Abstract
While avoidance of sick conspecifics is common among animals, little is known about how detecting diseased conspecifics influences an organism's physiological state, despite its implications for disease transmission dynamics. The avian pathogen Mycoplasma gallisepticum (MG) causes obvious visual signs of infection in domestic canaries (Serinus canaria domestica), including lethargy and conjunctivitis, making this system a useful tool for investigating how the perception of cues from sick individuals shapes immunity in healthy individuals. We tested whether disease-related social information can stimulate immune responses in canaries housed in visual contact with either healthy or MG-infected conspecifics. We found higher complement activity and higher heterophil counts in healthy birds viewing MG-infected individuals around 6–12 days post-inoculation, which corresponded with the greatest degree of disease pathology in infected stimulus birds. However, we did not detect the effects of disease-related social cues on the expression of two proinflammatory cytokines in the blood. These data indicate that social cues of infection can alter immune responses in healthy individuals and suggest that public information about the disease can shape how individuals respond to infection.
Keywords: social cues, disease, immunity, Mycoplasma gallisepticum, complement, white blood cells
1. Introduction
A common mechanism for preventing infection is to detect and avoid sick conspecifics [1–4]. Behavioural changes following the perception of disease can influence the capacity of pathogens to successfully invade and persist in a population [4–6] and have been implicated in shaping individual- and population-level differences in group structure and behaviour [7–9]. While perceiving a sick conspecific can result in behavioural changes such as avoidance behaviour, behavioural shifts may not be the only defense mechanism available to organisms when faced with an immune threat.
Viewing images associated with disease, such as sick individuals or rotting food, can stimulate immune responses in humans [10–12]. In insects, visual cues indicative of heightened infection risk, such as population density and crowding, can also alter immune function [13–15]. These studies imply that organisms can adjust investment in immune defenses to match the probability of exposure to an immune threat. However, no study has explored how individuals respond to conspecifics infected with a live replicating pathogen despite the clear implications for shaping individual susceptibility and disease dynamics. The avian bacterial pathogen Mycoplasma gallisepticum (MG) often causes obvious visual signs, including lethargy and conjunctivitis, in members of the finch family, making this system an ideal tool for investigating how the perception of social cues of disease shape the immune strategy of healthy individuals [16,17]. Here, we test whether social information transmitted by infected individuals alters complement activity, leucocyte counts and proinflammatory cytokine gene expression in domestic canaries (Serinus canaria domestica) housed in visual contact with either healthy or MG-infected conspecifics.
2. Material and methods
All birds were housed individually in cages with ad libitum access to water and a mixed seed diet on a 14 L : 10 D light cycle. Individual racks only housed birds of the same treatment and uninfected birds did not have physical contact with infected birds to prevent pathogen transmission, which occurs through direct contact and fomites. An opaque room divider separated control and control-focal birds from MG-infected and MG-focal birds (electronic supplementary material, figure S1). The divider isolated visual, but not auditory or odour cues between the respective treatment groups. Focal birds could see the entire rack of stimulus birds and were also in visual contact with two other focal birds that were housed on the same rack shelf. All stimulus birds (MG-infected, control) were female. Focal treatment groups included both females and males; however, we did not have a sufficient sample size to test for sex differences. To assess how social cues of disease shape immune function, we inoculated stimulus birds with either M. gallisepticum (MG-infected; VA1994, stock ID 2009.7994-1-7P; D. H. Ley, North Carolina State University) or Frey's media (control). We characterized the strength of the cue of infection by assessing disease pathology using eye score (conjunctival inflammation), body mass and fat score of the stimulus birds before and at several time points post-inoculation. To determine if seeing sick conspecifics can activate immune responses, we collected blood samples from all focal birds (MG-focal (N = 9; 4 females, 5 males): seeing cue of disease; control-focal (N = 9; 7 females, 2 males): not seeing cue of disease) prior to the experimental treatment and on days 2, 6, 12 and 24 post exposure to the cue to look at differential leucocyte counts, hemolytic complement activity and the expression of the proinflammatory cytokines IL-1β and IL-6. Detailed methods for each immune assay are provided as electronic supplementary material. Focal birds never developed clinical signs of MG and were seronegative for MG throughout the experiment (electronic supplementary material, figure S2).
