Abstract
Ants show remarkable ecological and evolutionary success due to their social life history and division of labour among colony members. In some lineages, the worker force became subdivided into morphologically distinct individuals (i.e. minor versus major workers), allowing for the differential performance of particular roles in the colony. However, the functional and ecological significance of these morphological differences are not well understood. Here, we applied finite element analysis (FEA) to explore the biomechanical differences between major and minor ant worker mandibles. Analyses were carried out on mandibles of two Pheidole species, a dimorphic ant genus. We tested whether major mandibles evolved to minimize stress when compared to minors using combinations of the apical tooth and masticatory margin bites under strike and pressure conditions. Majors performed better in pressure conditions yet, contrary to our expectations, minors performed better in strike bite scenarios. Moreover, we demonstrated that even small morphological differences in ant mandibles might lead to substantial differences in biomechanical responses to bite loading. These results also underscore the potential of FEA to uncover biomechanical consequences of morphological differences within and between ant workers.
Keywords: cuticle, division of labour, finite element analysis, mandible, trulleum, worker polymorphism
1. Introduction
The evolution of complex societies in ants followed the advent of reproductive division of labour into distinct castes, in which largely sterile and wingless individuals (i.e. workers) perform quotidian colony tasks, whereas winged individuals became specialized for colony reproduction (i.e. queens and males) [1,2]. These changes were accompanied by substantial morphological differences among reproductives and non-reproductives, with the latter giving up reproduction and dispersal capacities while experiencing both morphological and behavioural specialization [3–5]. In some ant lineages, the worker force became further subdivided into morphologically distinct subcastes (e.g. minor versus major workers), and such differences are thought to allow differential performance of particular roles in the colony, such as seed milling and defense [6,7]. In ants, worker polymorphism evolved in several lineages, and its role in facilitating task specialization is widely recognized [6–8]. Several studies explored the genetic [9,10], ecological [11–13] and developmental [14,15] determinants of worker polymorphism in distinct ant lineages [8].
The genus Pheidole shows an interesting pattern among its almost 1200 known species [16]: the development of dimorphic worker subcastes, represented by major and minor workers, where majors have a disproportionately larger head [6,15,17]. Pheidole species are distributed worldwide, but most of their diversity and abundance is concentrated in the tropics [18,19]. Although Pheidole species are typically considered diet generalists [17], some species might show some degree of dietary specialization [20]. Of all their food items, feeding on seeds evolved many independent times and has been indicated as an important factor to explain the lineage diversification due to behavioural and morphological adaptations related to seed harvesting and processing [21]. Since majors are specialized in tasks such as defense and food processing [17,22], their larger heads could be a consequence of evolutionary pressures towards the specialization to those tasks [23]. However, evidence gathered so far has been mixed (e.g. [24]).
Understanding the main trends in the morphological evolution of Pheidole has received considerable attention in the past decades. Different approaches were employed to understand the evolution of a variety of structures, showing contrasting results to the relative contributions of size and shape to the morphological diversity of the genus [23,25–28]. However, little is known about the evolution of mandibular morphology in Pheidole. The proximal articulations of ant mandibles are dicondylic, as expected for Pterygota [29], with both dorsal and ventral joints [30]. Ant worker mandibles are the primary structures used to interact with their environment (e.g. biting, carrying, excavating, cutting, fighting) [31]. Mandibular movement is powered by two muscles, the craniomandibularis internus (0md1), whose contraction closes the mandibles, and the craniomandibularis externus (0md3), responsible for the opening process [29,32]. The 0md1 fibres attach to the mandible through a mandibular cuticular projection called mandibular apodeme [32]. The angle of attachment to the apodeme, combined with sarcomere length, are directly related to the velocity and force of the mandibular movement [33], so that 0md1 is considered the key to the versatility of ant mandibles [34,35], being much more developed than the 0md3 [30,32,35]. In Pheidole majors, the 0md1 is remarkably large, with its increase in size compared with minors being achieved at the expense of the glandular, digestive, and nervous system in the head [36]. Fibres of the 0md1 also continue to develop even for days after the adult emergence in both subcastes, and this characteristic correlates to behavioural development in workers [37].
Regardless of the importance of mandibles to many aspects of ant life history, little is known about how morphological variation between species or worker subcastes relates to bite loading demands, except for one specialized snap-jaw species [38]. Worker polymorphism can lead to behavioural specialization, mainly through variation in mandible morphology [39–41], but biomechanical approaches to directly assess this relationship in ants are scarce [38]. To understand how mandible morphology relates to the biomechanical demands of biting, it is important to employ approaches that allow for the direct assessment of bite loading conditions. Finite element analysis (FEA) is a numerical method that approximates the mechanical simulation of loading conditions in structures of interest. By applying loads and defining the boundary conditions (movement restrictions) on the structure, FEA estimates the mechanical response, i.e. how stress flows along the structure according to its shape [42,43]. By employing FEA, one can assess how variation in mandibular morphology among ant species as well as between castes and subcastes translates into the capacity of mandibles to deal with bite loading demands [38], as also explored for the evolution of mandible form in dragonflies [44], stag beetles [45–47], and the functional morphology of the mouthparts of the reticulated beetle Priacma serrata (LeConte) [48]. Biomechanical approaches employing FEA have also revealed important aspects of the evolution of other insect structures, such as wings and the mechanics of flight [49,50] and the evolution of insect head capsules [51,52].
To improve our understanding of morphological evolution in Pheidole species, and the role of morphological differentiation to improve task specialization in polymorphic ants, we simulate several bite scenarios in silico by applying FEA [42,43] on three-dimensional (3D) models of minor and major mandibles of two Pheidole species. We hypothesize that major mandibles are better able to mitigate stress than those of minors, given their greater robustness. Alternatively, if each worker subcaste has mandibles optimized to perform different tasks, majors and minors could perform better in distinct biting scenarios. Interspecific differences are expected between the more distinct mandibles of majors, which can suggest changes in the capacity to deal with hard food items, given the specialized roles of those workers [17,22]. Alternatively, differences between species in minor worker mandibles will suggest that even small morphological distinctions can lead to biomechanical idiosyncrasies.
