpal tunnel syndrome (CTS), the most common entrapment neuropathy, affecting approximately 3.7% of the general population. 1 Traumatic peripheral nerve injuries, while less common, will frequently affect the upper extremity with an average annual incidence in the United States of 43.8 per 1 million people. 2 3 These conditions can present with a variety of symptoms, including functional deficits, neuropathic pain, and abnormal or deficient sensation, which can cause significant disability and have a severe impact on a patient's quality of life. 4 5 6 7 Additionally, a high economic burden can be placed on the patient, as the annual care for peripheral nerve injuries costs nearly $50,000 on average. 2 While the majority of patients being managed with these conditions tend to do well, a portion will develop situations prompting further intervention. The purpose of this article is to discuss the assessment and management of revision surgery in the setting of compression and traumatic neuropathy.
Preoperative Evaluation
Patients who present with abnormal sensation/deficits, neuropathic pain, or functional deficits after a history of a peripheral nerve injury or decompression surgery require an extensive preoperative assessment. This assessment is crucial for establishing an accurate diagnosis, determining an appropriate surgical management if applicable, and overall addressing patient concerns. Neuropathic pain is a relatively common indication for undergoing surgical revision from a prior operation. 8 Nevertheless, a thorough history, careful physical exam, diagnostic studies, and imaging are required to holistically address the patient's injury. To begin, the surgeon should inquire into the nature of the injury regarding its timing and etiology. 9 Additionally, the patient's past surgical history concerning the original peripheral nerve injury should be discussed, as prior surgical intervention may provide insight into the patient's current presentation. 10 11 Treatment options differ based on whether the patient presents with neuroma, nerve compression, nerve discontinuity, or musculoskeletal pain. Consequently, it is critical to assess the character and quality of the nerve pain as well as the level of activity of the affected limb, given that a missed diagnosis could result in worsening symptoms. 11 Use of standardized pain assessment tools, such as simple visual analogue scales, coupled with more comprehensive evaluations such as the DASH (Disability of the Arm, Shoulder, and Hand) and PROMIS (Patient-Reported Outcomes Measurement Information System) scales may provide the surgeon with a better understanding of the underlying condition specifically regarding pain and motor function. 11 12 13 In the setting of neuropathic pain, it is often helpful to have pain management involved to help optimize pain management prior to and after intervention. Neuropathic pain can often be exacerbated after intervention, and it is valuable to have a plan already in place for management.
The physical exam is an integral component of the preoperative assessment and necessitates a systematic approach to corroborate findings with the history. Before beginning the physical exam, attention should be placed on the initial encounter with the patient. 11 The overall appearance of the patient and visible signs of pain or guarding at rest will validate the story or provide insight into the severity of pathology. The surgeon should then meticulously perform a complete neuromuscular exam of the affected limb and compare it to the contralateral side. Edema, color changes, warmth, and any scars from prior surgeries should be noted, followed by the full sensory and motor exam. The sensory exam involves assessing for general sensation pattern differences by utilizing a Likert scale. If tolerated, two-point discrimination can also be performed. 11 14 Gentle percussion at the sight of traumatic scars may elicit a Tinel's sign. It is valuable to have the patient draw out the sensory nerve distribution of symptoms so that a baseline is available for future comparison, as complete resolution of neuropathic pain is not always possible. Motor function and range of motion should be systematically examined from the cervical region to the digits. It is helpful to inject a small amount of lidocaine 2% at the site of the Tinel's sign to assess if management of a neuroma at that site would likely result in complete resolution of the patient's symptoms.
In the setting of recurrent compression neuropathies, specific provocative maneuvers such as Phalen's/Durkan's test for median nerve entrapment, elbow flexion/compression at the cubital tunnel for proximal ulnar nerve entrapment, and Tinel's sign over Guyon's canal for distal ulnar nerve entrapment should be performed. 15 One must also consider the possibility of a more proximal compression and a double-crush phenomenon when assessing recurrent compression neuropathies. 16 Eason et al reported that 81% of their patients with suboptimal results following carpal tunnel release presented with cervical pain and abnormal imaging, supporting the double-crush phenomenon. 17 Wessel et al further demonstrated that patients with double-crush syndrome demonstrate higher pain and lower satisfaction scores following both nerve decompression and cervical spine surgery, compared with those with isolated peripheral nerve compression who similarly underwent surgical management. 18
Additional testing can provide supplemental objective information when the diagnosis is unclear. Nerve conduction studies and electromyograms (EMGs) can be performed to evaluate injury or compression to larger mixed motor/sensory nerves. In the setting of recurrent compression neuropathy, repeat nerve studies can be compared with previous nerve studies to evaluate for improvement of latency, conduction velocities, or amplitude. High-resolution ultrasonography is useful for analyzing peripheral nerve pathology, particularly in the acute and subacute setting. Ultrasound is a safe, inexpensive, and noninvasive imaging modality that can be performed to assess for the precise location, etiology, and extent of peripheral nerve injury. 19 20 21 Toia et al reported that high-resolution ultrasonography provided diagnostic value to the preoperative evaluation and confirmed electrodiagnostic findings in a significant portion of their patients regardless of pathology. 20 High-resolution ultrasonography has demonstrated 89% sensitivity and 95% specificity for diagnosing peripheral nerve injury. 22 Ultrasound can also help diagnose compression neuropathies such as carpal and cubital tunnel syndrome via enlargement of the median and ulnar nerves, respectively. 23 24 25 26 27 28 Duetzmann et al demonstrated utility of ultrasound for diagnosing recurrent CTS based on cross-sectional area (CSA) of the median nerve. 25 One study reported 95% sensitivity and 100% specificity for diagnosing CTS when utilizing a CSA cut-off of 9 mm 2 . 29 While imaging serves to complement the clinical and electrodiagnostic exams in the preoperative setting, ultrasound may be less effective for chronic nerve injuries due to the extent of fibrosis that may encompass the nerve.
Corticosteroid injections can be performed at the carpal tunnel to potentially support the diagnosis of recurrence if symptoms temporarily improve, as history, physical examination, and electrodiagnostic studies can sometimes be inconclusive.
Traumatic Injury
Management Options
Cutaneous peripheral neuromas affecting the hand and forearm can cause significant pain and morbidity to the patient as a result of disorganized axon regeneration sheathed in scar tissue. 30 31 32 Many surgical techniques have been described in the literature for neuroma management, underscoring the lack of a decisive treatment strategy that supersedes the rest; however, the technique of neuroma excision and transposition has been studied thoroughly due to its relative success. 31 33 34 35 36 Other options include coapting the nerve to its distal counterpart (if available), targeted muscle reinnervation (TMR), and regenerative peripheral nerve interfaces (RPNIs).
The surgical principles to consider while performing the local burial technique include avoiding tension to the proximal nerve end, placing the nerve away from the skin surface, and minimizing scar tissue formation surrounding the transected nerve. 36 The neuroma should be visualized and excised. The transected nerve should then be dissected further proximally to provide adequate length to transpose the nerve to a proximal anatomical site for deep burial. 37 It is helpful to also secure the nerves into their burial sites using microsutures (similar to techniques used in RPNI, discussed later), with or without the use of a fibrin glue. This helps prevent postoperative dislodgement of the nerve from the burial site.
TMR is a surgical technique that was originally designed to improve upper limb prosthetic control for amputees through nerve transfers, and has been used to help manage terminal neuromas associated with amputations. TMR has been principally performed for transhumeral and shoulder disarticulation patients, but its use has been extrapolated to other areas of the body, including distal upper extremity and lower extremity. 38 39 The surgical technique has been previously described in detail. 40 41 42 Briefly, nerves that previously innervated muscles of the upper extremity are transferred to reinnervate alternative muscles that have since been rendered functionless ( Fig. 1 ). 42 Reinnervation of the muscle results in amplification of the electrical signal from the transferred nerve. The EMG signals are then recorded and translated into motor function utilizing a myoelectric prosthesis.
Fig. 1.
