Abstract
Breast augmentation is consistently one of the most commonly performed aesthetic operations every year. Unfortunately, revision rates following primary augmentation remain as high as 36%. There are several causes for revision breast augmentation; however, the most common and challenging of these include capsular contracture, implant malposition, and ptosis of the aging breast following augmentation. Successful management of these problems requires knowledge on how to best treat the implant and capsule with the corresponding soft tissue simultaneously. While surgical management is important, understanding the pathological causes of these entities during the primary operation can reduce the need for revision. This article utilizes the most up-to-date literature to review the appropriate clinical evaluation and surgical management of these complex cases.
Keywords: revision breast augmentation, secondary breast augmentation, capsular contracture, implant malposition, breast ptosis
Breast augmentation remains one of the most commonly performed aesthetic procedures in the United States. According to the American Society of Plastic Surgery (ASPS) statistics, almost 300,000 breast augmentation cases were performed in 2019 alone. 1 However, studies continue to show a high percentage of reoperation following primary breast augmentation, with rates as high as 36% in some publications. 2 Therefore, plastic surgeons performing aesthetic or reconstructive breast procedures must maintain a working knowledge on the causes of revision implant surgery and their appropriate analysis and treatment options.
Some causes of reoperation are unavoidable, such as longitudinal patient factors, which include pregnancy, weight changes, and hormonal changes of the breast. However, many causes are avoidable, such as selection of oversized implants, failure to optimize soft-tissue coverage over the implant, traumatic nonhemostatic pocket dissection, improper handling of the implant, and failure to maintain a sterile surgical field. 2
This article will explore the most common reasons for breast augmentation revision, their causes, and proper patient analysis and treatment. There are enough causes of secondary breast augmentation to supply the contents for a book unto itself. Given the format of this publication as a article and not a book, this article will describe the most common and challenging topics of secondary breast augmentation: capsular contracture, implant malposition, and ptosis of the aging breast following breast augmentation. While each of these entities has specific differences, they also have similarities in terms of clinical approach. A detailed history is important with careful note of all previous surgeries and if possible previous operative notes from prior surgeons. Physical exam should include location of previous scars and quality and volume of soft tissues. The devised plan should avoid repeating procedures that have failed in the past. These patients have often undergone multiple operations, and everything should be done to make the next operation the last. Finally, revision patients may present with unexpected findings intraoperatively, and it is important to be prepared for this and discuss the possible scenarios with the patient before surgery and address any solutions with informed consent.
Capsular Contracture
Capsular contracture is a common cause of revision breast implant surgery. Its etiology is likely multifactorial and involves the formation of a thick periprosthetic capsule that can present as hardening, contour irregularities, and pain. 2 3 4 5 Surgical techniques to prevent capsular contracture are important as recurrence rates are high. 2 6 Extensive research shows patient, surgeon, and implant factors contribute to the development of capsular contracture. 3 Nonetheless, despite increasing scientific and clinical knowledge, capsular contracture remains a challenge for surgical management.
Cause
The formation of a fibrous capsule around an implant is a physiological response that involves macrophages, T-cells, and cytokines. 6 7 A normal capsule is soft, thin, and virtually undetectable on physical exam, but when the fibrous tissue becomes excessive or causes contracture, aesthetic irregularities or symptoms develop. 3 Many potential causes of capsular contracture have been suggested, including biofilm, subclinical infection, soft-tissue trauma, silicone implant rupture, hematoma, seroma, and radiation. 2 8 9 These all increase inflammation and immune system activation, leading to increased scar formation. 5 6
The biofilm theory is supported by both laboratory and clinical evidence and is the most widely accepted in the surgical literature. 10 11 12 13 Biofilm formation occurs in three stages by planktonic bacteria that are capable of forming biofilms: attachment, maturation, and dispersion. In cultured breast implant capsules, the most common bacteria detected is staphylococcus, primarily Staphylococcus epidermidis . 6 Breast implants are most likely contaminated at the time of insertion from native skin flora. 7 Once the biofilm is formed, an ongoing activation of host defenses and chronic inflammation occurs and leads to deposition of collagen and fibrosis and eventually capsular contracture. 2
Prevalence
Prevalence of capsular contracture varies greatly throughout the scientific literature. According to Bachour et al, capsular contracture occurred in 0 to 45% of patients who underwent implant surgery in studies between 1962 and 2016. 3 This wide range in variation is likely due to differences in the classification of Baker grades. Studies by Mentor and Inamed (McGhan, now Allergan) demonstrated a capsular contracture rate of 10 to 15% in aesthetic breast augmentation at 6-year follow-up. 14 15 16 The Sientra Core study demonstrated a capsular contracture rate of 13%. 8 17 18
Classification and Diagnosis
Capsular contracture is classified as early (<6 months) or late (>6 months). Early is typically related to intraoperative factors, whereas late is more likely associated with biofilm. 2 The majority of capsular contracture cases are classified as late. Nearly half (41%) of the cases present by 2 years and 80% present by 5 years. 9 The most commonly used classification system is the Baker classification system, which describes the severity of capsular contracture. 3 There are four increasing grades of severity from I to IV, with I being normal and IV being a firm and painful capsule. Grades III and IV are clinically significant and warrant clinical intervention. 8 Diagnosis is largely based on history and physical examination.
Prevention
Methods to prevent capsular contracture are important as capsular contracture can be difficult to treat. 2 4 Multiple strategies have been suggested, including optimal incision location, pocket location, implant fill and surface texture, surgical technique, and postoperative protocols. 2 19
The inframammary fold (IMF) incision avoids dissection through breast tissue and has been shown in multiple studies to have a lower relative risk of capsular contracture than other incision locations. 2 20 21 Bachour et al compared incision location to capsular contracture rates and found transaxillary and periareolar incisions to have the highest rates of capsular contracture. 3 Unlike transaxillary or periareolar approaches, the IMF incision likely has a lower capsular contracture rate as it reduces implant exposure to tissues such as mammary glands colonized with bacteria. 21
Pocket location and implant surface texture affect capsular contracture. Placement of smooth-surfaced implants in the submuscular pocket compared with subglandular placement has been shown in numerous studies to have decreased rates of capsular contracture. 3 9 Meanwhile, textured implants were found to have a protective effect against capsular contracture in the subglandular plane compared with smooth devices. 22 23 However, these studies were performed prior to today's now widely accepted surgical techniques meant to prevent capsular contracture. These studies also predate today's newer-generation silicone implants with less silicone gel bleed and increased cohesivity, which have correlated with a decreased incidence of capsular contracture. 24 25 26 More recent studies demonstrate low capsular contracture rates of smooth implants in a subglandular plane. Lista et al published a capsular contracture rate of 2.28% in 212 patients and found no statistically significant difference in capsular contracture rates between textured and smooth implants in the subglandular plane. Aseptic technique to minimize bacterial contamination and biofilm formation was thought to be more important than implant surface characteristics. 27 This is particularly important to consider as the risk of anaplastic large cell lymphoma (ALCL) continues to question the use of textured devices.