2. Methods
2.1. Studied species
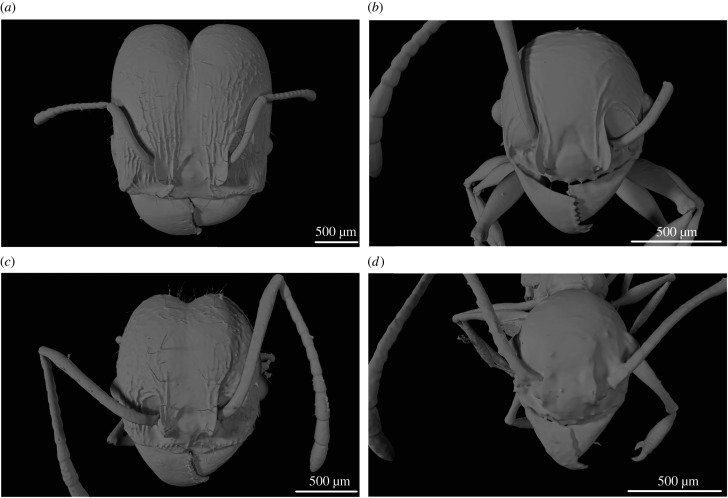
Colonies of Pheidole hetschkoi Emery and P. cf. lucretii were collected in an urban fragment of Atlantic Forest in Curitiba, Paraná, Brazil (25°26'45.9″ S 49°13'55.5″ W). Those species were selected due to their high abundance and ease of collection in the study area, as well as because of the morphological discrepancies observed mainly between major worker mandibles, which suggested possible biomechanical idiosyncrasies in the response to bite loading demands that could affect species dietary amplitudes. Majors of P. hetschkoi are sturdy, with larger heads (figure 1a) and more robust mandibles than P. cf. lucretii majors, which have also smaller heads and are slender (figure 1c). Minors differ little between species in mandible shape (figure 1b,d). Pheidole hetschkoi harvest and accumulate seeds in their nests, which was never recorded for P. cf. lucretii (author's personal observation). Voucher specimens are deposited at the Entomological Collection Padre Jesus Santiago Moure, Department of Zoology, Federal University of Paraná, Brazil.
Figure 1.
3D models of Pheidole workers heads. Pheidole hetschkoi (a) major and (b) minor worker. Pheidole cf. lucretii (c) major and (d) minor worker.
2.2. CT scanning and image processing
One specimen of each subcaste and species were scanned with a ZEISS Xradia 510 Versa X-ray microCT scanner at the Okinawa Institute of Science and Technology, using the software ZEISS Scout and Scan Control System. Exposure time of each specimen varied from 1 to 5 s, under an ‘Air' filter and 4× objective. The voltage was set between 30 and 50 keV, from 4 to 5 W of power, under a ‘normal' field mode and intensity levels of 15 000 and 17 000 across the whole specimen. Scan time varied from 27 to 30 min, generating 801 projections from full 360-degree rotations. Model reconstruction was performed with XMReconstructor, and mandibles segmentation was carried in ITK-snap 3.8.0 [53]. For mesh generation and simplification, we used the software MeshLab [54], and to generate 3D mandible models for FEA simulations, we used the software Fusion 360 (AUTODESK). Ant mandibles are internally hollow, and their cuticle varies in thickness along the mandible axis, characteristics that can influence mechanical behaviour and structure stiffness, and we incorporated these aspects in our 3D reconstructions to model realistic mandible morphologies (electronic supplementary material, file S1).
2.3. Finite element analysis simulations
FEA is a numerical method that approximates the mechanical responses of a structure submitted to loading demands [43] which, in the case of biological structures, could represent the demands of biting, running, jumping and so on [42]. Here, the structures of interest are the Pheidole worker mandibles, and the loading demands refer to different bite conditions. To quantify the mechanical response of a structure to external loading, FEA requires the discretization of the structure into small parts, resulting in the finite element mesh composed of elements of pre-defined shape and a specific number of points, called nodes, used to solve the equations [55,56]. Displacements on nodes are calculated to estimate stress and strain based on the structure's material properties and shape [42]. We used 10-node tetrahedral elements (C3D10) to generate the finite element mesh. The number of elements varied for each model, as well as the size of each element between subcastes, to adapt meshes to each morphology (table 1).
Table 1.
Size measurements of each worker and characteristics of each finite element mesh.
| specimen | mesh superficial area (mm2) | element edge length (mm) | number of elements | voxel size (µm) | mandible length (mm) | head width (mm) | |
|---|---|---|---|---|---|---|---|
| major | P. hetschkoi | 2.35 | 0.023 | 449 488 | 5.30876 | 1.10 | 1.84 |
| P. cf. lucretii | 1.011 | 0.023 | 278 634 | 4.49981 | 0.85 | 1.18 | |
| minor | P. hetschkoi | 0.503 | 0.0035 | 881 691 | 3.89985 | 0.65 | 0.80 |
| P. cf. lucretii | 0.25 | 0.0035 | 392 790 | 4.04989 | 0.47 | 0.58 |
We performed linear static simulations of four distinct biting scenarios for each species and subcastes, divided into two categories, namely strike and pressure, which reflect different aspects of mandible movement in terms of force and velocity. In all simulations, we defined the constrained and loaded regions to capture the mechanical response at the exact moment that the mandible hits or presses an object. Therefore, we did not intend to simulate the conditions during the mandibular closing movement. In strike scenarios, a condition associated with faster movements, we define the mandible articulations with the head (dorsal - dma and ventral - vma) as the constrained regions, applying static load on the apical tooth or the masticatory margin (at and mm, figure 2a). In pressure scenarios associated with slower mandible movements but powerful bites, in addition to the mandibular joints, we also constrained the apical tooth or the mm. We applied the load to the region of 0md1 insertion, following the direction of contraction (figure 2b) to simulate the use of mandibles for food compression. We constrained nodal displacement in x, y and z-directions and applied a 1 N load uniformly distributed among nodes in all simulations. We modelled the mandible cuticle as an isotropic and linearly elastic material, setting Young's modulus as 2.75 GPa and the Poisson's ratio as 0.3, based on measures from the cuticle of ant mandibles available in the literature [57]. Given that we intended to investigate how variation in mandible morphology affects the mechanical responses to the same loading demands in different biting conditions, the only source of variation for each biting simulation between species and workers was the morphology of the mandibles. Therefore, we can test if some morphologies are better suited for specific biting conditions. We present FEA stress results based on Tresca failure criterion, more suitable for brittle fracture, which determines an equivalent stress value under which the material will possibly fail when subjected to combined load [58]. We used Abaqus 6 (Dassault Systèmes) to run the FEA simulations. Mandible 3D solid models are available on electronic supplementary material, file S1.