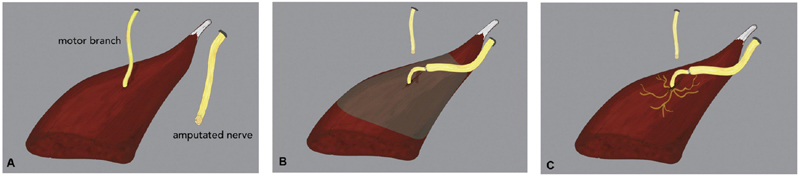
Illustration of targeted muscle reinnervation. ( A ) Motor nerve target and recently amputated nerve. ( B ) Motor nerve target is transected and coapted to the amputated nerve. ( C ) Reinnervation of the muscle results in neurotization and muscle regeneration. (Reproduced with permission from Valerio et al. 44 )
While initially designed to foster better control of prosthesis in amputees, TMR has evolved as a surgical technique to manage patients who develop postoperative pain. It has demonstrated success in treating phantom limb pain (PLP) and residual limb pain typically caused by terminal neuroma formation by providing the affected nerve with an end target. 30 34 In their randomized clinical trial, Dumanian et al reported that TMR reduced PLP and residual limb pain to a greater degree compared with conventional methods of neuroma excision and muscle burying techniques. 30 Furthermore, TMR has also served to prevent postoperative pain if performed at the time of limb amputation. 43 44 45 Valerio et al established that preemptive management of postamputation pain with TMR resulted in significantly reduced PLP and residual limb pain compared with the untreated amputee cohort. 44
Recently, TMR in the hand has been studied to examine motor entry points (MEPs) to the intrinsic muscles. In their anatomical study, Daugherty et al demonstrated that the MEPs had a consistent location in the intrinsic hand muscles with favorable sensory-to-MEP diameter ratios of less than 2:1. They reported 19 MEPs through the volar approach and 12 MEPs through the dorsal approach. Because ray amputation is typically approached dorsally, they advocate for performing TMR primarily via a dorsal approach in all digits but the thumb, due to unreliability of the MEPs in the thenar muscles dorsally. Secondary management of neuromas, however, can be approached either dorsally or volarly depending on location. 46 Elmaraghi et al. clinically examined outcomes for two cases following TMR in the hand: (1) at the time of ray amputation and (2) secondarily following neuroma formation. Both cases described resulted in successful relief and prevention of pain symptoms. 47
Despite the advancements in human-prosthetic interfacing via TMR, improvements in signal fidelity, long-term stability, and real-time feedback are still necessary. RPNI serves to address these particular factors to provide optimal prosthetic control; furthermore, its utilization of free muscle grafts is valuable in more distal aspects of the digit and hand where muscle bulk is absent to provide sufficient burial options for managing neuromas. 48 RPNI involves first uncovering and resecting the neuroma under tourniquet control. Free muscle grafts for each RPNI are then excised from a dispensable donor site parallel to the axis of the muscle fibers and transferred near the proximal peripheral nerve end. 48 49 Under loupe magnification, the epineurium of the nerve end is sutured to the epimysium at the center of the muscle graft. 48 49 50 The muscle graft is then completely enveloped around the severed end of the nerve via additional sutures and the RPNI is placed deep into the soft tissue ( Fig. 2 and Fig. 3 ). 48 49 51 Interestingly, both sensory and motor nerve endings can be utilized for the purposes of creating RPNIs with comparable outcomes. 49 52 Studies have shown that the muscle undergoes regeneration, revascularization, and reinnervation, which serves to amplify the EMG signals from the residual nerve. 53 54 55 If prosthetic use is anticipated, an electrode is sutured to the distal end of the relocated muscle, and remains functional throughout an extended timeframe. 50
Fig. 2.
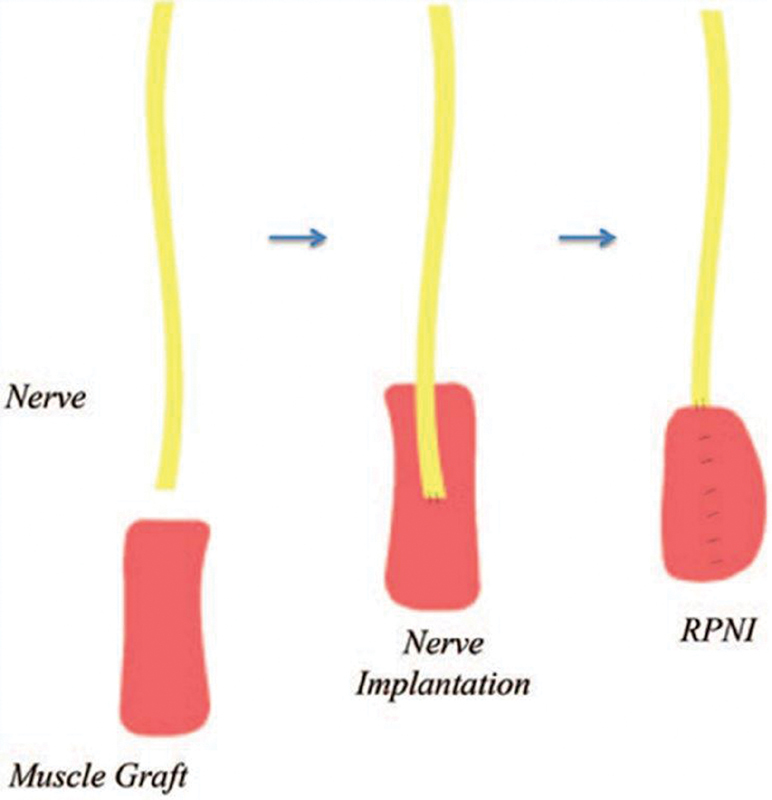
Schematic of a regenerative peripheral nerve interface (RPNI). (Reproduced with permission from Woo et al. 49 )
Fig. 3.
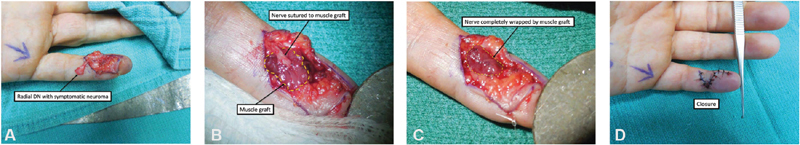
RPNI technique for surgical management of a symptomatic left fifth digit radial digital nerve neuroma. ( A ) Dissected radial digital nerve neuroma for planned neuroma excision. ( B ) Radial digital nerve sutured into transposed muscle graft. ( C ) Muscle graft wrapped circumferentially around the radial digital nerve. ( D ) Closure. (Reproduced with permission from Hooper et al. 48 )
Similar to TMR, RPNI has also been performed to manage peripheral neuromas following limb amputation. 48 49 56 Woo et al observed a considerable reduction in neuroma pain and PLP for major limb amputations following RPNI implantation. 49 Hooper et al noted that 85% of patients either were pain-free or experienced substantial pain improvement following treatment of neuroma pain with RPNIs after distal amputations of the hand and digits. 48 RPNI can also prevent neuroma formation via its ability to promote reinnervation of the free muscle graft and should be considered as a prophylactic procedure at the time of amputation. 49 56 57 In one report of 181 collective RPNI procedures, no patients clinically presented with symptoms of neuroma formation at their 1-year postoperative follow-up. 56
Most recently, TMR has been combined with an adapted vascularized RPNI technique to manage and prevent neuroma formation. This novel surgical variation involves coaptation of an amputated nerve to a proximal motor nerve target (TMR), followed by wrapping the neurorrhaphy with the surrounding, recently denervated, vascularized muscle (vascularized RPNI). Valerio et al argue that this technique may contribute to enhanced muscle neurotization and nerve regeneration. 58
Management Based on Location
Sood and Elliot originally classified neuroma formation within the hand and forearm into three zones ( Table 1 ). 59 Subsequent management options are based on which zone the neuroma lies in. Zone I injuries have several options for management, including burial within bone, RPNI, and more proximal TMR. First reported by Hazari and Elliot, terminal neuromas within zone I are commonly relocated to the lateral surface of the proximal phalanx or the dorsolateral aspect of the metacarpal bone. Drill holes should be placed prior to nerve transfer to obviate the risk of intraoperative nerve injury. 60 61 If pain recurs, nerve relocation to the pronator quadratus muscle can be performed as a respectable alternative with more proximal dissection. 35 60 Generally, however, zone I neuromas are not buried within muscle distally due to a greater risk of insufficient muscle bulk locally within the digit itself. 36
Table 1. Zones of the hand for nerve transposition.