Intraoperative techniques employed to decrease capsular contracture include atraumatic dissection to minimize inflammation and decrease the presence of blood and serous fluid in the pocket. 2 22 23 Subclinical hematomas are suspected to be a risk factor for the development of capsular contracture. Multiple studies found a significant increase in capsular contracture in patients who had postoperative hematomas. 3 24 25 Other intraoperative techniques include the use of nipple shields to help prevent local contamination. 2 26
Pocket irrigation prior to implant insertion is commonly used to reduce capsular contracture, although the choice of solution and their efficacy remain an area of debate. The most common solutions used are triple antibiotic solution, povidone-iodine (Betadine; Purdue Pharma, Stamford, CT) solution, or Betadine mixed with triple antibiotic solution. 2 3 4 28 Triple antibiotic solution consists of 50,000 units of bacitracin, 1 g of cephazolin, and 80 mg of gentamicin in 500 mL of normal saline. Adams et al reported a 1.8% capsular contracture rate in primary augmentation and a 9.5% rate in reconstruction patients with triple antibiotic irrigation. 29 Betadine mixed with triple antibiotic solution, or “Betadine triple,” has recently been advocated to reduce the risk of capsular contracture and ALCL. 28 30 Betadine-containing irrigations have greater coverage of gram-negative bacteria including Ralstonia pickettii and therefore may be more protective against ALCL. 31 32 33 Based on systematic review and meta-analysis by Yalanis et al, povidone-iodine irrigation in the breast implant pocket is effective at reducing capsular contracture in aesthetic breast augmentation. 18 A consensus does not exist on the superiority of one irrigation solution over another. In fact, recent systematic reviews and meta-analysis offer conflicting conclusions on the efficacy of the use antimicrobial irrigation for prevention of capsular contracture. 34 35 However, a recent survey of ASPS members found 41.2% of plastic surgeons used triple antibiotic solution, 16.5% used triple antibiotic with Betadine, less than 10% used dilute Betadine, and only 4.1% used normal saline without antibiotic irrigation. 36
The “no-touch technique”—which involves some variation of placing new gloves, using a sleeve/funnel for insertion, and minimal manipulation once inserted—is thought to decrease contamination of the implant by the surgeon's gloves and the patient's skin flora, and avoid contamination of the implant once in situ. 37 The Keller Funnel (Keller Medical, Inc., Stuart, FL) was introduced in 2009 as an adjunct to the no-touch technique. It is made of polymeric vinyl and was developed to make silicone gel implant insertion easier, minimize implant trauma during insertion, and allow for minimal contact with the surgeon's hands or the patient's skin. 4 Flugstad et al showed 54.4% reduction of grade III and IV capsular contracture with funnel use. 4 This is also supported by Newman and Davison, who showed a reduced capsular contracture rate in periareolar insertion from 10 to 1.3%, an 87% reduction in incidence ( p < 0.05) using the Keller Funnel. 38
Many surgeons employ specific postoperative protocols to reduce capsular contracture. These include displacement massage for smooth devices and the use of leukotriene inhibitors. Although displacement massage is common practice, there is lack of evidence-based information to support its efficacy. 2 5 Leukotriene inhibitors are accepted by many as a potential treatment for early-stage capsular contracture. Some studies also promote their use for capsular contracture prophylaxis. 5 The medication is not without risk of side effects, especially hepatoxicity, and therefore its risk and benefits must be discussed with the patient prior to use.
Adams et al described their 14-point plan in 2014 intended to reduce bacteria/biofilm on breast implant surfaces and thus reduce the risk of capsular contracture and ALCL. Following this plan, the overall capsular contracture rate was 2.2% and there were no cases of ALCL in 42,035 Biocell implants with an average follow-up of 11.7 years. The 14-point plan largely encompasses the above-mentioned points and also includes use of intravenous antibiotic prophylaxis at the time of anesthetic induction, avoiding dissection into the breast parenchyma, minimizing implant open time and replacement, and avoiding use of drainage tubes. 28
Treatment
Treatment of capsular contracture is a significant challenge for the plastic surgeon as recurrence rates are high. Goals for treatment are for optimization of aesthetics and/or alleviation of symptoms, such as pain. Conservative therapy is the mainstay for Baker II. Baker III/IV classification surgery remains the gold standard. 2 3
Surgical options are all-encompassing with the goal to treat and prevent recurrence. 2 If the patient desires removal of the implants alone, then explantation with or without soft-tissue modification is an option. In this situation, capsulectomy is warranted if the capsule is thick or calcified, has a mass present, or is embedded with silicone. 39 Calcified or thick capsules should be excised to minimize breast distortion and interference with future screening mammograms. 40 A soft capsule can be left intact as capsulectomy is not without risks such as hematoma, pneumothorax, and possible undesirable aesthetic outcome. 39
If implant retention is desired, then strong consideration should be made for placing a new implant in a new site or pocket. 2 The gold standard of treatment is capsulectomy, implant exchange, and possible site change. 40 The reasoning behind this is to address the underlying cause of biofilm presence and therefore place the new implant in a new site by performing a capsulectomy with or without site change. 2 However, the benefit of capsulectomy and its subsequent tissue trauma must outweigh the risk. 2 A 2016 systematic review of capsular contracture found site change and implant exchange to be associated with lower rates of capsular contracture, but no difference when comparing capsulectomy versus capsulotomy. Swanson described his algorithm and technique for capsulotomy with a 22.7% recurrence rate after the first capsulotomy and a 13.3% recurrence rate after the second. 41 While there are some advocates for capsulotomy alone, capsulectomy remains the gold standard.
The following are general guidelines for management of capsular contracture with an implant in the subglandular plane. If the breast tissue is adequate and the pectoralis major muscle is normal, a total capsulectomy with conversion to a subpectoral or dual plane is recommended. However, if the pectoralis is atrophied, then anterior capsulectomy versus possible total capsulectomy if safe, with the implant remaining subglandular, is the best approach. If the breast tissue is thin with a normal pectoralis major, a posterior capsulectomy versus possible total capsulectomy if safe, with conversion to subpectoral or dual plane, is warranted. Conversely, if the breast tissue and the pectoralis are atrophied, open capsulotomy with the implant remaining subglandular is recommended. 40
The following are general guidelines for management of capsular contracture with an implant in the subpectoral plane. If the pectoralis is normal and no unwanted movement is present, then anterior capsulectomy and implant placement either remaining subpectoral or in a dual plane position is warranted. When the pectoralis and the breast tissue are atrophied, open capsulotomy versus anterior capsulectomy if safe and keeping the implant subpectoral is recommended. If there is implant malposition or unwanted pectoralis movement, the best approach is anterior capsulectomy with suture obliteration of the capsule and conversion to subglandular. 40 Given the risk of pneumothorax with posterior capsulectomy, the posterior capsule can instead be obliterated with cauterization. 39 Fig. 1 shows a patient with a smooth silicone implants in the subpectoral plane with bilateral grade III capsular contracture. The patient underwent bilateral total capsulectomy and implant exchange.
Fig. 1.
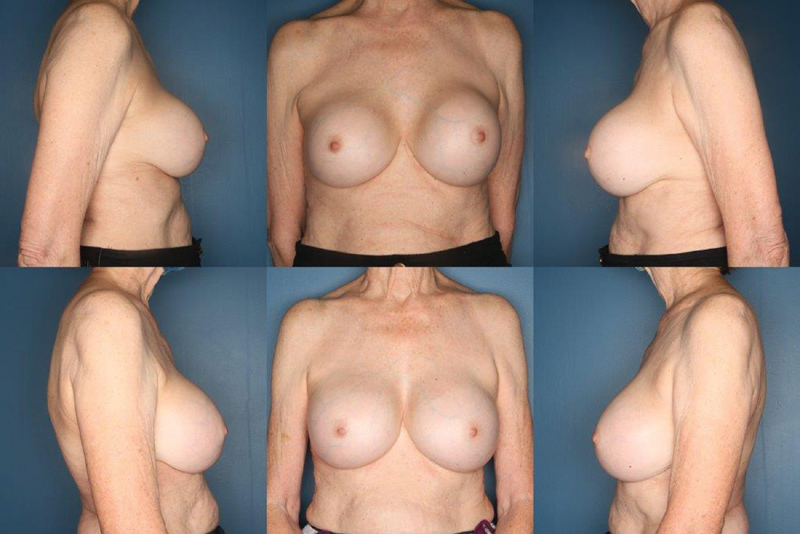
A 76 year-old woman with a history of submuscular silicone implants placed in 1986 with recent changes and hardening of her left breast and an ultrasound suggestive of implant rupture. She underwent bilateral total capsulectomies and implant exchange. Her before picture is the top row and after picture bottom row at 1-year follow-up.