Figure 2.
Loaded and constrained regions in strike (a) and pressure (b) biting simulations. In (a,b), black arrows indicate the direction and region of load, and dashed lines enclose the constrained regions for each simulation. al, Atala; at, apical tooth; bm, basal margin; dma, dorsal mandibular articulation; ef, external mandibular face; em, external margin; if, internal face of the mandible; mm, masticatory margin; vma, ventral mandibular articulation; 0md1, muscle craniomandibularis internus.
3. Results
3.1. Finite element analysis simulations
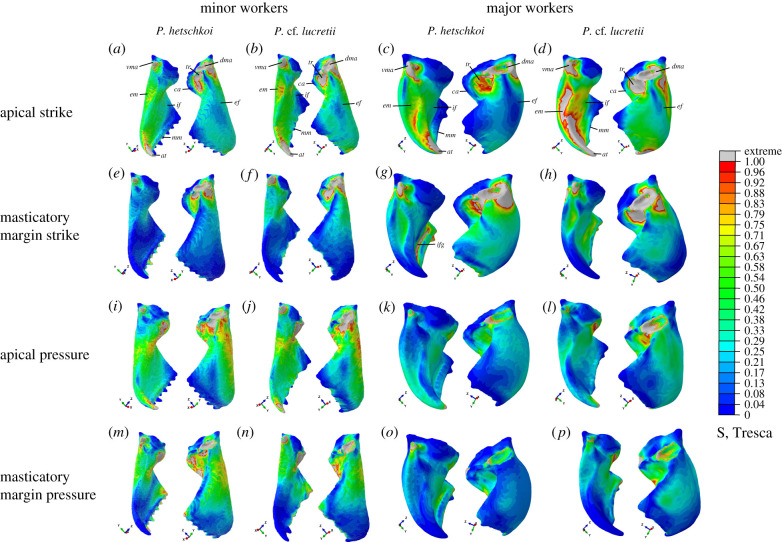
Stress distribution results are shown in figure 3. Given that the size of each model varies, and that we used idealized loads and material properties, we chose not to interpret absolute stress values. Instead, we will focus on qualitative differences among simulations by rescaling the stress ranges based on a reference model to facilitate comparisons between species, subcastes and biting scenarios (electronic supplementary material, figure S1). Therefore, relative differences in stress distribution between simulations indicate mandibular biomechanical distinctions to assimilate loading conditions.
Figure 3.
Tresca stress results (rescaled to range between 0 and 1) for the four biting scenarios (rows), from minors and majors of both Pheidole species (columns). Each letter depicts a distinct simulation. Colour represents a proportional value of stress in relation to the maximum value considered for each simulation, indicated as 1.00, and grey represents extremes values above the maximum considered. at, Apical tooth; ca, canthellus; dma, dorsal mandibular articulation; ef, external mandibular face; em, external margin; if, internal face of the mandible; ifg, groove on the internal face of the mandible; mm, masticatory margin; tr, trulleum; vma, ventral mandibular articulation.
3.2. Major worker mandibles
When displacement restrictions were applied on the mandibular joints, those regions expectedly showed high-stress levels, but stresses had to spread to other regions to be effectively absorbed. Starting from the dma, stresses dissipate mainly along the mandible's external face (ef) and trulleum (tr, figure 3c,d,g,h,k,l,o,p). Indeed, the trulleum and the canthellus (ca, figure 3c,d) were important to concentrate stresses coming from the dma in all simulations. Stresses from the vma spread mainly along the external margin (em) and through its surroundings along the internal (if) and external faces of the mandible (figure 3c,d,g,h,k,l,o,p). Contrasting different biting scenarios, higher stresses are found when only the apical tooth is employed, mainly at strike (figure 3c,d,k,l). This result indicates that ants face marked mechanical restrictions whether they only use the apical tooth. Pressure scenarios generated higher stresses around the basal region of the if (figure 3k,l,o,p), whereas strike scenarios concentrated more stress near the mm, an expected consequence of load application (figure 3c,d,g,h). However, the key aspect related to the different biting scenarios is the higher stress levels in dma and vma in the strike (figure 3c,d,g,h) versus pressure simulations (figure 3k,l,o,p), which indicates that strike causes higher mechanical demands in the mandibular joints than pressure.
The main aspect that influences stress dissipation differences between species is the presence of a groove in the if. When applying a load or constraining the mm of majors, the mm concavity (defined by the mm and a parallel carina ventrolaterally) of both species concentrates stress, but much of the stress spreads in direction to the if. Pheidole hetschkoi has a deeper groove near the mm, which acts as an important stress concentrator, mainly in strike scenarios on the mm (ifg, figure 3g). While P. cf. lucretii also shows stress concentration at the same region in this biting scenario, those stresses spread more extensively along the if (figure 3h), which suggests that its groove is shallow and does not act as a stress concentrator. The ef curvature also differs between species, but there are no substantial differences in stress dissipation patterns (figure 3c,d,g,h,k,l,o,p). The dissipation through the ef is more restricted to the articulation surroundings, given the robustness of the mandibular base, which could explain why there is not a conspicuous effect of the ef curvature in the stress dissipation pattern between species. Stresses were proportionately higher in the P. cf. lucretii mandible, through most mandibular regions and all biting scenarios, but the differences are more striking in pressure scenarios (figure 3l,p).