All statistical analyses were run in SAS 9.1 (SAS Institute Inc., Cary, NC, USA) and R v. 3.6.1 (The R Project for Statistical Computing, Vienna, Austria). To characterize the cue of infection, we ran separate mixed models (PROC MIXED) to test whether experimental inoculation influenced body mass, fat score and eye inflammation score in stimulus birds. Each model included treatment, time post-inoculation and a treatment by time interaction. We ran separate mixed models (PROC MIXED) to test whether exposure to a social cue of disease influenced body mass, fat score, complement activity, heterophil/lymphocyte ratios and cytokine gene expression of focal birds and a repeated measures MANOVA (SAS PROC GLM) to examine the effect of social cues of disease on the type of white blood cells present. To meet parametric requirements for normality and homoscedasticity, body mass and heterophil/lymphocyte ratios were log transformed and complement activity data were square-root transformed. Each model included treatment (control-focal or MG-focal), time post-cue and a treatment by time interaction. Bird identity was included as a random effect in all models since individuals were repeatedly sampled over time. To gain insight into the complex relationships among immune parameters, we performed a multifactor analysis using the FactorMineR (v. 1.42) package in R [18] to assess how a perceived immune threat altered the overall immune profiles of birds (see electronic supplementary material). Cytokine gene expression values were not included in the multifactor analysis as some birds had missing time points due to low mRNA yields.
3. Results
(a) . Characterization of social cue of disease
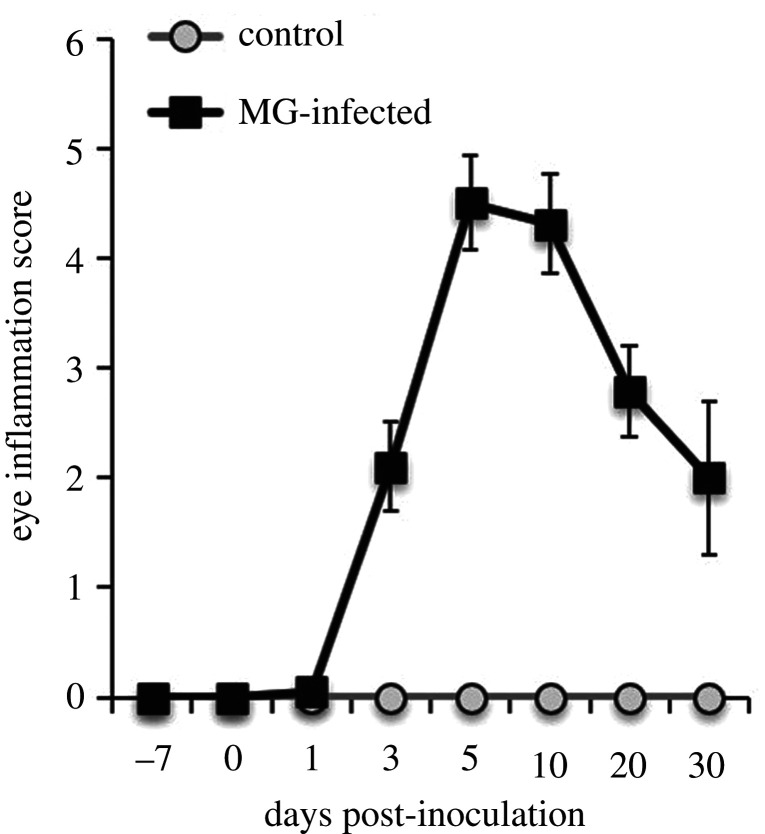
The visual symptoms of stimulus birds varied based on treatment and with time post-infection (day * treatment: F7,110 = 20.98, p < 0.0001). Control stimulus birds never developed conjunctivitis, while peak conjunctival swelling occurred around days 5–10 post-inoculation in MG-infected stimulus birds (figure 1). This timeframe was also when peak lethargy (reduced activity, not moving when the cage was approached) occurred for MG-infected birds (ACL 2017, personal observation) and coincides with peak sickness behaviour reported in other studies of passerines infected with MG [17,19]. Lethargic behaviour was never observed in control individuals. MG-infected individuals also had reduced body mass (electronic supplementary material, figure S3; day * treatment: F7,110 = 5.72, p < 0.0001) and lower furcular fat scores (electronic supplementary material, figure S3; treatment: F1,18 = 13.47, p = 0.002) when compared with control birds post-inoculation. Unexpectedly, three mortalities occurred during the experiment, where two MG-infected individuals died on day 9 post-infection and an additional MG-infected bird died on day 19 post-infection.