The above guidelines are conventional surgical therapies and unfortunately have a reported failure rate as high as 54%. 42 Use of acellular dermal matrix (ADM) in the surgical management of capsular contracture has proven in numerous studies to have very low recurrence rates. Theoretically, capsule formation does not occur in areas where the implant contacts the ADM surface. Hidalgo and Weinstein reported a 96.9% success rate for surgical treatment of capsular contracture with ADM. 42 Their study describes an algorithm in which ADM is used as the first-line therapy for primary bilateral capsular contracture. Conventional therapy is utilized in the setting of unilateral capsular contracture and no previous history of treatment failure. Other benefits of ADM include stabilization of the position of the pectoralis muscle, support of the implant under the muscle, and additional coverage in patients with atrophic breasts. 42 There are, however, disadvantages and risks to the use of ADM such as the additional cost, infection, inflammation, seroma, and malposition. 2
Implant Malposition
Implant malposition also remains a leading cause of revisionary aesthetic breast surgery. Studies show an average rate of 5% for malposition in primary augmentation and as high as 10% in secondary augmentation. 16 43 44 These statistics indicate implant malposition's significance and that implant malposition can lead to patient dissatisfaction. 45 Understanding the factors that contribute to implant malposition will help not only with prevention but also with successful treatment. Brown et al described several key pillars to understanding this complex process: patient factors, procedural selection, implant selection, surgical technique, and postoperative management. 2 We will discuss these key factors in detail as well as treatment options including pocket adjustment and pocket change.
Types of Implant Malposition
Implant malposition is defined as incorrect position of an implant. The pocket is either too small or too large, or the implant is improperly positioned. This must be distinguished from other processes such as capsular contracture and ptosis, which can often be confused with implant malposition. Implant malposition can be classified as inferior, medial, superior, and lateral. 46
Inferior malposition is the most common type of implant malposition. It occurs when the implant descends below the IMF. 46 The IMF is created by fusion of the superficial and deep fascia at the level of the IMF based on anatomical and histologic studies performed in cadavers. 47 Overdissection of this structure can lead to inferior malposition and bottoming out of the implant or the double-bubble deformity. Bottoming out occurs when the IMF is disrupted and the implant lacks lower pole support. The result is a longer nipple-to-fold distance with a longer inferior pole and high-riding nipple. 48 The double-bubble deformity involves two parallel folds beneath the breast. The superior fold is the original IMF and the lower fold is the level to which implant has descended. Double bubble commonly occurs in two clinical scenarios. The first is overdissection of the IMF as the implant extends beneath the breast into the upper abdominal tissues. The second scenario is in patients who have a constricted IMF, short nipple-to-fold distance, or those with tuberous breast deformity. 49 These patients are high risk for double bubble because failure to fully release the ligamentous attachments between the pectoralis fascia and the undersurface of the dermis of the IMF predisposes to its formation. In all cases, double bubble is most prevalent in the setting of a subpectorally placed implant. 49 Optimal treatment is often conversion to a subglandular plane, which reduces the risk of recurrence. Fig. 2 shows a patient with left breast double bubble and subsequent correction with conversion of the implant plane from a subpectoral to subglandular plane.
Fig. 2.
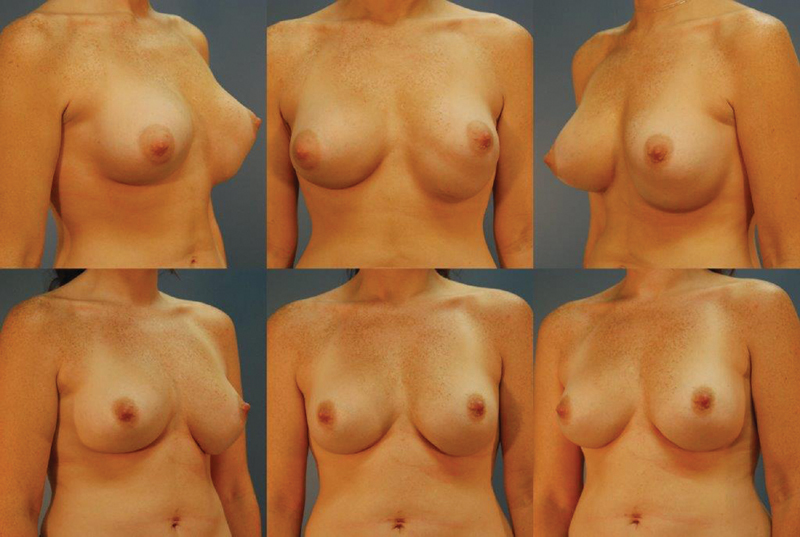
A 41-year-old woman with a history of silicone, submuscular implants. She underwent a site change with conversion of her implants to a subglandular plane and fat transfer to her breasts. Her before picture is the top row and after picture bottom row at 1-year follow-up.
Medial malposition can range from slight medial displacement to violation of the midsternal fascia and parasternal skin with the implant crossing midline due to violation of the intermammary sulcus, otherwise known as symmastia. 46 50 Wong et al described the etiologies of medial malposition as medial overdissection above the sternum in the subglandular plane, disruption of the pectoralis major fibers medially in the submuscular plane, excessively large implants in volume or diameter, and congenital. 51 Moliver et al described contraction of aberrant lateral pectoralis major and minor fibers as an additional contributor to medial implant malposition in submuscular position. 50
Superior malposition is also known as a “high-riding implant” and can contribute to the effect of waterfall ptosis. This is common with subpectoral implant placement via a transaxillary incision if the inferior origins of the pectoralis are inadequately released. 46 52
Lateral implant malposition occurs when overdissection occurs laterally. This often leads to widely separated implants and is also known as telemastia. 46
Patient Factors Contributing to Implant Malposition
Performing a thorough physical exam prior to primary aesthetic breast surgery can decrease the incidence of implant malposition. Patient factors contributing to implant malposition can be divided into musculoskeletal and soft-tissue factors.
Musculoskeletal factors are most apparent with chest wall examination, which may reveal such pathologies as pectus excavatum or pectus carinatum. Excavatum can lead to medial displacement of an implant, while carinatum can lead to lateral displacement. 2 Likewise, patients with a rounder chest wall can have increased telemastia postoperatively, while those with a rectangular thorax can have increased chances of medialization. 53 In addition, hyperactive pectoralis major muscles can lead to superior malposition. 2
Soft-tissue factors that contribute to implant malposition include a high or tight IMF. Release of this can lead to inferior malposition and double-bubble deformity or bottoming out. Tuberous breast deformity and short nipple-to-IMF distance (<4 cm) can also lead to inferior implant malposition and are risk factors for double bubble. Changes in the breast parenchyma and skin envelope due to pregnancy, weight changes, or aging can also lead to implant malposition. 2 46
Procedural Selection Contributing to Implant Malposition
Proper incision selection and pocket selection during primary aesthetic breast surgery can also decrease the risk of implant malposition.
While there are several approaches to implant placement, transaxillary has been associated with risk of superior implant malposition. This is often due to inadequate visualization of the IMF and blind dissection of the inferior pectoralis major fibers, leading to inadequate inferior pole dissection and superior migration of the implant. 54 In addition, use of the IMF incision can lead to disruption of the fold and inferior malposition. As a result, IMF approach should heed notice of possible need for IMF repair at the time of augmentation. 22 Likewise, with the periareolar approach, subcutaneous dissection to the IMF to avoid parenchymal transection can lead to detachment of the breast mound and an implant located in the subcutaneous position inferiorly. 55 However, according to Namnoum et al, the IMF incision is associated with decreased rates of implant malposition compared with the periareolar or transaxillary incision. 21
Pocket selection for an implant can be subpectoral, subfascial, subglandular, or dual plane. While all types of implant malposition can be seen with any of these pocket selections, Namnoum et al found that implants placed in the subglandular position resulted in the highest rate of implant malposition. 21
Selection of the appropriate implant from a size and surface standpoint is imperative. Implants that are too large will distort the pocket and stretch the breast parenchyma and skin, leading to a suboptimal outcome and implant malposition. Several algorithms have been developed to aid with proper implant selection. One of these algorithms is the High Five approach as described by Tebbetts and Adams. 19 56 This algorithm is objective and selects implants based on soft-tissue coverage, implant size (volume/weight), implant dimensions, location of the IMF, and incision location.