3.3. Minor worker mandibles
There is a distinguished stress concentration around the more constricted region of the if, a trend that occurs mainly in strike simulations, especially when the load was applied on the mm (figure 3e,f). This constriction acts as a stress concentrator in minors due to their slender mandibles in comparison to majors. When the results of different species are compared, P. cf. lucretii simulations show proportionately higher stresses than P. hetschkoi in general (figure 3b,f,j,n), contrary to the expectation that minor mandibles would not differ in mechanical performance. The overall lower stress levels found in mm strike simulations of the P. hetschkoi minor seems to reflect the presence of well-developed teeth along its mm. It is noticeable that the mm teeth absorb great levels of stress (figure 3e) so that their absence leads to higher stress levels along the mandible surfaces in strike simulations of P. cf. lucretii minor, as well as in majors of both species. The higher stresses along the if in P. cf. lucretii minor mandible, compared to P. hetschkoi minor mandible, draw attention to the mechanical limitations associated with worn mandibles, as is the case of the P. cf. lucretii minor mandible modelled, which can lead to behavioural switches in task performance along the worker lifetime.
Regarding the biting scenarios, pressure in minors results in higher stresses on the mandibular internal and external faces of both species when compared to majors (figure 3i,j,m,n). As occurred in pressure scenarios for majors, stresses along the if concentrate near the base of the mandible, where the load was applied. However, in minors, the mandible base is slender, which can explain why the mandibular surfaces in minors are proportionately more stressed in pressure than in strike simulations.
4. Discussion
In this study, we apply FEA in mandibles of Pheidole workers to simulate different biting scenarios and investigate how morphological differences in mandible morphology reflect their responses to those bite loading demands. Our results demonstrate how the mandible morphology of dimorphic workers can be optimized for particular tasks and draws attention to the role of specific mandibular regions or structures to deal with the stresses generated by their bite. Most extant lineages preserve the primitive ant mandible shape, which consists on a blade whose mm possess a row of teeth for cutting and grasping [59], and that can be divided into two components, a basal thick stem, and a distal triangular blade [32]. Our results indicate that the increased thickness of the mandible basal region may conform to the high loading demands experienced by the mandibular articulations with the head. Most of the stresses generated on the apical tooth dissipate along the external margin towards the mandibular base, in both species and subcastes, avoiding the spread of considerable stresses through the more delicate mandibular surfaces. In strike simulations on the mm, the presence of well-developed teeth results in stresses being concentrated on the teeth instead of spreading through the internal face of the mandible. Majors of Pheidole, in which the mm is toothless, show high levels of stress in the mm concavity that is not entirely absorbed in this region. Interestingly, they have a deeper groove on their mandible internal face, especially P. hetschkoi, which helps to concentrate stresses near the more robust mm instead of spreading through the internal face of the mandible. Although alleviating the stress level in the mandibular articulations, such stress concentration can be harmful in cases in which the structure is submitted to cycles of loading, leading to structural failure due to material fatigue [60].
An important aspect of Pheidole mandibular morphology to bite mechanics is the role of the trulleum and the canthellus on stress concentration. The trulleum is a concavity near the dma that is delimited by a cuticular ridge called canthellus, a configuration that is present only in some myrmicine ants [30]. The function of the trulleum was hitherto unknown, although it was suggested that it could act as an additional stabilization of the mandible [32]. Here, we demonstrate for the first time the importance of the trulleum and the canthellus to assist in stress concentration along the dma, avoiding the spread of stresses through the more delicate mandibular surfaces. This is an interesting discovery, given that the dma seems to concentrate higher stresses in general than the vma. Given the suggested functional role of those mandibular regions outlined by our results, it would be interesting to investigate the biomechanical responses of mandibles that lack the development of this structure to understand how stresses dissipate from dma without those important stress concentrators, especially in ant species with similar loading demands as Pheidole mandibles. Although many ant lineages share the common mandible triangular shape as Pheidole species [32], other subtle morphological characteristics could assist in stress concentration (e.g. the cuticular thickness around this region and the mandible curvature pattern). Differences in mandible use and diet can also influence the amount of stress in the mandibular articulations (e.g. a diet rich in liquid food represents much lower bite loading demands than one composed of seeds or arthropods).
Our results also underscore how more robust major mandibles are better suited to deal with pressure biting than the minors' slender mandibles, which surprisingly show higher performance in strike scenarios. These results agree with the specialized roles played by major workers in the colony. The behavioural repertoire of major workers is particularly limited, being frequently restricted to defense and/or food processing [17,22]. Indeed, when minors are experimentally removed from the colony, major workers take over many of their tasks, although with decreased efficiency [22,61]. Major mandibles meet the demands to deal with the processing of hard food items through pressure, with their toothless mm spreading bite forces evenly around the food item. Seed consumption is considered an important aspect in the evolution of several myrmicine genera, such as Pheidole, Pogonomyrmex and Solenopsis [21,62]. However, the influence of granivory on morphological evolution, especially regarding the dimorphism in the Pheidole worker caste, is still poorly known [24]. Here, we demonstrate for the first time how ant mandible morphology can be tuned to deal with the mechanical demands of processing hard food items, such as seeds and arthropod cuticles, through the better performance of majors' mandibles in pressure biting conditions. Also, mandibles of P. hetschkoi majors show an even better performance in pressure bite than P. cf. lucretii, suggesting that majors of P. hetschkoi can deal better with harder food items than P. cf. lucretii. These results may lead to the possibility of food partitioning among Pheidole coexisting species and agree with the habit of seed consumption by P. hetschkoi, which demands higher bite forces and consequently leads to higher stress levels on the mandibles.
In general, Pheidole minor mandibles show a more serrated and sharped mm, with well-developed teeth, whereas majors have mandibles that are blunter and that show broader mm [39]. However, the particular specimen of P. cf. lucretii minor worker included in our study showed high levels of teeth wear, allowing us to assess the consequences of teeth wear on bite loadings. Teeth concentrate the forces generated by the masticatory muscles on smaller areas, with the potential to improve the initiation of fracture in the gripped object [63]. The importance of teeth on task efficiency was demonstrated for leaf-cutting ants, in which workers specialized to cut leaves switch to carrying them once their teeth are worn to a certain degree, reducing their cutting efficiency [64]. In Pheidole, minors perform a wide range of tasks in the colony [17,22], but information on the role of teeth wear on the probability of task switch in minors is scarce. Here we demonstrate the possible mechanical consequences of teeth wear in ant mandibles, comparing the relative amount of stress generated during mm strike simulations in P. hetschkoi and P. cf. lucretii minors. Our results indicated that P. cf. lucretii has relatively higher stresses than P. hetschkoi, mainly along its internal face of the mandible, which drives higher stresses at the mandibular articulations with the head. Further studies on task allocation and mandible morphology in dimorphic ant species can address if teeth wear generates task switch, and biomechanical studies can reveal how teeth wear reduces task efficiency [64]. Also important is to understand if cuticle hardening by heavy metal bioaccumulation in the mandible mm [57,65] could help mitigate the stress levels reaching the mandibular faces and articulations, as suggested for genital damage in bush crickets [66].