| Zone involvement | Nerve involvement |
|---|---|
| Zone I | Digital nerves Terminal sensory branches of nerves innervating dorsal hand |
| Zone II | Common digital nerves Palmar cutaneous branches of the median nerve Palmar cutaneous branches of the ulnar nerves Dorsal branches of the ulnar nerve |
| Zone III | Superficial radial nerve Medial antebrachial cutaneous nerve Lateral antebrachial cutaneous nerve Posterior cutaneous nerve of the forearm |
Zone II neuromas have several options for local burial. Neuromas can be buried within the pronator quadratus or beneath the thenar or hypothenar musculature. 36 59 62 63 When relocating within the pronator quadratus, dissection is performed 5 to 10 cm proximal to the wrist to allow for sufficient length for transposition. The nerve is then buried deep in the muscle and sutured to its epimysium. 63 In an extension of Sood and Elliot's original paper, Atherton et al reported greater than 90% control in all pain subgroup categories following nerve relocation to the pronator quadratus muscle. 59 63 Transposition to the underside of the thenar and hypothenar muscles can also be performed; however, its overall performance has not been well accepted in the literature. 36
Zone III neuromas can be most difficult to manage due to appreciable overlap of the nerve territories within the forearm; however, more local muscle options are available proximally to bury the nerve within. 64 65 Moreover, neuromas in continuity of the major peripheral nerves tend not to have a sensitivity component to them, as the median and ulnar nerves tend to lie beneath musculature, which provide sufficient padding. For the more superficial nerves, however, similar to surgical treatment of zone I and II neuromas, the neuroma is resected and the affected nerve is dissected proximally. The superficial radial nerve is dissected to its exit point at the brachioradialis muscle and is transferred to this muscle. 64 The lateral antebrachial cutaneous nerve is traced through the forearm fascia, then lateral to the biceps tendon, and finally to the brachialis muscle, where it is transected just distal to its muscle belly for transposition to the brachialis muscle. 64 66 The medial antebrachial cutaneous nerve branches can be traced to where they converge along the medial aspect of the elbow and can be relocated to the biceps muscle. The posterior cutaneous nerve of the forearm is commonly dissected and transferred to muscle located proximal to the injury such as the brachioradialis muscle or the triceps muscle. 37 64 While zone III neuromas are complex, muscle burial within the forearm and arm muscles has demonstrated reliable results with significantly reduced pain outcomes postoperatively. 64 66 Though these surgical techniques have demonstrated the adaptability of the muscle burial method, pain recurrence at the original and relocation site is still possible and may require revisional operations. Additionally, a drawback to this operative technique is the loss of sensory input distal to the relocated nerves. 35 36 64 These complications should be discussed with the patient prior to undergoing the procedure to ensure realistic expectations.
Revision Nerve Compression Surgery
Carpal Tunnel Revision
CTS is the most common compression neuropathy of the upper extremity and consequently carpal tunnel decompression is the most frequently performed hand procedure. 67 68 However, up to 25% of patients who undergo carpal tunnel decompression will experience treatment failure. 69 The open approach, limited incision, and endoscopic method have all been utilized to surgically manage CTS. 70 71 72 While the endoscopic technique has resulted in faster recovery times and reduced risk of scar tenderness compared with open carpal tunnel release, it has demonstrated higher risk of intraoperative nerve injury and revision surgery (2–4% for endoscopic vs. 1–2% for open release). 73 74 75 76 These increased rates of iatrogenic injuries are likely due to anatomical variants of the median nerves, which may be as high as 25%. 77 78 Ancillary findings, such as amyloidosis, calcific tendonitis, and ganglion cysts, may also render endoscopic carpal tunnel release a futile endeavor ( Fig. 4 ). 77 Furthermore, patients who present with systemic disease such as diabetes (particularly diabetic neuropathy), hypothyroidism, and rheumatoid arthritis typically display worse outcomes than those with isolated compression neuropathies. 76 79 Patients with preoperative severe findings (based on muscle atrophy or electrodiagnostics) may also portend to delayed, slowed, or incomplete recovery due to the longstanding nature of the compression injury to the nerve. Generally, 6 months or more are allowed to determine the true result or outcome from nerve decompression.
Fig. 4.
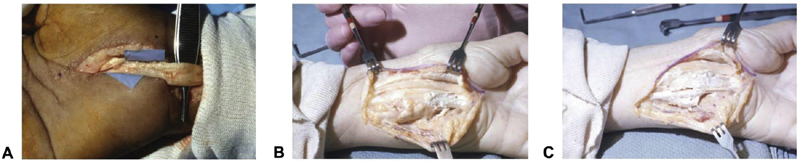
Ancillary findings that are contraindications to endoscopic carpal tunnel release. ( A ) Patient with renal failure and systemic lupus erythematosus presenting with amyloidosis of the tendon. ( B ) A second patient with calcific tendinitis. ( C ) This same patient following excision of the calcified substance. (Reproduced with permission from Gould et al. 77 )
The subjective history from the patient following their original release surgery can provide helpful clues as to the etiology of their continued symptomatology. Tung and Mackinnon classified patients into persistent, recurrent, and new pain. 68 Persistence of symptoms/pain usually occurs due to incomplete release of the transverse carpal ligament distally or the antebrachial fascia more proximally at the wrist. 68 80 Patients never experience complete relief of pain following the operation.
Recurrent cases have symptoms resolve after surgery for some period of time, but with eventual recurrence of symptoms. It is important at this point to characterize whether the symptoms are exactly the same as prior to surgery or different. Recurrence frequently results from pathology at the previous surgery site, including scar tethering of the nerve, fibrosis circumferentially, or double-crush syndrome in which release of the median nerve unmasks a more proximal injury. Scarring involving the median nerve may, to some degree, have to do with where the previous release on the transverse retinacular ligament was performed. If the ligament was released directly over the median nerve versus adjacent to this, there would be a greater tendency for the resulting healing scar to involve the median nerve.
New symptoms usually arise due to intraoperative complications such as injury to the surrounding nerve branches, vasculature, and tendons. Patients generally display symptoms that were not originally present prior to their index operation. 68 80
Surgical management for a revisional procedure will vary depending on the etiology of the patient presentation. Importantly, reoperation commonly results in worse outcomes compared with the primary procedure, which should be explained to the patient prior to undergoing surgical intervention. 81 Additionally, the surgery itself tends to be more extensive than the original operation, with the carpal tunnel release being performed in an extended fashion, with or without the use of hypothenar fat pad flap (HTFPF). 82 83 During the surgery, the transverse carpal ligament/antebrachial fascia will seem to be intact and not completely released, but this will always be present and may just be a representation of the ligament or fascia healing in a more elongated position. A more extensive evaluation of the carpal tunnel contents is also performed to identify scarring around the median nerve itself, significant synovitis of the adjacent flexor tendons, or ganglion cysts along the floor of the carpal tunnel. 77 It is important to note that adjuvant flexor tenosynovectomy does not result in better outcomes compared with isolated carpal tunnel release, 84 but biopsy may provide additional information regarding potentiating underlying diagnoses, such as amyloidosis or rheumatoid arthritis.
The HTFPF was developed as an interposition flap to prevent additional scar formation and fibrosis around the median nerve. 85 86 87 The procedure involves the performance of the carpal tunnel release, and subsequently after identifying the median nerve, an external neurolysis is performed if significant perineural fibrosis is noted. 85 87 The hypothenar fat pad is then raised with its pedicle off of the ulnar artery and the flap is transposed volar to the median nerve and deep to the radial leaf of the transverse carpal ligament. 82 85 Chrysopoulo et al delineated modifications to the traditional surgical technique. In their operative method, dissection of the fat pad continues medially even after visualization of the ulnar neurovascular bundle, which provides enhanced flap mobility without jeopardizing the vascular supply ( Fig. 5 and Fig. 6 ). 88 In their systematic review on surgical revision techniques for recalcitrant CTS, Soltani et al reported that decompression with flap interposition resulted in enhanced outcomes compared with isolated open decompression (86 and 75%, respectively). 89 Craft et al corroborated their results with previous studies demonstrating that the HTFPF combined with microneurolysis was an effective method for reducing pain, numbness, and paresthesias in patients with recalcitrant symptoms. 85 90 91 They reported that pain and numbness were completely resolved postoperatively in 83 and 42% of their patients, respectively. 90 Conversely, there are data to suggest that there may be no significant benefit to performing the fat pad flap. 92 Specifically, Pace et al reported that there was no significant difference in self-reported symptom and function severity when queried via telephone and a Boston Carpal Tunnel Questionnaire. 92
Fig. 5.
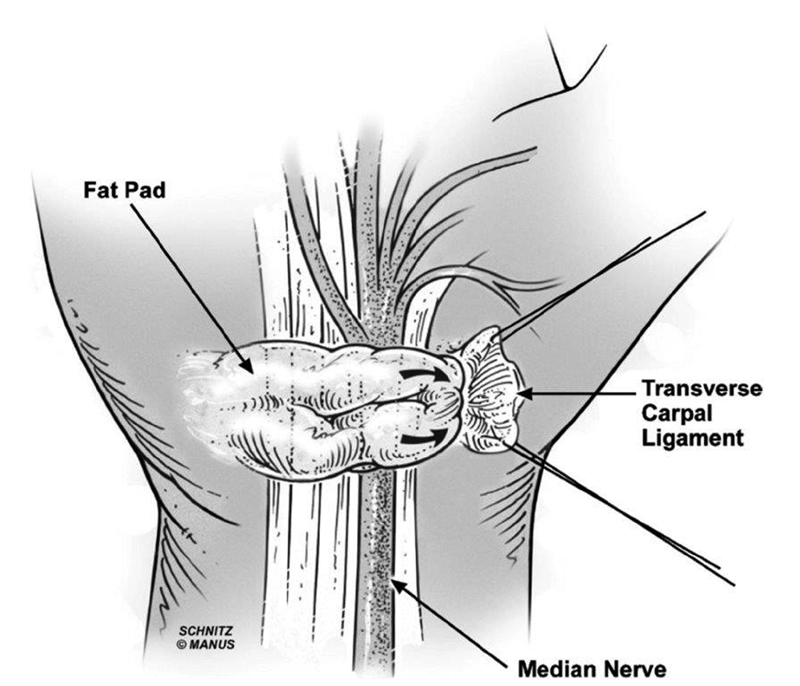
Schematic illustrating the vascularized hypothenar fat pad transposition flap overlying and protecting the neurolyzed median nerve. (Reproduced with permission from Chrysopoulo et al. 88 )
Fig. 6.