Likewise, implant surface has also been shown to be important in preventing implant malposition. Overall, textured devices have been shown to have a decreased rate of implant malposition compared with smooth implants due to friction between the soft tissue and implant. However, Derby and Codner noted that precise pocket dissection is needed to optimize this contact between the implant and soft tissue to decrease implant malposition. 57 Calobrace et al also found that textured implants promote pocket and positional stability. However, they also recognize that breast implant–associated ALCL is associated with the use of textured devices and that proper patient education on the risk and symptoms of this disease process is imperative. 58
Surgical Techniques Leading to Implant Malposition
Technical errors with dissection can lead to implant malposition. Insufficient pocket dissection can lead to superior malposition of the implant. Overdissection in any plane can lead to inferior, lateral, or medial malposition. 46 In addition, trauma to the rib perichondrium can lead to postoperative inflammation and implant malposition. Similarly, fluid collections such as hematoma or seroma can expand the pocket, leading to implant malposition. Sizers are often used to determine final implant selection but these should be utilized carefully to avoid inadvertent pocket overdissection, which can lead to implant malposition. Finally, any fascial disruption, especially the IMF, should be repaired to prevent bottoming out or double bubble. 22
Postoperative Risk Factors Leading to Implant Malposition
While there is no consensus on postoperative management following implant placement in aesthetic plastic surgery, postoperative garments, bras, and tape are often used to maintain proper implant position. However, improper use of these as well as postoperative massage can lead to implant malposition. In addition, anterior chest pressure in the early postoperative period as well as excess activity can lead to implant malposition. 2 53
Surgical Correction of Implant Malposition
Repair of implant malposition can be divided into adjusting the existing pocket or creating a new pocket. 2 46
Spear and Little in 1988 and later Chasan and Francis in 2008 described the technique of suture capsulorrhaphy to correct implant malposition. 59 60 Chasan and Francis described suture capsulorrhaphy in addition to a mirror-image capsulotomy to decrease tension on the repair. In their study of 75 patients, most patients underwent inferolateral capsulorrhaphy. This was often accompanied by exchange of implants that were either smaller, larger, or equivalent in size to the original implants. 60
Harris et al described the use of thermal capsulorrhaphy in 2014 in 157 breast revisions. Electrocautery is used to contract the capsule prior to suture capsulorrhaphy with quilled suture. They also advocated for mirror-image capsulotomy to offload tension on the repair. The advantages of this technique are that the cautery decreases the surface area needed for suture capsulorrhaphy and thickens atrophic capsular tissue, while the suture capsulorrhaphy component helps support the cauterized tissue until scarring occurs. In their study, mean follow-up was 2 years, with 89.9% patients having complete correction of implant malposition at 1 year, 2.5% partially successful, and 7.6% failure. 61
Suture capsulorrhaphy can be challenging, as it can lead to dimpling of the skin laterally, or less successful and with high recurrence rates due to thin, fragile capsules. In addition, the thermal capsulorrhaphy technique risks cutaneous burns. As a result, Calobrace et al described the technique of popcorn capsulorrhaphy. Whereas thermal capsulorrhaphy applies electrocautery directly to the capsule to induce capsular thickening, popcorn capsulorrhaphy uses insulated forceps to grasp the capsule and pull it into the pocket away from the skin, reducing the risk of burns. The forceps are then touched by the electrocautery unit to create full-thickness capsular injury, signified by a popping sound. The full-thickness injury effectively thickens the capsule for a stronger, more stable capsule. Suture reinforcement is used if the pocket does not adequately shrink with popcorn capsulorrhaphy, and mesh reinforcement is used in cases with high risk of recurrence, such as severe chest wall abnormalities or previous failed repairs. The rate of recurrence was 5.2% (13 of 246, with 4 of these 13 requiring revisions). 62
In 2001, Voice and Carlsen described the use of a capsular flap to correct implant malposition or to support capsulorrhaphy. Advantages to this technique are that it creates a vascularized sling of tissue that places the suture line away from the maximum weight of the implant. The flap can be raised from the anterior or posterior surface of the pocket on a small pedicle. Disadvantages include need for sufficient quality and quantity of tissue. 63 64 Due to these disadvantages, the use of techniques involving ADMs, synthetic meshes, and autologous dermal grafts has been developed.
ADM has long been used in reconstructive surgery to help define the inframammary and lateral folds and allow for pocket control. Advantages of ADM include decreased risk of capsular contracture, decreased rippling, tissue incorporation, and better implant control with improved shape and projection. Disadvantages include the increased risk of seroma formation and also the higher cost. 65 66 Spear et al evaluated the use of ADM in the treatment and prevention of implant-associated breast deformities. In their study, ADM was indicated in 32 cases of implant malposition in both reconstructive and aesthetic breast surgery. Failure occurred in 1 of 32 patents, or in 3.1%, and was due to inadequate ADM size selection leading to bottoming out in a revisionary breast augmentation. 67 Hartzell et al reviewed 23 patients (38 breasts) undergoing revisionary aesthetic breast surgery with the use of ADM. Twenty-two breasts had implant malposition and were corrected with the use of ADM. In their series, only one complication occurred and was infection due to layering of multiple sheets of ADM. They advised against layering ADM to prevent lack of incorporation and seroma formation and instead using a thicker sheet of ADM if needed. 68
Due to the increased cost of utilizing ADM, the use of autologous dermal grafting has gained popularity as an alternative. According to Davis et al, autologous dermal grafting leads to improved biocompatibility and decreased immunogenicity as it is the patient's native tissue and reduced costs. 69 Likewise, Colwell and Breuing described their technique of autologous dermal grafting primarily in mastopexy and designed an algorithm for utilization of this technique. Use of autologous dermal grafting is predicated on having sufficient quality and quantity of donor-site tissue. 70
Due to potential disadvantages with the use of ADMs and autologous dermal grafting, the use of synthetic mesh emerged in the field of aesthetic breast surgery. While permanent meshes were originally applied, such as polytetrafluoroethylene (PTFE), they were found to be too rigid. However, when absorbable mesh, such as Vicryl (Ethicon Inc., Bridgewater, NJ), was utilized, rapid absorption led to implant malposition. This has led to the use of long-term, resorbable synthetic meshes, which have promising advantages such as decreased cost, absence of bacterial or viral transmission, improved incorporation, and decreased risk of seroma or infection. 71 GalaFLEX (Galatea Surgical, Lexington, MA) is a macroporous monofilament synthetic mesh made of poly-4-hydroxybutyrate (P4HB) that is completely bioresorbable. It has gained in popularity recently in aesthetic breast revision cases as it is safely absorbed and degraded into carbon dioxide and water, provides reliably strong soft-tissue support, and is about half the cost of ADM and less prone to seroma formation. It retains 70% of its strength after 12 weeks and fully resorbed by 18 to 24 months. Uses in revision breast surgery include capsular contracture for pocket control and for malposition cases by reinforcing capsulorrhaphy techniques. The use of GalaFLEX for revision breast cases is off-label; therefore, patient consent must acknowledge this prior to use. 72
If pocket adjustment with the above-mentioned techniques is not feasible, pocket change can be performed. The advantage of this technique is that it completely eliminates the problematic pocket and allows for the creation of a new pocket with the correct dimensions of the desired implant. 2 Pocket change can also be accompanied by supplementary materials such as ADM, autologous dermal grafting, fat grafting, or use of synthetic meshes.
If implant malposition occurs in the subpectoral plane, the pocket can be converted to a subglandular plane, subfascial plane, dual plane, or neosubpectoral plane. Converting to a subglandular plane often relieves the distorting forces of the muscle. 46 Lesavoy et al performed a technique in which they converted a subpectoral plane to subglandular and resuspended the pectoralis muscle, preventing implant migration into the previous subpectoral plane. In their study, 36 patients underwent revision surgery with average follow-up of 20.2 months. The most common indication for conversion to the subglandular plane was implant malposition. They found that unwanted implant movement, symmastia, and implant malposition had 100% success rate. 73 Similarly, a malpositioned subpectoral implant can be converted to a subfascial plane. Both of these techniques require adequate soft-tissue coverage over the implant. Junior et al found no difference in outcomes between subglandular and subfascial, and suggested that selection should be based on surgeon experience. 74 Fig. 3 demonstrates subpectoral implants with inferior and lateral malposition. Surgical correction was achieved with conversion of the implants to a neosubmuscular plane and optimized nipple position with a vertical mastopexy.
Fig. 3.