The morphological evolution of Pheidole might be strongly driven by differences in size [23], which tends to evolve at higher rate than shape [18,25,27]. More recently, studies applying geometric morphometrics approaches validated the prominence of size to explain the morphological disparity in the genus but also pointed to different evolutionary rates and levels of integration between head and mesosoma shape and size [27,28]. Pheidole morphological diversification seems to be very constrained [23], in contrast to their ecological disparity [18,67], as reflected in the widespread distribution of the genus throughout most of the terrestrial ecosystems [19]. Field observations demonstrate that, despite the relative morphological resemblance in Pheidole species, they can show considerable ecological and behavioural diversity [68,69]. Here, we demonstrate that even small morphological differences in mandible shape between species can lead to biomechanical specialization, mainly in the food processing capacity of majors, as suggested by descriptions of the mandibular morphology variation in Pheidole species [39]. This biomechanical specialization can expand the diet range of species and contribute to food partitioning [20,70,71], decreasing the degree of competition and allowing for species coexistence [72].
Our results provide a biomechanical basis to understand how mandible morphological evolution can improve task specialization in polymorphic ants and help develop a general understanding of form–function relationships in ant mandibles. Morphological polymorphism in the worker caste can expand the range of prey items that a species can handle, as demonstrated for some army ants in the genus Eciton [11,12]. In the highly polymorphic genus Cephalotes, which together with Procryptocerus is Pheidole's sister lineage, some workers have the head modified into a flat surface used to obstruct and close the nest entrance, protecting the colony against invasion [73]. In some fire ants, such as Solenopsis geminata (Fabricius), the degree of worker polymorphism is associated with higher levels of division of labour, with major workers being specialized in seed milling [62,74]. Division of labour in leaf-cutting ants is associated with morphological distinctions among worker mandibles, as demonstrated for the polymorphic genus Atta [41]. In addition, refined morphological descriptions of Pheidole worker mandibles suggest that differences in the mm can improve task specialization [39].
Although the role of worker polymorphism for division of labour in ants is well established [8], we show that, by applying biomechanical approaches we can advance our understanding about the functional role of morphological disparity, as demonstrated here for Pheidole workers. Polymorphic ant lineages are ideal models to investigate form–function relationships, and the morphological differentiation of their mandibles should be studied in detail, given the importance of this structure to worker interactions with the environment. Future studies can contribute to our knowledge on the evolution of ant mandibles morphology by investigating the role of metal bioaccumulation [75–77] and the consequent cuticle hardening [57,65] in the mechanical response of mandibles to bite loading demands. Additionally, biomechanical approaches investigating the variation in bite force in morphologically polymorphic ant lineages can provide valuable information for the evolution of task specialization [39], and to understand how mandible morphology can be optimized to deal with powerful or fast movements [38].
Acknowledgements
The authors acknowledge Dr Emily Rayfield, Dr Mauricio Moura, Dr Roberto Keller, and two anonymous reviewers for valuable comments on the manuscript. The authors thank the OIST Imaging section for access to the CT scanner.
Data accessibility
Three-dimensional reconstructions of the Pheidole workers mandibles used in the finite element analysis simulations are available as electronic supplementary material File S1 at rs.figshare.com [78].
Authors' contributions
A.C.F. and E.P.E. collected the raw data; C.L.K., A.C.F., M.A.A. and M.R.P. contributed on the study design; C.L.K., A.C.F. and M.A.A. contributed on data analysis; C.L.K. and M.R.P. drafted the manuscript; A.C.F., E.P.E. and M.A.A. critically revised the manuscript; M.R.P. coordinated the study.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) – doctorate scholarship to C.L.K. (financial code 001) and PDSE grant no. 88881.189085/2018-01 to A.C.F., and E.P.E. was supported by subsidy funding to OIST.
References
- 1.Wilson EO. 1971. The insect societies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 3.Keller RA, Peeters C, Beldade P. 2014. Evolution of thorax architecture in ant castes highlights trade-off between flight and ground behaviors. eLife 3, e01539. ( 10.7554/eLife.01539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Divieso R, Silva TSR, Pie MR. 2020. Morphological evolution in the ant reproductive caste. Biol. J. Linnean Soc. 131, 465-475. ( 10.1093/biolinnean/blaa138) [DOI] [Google Scholar]
- 5.Peeters C, Keller RA, Khalife A, Fischer G, Katzke J, Blanke A, Economo EP. 2020. The loss of flight in ant workers enabled an evolutionary redesign of the thorax for ground labour. Front. Zool. 17, 33. ( 10.1186/s12983-020-00375-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson EO. 1953. The origin and evolution of polymorphism in ants. Q Rev. Biol. 28, 136-156. ( 10.1086/399512) [DOI] [PubMed] [Google Scholar]
- 7.Oster GF, Wilson EO. 1978. Caste and ecology in the social insects. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 8.Wills BD, Powell S, Rivera MD, Suarez AV. 2018. Correlates and consequences of worker polymorphism in ants. Annu. Rev. Entomol. 63, 575-598. ( 10.1146/annurev-ento-020117-043357) [DOI] [PubMed] [Google Scholar]
- 9.Gadagkar R. 1997. The evolution of caste polymorphism in social insects: genetic release followed by diversifying evolution. J. Genet. 76, 167-179. ( 10.1007/BF02932215) [DOI] [Google Scholar]
- 10.Huang MH, Wheeler DE, Fjerdingstad EJ. 2013. Mating system evolution and worker caste diversity in Pheidole ants. Mol. Ecol. 22, 1998-2010. ( 10.1111/mec.12218) [DOI] [PubMed] [Google Scholar]
- 11.Powell S, Franks NR. 2005. Caste evolution and ecology: a special worker for novel prey. Proc. R. Soc. B 272, 2173-2180. ( 10.1098/rspb.2005.3196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell S, Franks NR. 2006. Ecology and the evolution of worker morphological diversity: a comparative analysis with Eciton army ants. Funct. Ecol. 20, 1105-1114. ( 10.1111/j.1365-2435.2006.01184.x) [DOI] [Google Scholar]
- 13.Powell S. 2009. How ecology shapes caste evolution: linking resource use, morphology, performance and fitness in a superorganism. J. Evol. Biol. 22, 1004-1013. ( 10.1111/j.1420-9101.2009.01710.x) [DOI] [PubMed] [Google Scholar]
- 14.Wheeler DE. 1991. The developmental basis of worker caste polymorphism in ants. Am. Nat. 138, 1218-1238. ( 10.1086/285279) [DOI] [Google Scholar]
- 15.Rajakumar, R, et al. 2018. Social regulation of a rudimentary organ generates complex worker-caste systems in ants. Nature 562, 574-577. [DOI] [PubMed] [Google Scholar]
- 16.Bolton B. 2020. An online catalog of the ants of the world. See antcat.org (accessed on 3 September 2020).