Figure 1.
Characterization of disease severity in stimulus birds exposed to Mycoplasma gallisepticum (MG-infected, cue of disease) or a control media (control, no cue of disease). The points denote the average eye inflammation score (±s.e.) of birds in the control (n = 10) and infected (n = 7–10) treatments.
(b) . Physiological responses to social cue of disease
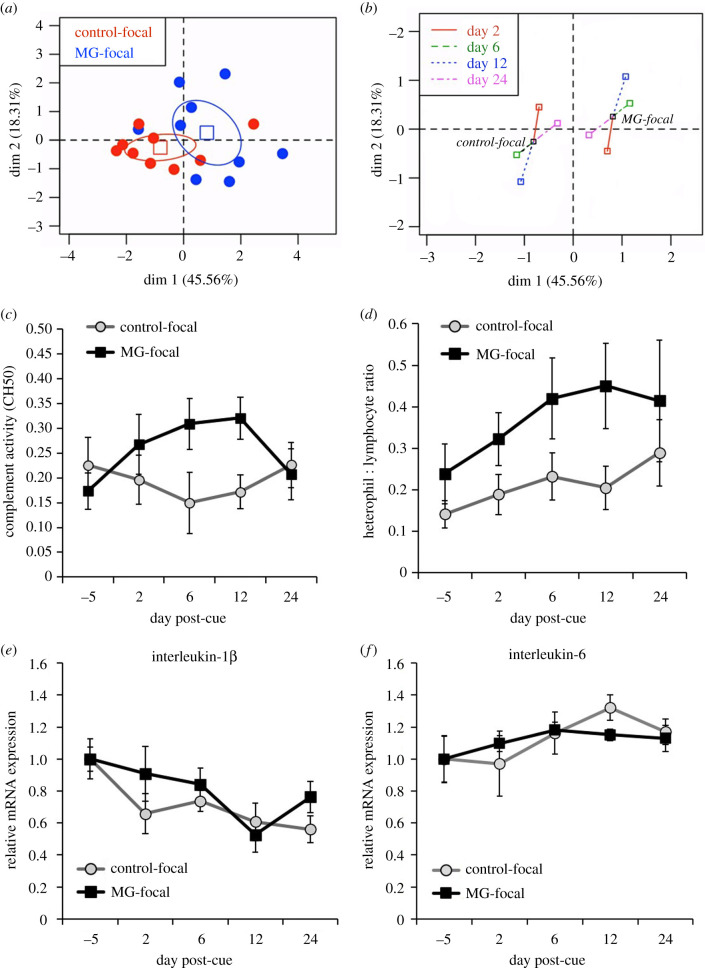
Body mass of focal birds was not affected by viewing a cue of infection and did not differ over time (electronic supplementary material, figure S4; all F ≤ 1.18, all p ≥ 0.329). Fat score did not vary by treatment (F1,16 = 0.50, p = 0.488) but did fluctuate over time in both control-focal and cue of disease birds (electronic supplementary material, figure S4; day: F4,68 = 7.22, p < 0.0001). For the multifactor analysis examining whether a perceived immune threat altered the overall immune profiles of birds, the first two dimensions had eigenvalues greater than or equal to 1.0 that explained 63.9% of the variance (electronic supplementary material, table S1). The coordinates, contributions and correlations for each immune parameter in the multifactor analysis are shown in electronic supplementary material, table S2. MG-focal and control-focal birds had significantly different immune profiles for dimension 1 of the multifactor analysis (test value: 2.061). To visualize differences between treatment groups, we plotted dimension 1 against dimension 2. The 95% confidence ellipses indicate there is shift in the overall immune profiles (figure 2a) of MG-focal and control-focal birds and these differences were largely driven by shifts in immunity on days 6 and 12 following treatment (figure 2b).