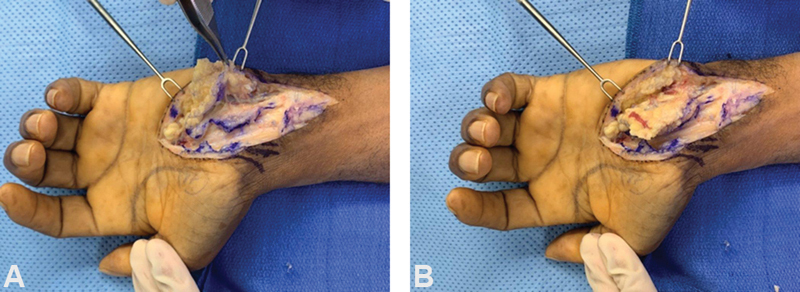
The hypothenar fat pad transposition flap technique for patient with recurrent carpal tunnel syndrome. ( A ) Fat pad raised off the hypothenar muscles and transverse carpal ligament. ( B ) Fat pad is transposed over the median nerve. (The images are provided courtesy of Dr. David Netscher.)
In addition to the HTFPF, other muscle flaps have also been employed to treat recurrence of symptoms, including the pronator quadratus, abductor digiti minimi, and palmaris brevis muscles. 93 94 95 The pronator quadratus muscle is particularly helpful in situations where the Tinel's sign and circumferential scarring are located proximal to the distal palmar crease, as its ability to translate distally will be limited by its vascular pedicle ( Fig. 7 ). In addition to muscle and adipofascial flaps, Dy et al reviewed the myriad of nerve barrier options, including autologous vein grafts, human amniotic fluid membrane wraps, and xenografts largely composed of type 1 collagen (e.g., bovine tendon). They concluded their review by recommending use of the HTFPF for recurrent CTS if perineural fibrosis was present, and recommending a submuscular transposition in the setting of recurrent cubital tunnel syndrome. If a submuscular transposition had previously been performed, then they would perform an autologous vein wrap if perineural fibrosis was also present ( Fig. 8 ). 96 They stated that, regardless of choice, the barrier should minimize risk of inflammation or rejection, provide adequate permeability to promote diffusion of nutrients while preventing axonal escape, avert scar-induced ischemia, facilitate nerve gliding, and avoid or reduce donor-site morbidity. 96
Fig. 7.
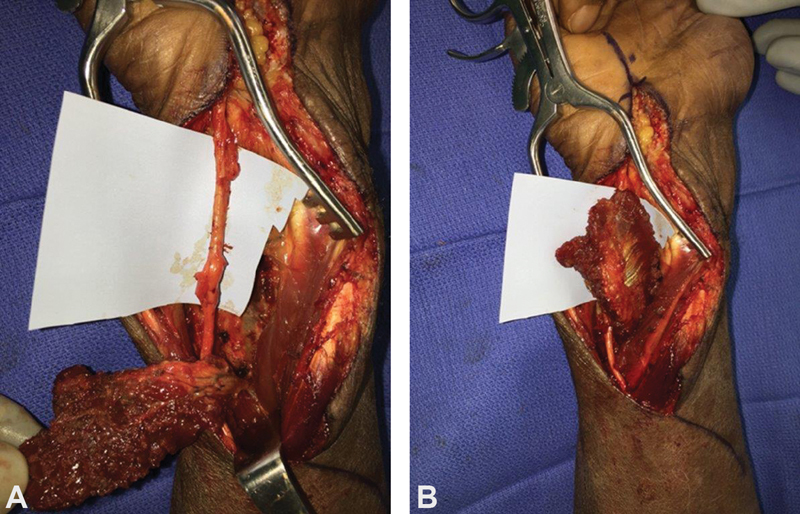
The pronator quadratus flap technique for patient with recurrent carpal tunnel syndrome proximal to distal palmar crease. ( A ) Neurolyzed median nerve and elevated pronator quadratus flap. ( B ) Transposed pronator quadratus flap wrapped circumferentially around median nerve.
Fig. 8.

Autologous saphenous vein wrap technique for peripheral nerve compression. ( A ) Incisional markings and superficial veins are outlined. ( B ) The peripherally compressed nerve is dissected and exposed. ( C ) The saphenous vein is harvested and transected longitudinally. ( D ) The saphenous vein graft is circumferentially wrapped around the peripherally compressed nerve. ( E ) The graft should be wrapped loosely around the nerve as appears from placement of the forceps between the nerve and vein wrap. (Reproduced with permission from Dy et al. 96 )
In addition to compression at the carpal tunnel, the median nerve can be compressed more proximally ( Fig. 9 ). As such, possible “recurrent” CTS requires assessment for weakness of the flexor digitorum profundus of the index finger and sensory changes involving the palmar cutaneous branch of the median nerve. These findings would point toward a more proximal point of compression. Median nerve entrapment along the proximal forearm is commonly termed pronator syndrome. In the proximal forearm, the median nerve is most commonly compressed by the two heads of the pronator teres muscle but can also be compressed by the bicipital aponeurosis, the flexor digitorum superficialis muscle, and the ligament of Struthers in patients who have a supracondylar process at the distal humerus. 97 98 99 Surgical decompression results in improvement of symptoms in up to 93% of patients with no significant differences between the open and minimally invasive approaches, suggesting that limited decompression may address most cases of pronator syndrome. 99 Entrapment of the anterior interosseous nerve (AIN) is typically termed anterior interosseous nerve syndrome , and presents with muscle weakness and pain in the forearm with characteristic weakness along the second and third digits. While most commonly due to entrapment at the deep head of the pronator teres muscle, AIN syndrome can occur along the length of the nerve within the forearm. 100 101
Fig. 9.
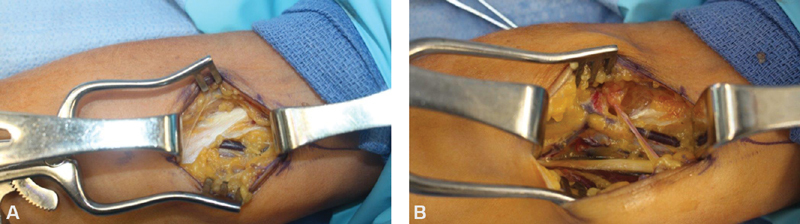
Patient with previous median nerve open carpal tunnel release performed 5 years ago with Tinel's sign and recurrence of symptoms with resisted elbow flexion. ( A ) Prominent lacertus fibrosus visualized demonstrating median nerve compression. ( B ) After complete release of all compression points.