- 17.Wilson EO. 2003. Pheidole in the New World: a dominant, hyperdiverse ant genus. Cambridge, MA: Harvard University Press. [Google Scholar]
- 18.Economo EP, Klimov P, Sarnat EM, Guénard B, Weiser MD, Lecroq B, Knowles LL. 2015. Global phylogenetic structure of the hyperdiverse ant genus Pheidole reveals the repeated evolution of macroecological patterns. Proc. R. Soc. B 282, 20141416. ( 10.1098/rspb.2014.1416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Economo EP, Huang J-P, Fischer G, Sarnat EM, Narula N, Janda M, Guénard B, Longino JT, Knowles LL. 2019. Evolution of the latitudinal diversity gradient in the hyperdiverse ant genus Pheidole. Global Ecol. Biogeogr. 28, 456-470. ( 10.1111/geb.12867) [DOI] [Google Scholar]
- 20.Rosumek FB. 2017. Natural history of ants: what we (do not) know about trophic and temporal niches of neotropical species. Sociobiology 64, 244. ( 10.13102/sociobiology.v64i3.1623) [DOI] [Google Scholar]
- 21.Moreau CS. 2008. Unraveling the evolutionary history of the hyperdiverse ant genus Pheidole (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 48, 224-239. ( 10.1016/j.ympev.2008.02.020) [DOI] [PubMed] [Google Scholar]
- 22.Wilson EO. 1984. The relation between caste ratios and division of labor in the ant genus Pheidole (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 16, 89-98. ( 10.1007/BF00293108) [DOI] [Google Scholar]
- 23.Pie MR, Traniello JFA. 2007. Morphological evolution in a hyperdiverse clade: the ant genus Pheidole . J. Zool. 271, 99-109. ( 10.1111/j.1469-7998.2006.00239.x) [DOI] [Google Scholar]
- 24.Holley J-AC, Moreau CS, Laird JG, Suarez AV. 2016. Subcaste-specific evolution of head size in the ant genus Pheidole. Biol. J. Linn. Soc. 118, 472-485. ( 10.1111/bij.12769) [DOI] [Google Scholar]
- 25.Pie MR, Tschá MK. 2013. Size and shape in the evolution of ant worker morphology. PeerJ 1, e205. ( 10.7717/peerj.205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarnat EM, Friedman NR, Fischer G, Lecroq-Bennet B, Economo EP. 2017. Rise of the spiny ants: diversification, ecology and function of extreme traits in the hyperdiverse genus Pheidole (Hymenoptera: Formicidae). Biol. J. Linnean Soc. 122, 514-538. ( 10.1093/biolinnean/blx081) [DOI] [Google Scholar]
- 27.Friedman NR, Remeš V, Economo EP. 2019. A morphological integration perspective on the evolution of dimorphism among sexes and social insect castes. Integr. Comp. Biol. 59, 410-419. [DOI] [PubMed] [Google Scholar]
- 28.Friedman NR, Lecroq Bennet B, Fischer G, Sarnat EM, Huang J, Knowles LLK, Economo EP. 2020. Macroevolutionary integration of phenotypes within and across ant worker castes. Ecol. Evol. 10, 9371-9383. ( 10.1002/ece3.6623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snodgrass RE, 1935. Principles of insect morphology. New York, NY: Cornell University Press. [Google Scholar]
- 30.Richter A, Keller RA, Rosumek FB, Economo EP, Hita Garcia F, Beutel RG. 2019. The cephalic anatomy of workers of the ant species Wasmannia affinis (Formicidae, Hymenoptera, Insecta) and its evolutionary implications. Arthropod. Struct. Dev. 49, 26-49. ( 10.1016/j.asd.2019.02.002) [DOI] [PubMed] [Google Scholar]
- 31.Wheeler WM. 1910. Ants: their structure, development and behavior. New York, NY: Columbia University Press. [Google Scholar]
- 32.Richter A, Hita Garcia F, Keller RA, Billen J, Economo EP, Beutel RG. 2020. Comparative analysis of worker head anatomy of Formica and Brachyponera (Hymenoptera: Formicidae). Arthropod Syst. Phylogeny 78, 133-170. ( 10.26049/ASP78-1-2020-06) [DOI] [Google Scholar]
- 33.Paul J, Gronenberg W. 1999. Optimizing force and velocity: mandible muscle fibre attachments in ants. J. Exp. Biol. 202, 797-808. [DOI] [PubMed] [Google Scholar]
- 34.Gronenberg W, Paul J, Just S, Hölldobler B. 1997. Mandible muscle fibers in ants: fast or powerful? Cell Tissue Res. 289, 347-361. ( 10.1007/s004410050882) [DOI] [PubMed] [Google Scholar]
- 35.Paul J. 2001. Mandible movements in ants. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 131, 7-20. ( 10.1016/S1095-6433(01)00458-5) [DOI] [PubMed] [Google Scholar]
- 36.Lillico-Ouachour A, Metscher B, Kaji T, Abouheif E. 2018. Internal head morphology of minor workers and soldiers in the hyperdiverse ant genus Pheidole. Can. J. Zool. 96, 383-392. ( 10.1139/cjz-2017-0209) [DOI] [Google Scholar]
- 37.Muscedere ML, Traniello JFA, Gronenberg W. 2011. Coming of age in an ant colony: cephalic muscle maturation accompanies behavioral development in Pheidole dentata. Naturwissenschaften 98, 783-793. ( 10.1007/s00114-011-0828-6) [DOI] [PubMed] [Google Scholar]
- 38.Larabee FJ, Smith AA, Suarez AV. 2018. Snap-jaw morphology is specialized for high-speed power amplification in the Dracula ant, Mystrium camillae . R. Soc. Open Sci. 5, 181447. ( 10.1098/rsos.181447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang MH. 2012. Extreme worker polymorphism in the big-headed Pheidole ants. Tucson, AZ: The University of Arizona. [Google Scholar]