Figure 2.
Effects of disease-related social information on immune profiles in domestic canaries. (a) Multifactor analysis plot of dimensions 1 and 2 for immune profiles (heterophil/lymphocyte ratio, complement activity) in birds viewing sick conspecifics (MG-focal: N = 9) or healthy conspecifics (control-focal: N = 9). Individual points represent individual birds and ellipses represent 95% confidence regions for each treatment. (b) Partial points plot showing the contributions of each timepoint (days 2, 6, 12 and 24) to the divergence in immune profiles between each treatment. (c) Hemolytic complement activity (CH50), (d) heterophil/lymphocyte ratio and relative mRNA gene expression of (e) interleukin-1β and (f) interleukin-6 in birds exposed to a cue of infection (MG-focal) or healthy conspecifics (control-focal). The points denote average values (±s.e.) of birds in the control-focal (n = 9) and MG-focal (n = 9) treatments.
Birds viewing sick conspecifics temporarily increased CH50 complement activity, which eventually returned to baseline levels as pathology in the stimulus birds began to subside (figure 2c; day * treatment: F4,64 = 2.66, p = 0.041). Leucocyte counts also differed between birds seeing a diseased conspecific and birds seeing healthy conspecifics (table 1; treatment: F5,80 = 2.96, p = 0.025). Among specific cell types, birds seeing a cue of disease had more heterophils (treatment: F1,16 = 10.49, p = 0.002) and fewer lymphocytes (treatment: F1,16 = 7.88, p = 0.006). Consequently, there was a non-significant trend for birds viewing a diseased conspecific to have an increased heterophil/lymphocyte ratio (figure 2d; treatment: F1,16 = 4.00, p = 0.063). There was also a trend for monocyte counts to be lower in MG-focal birds (treatment: F1,16 = 3.92, p = 0.051). Eosinophils and basophils were rarely observed in blood smears and did not differ by treatment or over time (all F ≤ 1.12, all p ≥ 0.320).
Table 1.
White blood cell differentials (mean ± s.e. for each cell type) for birds viewing healthy conspecifics (control-focal, n = 9) or a cue of infection (MG-focal, n = 9).
| treatment | day | lymphocytes (%) | heterophils (%) | monocytes (%) | eosinophils (%) | basophils (%) |
|---|---|---|---|---|---|---|
| control-focal | −5 | 84.6 ± 2.5 | 11.4 ± 2.3 | 3.7 ± 1.8 | 0.3 ± 0.2 | 0 ± 0 |
| 2 | 82.1 ± 2.6 | 14.6 ± 3.3 | 3.1 ± 1.0 | 0.2 ± 0.1 | 0 ± 0 | |
| 6 | 78.8 ± 2.6 | 17.1 ± 3.6 | 3.9 ± 1.2 | 0.2 ± 0.1 | 0 ± 0 | |
| 12 | 82.9 ± 3.3 | 15.6 ± 3.3 | 1.4 ± 0.6 | 0 ± 0 | 0 ± 0 | |
| 24 | 76.8 ± 4.1 | 19.7 ± 3.9 | 2.8 ± 1.0 | 0.7 ± 0.3 | 0.1 ± 0.1 | |
| MG-focal | −5 | 81.2 ± 4.3 | 17.0 ± 4.1 | 1.7 ± 0.8 | 0.1 ± 0.1 | 0 ± 0 |
| 2 | 74.7 ± 3.7 | 22.3 ± 3.6 | 2.7 ± 1.2 | 0.3 ± 0.2 | 0 ± 0 | |
| 6 | 72.3 ± 4.9 | 26.7 ± 5.0 | 0.8 ± 0.4 | 0.2 ± 0.1 | 0 ± 0 | |
| 12 | 70.1 ± 4.3 | 28.1 ± 4.6 | 1.6 ± 0.7 | 0.2 ± 0.1 | 0 ± 0 | |
| 24 | 73.2 ± 5.0 | 24.7 ± 5.3 | 1.9 ± 0.8 | 0.2 ± 0.1 | 0 ± 0 |
Social cues of disease did not influence the expression of the proinflammatory cytokines measured in the present study. Specifically, IL-1β expression did not vary by treatment (F1,16 = 0.03, p = 0.875) but did decrease over time in both control-focal and MG-focal birds (figure 2e; day: F4,59 = 4.20, p = 0.005). IL-6 expression was also not affected by whether birds were seeing a cue of infection and did not differ over time during the experiment (figure 2f; all F ≤ 1.35, all p ≥ 0.263).