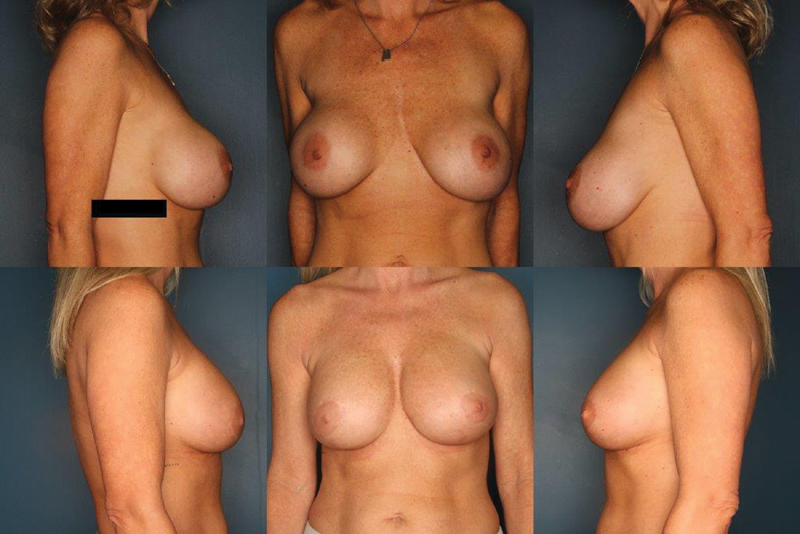
A 44-year-old woman with a history of a saline, subpectoral breast augmentation performed 10 years ago. She was bothered by implant malposition and pseudoptosis. She underwent correction with a neosubmuscular pocket, exchange of her implants for silicone devices, and vertical mastopexy. Her before picture is the top row and after picture bottom row at 2-year follow-up.
If the implant in the subpectoral plane is superiorly malpositioned, conversion to a dual plane pocket may be beneficial. Dissection is performed over the old capsule until the inferior edge of the pectoralis muscle is identified. An anterior capsulotomy is then made, allowing the implant to fall into the dual plane. 2
Likewise, Maxwell and Gabriel described the use of the neosubpectoral pocket in 2008. A new pocket is created superficial to the anterior capsule but deep to the pectoralis major. This was described for 15 patients with malposition, and all patients had successful correction of implant malposition at 26.2 months. 75 Similarly, Spear et al described the use of this same technique for correction of symmastia in 23 patients, all of which were successful at 22-month follow-up. 76 There are several advantages to the neosubpectoral pocket, including providing pectoral coverage to decrease contour irregularities and rippling, and avoiding the inherent risks and soft-tissue trauma of capsulectomy. 75
Implant malposition that occurs in the subglandular plane can be converted to a submuscular, subfascial, or neosubglandular plane. Conversion to a complete submuscular plane can oftentimes lead to animation deformity or increased pain compared with the subfascial plane. However, with the subfascial plane, there has to be adequate soft-tissue coverage over the implant. Regardless of technique chosen, the subglandular plane must be closed to prevent implant migration into the previous pocket and the pectoralis major muscle must be secured inferiorly to prevent the window shading effect. 2 Finally, implant malposition in the subglandular pocket may be converted to a neosubglandular pocket either above or below the existing capsule, provided there is adequate soft-tissue coverage.
Breast Ptosis and Aging Breasts following Breast Augmentation
Gradual progression of breast ptosis is a common problem many years after the initial breast augmentation procedure. Etiology contributing to gradual breast ptosis includes breast enlargement followed by postpartum atrophy associated with pregnancy, weight gain, weight loss, and years of the weight of the implant pressing against the breast tissue resulting in atrophy and gradual ptosis. 77
Evaluation should include previous scars and knowledge of any previous pedicle used for mastopexy to plan accordingly to avoid issues with blood supply to the nipple–areola complex (NAC) or breast skin and soft tissue. These patients often have had implants for as long as 10 to 20 years and therefore capsular contracture may also be present. This is important to note as the appropriate treatment may include correction of capsular contracture and/or implant malposition, in addition to ptosis. 49
There are two major options for these patients. The first is removal and replacement of the implant with simultaneous mastopexy. The second is removal of the implant without replacement with the option for tissue modification. Tissue modification could include mastopexy with or without fat grafting. Decision-making can be challenging as the final breast shape can be difficult to predict once the implant is removed. In patients with saline implants, Grotting et al described a technique to aid patient decision-making. The implant is deflated percutaneously in the office under local anesthesia with an 18-gauge needle. The patient is then re-evaluated 1 month later to allow the tissues to contract and become firm. At this time, the patient can decide if she does or does not have enough breast volume and decide between implant removal and mastopexy alone versus mastopexy with replacement of the implant. 77
When the patient has decided to remove and replace the implant with simultaneous mastopexy, the operative sequence starts with demarcation of the NAC with a cookie cutter followed by peripheral de-epithelialization. Most often, a vertical mastopexy is required, allowing for access to the breast tissue which is incised to the implant capsule. The implant is removed and examined for possible rupture. The capsule is then evaluated and a decision is made regarding capsulectomy and/or site change, as described previously in this article. Once the pocket has been adjusted and dimensional stabilization achieved, the base width of the pocket is measured and the new implant is selected based on these measurements and preoperative discussion with the patient. The pocket is irrigated with antibiotic solution and the new implant placed by sterile technique. A vertical mastopexy is then configured with tailor tack method. The skin is closed in a multilayer closure. 49 77 Fig. 4 demonstrates a patient with breast ptosis several years after augmentation. Surgical management consisted of bilateral subtotal capsulectomy with a vertical mastopexy for optimal nipple position.
Fig. 4.
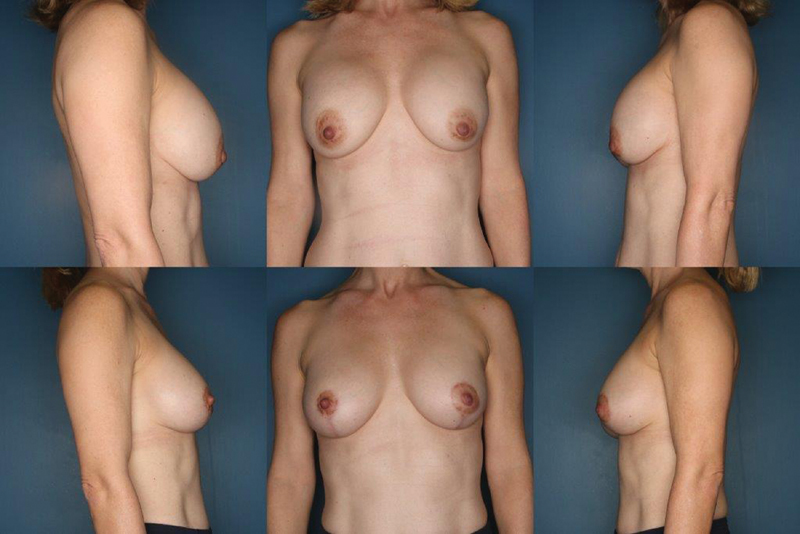
A 48-year-old woman with a history of saline, submuscular breast augmentation performed 15 years ago. She was bothered by postpartum changes to her breast and changes following moderate weight loss. She underwent subtotal capsulectomy with exchange of her implants for silicone devices and vertical mastopexy. Her before picture is the top row and after picture bottom row at 1-year follow-up.