- 40.Camargo RS, Hastenreiter IN, Forti LC, Lopes JFS. 2015. Relationship between mandible morphology and leaf preference in leaf-cutting ants (Hymenoptera: Formicidae). Rev. Colomb. Entomol. 41, 241-244. [Google Scholar]
- 41.Silva LC, Camargo RS, Lopes JFS, Forti LC. 2016. Mandibles of leaf-cutting ants: morphology related to food preference. Sociobiology 63, 881. ( 10.13102/sociobiology.v63i3.1014) [DOI] [Google Scholar]
- 42.Rayfield EJ. 2007. Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annu. Rev. Earth Planet. Sci. 35, 541-576. ( 10.1146/annurev.earth.35.031306.140104) [DOI] [Google Scholar]
- 43.Kupczik K. 2008. Virtual biomechanics: basic concepts and technical aspects of finite element analysis in vertebrate morphology. JASs 86, 193-198. [PubMed] [Google Scholar]
- 44.Blanke A, Schmitz H, Patera A, Dutel H, Fagan MJ. 2017. Form–function relationships in dragonfly mandibles under an evolutionary perspective. J. R. Soc. Interface 14, 20161038. ( 10.1098/rsif.2016.1038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyens J, Soons J, Aerts P, Dirckx J. 2014. Finite-element modelling reveals force modulation of jaw adductors in stag beetles. J. R. Soc. Interface 11, 20140908. ( 10.1098/rsif.2014.0908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goyens J, Dirckx J, Aerts P. 2015. Built to fight: variable loading conditions and stress distribution in stag beetle jaws. Bioinspir. Biomim. 10, 046006. ( 10.1088/1748-3190/10/4/046006) [DOI] [PubMed] [Google Scholar]
- 47.Goyens J, Dirckx J, Aerts P. 2016. Jaw morphology and fighting forces in stag beetles. J. Exp. Biol. 219, 2955-2961. ( 10.1242/jeb.141614) [DOI] [PubMed] [Google Scholar]
- 48.Hörnschemeyer T, Bond J, Young PG. 2013. Analysis of the functional morphology of mouthparts of the Beetle Priacma serrata, and a discussion of possible food sources. J. Insect Sci. 13, 1-14. ( 10.1673/031.013.12601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajabi H, Ghoroubi N, Darvizeh A, Dirks J-H, Appel E, Gorb SN. 2015. A comparative study of the effects of vein-joints on the mechanical behaviour of insect wings: I. Single joints. Bioinspir. Biomim. 10, 056003. ( 10.1088/1748-3190/10/5/056003) [DOI] [PubMed] [Google Scholar]
- 50.Rajabi H, Ghoroubi N, Darvizeh A, Appel E, Gorb SN. 2016. Effects of multiple vein microjoints on the mechanical behaviour of dragonfly wings: numerical modelling. R. Soc. Open Sci. 3, 150610. ( 10.1098/rsos.150610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanke A, Watson PJ, Holbrey R, Fagan MJ. 2017. Computational biomechanics changes our view on insect head evolution. Proc. R. Soc. B 284, 20162412. ( 10.1098/rspb.2016.2412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanke A, Pinheiro M, Watson PJ, Fagan MJ. 2018. A biomechanical analysis of prognathous and orthognathous insect head capsules: evidence for a many-to-one mapping of form to function. J. Evol. Biol. 31, 665-674. ( 10.1111/jeb.13251) [DOI] [PubMed] [Google Scholar]
- 53.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. 2006. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116-1128. ( 10.1016/j.neuroimage.2006.01.015) [DOI] [PubMed] [Google Scholar]
- 54.Cignoni P, Callieri M, Corsini M, Dellepiane M, Ganovelli F, Ranzuglia G.. 2008. Meshlab: an open-source mesh processing tool. In Eurographics Italian chapter conference, Salerno, Italy, pp. 129-136. Aire-la-Ville, Switzerland: The Eurographics Association. ( 10.2312/LocalChapterEvents/ItalChap/ItalianChapConf2008/129-136) [DOI] [Google Scholar]
- 55.Bathe KJ. 1995. Finite element procedures. London, UK: Pearson. [Google Scholar]
- 56.Marcé-Nogué J, Fortuny J, Gil L, Sánchez M. 2015. Improving mesh generation in finite element analysis for functional morphology approaches. Spanish J. Palaeontol. 30, 117-132. ( 10.7203/sjp.30.1.17227) [DOI] [Google Scholar]
- 57.Brito TO, Elzubair A, Araújo LS, Camargo SA de S, Souza JLP, Almeida LH. 2017. Characterization of the mandible Atta Laevigata and the bioinspiration for the development of a biomimetic surgical clamp. Mat. Res. 20, 1525-1533. ( 10.1590/1980-5373-mr-2016-1137) [DOI] [Google Scholar]
- 58.Özkaya N, Leger D, Goldsheyder D, Nordin M. 2017. Multiaxial deformations and stress analyses. In Fundamentals of biomechanics: equilibrium, motion, and deformation, pp. 317-360. New York, NY: Springer. [Google Scholar]
- 59.Wilson EO. 1987. Causes of ecological success: the case of the ants. J. Anim. Ecol. 56, 1. ( 10.2307/4795) [DOI] [Google Scholar]
- 60.Dirks J-H, Parle E, Taylor D. 2013. Fatigue of insect cuticle. J. Exp. Biol. 216, 1924-1927. ( 10.1242/jeb.083824) [DOI] [PubMed] [Google Scholar]