Cubital Tunnel Revision
Cubital tunnel syndrome is the second most common compression neuropathy in the upper extremity. 102 103 Numerous surgical techniques exist for the management of cubital tunnel syndrome, with no single method definitively superior to the rest. These techniques primarily include simple decompression and anterior transposition (subcutaneous, intramuscular, submuscular). 104 105 106 Similar to primary carpal tunnel decompression, however, surgical failure rates have been reported, ranging from 10 to 25%. 104 107 108 Furthermore, reoperation does not typically result in better outcomes compared with the index procedure. 109 110 Aleem et al showed that while 79% of patients undergoing revision cubital tunnel surgery reported some amount of symptomatic improvement, they demonstrated worse outcomes compared with patients undergoing primary repair, including significantly elevated rates of persistent symptoms and weakness. 110
Persistence and recurrence of symptoms arise due to a variety of etiologies. While a careful preoperative evaluation via history, physical exam, and electrodiagnostic studies is required, oftentimes the specific cause is not elucidated until the surgeon intraoperatively assesses the patient. 111 Symptoms following the primary procedure commonly result from incomplete decompression, new sites of compression, nerve subluxation, injury to surrounding nerves, and scarring or fibrosis of the ulnar nerve. 112 Interestingly, Amadio delineated five anatomical locations where the ulnar nerve can be compressed, namely, the arcade of Struthers, the medial intermuscular septum, the medial epicondyle, the cubital tunnel, and the deep aponeurosis of the flexor carpi ulnaris. 113 New anatomical sites of compression typically occur due to utilization of the anterior transposition technique. For example, transposition without release of the arcade of Struthers or medial intermuscular septum can result in secondary compression points at these proximal locations. 114 115 Nerve subluxation can typically arise following simple decompression. 116 Injury to the ulnar nerve or antebrachial nerve can also occur intraoperatively, contributing to postoperative neuroma formation. 117
Submuscular transposition is the most commonly performed technique for revision surgery. Common to most surgical options, the prior incisions should be extended to entirely expose all potential compression sites. 112 Gabel and Amadio reported that an average of at least two compression sites were observed in each patient during revision surgery, highlighting the importance of extending the operative field for maximal visualization. 111 The ulnar nerve should then be located proximal to the olecranon notch and dissected distally past the two heads of the flexor carpi ulnaris muscle, which are divided to expose the nerve. Proximally, the surgeon should split the arcade of Struthers and medial intermuscular septum to prevent impingement of the nerve. The pronator teres muscle is then cut, making sure to leave a cuff of muscle around the medial epicondyle to allow for closure following transposition. A trough is developed anteriorly, and the ulnar nerve can then be transposed. 118
While submuscular transposition is generally well supported in the literature, it has not displayed significant superiority to other operative methods for surgical revision. Other commonly performed techniques employed with demonstrably similar outcomes include subcutaneous transposition, neurolysis, and nerve wrapping. 119 120 121 122 123 Danoff et al described use of a vascularized adipose flap to prevent ulnar nerve subluxation when performing subcutaneous transposition. This technique is performed by elevating the subcutaneous tissue superficial to the nerve, ensuring visualization of the vascular pedicle, and circumferentially wrapping the flap around the nerve. 124 This can and has been used in revisionary procedures, as well. Regardless of surgical technique, the most important principles to remember include ensuring adequate release of the nerve along all compression sites and placing the nerve in a stable anatomical region to prevent subluxation. 112
Though ulnar nerve entrapment frequently arises at one of the compression sites located around the elbow, the ulnar nerve can be compressed distally at the wrist as well, in Guyon's canal. The distal sites of ulnar nerve compression can be classified into three zones. Zone 1 is located most proximally and is found before the bifurcation of the ulnar nerve into its motor and sensory branches. Zones 2 and 3 follow distally in that order. 125 Zone 1 compression results in mixed motor and sensory nerve deficits; zone 2 compression results in isolated motor deficits; and zone 3 compression results in isolated sensory deficits. Zones 1 and 2 entrapment are most commonly caused by ganglia and hook of hamate fractures, while zone 3 compression generally occurs from ulnar artery pathology such as thrombosis or aneurysm. 126 127 If a patient has concomitant carpal tunnel and concern for compression in Guyon's canal, then one can perform the carpal tunnel release in isolation with improvement in volume of Guyon's canal. 128 129 Ginanneschi et al demonstrated both a significant increase in ulnar nerve CSA postoperatively (3.48 to 4.16 mm 2 ) and change in geometric shape from a flattened to oval form, illustrating the effect on volume of Guyon's canal following carpal tunnel release. 128
Conclusion
Peripheral nerve injuries of the upper extremity cause significant morbidity and greatly impact quality of life. Consequently, surgical management is often necessary to treat the afflicted patient. Not infrequently, however, symptoms persist or recur following the index operation, requiring revision surgery. Numerous revision methods exist for surgically treating both traumatic and compression injuries, each with their respective advantages and drawbacks. The surgeon should thus thoroughly assess the patient preoperatively to determine optimal operative management. While recurrence of symptoms is more difficult to treat, revision operations have continued to evolve to incorporate the strengths and improve on the flaws noted from previous modalities.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Papanicolaou G D, McCabe S J, Firrell J. The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg Am. 2001;26(03):460–466. doi: 10.1053/jhsu.2001.24972. [DOI] [PubMed] [Google Scholar]
- 2.Karsy M, Watkins R, Jensen M R, Guan J, Brock A A, Mahan M A. Trends and cost analysis of upper extremity nerve injury using the national (nationwide) inpatient sample. World Neurosurg. 2019;123:e488–e500. doi: 10.1016/j.wneu.2018.11.192. [DOI] [PubMed] [Google Scholar]
- 3.Kouyoumdjian J A. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve. 2006;34(06):785–788. doi: 10.1002/mus.20624. [DOI] [PubMed] [Google Scholar]
- 4.Miranda G E, Torres R Y. Epidemiology of traumatic peripheral nerve injuries evaluated with electrodiagnostic studies in a tertiary care hospital clinic. P R Health Sci J. 2016;35(02):76–80. [PubMed] [Google Scholar]
- 5.Grinsell D, Keating C P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapadia M, Mozaffar T, Gupta R. Compressive neuropathies of the upper extremity: update on pathophysiology, classification, and electrodiagnostic findings. J Hand Surg Am. 2010;35(04):668–677. doi: 10.1016/j.jhsa.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menorca R MG, Fussell T S, Elfar J C. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29(03):317–330. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decrouy-Duruz V, Christen T, Raffoul W. Evaluation of surgical treatment for neuropathic pain from neuroma in patients with injured peripheral nerves. J Neurosurg. 2018;128(04):1235–1240. doi: 10.3171/2017.1.JNS161778. [DOI] [PubMed] [Google Scholar]
- 9.Griffin M F, Malahias M, Hindocha S, Wasim S K. Peripheral nerve injury: principles for repair and regeneration. Open Orthop J. 2014;8(01):199–203. doi: 10.2174/1874325001408010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vora A M, Schon L C. Revision peripheral nerve surgery. Foot Ankle Clin. 2004;9(02):305–318. doi: 10.1016/j.fcl.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Poppler L H, Mackinnon S E. The role of the peripheral nerve surgeon in the treatment of pain. Neurotherapeutics. 2019;16(01):9–25. doi: 10.1007/s13311-018-00695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waljee J F, Carlozzi N, Franzblau L E, Zhong L, Chung K C. Applying the patient-reported outcomes measurement information system to assess upper extremity function among children with congenital hand differences. Plast Reconstr Surg. 2015;136(02):200e–207e. doi: 10.1097/PRS.0000000000001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Upper Extremity Collaborative Group (UECG) . Hudak P L, Amadio P C, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected] Am J Ind Med. 1996;29(06):602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Moore A M, Wagner I J, Fox I K. Principles of nerve repair in complex wounds of the upper extremity. Semin Plast Surg. 2015;29(01):40–47. doi: 10.1055/s-0035-1544169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackinnon S, Novak C. 7th ed. New York, NY: Elsevier; 2016. Compression neuropathies; pp. 921–958. [Google Scholar]