If the patient decides not to replace the implant, options to optimize aesthetics include mastopexy with or without fat grafting or fat grafting alone. With implant removal and mastopexy, the final result will depend on the residual breast tissue volume. The mastopexy pedicle should be chosen carefully based on previous breast operations and should remain as broad as possible to ensure viability. The mastopexy is initiated only after the implant has been removed and careful judicious skin excision determined by tailor tacking or intraoperative sequencing. Fisher described his method of intraoperative sequencing, in which both a vertical and traditional T-pattern mastopexy are planned based on an inferior and superior pedicle. At each step, tailor tacking of the skin is performed to assess the contour and shape with gradual stepwise skin excision until optimal result is achieved. 49 Several authors advocate auto-augmentation flaps with mastopexy to provide core projection. 78 79 80 The technique involves a superiorly based mastopexy with a de-epithelialized inferior dermoglandular flap that is flipped under the areola and breast and sutured to the overlying breast tissue. 39
Fat grafting can be applied with or without implant replacement and with or without mastopexy. Fat grafting can improve global volume, including upper pole and cleavage volume, but is poor at creating core projection. Therefore, it is a powerful adjunct in patients in whom mastopexy or implant replacement alone may require additional breast volume. Patients must be counseled that fat grafting cannot give the same volume guarantees as an implant due to the inevitable absorption of fat, which is on average 50%. Retention of fat is based on variables such as flap vascularity and quality of the fat injected, and patients should be counseled that additional fat grafting sessions may be warranted to achieve desired goals. 39 Numerous studies have shown fat grafting does not increase the risk of breast cancer 81 82 83 84 85 and a blinded clinical study showed fat grafting to the breast has less mammographic abnormalities requiring biopsy than breast reduction. 86
A novel method for large-volume fat grafting to the breast following breast implant explantation is expansion vibration lipofilling (EVL). This technique was originally described by Del Vecchio and Wall in 2018 for gluteal fat grafting. EVL allows for recipient-site expansion by using exploded tip injection cannulas vibrating rapidly at high speeds, thus creating a wider potential space and ability to separate and equalize tissue in which the fat is then deposited into the expanded area. 87 Abboud et al applied these concepts to immediate large-volume grafting of autologous fat to the breast following implant removal. Their study described 80 breast augmentation patients; all patients underwent explantation and fat transfer in one session, and at 6 months postoperatively all patients had maintenance of bra cup size. Potential complications include cyst formation, which occurred at a rate of 5.6% in sample size of 160 breasts. Of these, all but one were treated conservatively with careful observation and the cysts resolved without surgical intervention. 88
Conclusion
Capsular contracture, implant malposition, and aging breasts following augmentation are some of the most common and challenging cases of revision breast augmentation. Understanding the causes of these problems can aid in prevention during index breast augmentation. Key tenets for treatment include understanding how to best address the capsule and the associated soft tissue. Options for the capsule and pocket include pocket modification with capsulorrhaphy, ADM, dermal grafting, or synthetic meshes, or pocket change with capsulectomy, capsulotomy, or site change. Soft-tissue modification may include mastopexy, fat grafting, or auto-augmentation flaps coupled with a decision to remove or replace the implant. This article provides the most up-to-date recommendations and description of the most recent surgical techniques to provide a framework for optimal management of these complex causes of secondary breast augmentation.
Footnotes
Conflict of Interest None declared.
References
- 1.Plastic Surgery Statistics Report . 2019. ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. American Society of Plastic Surgeons [Google Scholar]
- 2.Brown M H, Somogyi R B, Aggarwal S. Secondary breast augmentation. Plast Reconstr Surg. 2016;138(01):119e–135e. doi: 10.1097/PRS.0000000000002280. [DOI] [PubMed] [Google Scholar]
- 3.Bachour Y, Bargon C A, de Blok C JM, Ket J CF, Ritt M JPF, Niessen F B. Risk factors for developing capsular contracture in women after breast implant surgery: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2018;71(09):e29–e48. doi: 10.1016/j.bjps.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Flugstad N A, Pozner J N, Baxter R A. Does implant insertion with a funnel decrease capsular contracture? A preliminary report. Aesthet Surg J. 2016;36(05):550–556. doi: 10.1093/asj/sjv237. [DOI] [PubMed] [Google Scholar]
- 5.Graf R, Ascenço A SK, Freitas R DS. Prevention of capsular contracture using leukotriene antagonists. Plast Reconstr Surg. 2015;136(05):592e–596e. doi: 10.1097/PRS.0000000000001683. [DOI] [PubMed] [Google Scholar]
- 6.Bachour Y, Verweij S P, Gibbs S. The aetiopathogenesis of capsular contracture: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2018;71(03):307–317. doi: 10.1016/j.bjps.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Ajdic D, Zoghbi Y, Gerth D, Panthaki Z J, Thaller S. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthet Surg J. 2016;36(03):297–309. doi: 10.1093/asj/sjv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner D S, Mirhaidari S J. Capsulectomy, implant exchange, and placement of acellular dermal matrix is effective in treating capsular contracture in breast augmentation patients. Aesthet Surg J. 2021;41(03):304–312. doi: 10.1093/asj/sjz358. [DOI] [PubMed] [Google Scholar]
- 9.Calobrace M B, Stevens W G, Capizzi P J, Cohen R, Godinez T, Beckstrand M.Risk factor analysis for capsular contracture: a 10-year Sientra study using round, smooth, and textured implants for breast augmentation Plast Reconstr Surg 2018141(4S Sientra Shaped and Round Cohesive Gel Implants, 4S):20S–28S. [DOI] [PubMed] [Google Scholar]
- 10.Tamboto H, Vickery K, Deva A K. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126(03):835–842. doi: 10.1097/PRS.0b013e3181e3b456. [DOI] [PubMed] [Google Scholar]
- 11.Deva A K, Adams W P, Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132(05):1319–1328. doi: 10.1097/PRS.0b013e3182a3c105. [DOI] [PubMed] [Google Scholar]
- 12.Pajkos A, Deva A K, Vickery K, Cope C, Chang L, Cossart Y E. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111(05):1605–1611. doi: 10.1097/01.PRS.0000054768.14922.44. [DOI] [PubMed] [Google Scholar]
- 13.Adams W P, Jr, Haydon M S, Raniere J., JrA rabbit model for capsular contracture: development and clinical implications Plast Reconstr Surg 2006117041214–1219., discussion 1220–1221 [DOI] [PubMed] [Google Scholar]
- 14.Cunningham B. The Mentor Core study on silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(07) 01:19S–29S. doi: 10.1097/01.prs.0000286574.88752.04. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg. 2009;33(03):440–444. doi: 10.1007/s00266-009-9364-6. [DOI] [PubMed] [Google Scholar]
- 16.Inamed Silicone Breast Implant U.S. Study Group . Spear S L, Murphy D K, Slicton A, Walker P S. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(07) 01:8S–16S. doi: 10.1097/01.prs.0000286580.93214.df. [DOI] [PubMed] [Google Scholar]
- 17.Stevens W G, Calobrace M B, Harrington J, Alizadeh K, Zeidler K R, d'Incelli R C. Nine-year core study data for Sientra's FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthet Surg J. 2016;36(04):404–416. doi: 10.1093/asj/sjw015. [DOI] [PubMed] [Google Scholar]