- 61.Mertl AL, Traniello JFA. 2009. Behavioral evolution in the major worker subcaste of twig-nesting Pheidole (Hymenoptera: Formicidae): does morphological specialization influence task plasticity? Behav. Ecol. Sociobiol. 63, 1411-1426. ( 10.1007/s00265-009-0797-3) [DOI] [Google Scholar]
- 62.Ferster B, Pie MR, Traniello JFA. 2006. Morphometric variation in North American Pogonomyrmex and Solenopsis ants: caste evolution through ecological release or dietary change? Ethol. Ecol. Evol. 18, 19-32. ( 10.1080/08927014.2006.9522723) [DOI] [Google Scholar]
- 63.Clissold FJ. 2007. The biomechanics of chewing and plant fracture: mechanisms and implications. Adv. Insect. Phys. 34, 317-372. [Google Scholar]
- 64.Schofield RMS, Emmett KD, Niedbala JC, Nesson MH. 2011. Leaf-cutter ants with worn mandibles cut half as fast, spend twice the energy, and tend to carry instead of cut. Behav. Ecol. Sociobiol. 65, 969-982. ( 10.1007/s00265-010-1098-6) [DOI] [Google Scholar]
- 65.Schofield RMS, Nesson MH, Richardson KA. 2002. Tooth hardness increases with zinc-content in mandibles of young adult leaf-cutter ants. Naturwissenschaften 89, 579-583. ( 10.1007/s00114-002-0381-4) [DOI] [PubMed] [Google Scholar]
- 66.Matsumura Y, Jafarpour M, Ramm SA, Reinhold K, Gorb SN, Rajabi H. 2020. Material heterogeneity of male genitalia reduces genital damage in a bushcricket during sperm removal behaviour. Sci. Nat. 107, 52. ( 10.1007/s00114-020-01706-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Economo EP, et al. 2015. Breaking out of biogeographical modules: range expansion and taxon cycles in the hyperdiverse ant genus Pheidole. J. Biogeogr. 42, 2289-2301. ( 10.1111/jbi.12592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mertl AL, Sorenson MD, Traniello JFA. 2010. Community-level interactions and functional ecology of major workers in the hyperdiverse ground-foraging Pheidole (Hymenoptera, Formicidae) of Amazonian Ecuador. Insect. Soc. 57, 441-452. ( 10.1007/s00040-010-0102-5) [DOI] [Google Scholar]
- 69.Tschá MK, Pie MR. 2019. Correlates of ecological dominance within Pheidole ants (Hymenoptera: Formicidae). Ecol. Entomol. 44, 163-171. ( 10.1111/een.12685) [DOI] [Google Scholar]
- 70.Blüthgen N, Gebauer G, Fiedler K. 2003. Disentangling a rainforest food web using stable isotopes: dietary diversity in a species-rich ant community. Oecologia 137, 426-435. ( 10.1007/s00442-003-1347-8) [DOI] [PubMed] [Google Scholar]
- 71.Rosumek FB, Blüthgen N, Brückner A, Menzel F, Gebauer G, Heethoff M. 2018. Unveiling community patterns and trophic niches of tropical and temperate ants using an integrative framework of field data, stable isotopes and fatty acids. PeerJ 6, e5467. ( 10.7717/peerj.5467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blüthgen N, Feldhaar H. 2010. Food and shelter: how resources influence ant ecology. In Ant ecology, pp. 115-136. New York, NY: Oxford University Press. [Google Scholar]
- 73.Powell S. 2008. Ecological specialization and the evolution of a specialized caste in Cephalotes ants. Funct. Ecol. 22, 902-911. ( 10.1111/j.1365-2435.2008.01436.x) [DOI] [Google Scholar]
- 74.Wilson EO. 1978. Division of labor in fire ants based on physical castes (Hymenoptera: Formicidae: Solenopsis). J. Kansas Entomol. Soc. 51, 615-636. [Google Scholar]
- 75.Hillerton JE, Vincent JFV. 1982. The specific location of zinc in insect mandibles. J. Exp. Biol. 101, 333-336. [Google Scholar]
- 76.Schofield RMS, Nesson MH, Richardson KA, Wyeth P. 2003. Zinc is incorporated into cuticular ‘tools’ after ecdysis: the time course of the zinc distribution in ‘tools’ and whole bodies of an ant and a scorpion. J. Insect. Physiol. 49, 31-44. ( 10.1016/S0022-1910(02)00224-X) [DOI] [PubMed] [Google Scholar]
- 77.Polidori C, Jorge A, Keller A, Ornosa C, Tormos J, Asís JD, Nieves-Aldrey JL. 2020. Strong phylogenetic constraint on transition metal incorporation in the mandibles of the hyper-diverse Hymenoptera (Insecta). Org. Divers. Evol. 20, 511-526. ( 10.1007/s13127-020-00448-x) [DOI] [Google Scholar]
- 78.Klunk CL, Argenta MA, Casadei-Ferreira A, Economo EP, Pie MR. 2021. Supplementary material from “Mandibular morphology, task specialization and bite mechanics in Pheidole ants (hymenoptera: formicidae)”. The Royal Society. Collection. 10.6084/m9.figshare.c.5438938.v1. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Klunk CL, Argenta MA, Casadei-Ferreira A, Economo EP, Pie MR. 2021. Supplementary material from “Mandibular morphology, task specialization and bite mechanics in Pheidole ants (hymenoptera: formicidae)”. The Royal Society. Collection. 10.6084/m9.figshare.c.5438938.v1. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Three-dimensional reconstructions of the Pheidole workers mandibles used in the finite element analysis simulations are available as electronic supplementary material File S1 at rs.figshare.com [78].