- 16.Hsiao C W, Shih J T, Hung S T. Concurrent carpal tunnel syndrome and pronator syndrome: a retrospective study of 21 cases. Orthop Traumatol Surg Res. 2017;103(01):101–103. doi: 10.1016/j.otsr.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Eason S Y, Belsole R J, Greene T L. Carpal tunnel release: analysis of suboptimal results. J Hand Surg [Br] 1985;10(03):365–369. doi: 10.1016/s0266-7681(85)80063-2. [DOI] [PubMed] [Google Scholar]
- 18.Wessel L E, Fufa D T, Canham R B, La Bore A, Boyer M I, Calfee R P. Outcomes following peripheral nerve decompression with and without associated double crush syndrome: a case control study. Plast Reconstr Surg. 2017;139(01):119–127. doi: 10.1097/PRS.0000000000002863. [DOI] [PubMed] [Google Scholar]
- 19.Toros T, Karabay N, Özaksar K, Sugun T S, Kayalar M, Bal E. Evaluation of peripheral nerves of the upper limb with ultrasonography: a comparison of ultrasonographic examination and the intra-operative findings. J Bone Joint Surg Br. 2009;91(06):762–765. doi: 10.1302/0301-620X.91B6.22284. [DOI] [PubMed] [Google Scholar]
- 20.Toia F, Gagliardo A, D'Arpa S, Gagliardo C, Gagliardo G, Cordova A. Preoperative evaluation of peripheral nerve injuries: what is the place for ultrasound? J Neurosurg. 2016;125(03):603–614. doi: 10.3171/2015.6.JNS151001. [DOI] [PubMed] [Google Scholar]
- 21.Khachi G, Skirgaudes M, Lee W PA, Wollstein R. The clinical applications of peripheral nerve imaging in the upper extremity. J Hand Surg Am. 2007;32(10):1600–1604. doi: 10.1016/j.jhsa.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Cartwright M S, Chloros G D, Walker F O, Wiesler E R, Campbell W W. Diagnostic ultrasound for nerve transection. Muscle Nerve. 2007;35(06):796–799. doi: 10.1002/mus.20761. [DOI] [PubMed] [Google Scholar]
- 23.Tan T C, Yeo C J, Smith E W. High definition ultrasound as diagnostic adjunct for incomplete carpal tunnel release. Hand Surg. 2011;16(03):289–294. doi: 10.1142/S0218810411005564. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro S A, Alkhamisi A, Pujalte G G. Sonographic appearance of the median nerve following revision carpal tunnel surgery. J Clin Imaging Sci. 2016;6(01):11. doi: 10.4103/2156-7514.179419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duetzmann S, Tas S, Seifert V, Marquardt G, Dombert T, Staub F. Cross-sectional area of the median nerve before revision carpal tunnel release-a cross-sectional study. Oper Neurosurg (Hagerstown) 2018;14(01):20–25. doi: 10.1093/ons/opx079. [DOI] [PubMed] [Google Scholar]
- 26.Vögelin E, Nüesch E, Jüni P, Reichenbach S, Eser P, Ziswiler H R. Sonographic follow-up of patients with carpal tunnel syndrome undergoing surgical or nonsurgical treatment: prospective cohort study. J Hand Surg Am. 2010;35(09):1401–1409. doi: 10.1016/j.jhsa.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Rayegani S M, Raeissadat S A, Kargozar E, Rahimi-Dehgolan S, Loni E. Diagnostic value of ultrasonography versus electrodiagnosis in ulnar neuropathy. Med Devices (Auckl) 2019;12:81–88. doi: 10.2147/MDER.S196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grandizio L C, Maschke S, Evans P J. The management of persistent and recurrent cubital tunnel syndrome. J Hand Surg Am. 2018;43(10):933–940. doi: 10.1016/j.jhsa.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal P, Jirankali V, Garg S K. Accuracy of high-resolution ultrasonography in establishing the diagnosis of carpal tunnel syndrome. ANZ J Surg. 2020;90(06):1057–1061. doi: 10.1111/ans.15704. [DOI] [PubMed] [Google Scholar]
- 30.Dumanian G A, Potter B K, Mioton L M. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270(02):238–246. doi: 10.1097/SLA.0000000000003088. [DOI] [PubMed] [Google Scholar]
- 31.Guse D M, Moran S L. Outcomes of the surgical treatment of peripheral neuromas of the hand and forearm: a 25-year comparative outcome study. Ann Plast Surg. 2013;71(06):654–658. doi: 10.1097/SAP.0b013e3182583cf9. [DOI] [PubMed] [Google Scholar]
- 32.Curtin C, Carroll I. Cutaneous neuroma physiology and its relationship to chronic pain. J Hand Surg Am. 2009;34(07):1334–1336. doi: 10.1016/j.jhsa.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood V E, Mudge M K. Treatment of neuromas about a major amputation stump. J Hand Surg Am. 1987;12(02):302–306. doi: 10.1016/s0363-5023(87)80297-6. [DOI] [PubMed] [Google Scholar]
- 34.Souza J M, Cheesborough J E, Ko J H, Cho M S, Kuiken T A, Dumanian G A. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014;472(10):2984–2990. doi: 10.1007/s11999-014-3528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliot D. Surgical management of painful peripheral nerves. Clin Plast Surg. 2014;41(03):589–613. doi: 10.1016/j.cps.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Dellon A L, Mackinnon S E. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg. 1986;77(03):427–438. doi: 10.1097/00006534-198603000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Regal S, Tang P. Surgical management of neuromas of the hand and wrist. J Am Acad Orthop Surg. 2019;27(10):356–363. doi: 10.5435/JAAOS-D-17-00398. [DOI] [PubMed] [Google Scholar]
- 38.Morgan E N, Kyle Potter B, Souza J M, Tintle S M, Nanos G P., III Targeted muscle reinnervation for transradial amputation: Description of operative technique. Tech Hand Up Extrem Surg. 2016;20(04):166–171. doi: 10.1097/BTH.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 39.Bowen J B, Ruter D, Wee C, West J, Valerio I L. Targeted muscle reinnervation technique in below-knee amputation. Plast Reconstr Surg. 2019;143(01):309–312. doi: 10.1097/PRS.0000000000005133. [DOI] [PubMed] [Google Scholar]
- 40.Hijjawi J B, Kuiken T A, Lipschutz R D, Miller L A, Stubblefield K A, Dumanian G A. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118(07):1573–1578. doi: 10.1097/01.prs.0000242487.62487.fb. [DOI] [PubMed] [Google Scholar]
- 41.Gart M S, Souza J M, Dumanian G A. Targeted muscle reinnervation in the upper extremity amputee: a technical roadmap. J Hand Surg Am. 2015;40(09):1877–1888. doi: 10.1016/j.jhsa.2015.06.119. [DOI] [PubMed] [Google Scholar]
- 42.Dumanian G A, Ko J H, O'Shaughnessy K D, Kim P S, Wilson C J, Kuiken T A. Targeted reinnervation for transhumeral amputees: current surgical technique and update on results. Plast Reconstr Surg. 2009;124(03):863–869. doi: 10.1097/PRS.0b013e3181b038c9. [DOI] [PubMed] [Google Scholar]
- 43.Frantz T L, Everhart J S, West J M, Ly T V, Phieffer L S, Valerio I L. Targeted muscle reinnervation at the time of major limb amputation in traumatic amputees: early experience of an effective treatment strategy to improve pain. JBJS Open Access. 2020;5(02):e0067. doi: 10.2106/JBJS.OA.19.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valerio I L, Dumanian G A, Jordan S W. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019;228(03):217–226. doi: 10.1016/j.jamcollsurg.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Cheesborough J E, Souza J M, Dumanian G A, Bueno R A., Jr Targeted muscle reinnervation in the initial management of traumatic upper extremity amputation injury. Hand (N Y) 2014;9(02):253–257. doi: 10.1007/s11552-014-9602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daugherty T HF, Mailey B A, Bueno R A, Jr, Neumeister M W. Targeted muscle reinnervation in the hand: an anatomical feasibility study for neuroma treatment and prevention. J Hand Surg Am. 2020;45(09):802–812. doi: 10.1016/j.jhsa.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Elmaraghi S, Albano N J, Israel J S, Michelotti B F. Targeted muscle reinnervation in the hand: treatment and prevention of pain after ray amputation. J Hand Surg Am. 2020;45(09):8840–8.84E8. doi: 10.1016/j.jhsa.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Hooper R C, Cederna P S, Brown D L. Regenerative peripheral nerve interfaces for the management of symptomatic hand and digital neuromas. Plast Reconstr Surg Glob Open. 2020;8(06):e2792. doi: 10.1097/GOX.0000000000002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo S L, Kung T A, Brown D L, Leonard J A, Kelly B M, Cederna P S. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open. 2016;4(12):e1038. doi: 10.1097/GOX.0000000000001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kung T A, Langhals N B, Martin D C, Johnson P J, Cederna P S, Urbanchek M G. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. 2014;133(06):1380–1394. doi: 10.1097/PRS.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 51.Herr H M, Clites T R, Srinivasan S. Reinventing extremity amputation in the era of functional limb restoration. Ann Surg. 2021;273(02):269–279. doi: 10.1097/SLA.0000000000003895. [DOI] [PubMed] [Google Scholar]
- 52.Nedic A, Moon J D, Kung T A, Langhals N B, Cederna P S, Urbanchek M G.Von Frey monofilament testing successfully discriminates between sensory function of mixed nerve and sensory nerve regenerative peripheral nerve interfacesIn: International IEEE/EMBS Conference on Neural Engineering, NER;2013255–258.