- 18.Yalanis G C, Liu E W, Cheng H T. Efficacy and safety of povidone-iodine irrigation in reducing the risk of capsular contracture in aesthetic breast augmentation: a systematic review and meta-analysis. Plast Reconstr Surg. 2015;136(04):687–698. doi: 10.1097/PRS.0000000000001576. [DOI] [PubMed] [Google Scholar]
- 19.Adams W P., Jr The process of breast augmentation: four sequential steps for optimizing outcomes for patients. Plast Reconstr Surg. 2008;122(06):1892–1900. doi: 10.1097/PRS.0b013e31818d20ec. [DOI] [PubMed] [Google Scholar]
- 20.Henriksen T F, Fryzek J P, Hölmich L R. Surgical intervention and capsular contracture after breast augmentation: a prospective study of risk factors. Ann Plast Surg. 2005;54(04):343–351. doi: 10.1097/01.sap.0000151459.07978.fa. [DOI] [PubMed] [Google Scholar]
- 21.Namnoum J D, Largent J, Kaplan H M, Oefelein M G, Brown M H. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66(09):1165–1172. doi: 10.1016/j.bjps.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 22.Somogyi R B, Brown M H. Outcomes in primary breast augmentation: a single surgeon's review of 1539 consecutive cases. Plast Reconstr Surg. 2015;135(01):87–97. doi: 10.1097/PRS.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 23.Tebbetts J B. Achieving a zero percent reoperation rate at 3 years in a 50-consecutive-case augmentation mammaplasty premarket approval study. Plast Reconstr Surg. 2006;118(06):1453–1457. doi: 10.1097/01.prs.0000239602.99867.07. [DOI] [PubMed] [Google Scholar]
- 24.Handel N, Jensen J A, Black Q, Waisman J R, Silverstein M J. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg. 1995;96(07):1521–1533. doi: 10.1097/00006534-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Codner M A, Mejia J D, Locke M B. A 15-year experience with primary breast augmentation. Plast Reconstr Surg. 2011;127(03):1300–1310. doi: 10.1097/PRS.0b013e318205f41b. [DOI] [PubMed] [Google Scholar]
- 26.Wixtrom R N, Stutman R L, Burke R M, Mahoney A K, Codner M A. Risk of breast implant bacterial contamination from endogenous breast flora, prevention with nipple shields, and implications for biofilm formation. Aesthet Surg J. 2012;32(08):956–963. doi: 10.1177/1090820X12456841. [DOI] [PubMed] [Google Scholar]
- 27.Lista F, Austin R E, Saheb-Al-Zamani M, Ahmad J. Does implant surface texture affect the risk of capsular contracture in subglandular breast augmentation and breast augmentation-mastopexy? Aesthet Surg J. 2020;40(05):499–512. doi: 10.1093/asj/sjz241. [DOI] [PubMed] [Google Scholar]
- 28.Adams W P, Jr, Culbertson E J, Deva A K. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg. 2017;140(03):427–431. doi: 10.1097/PRS.0000000000003575. [DOI] [PubMed] [Google Scholar]
- 29.Adams W P, Jr, Rios J L, Smith S J.Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study Plast Reconstr Surg 2006118(7, Suppl):46S–52S. [DOI] [PubMed] [Google Scholar]
- 30.Culbertson E J, Felder-Scott C, Deva A K, Greenberg D E, Adams W P., Jr Optimizing breast pocket irrigation: the breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) era. Aesthet Surg J. 2020;40(06):619–625. doi: 10.1093/asj/sjz246. [DOI] [PubMed] [Google Scholar]
- 31.Hu H, Johani K, Almatroudi A. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137(06):1659–1669. doi: 10.1097/PRS.0000000000002010. [DOI] [PubMed] [Google Scholar]
- 32.Jewell M L, Adams W P., Jr Betadine and breast implants. Aesthet Surg J. 2018;38(06):623–626. doi: 10.1093/asj/sjy044. [DOI] [PubMed] [Google Scholar]
- 33.Ryan M P, Adley C C.The antibiotic susceptibility of water-based bacteria Ralstonia pickettii and Ralstonia insidiosa J Med Microbiol 201362(Pt 7):1025–1031. [DOI] [PubMed] [Google Scholar]
- 34.Drinane J J, Chowdhry T, Pham T H, Ritter E. Examining the role of antimicrobial irrigation and capsular contracture: a systematic review and meta-analysis. Ann Plast Surg. 2017;79(01):107–114. doi: 10.1097/SAP.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 35.Lynch J M, Sebai M E, Rodriguez-Unda N A, Seal S, Rosson G D, Manahan M A. Breast pocket irrigation with antibiotic solution at implant insertion: a systematic review and meta-analysis. Aesthetic Plast Surg. 2018;42(05):1179–1186. doi: 10.1007/s00266-018-1166-2. [DOI] [PubMed] [Google Scholar]
- 36.Epps M T, Langsdon S, Pels T K.Pocket irrigation and technique during reconstructive surgery: an American Society of Plastic Surgery Survey of Current Practice Ann Plast Surg 201982(6S):05S427–S432. [DOI] [PubMed] [Google Scholar]
- 37.Mladick R A. “No-touch” submuscular saline breast augmentation technique. Aesthetic Plast Surg. 1993;17(03):183–192. doi: 10.1007/BF00636260. [DOI] [PubMed] [Google Scholar]
- 38.Newman A N, Davison S P. Effect of Keller Funnel on the rate of capsular contracture in periareolar breast augmentation. Plast Reconstr Surg Glob Open. 2018;6(06):e1834. doi: 10.1097/GOX.0000000000001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calobrace M B, Mays C. An algorithm for the management of explantation surgery. Clin Plast Surg. 2021;48(01):1–16. doi: 10.1016/j.cps.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Wan D, Rohrich R J. Revisiting the management of capsular contracture in breast augmentation: a systematic review. Plast Reconstr Surg. 2016;137(03):826–841. doi: 10.1097/01.prs.0000480095.23356.ae. [DOI] [PubMed] [Google Scholar]
- 41.Swanson E. Open capsulotomy: an effective but overlooked treatment for capsular contracture after breast augmentation. Plast Reconstr Surg Glob Open. 2016;4(10):e1096. doi: 10.1097/GOX.0000000000001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hidalgo D A, Weinstein A L. Surgical treatment for capsular contracture: a new paradigm and algorithm. Plast Reconstr Surg. 2020;146(03):516–525. doi: 10.1097/PRS.0000000000007079. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell G P, Van Natta B W, Bengtson B P, Murphy D K. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J. 2015;35(02):145–155. doi: 10.1093/asj/sju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGuire P, Reisman N R, Murphy D K. Risk factor analysis for capsular contracture, malposition, and late seroma in subjects receiving Natrelle 410 form-stable silicone breast implants. Plast Reconstr Surg. 2017;139(01):1–9. doi: 10.1097/PRS.0000000000002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y J, Kim Y W, Cheon Y W. Prevention of implant malposition in inframammary augmentation mammaplasty. Arch Plast Surg. 2014;41(04):407–413. doi: 10.5999/aps.2014.41.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chopra K, Gowda A U, Kwon E, Eagan M, Grant Stevens W. Techniques to repair implant malposition after breast augmentation: a review. Aesthet Surg J. 2016;36(06):660–671. doi: 10.1093/asj/sjv261. [DOI] [PubMed] [Google Scholar]
- 47.Muntan C D, Sundine M J, Rink R D, Acland R D.Inframammary fold: a histologic reappraisal Plast Reconstr Surg 200010502549–556., discussion 557 [DOI] [PubMed] [Google Scholar]
- 48.Salgarello M, Visconti G. Staying out of double-bubble and bottoming-out deformities in dual-plane breast augmentation: anatomical and clinical study. Aesthetic Plast Surg. 2017;41(05):999–1006. doi: 10.1007/s00266-017-0918-8. [DOI] [PubMed] [Google Scholar]
- 49.Fisher J. Boca Raton, FL: CRC Press; 2014. Mastopexy without implant exchange; pp. 329–334. [Google Scholar]
- 50.Moliver C L, Sanchez E R, Kaltwasser K, Sanchez R J. A muscular etiology for medial implant malposition following subpectoral augmentation. Aesthet Surg J. 2015;35(07):NP203–NP210. doi: 10.1093/asj/sjv072. [DOI] [PubMed] [Google Scholar]
- 51.Wong M T, Cheong E C, Lim J, Lim T C. Creation of an intermammary sulcus in congenital synmastia. Singapore Med J. 2007;48(01):e29–e31. [PubMed] [Google Scholar]