- 53.Vu P P, Vaskov A K, Irwin Z T. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci Transl Med. 2020;12(533):eaay2857. doi: 10.1126/scitranslmed.aay2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urbanchek M G, Wei B, Baghmanli Z, Sugg K, Cederna P S. Long-term stability of regenerative peripheral nerve interfaces (RPNI) Plast Reconstr Surg. 2011;128:88–89. [Google Scholar]
- 55.Urbanchek M G, Baghmanli Z, Moon J D, Sugg K B, Langhals N B, Cederna P S. Quantification of regenerative peripheral nerve interface signal transmission. Plast Reconstr Surg. 2012;130:55–56. [Google Scholar]
- 56.Kubiak C A, Kemp S WP, Cederna P S. Regenerative peripheral nerve interface for management of postamputation neuroma. JAMA Surg. 2018;153(07):681–682. doi: 10.1001/jamasurg.2018.0864. [DOI] [PubMed] [Google Scholar]
- 57.Ives G C, Kung T A, Nghiem B T. Current state of the surgical treatment of terminal neuromas. Neurosurgery. 2018;83(03):354–364. doi: 10.1093/neuros/nyx500. [DOI] [PubMed] [Google Scholar]
- 58.Valerio I, Schulz S A, West J, Westenberg R F, Eberlin K R. Targeted muscle reinnervation combined with a vascularized pedicled regenerative peripheral nerve interface. Plast Reconstr Surg Glob Open. 2020;8(03):e2689. doi: 10.1097/GOX.0000000000002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sood M K, Elliot D. Treatment of painful neuromas of the hand and wrist by relocation into the pronator quadratus muscle. J Hand Surg [Br] 1998;23(02):214–219. doi: 10.1016/s0266-7681(98)80177-0. [DOI] [PubMed] [Google Scholar]
- 60.Hazari A, Elliot D. Treatment of end-neuromas, neuromas-in-continuity and scarred nerves of the digits by proximal relocation. J Hand Surg [Br] 2004;29(04):338–350. doi: 10.1016/j.jhsb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Mass D P, Ciano M C, Tortosa R, Newmeyer W L, Kilgore E S., Jr Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg. 1984;74(02):182–185. doi: 10.1097/00006534-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Evans G RD, Dellon A L. Implantation of the palmar cutaneous branch of the median nerve into the pronator quadratus for treatment of painful neuroma. J Hand Surg Am. 1994;19(02):203–206. doi: 10.1016/0363-5023(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 63.Atherton D D, Leong J CS, Anand P, Elliot D. Relocation of painful end neuromas and scarred nerves from the zone II territory of the hand. J Hand Surg Eur Vol. 2007;32(01):38–44. doi: 10.1016/j.jhsb.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Atherton D D, Fabre J, Anand P, Elliot D. Relocation of painful neuromas in zone III of the hand and forearm. J Hand Surg Eur Vol. 2008;33(02):155–162. doi: 10.1177/1753193408087107. [DOI] [PubMed] [Google Scholar]
- 65.Mackinnon S E, Dellon A L. The overlap pattern of the lateral antebrachial cutaneous nerve and the superficial branch of the radial nerve. J Hand Surg Am. 1985;10(04):522–526. doi: 10.1016/s0363-5023(85)80076-9. [DOI] [PubMed] [Google Scholar]
- 66.Atherton D D, Elliot D. Relocation of neuromas of the lateral antebrachial cutaneous nerve of the forearm into the brachialis muscle. J Hand Surg Eur Vol. 2007;32(03):311–315. doi: 10.1016/J.JHSB.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282(02):153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 68.Tung T HH, Mackinnon S E. Vol 107. Berlin Heidelberg: Springer; 2007. Secondary carpal tunnel surgery; pp. 307–318. [Google Scholar]
- 69.Neuhaus V, Christoforou D, Cheriyan T, Mudgal C S. Evaluation and treatment of failed carpal tunnel release. Orthop Clin North Am. 2012;43(04):439–447. doi: 10.1016/j.ocl.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 70.Pajardi G, Pegoli L, Pivato G, Zerbinati P. Endoscopic carpal tunnel release: our experience with 12,702 cases. Hand Surg. 2008;13(01):21–26. doi: 10.1142/S0218810408003815. [DOI] [PubMed] [Google Scholar]
- 71.Bromley G S. Minimal-incision open carpal tunnel decompression. J Hand Surg Am. 1994;19(01):119–120. doi: 10.1016/0363-5023(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 72.Badger S A, O'Donnell M E, Sherigar J M, Connolly P, Spence R A. Open carpal tunnel release--still a safe and effective operation. Ulster Med J. 2008;77(01):22–24. [PMC free article] [PubMed] [Google Scholar]
- 73.Sayegh E T, Strauch R J. Open versus endoscopic carpal tunnel release: a meta-analysis of randomized controlled trials. Clin Orthop Relat Res. 2015;473(03):1120–1132. doi: 10.1007/s11999-014-3835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hankins C L, Brown M G, Lopez R A, Lee A K, Dang J, Harper R D. A 12-year experience using the Brown two-portal endoscopic procedure of transverse carpal ligament release in 14,722 patients: defining a new paradigm in the treatment of carpal tunnel syndrome. Plast Reconstr Surg. 2007;120(07):1911–1921. doi: 10.1097/01.prs.0000287287.85044.87. [DOI] [PubMed] [Google Scholar]
- 75.Zhang D, Blazar P, Earp B E. Rates of complications and secondary surgeries of mini-open carpal tunnel release. Hand (N Y) 2019;14(04):471–476. doi: 10.1177/1558944718765226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Westenberg R F, Oflazoglu K, de Planque C A, Jupiter J B, Eberlin K R, Chen N C. Revision carpal tunnel release: risk factors and rate of secondary surgery. Plast Reconstr Surg. 2020;145(05):1204–1214. doi: 10.1097/PRS.0000000000006742. [DOI] [PubMed] [Google Scholar]
- 77.Gould D, Kulber D, Kuschner S, Dellamaggiorra R, Cohen M. Our surgical experience: open versus endoscopic carpal tunnel surgery. J Hand Surg Am. 2018;43(09):853–861. doi: 10.1016/j.jhsa.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 78.Henry B M, Zwinczewska H, Roy J. The prevalence of anatomical variations of the median nerve in the carpal tunnel: a systematic review and meta-analysis. PLoS One. 2015;10(08):e0136477. doi: 10.1371/journal.pone.0136477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmerman M, Dahlin E, Thomsen N OB, Andersson G S, Björkman A, Dahlin L B. Outcome after carpal tunnel release: impact of factors related to metabolic syndrome. J Plast Surg Hand Surg. 2017;51(03):165–171. doi: 10.1080/2000656X.2016.1210521. [DOI] [PubMed] [Google Scholar]
- 80.Jones N F, Ahn H C, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(03):683–692. doi: 10.1097/PRS.0b013e3182402c37. [DOI] [PubMed] [Google Scholar]
- 81.Botte M J, von Schroeder H P, Abrams R A, Gellman H. Recurrent carpal tunnel syndrome. Hand Clin. 1996;12(04):731–743. [PubMed] [Google Scholar]
- 82.Mosier B A, Hughes T B. Recurrent carpal tunnel syndrome. Hand Clin. 2013;29(03):427–434. doi: 10.1016/j.hcl.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 83.Zieske L, Ebersole G C, Davidge K, Fox I, Mackinnon S E. Revision carpal tunnel surgery: a 10-year review of intraoperative findings and outcomes. J Hand Surg Am. 2013;38(08):1530–1539. doi: 10.1016/j.jhsa.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shum C, Parisien M, Strauch R J, Rosenwasser M P. The role of flexor tenosynovectomy in the operative treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2002;84(02):221–225. doi: 10.2106/00004623-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Strickland J W, Idler R S, Lourie G M, Plancher K D. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(05):840–848. doi: 10.1016/S0363-5023(96)80201-2. [DOI] [PubMed] [Google Scholar]
- 86.Cramer L.Local fat coverage for the median nerve. ASSH Correspondence Newsletter 198535
- 87.Abzug J M, Jacoby S M, Osterman A L. Surgical options for recalcitrant carpal tunnel syndrome with perineural fibrosis. Hand (N Y) 2012;7(01):23–29. doi: 10.1007/s11552-012-9391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chrysopoulo M T, Greenberg J A, Kleinman W B. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(03):150–156. doi: 10.1097/01.bth.0000225004.56982.42. [DOI] [PubMed] [Google Scholar]
- 89.Soltani A M, Allan B J, Best M J, Mir H S, Panthaki Z J. A systematic review of the literature on the outcomes of treatment for recurrent and persistent carpal tunnel syndrome. Plast Reconstr Surg. 2013;132(01):114–121. doi: 10.1097/PRS.0b013e318290faba. [DOI] [PubMed] [Google Scholar]
- 90.Craft R O, Duncan S FM, Smith A A. Management of recurrent carpal tunnel syndrome with microneurolysis and the hypothenar fat pad flap. Hand (N Y) 2007;2(03):85–89. doi: 10.1007/s11552-007-9025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mathoulin C, Bahm J, Roukoz S. Pedicled hypothenar fat flap for median nerve coverage in recalcitrant carpal tunnel syndrome. Hand Surg. 2000;5(01):33–40. doi: 10.1142/s0218810400000120. [DOI] [PubMed] [Google Scholar]