- 52.Frame J. The waterfall effect in breast augmentation. Gland Surg. 2017;6(02):193–202. doi: 10.21037/gs.2016.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hidalgo D A, Spector J A. Breast augmentation. Plast Reconstr Surg. 2014;133(04):567e–583e. doi: 10.1097/PRS.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 54.Kolker A R, Austen W G, Jr, Slavin S A. Endoscopic-assisted transaxillary breast augmentation: minimizing complications and maximizing results with improvements in patient selection and technique. Ann Plast Surg. 2010;64(05):667–673. doi: 10.1097/SAP.0b013e3181d9aa3d. [DOI] [PubMed] [Google Scholar]
- 55.Teitelbaum S.The inframammary approach to breast augmentation Clin Plast Surg 2009360133–43., v–vi [DOI] [PubMed] [Google Scholar]
- 56.Tebbetts J B, Adams W P.Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process Plast Reconstr Surg 2006118(7, Suppl):35S–45S. [DOI] [PubMed] [Google Scholar]
- 57.Derby B M, Codner M A. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg. 2015;135(01):113–124. doi: 10.1097/PRS.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 58.Calobrace M B, Schwartz M R, Zeidler K R, Pittman T A, Cohen R, Stevens W G. Long-term safety of textured and smooth breast implants. Aesthet Surg J. 2017;38(01):38–48. doi: 10.1093/asj/sjx157. [DOI] [PubMed] [Google Scholar]
- 59.Spear S L, Little J W.3rd.Breast capsulorrhaphy Plast Reconstr Surg 19888102274–279. [DOI] [PubMed] [Google Scholar]
- 60.Chasan P E, Francis C S. Capsulorrhaphy for revisionary breast surgery. Aesthet Surg J. 2008;28(01):63–69. doi: 10.1016/j.asj.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Harris R, Raphael P, Harris S W. Thermal capsulorrhaphy: a modified technique for breast pocket revision. Aesthet Surg J. 2014;34(07):1041–1049. doi: 10.1177/1090820X14542650. [DOI] [PubMed] [Google Scholar]
- 62.Calobrace M B, Mays C, Wilson R, Wermeling R. Popcorn capsulorrhaphy in revision aesthetic breast surgery. Aesthet Surg J. 2020;40(01):63–74. doi: 10.1093/asj/sjy324. [DOI] [PubMed] [Google Scholar]
- 63.Voice S D, Carlsen L N. Using a capsular flap to correct breast implant malposition. Aesthet Surg J. 2001;21(05):441–444. doi: 10.1067/maj.2001.119123. [DOI] [PubMed] [Google Scholar]
- 64.Yoo G, Lee P K. Capsular flaps for the management of malpositioned implants after augmentation mammoplasty. Aesthetic Plast Surg. 2010;34(01):111–115. doi: 10.1007/s00266-009-9456-3. [DOI] [PubMed] [Google Scholar]
- 65.Nahabedian M Y, Spear S L.Acellular dermal matrix for secondary procedures following prosthetic breast reconstruction Aesthet Surg J 201131(7, Suppl):38S–50S. [DOI] [PubMed] [Google Scholar]
- 66.Maxwell G P, Gabriel A.Acellular dermal matrix in aesthetic revisionary breast surgery Aesthet Surg J 201131(7, Suppl):65S–76S. [DOI] [PubMed] [Google Scholar]
- 67.Spear S L, Seruya M, Clemens M W, Teitelbaum S, Nahabedian M Y. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg. 2011;127(03):1047–1058. doi: 10.1097/PRS.0b013e31820436af. [DOI] [PubMed] [Google Scholar]
- 68.Hartzell T L, Taghinia A H, Chang J, Lin S J, Slavin S A. The use of human acellular dermal matrix for the correction of secondary deformities after breast augmentation: results and costs. Plast Reconstr Surg. 2010;126(05):1711–1720. doi: 10.1097/PRS.0b013e3181ef900c. [DOI] [PubMed] [Google Scholar]
- 69.Davis C, Boyd C, Mateo de Acosta Andino D A. Dermal autografts in breast reconstruction: a review of past and current trends. Ann Plast Surg. 2020;84(05):618–622. doi: 10.1097/SAP.0000000000002128. [DOI] [PubMed] [Google Scholar]
- 70.Colwell A S, Breuing K H. Improving shape and symmetry in mastopexy with autologous or cadaveric dermal slings. Ann Plast Surg. 2008;61(02):138–142. doi: 10.1097/SAP.0b013e31815bfe7c. [DOI] [PubMed] [Google Scholar]
- 71.Becker H, Lind J G., II The use of synthetic mesh in reconstructive, revision, and cosmetic breast surgery. Aesthetic Plast Surg. 2013;37(05):914–921. doi: 10.1007/s00266-013-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nair N M, Mills D C. Poly-4-hydroxybutyrate (P4HB) scaffold internal support: preliminary experience with direct implant opposition during complex breast revisions. Aesthet Surg J. 2019;39(11):1203–1213. doi: 10.1093/asj/sjy276. [DOI] [PubMed] [Google Scholar]
- 73.Lesavoy M A, Trussler A P, Dickinson B P. Difficulties with subpectoral augmentation mammaplasty and its correction: the role of subglandular site change in revision aesthetic breast surgery. Plast Reconstr Surg. 2010;125(01):363–371. doi: 10.1097/PRS.0b013e3181c2a4b0. [DOI] [PubMed] [Google Scholar]
- 74.Junior I M, Graf R M, Ascenço A SK. Is there a breast augmentation outcome difference between subfascial and subglandular implant placement? A prospective randomized double-blinded study. Aesthetic Plast Surg. 2019;43(06):1429–1436. doi: 10.1007/s00266-019-01465-8. [DOI] [PubMed] [Google Scholar]
- 75.Maxwell G P, Gabriel A. The neopectoral pocket in revisionary breast surgery. Aesthet Surg J. 2008;28(04):463–467. doi: 10.1016/j.asj.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Spear S L, Dayan J H, Bogue D. The “neosubpectoral” pocket for the correction of symmastia. Plast Reconstr Surg. 2009;124(03):695–703. doi: 10.1097/PRS.0b013e3181a8c89d. [DOI] [PubMed] [Google Scholar]
- 77.Grotting J C, Gardner P M, Cohn A B. 2nd ed. St. Louis, MO: Quality Medical Publishing; 2007. Reoperative surgery following breast augmentation; pp. 1261–1311. [Google Scholar]
- 78.Netscher D T. Aesthetic outcome of breast implant removal in 85 consecutive patients. Plast Reconstr Surg. 2004;113(03):1057–1059. doi: 10.1097/01.prs.0000105686.02437.74. [DOI] [PubMed] [Google Scholar]
- 79.Graf R M, Closs Ono M C, Pace D, Balbinot P, Pazio A LB, de Paula D R. Breast auto-augmentation (mastopexy and lipofilling): an option for quitting breast implants. Aesthetic Plast Surg. 2019;43(05):1133–1141. doi: 10.1007/s00266-019-01387-5. [DOI] [PubMed] [Google Scholar]
- 80.Rohrich R J, Parker T H., III Aesthetic management of the breast after explantation: evaluation and mastopexy options. Plast Reconstr Surg. 2007;120(01):312–315. doi: 10.1097/01.prs.0000264400.42376.e9. [DOI] [PubMed] [Google Scholar]
- 81.Maxwell G P, Gabriel A. Efficacy of acellular dermal matrices in revisionary aesthetic breast surgery: a 6-year experience. Aesthet Surg J. 2013;33(03):389–399. doi: 10.1177/1090820X13478967. [DOI] [PubMed] [Google Scholar]
- 82.Pozner J N, White J B, Newman M I. Use of porcine acellular dermal matrix in revisionary cosmetic breast augmentation. Aesthet Surg J. 2013;33(05):681–690. doi: 10.1177/1090820X13491279. [DOI] [PubMed] [Google Scholar]
- 83.Spear S L, Sinkin J C, Al-Attar A. Porcine acellular dermal matrix (strattice) in primary and revision cosmetic breast surgery. Plast Reconstr Surg. 2013;131(05):1140–1148. doi: 10.1097/PRS.0b013e3182865d0c. [DOI] [PubMed] [Google Scholar]
- 84.Barker D E, Retsky M I, Schultz S. “Bleeding” of silicone from bag-gel breast implants, and its clinical relation to fibrous capsule reaction. Plast Reconstr Surg. 1978;61(06):836–841. [PubMed] [Google Scholar]
- 85.Baker J L, Jr, Chandler M L, LeVier R R. Occurrence and activity of myofibroblasts in human capsular tissue surrounding mammary implants. Plast Reconstr Surg. 1981;68(06):905–912. doi: 10.1097/00006534-198112000-00010. [DOI] [PubMed] [Google Scholar]
- 86.Hwang K, Sim H B, Huan F, Kim D J. Myofibroblasts and capsular tissue tension in breast capsular contracture. Aesthetic Plast Surg. 2010;34(06):716–721. doi: 10.1007/s00266-010-9532-8. [DOI] [PubMed] [Google Scholar]
- 87.Del Vecchio D, Wall S., Jr Expansion vibration lipofilling: a new technique in large-volume fat transplantation. Plast Reconstr Surg. 2018;141(05):639e–649e. doi: 10.1097/PRS.0000000000004338. [DOI] [PubMed] [Google Scholar]
- 88.Abboud M H, Dibo S A, Abboud N M. Power-assisted liposuction and lipofilling: techniques and experience in large-volume fat grafting. Aesthet Surg J. 2020;40(02):180–190. doi: 10.1093/asj/sjz019. [DOI] [PubMed] [Google Scholar]