- 92.Pace G I, Zale C L, Gendelberg D, Taylor K F. Self-reported outcomes for patients undergoing revision carpal tunnel surgery with or without hypothenar fat pad transposition. Hand (N Y) 2018;13(03):292–295. doi: 10.1177/1558944717701243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dellon A L, Mackinnon S E. The pronator quadratus muscle flap. J Hand Surg Am. 1984;9(03):423–427. doi: 10.1016/s0363-5023(84)80236-1. [DOI] [PubMed] [Google Scholar]
- 94.Cheung K, Klausmeyer M A, Jupiter J B. Abductor digiti minimi flap for vascularized coverage in the surgical management of complex regional pain syndrome following carpal tunnel release. Hand (N Y) 2017;12(06):546–550. doi: 10.1177/1558944716681977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rose E H, Norris M S, Kowalski T A, Lucas A, Flegler E J. Palmaris brevis turnover flap as an adjunct to internal neurolysis of the chronically scarred median nerve in recurrent carpal tunnel syndrome. J Hand Surg Am. 1991;16(02):191–201. doi: 10.1016/s0363-5023(10)80096-6. [DOI] [PubMed] [Google Scholar]
- 96.Dy C J, Aunins B, Brogan D M. Barriers to epineural scarring: role in treatment of traumatic nerve injury and chronic compressive neuropathy. J Hand Surg Am. 2018;43(04):360–367. doi: 10.1016/j.jhsa.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.El-Haj M, Ding W, Sharma K, Novak C, Mackinnon S E, Patterson J MM. Median nerve compression in the forearm: a clinical diagnosis. Hand (N Y) 2019:1.558944719874137E15. doi: 10.1177/1558944719874137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olehnik W K, Manske P R, Szerzinski J. Median nerve compression in the proximal forearm. J Hand Surg Am. 1994;19(01):121–126. doi: 10.1016/0363-5023(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 99.Adler J A, Wolf J M. Proximal median nerve compression: pronator syndrome. J Hand Surg Am. 2020;45(12):1157–1165. doi: 10.1016/j.jhsa.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Akhondi H, Varacallo M.Anterior interosseous syndrome. Published online August 10, 2020 https://www.ncbi.nlm.nih.gov/books/NBK525956/. Accessed December 23, 2020
- 101.Dydyk A M, Negrete G, Cascella M.Median nerve injury. StatPearls. Published online 2020. Accessed December 23, 2020https://pubmed.ncbi.nlm.nih.gov/31971749/
- 102.Natroshvili T, Walbeehm E T, van Alfen N, Bartels R HMA. Results of reoperation for failed ulnar nerve surgery at the elbow: a systematic review and meta-analysis. J Neurosurg. 2018;130(03):686–701. doi: 10.3171/2017.8.JNS17927. [DOI] [PubMed] [Google Scholar]
- 103.Mowlavi A, Andrews K, Lille S, Verhulst S, Zook E G, Milner S. The management of cubital tunnel syndrome: a meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(02):327–334. doi: 10.1097/00006534-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 104.Bartels R HMA, Grotenhuis J A. Anterior submuscular transposition of the ulnar nerve. J Bone Joint Surg Br. 2004;86(07):998–1001. doi: 10.1302/0301-620x.86b7.14877. [DOI] [PubMed] [Google Scholar]
- 105.Göbel F, Musgrave D S, Vardakas D G, Vogt M T, Sotereanos D G. Minimal medial epicondylectomy and decompression for cubital tunnel syndrome. Clin Orthop Relat Res. 2001;(393):228–236. doi: 10.1097/00003086-200112000-00025. [DOI] [PubMed] [Google Scholar]
- 106.Catalano L W, III, Barron O A.Anterior subcutaneous transposition of the ulnar nerve Hand Clin 20072303339–344., vi [DOI] [PubMed] [Google Scholar]
- 107.Zlowodzki M, Chan S, Bhandari M, Kalliainen L, Schubert W. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome: a meta-analysis of randomized, controlled trials. J Bone Joint Surg. 2007;89A(12):2591–2598. doi: 10.2106/JBJS.G.00183. [DOI] [PubMed] [Google Scholar]
- 108.Kholinne E, Alsharidah M M, Almutair O. Revision surgery for refractory cubital tunnel syndrome: a systematic review. Orthop Traumatol Surg Res. 2019;105(05):867–876. doi: 10.1016/j.otsr.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 109.Vogel R B, Nossaman B C, Rayan G M. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(04):311–316. doi: 10.1016/j.bjps.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 110.Aleem A W, Krogue J D, Calfee R P. Outcomes of revision surgery for cubital tunnel syndrome. J Hand Surg Am. 2014;39(11):2141–2149. doi: 10.1016/j.jhsa.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 111.Gabel G T, Amadio P C. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(02):213–219. [PubMed] [Google Scholar]
- 112.Nellans K, Tang P. Evaluation and treatment of failed ulnar nerve release at the elbow. Orthop Clin North Am. 2012;43(04):487–494. doi: 10.1016/j.ocl.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 113.Amadio P C. Anatomical basis for a technique of ulnar nerve transposition. Surg Radiol Anat. 1986;8(03):155–161. doi: 10.1007/BF02427843. [DOI] [PubMed] [Google Scholar]
- 114.Sunderland S. Baltimore, MD: Williams and Wilkins Co; 1968. Nerves and Nerve Injuries. [Google Scholar]
- 115.Spinner M, Kaplan E B. The relationship of the ulnar nerve to the medial intermuscular septum in the arm and its clinical significance. Hand. 1976;8(03):239–242. doi: 10.1016/0072-968x(76)90008-5. [DOI] [PubMed] [Google Scholar]
- 116.Davidge K M, Ebersole G C, Mackinnon S E. Pain and function following revision cubital tunnel surgery. Hand (N Y) 2019;14(02):172–178. doi: 10.1177/1558944717743593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dellon A L, MacKinnon S E. Injury to the medial antebrachial cutaneous nerve during cubital tunnel surgery. J Hand Surg [Br] 1985;10(01):33–36. doi: 10.1016/s0266-7681(85)80011-5. [DOI] [PubMed] [Google Scholar]
- 118.Janjua R M, Fernandez J, Tender G, Kline D G.Submuscular transposition of the ulnar nerve for the treatment of cubital tunnel syndrome Neurosurgery 2008630402321–324., discussion 324–325 [DOI] [PubMed] [Google Scholar]
- 119.Charles Y P, Coulet B, Rouzaud J C, Daures J P, Chammas M. Comparative clinical outcomes of submuscular and subcutaneous transposition of the ulnar nerve for cubital tunnel syndrome. J Hand Surg Am. 2009;34(05):866–874. doi: 10.1016/j.jhsa.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 120.Caputo A E, Watson H K. Subcutaneous anterior transposition of the ulnar nerve for failed decompression of cubital tunnel syndrome. J Hand Surg Am. 2000;25(03):544–551. doi: 10.1053/jhsu.2000.6005. [DOI] [PubMed] [Google Scholar]
- 121.Kokkalis Z T, Jain S, Sotereanos D G.Vein wrapping at cubital tunnel for ulnar nerve problems J Shoulder Elbow Surg 201019(2, Suppl):91–97. [DOI] [PubMed] [Google Scholar]
- 122.Gaspar M P, Abdelfattah H M, Welch I W, Vosbikian M M, Kane P M, Rekant M S. Recurrent cubital tunnel syndrome treated with revision neurolysis and amniotic membrane nerve wrapping. J Shoulder Elbow Surg. 2016;25(12):2057–2065. doi: 10.1016/j.jse.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 123.Papatheodorou L K, Williams B G, Sotereanos D G. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J Hand Surg Am. 2015;40(05):987–992. doi: 10.1016/j.jhsa.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 124.Danoff J R, Lombardi J M, Rosenwasser M P. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(03):552–555. doi: 10.1016/j.jhsa.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 125.Gross M S, Gelberman R H. The anatomy of the distal ulnar tunnel. Clin Orthop Relat Res. 1985;(196):238–247. [PubMed] [Google Scholar]
- 126.Waugh R P, Pellegrini V D. Ulnar tunnel syndrome. Hand Clin. 2007;23(03):301–310. doi: 10.1016/j.hcl.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 127.Earp B E, Floyd W E, Louie D, Koris M, Protomastro P. Ulnar nerve entrapment at the wrist. J Am Acad Orthop Surg. 2014;22(11):699–706. doi: 10.5435/JAAOS-22-11-699. [DOI] [PubMed] [Google Scholar]
- 128.Ginanneschi F, Filippou G, Reale F, Scarselli C, Galeazzi M, Rossi A. Ultrasonographic and functional changes of the ulnar nerve at Guyon's canal after carpal tunnel release. Clin Neurophysiol. 2010;121(02):208–213. doi: 10.1016/j.clinph.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 129.Ablove R H, Moy O J, Peimer C A, Wheeler D R, Diao E. Pressure changes in Guyon's canal after carpal tunnel release. J Hand Surg [Br] 1996;21(05):664–665. doi: 10.1016/s0266-7681(96)80155-0. [DOI] [PubMed] [Google Scholar]