4. Discussion
In this study, we found that birds housed in visual contact with MG-infected conspecifics had immune profiles that were distinct from birds housed across from healthy conspecifics. Specifically, we detected an increase in complement activity and a shift in white blood cell profiles in MG-focal birds that was temporally concomitant with peak disease severity in the stimulus birds infected with MG. This suggests that healthy individuals can detect sick conspecifics infected with MG and that the perception of diseased conspecifics can alter physiological responses relevant to responding to infection. Specifically, the increase in CH50 activity detected in MG-focal birds indicates the activity of the complement system, which is responsible for the opsonization of pathogens and inducing inflammatory responses that help fight infection [20,21]. We also found that birds exposed to a cue of infection had increased numbers of heterophils and reduced numbers of lymphocytes circulating in the bloodstream. Heterophils are phagocytic cells that play an important role in inflammation and shaping host resistance and susceptibility to pathogens [22]. For example, chickens with heterophils that are less functionally active are more susceptible to infections than chickens with highly functional heterophils [22,23]. Thus, an increase in heterophils and complement activity following exposure to a cue of disease could provide protection against a perceived immune threat.
We did not find evidence that birds seeing a cue of infection had altered expression of either IL-1β or IL-6. This is contrary to work in humans showing that people have greater IL-6 responses to lipopolysaccharide (LPS) stimulation after viewing images of sick individuals [10]. It should be noted that we measured IL-6 mRNA levels, which do not always correlate with circulating cytokine levels [24,25]. Additionally, our study looked at baseline changes in proinflammatory gene expression during a perceived immune threat (without a direct immune stimulus like LPS). Further, because the immune system is complex and immune responses are metabolically expensive [26–28], it is expected that some immune endpoints might be upregulated in response to a signal (as observed with complement and heterophil counts), while others remain unchanged, are suppressed, or exhibit latent responses.
Unexpectedly, we had three mortalities occur during the experiment, and all of these individuals were MG-infected stimulus birds. While a dead conspecific presumably acts as a strong cue indicating that a threat like infection or predation is present [29], we started to see shifts in immunity in MG-focal birds before these mortalities occurred (around day 6 post-infection), suggesting that conspecific mortality was not the primary cue driving the initial shifts in immune physiology. It is possible that infection-induced changes in call/song rate and odour could have also served as social cues of infection to the MG-focal individuals. Traditionally, olfactory cues were not thought to play a prominent role in birds, however, recent research is challenging this assumption [30]. Infection is known to influence acoustic communication in some avian species, including canaries [31,32]. However, because all treatments were housed in the same room and were in olfactory and auditory contact with one another, it is unlikely these cues played a prominent role in driving the observed shift in immune responses in MG-focal birds.
The finding that immune activation in healthy individuals corresponds temporally with increasing disease severity of sick conspecifics could have interesting implications for the spread of disease, as MG-infected birds with higher disease pathology deposit more pathogens onto fomites such as bird feeders [33]. If healthy birds can detect and respond more strongly to birds with higher disease pathology, this could reduce their chances of becoming infected when the risk of infection is high. Interestingly, male (but not female) house finches preferentially feed near MG-infected conspecifics despite the increased risk of infection, presumably because these sick individuals are less aggressive at feeders [34]. If viewing sick conspecifics alters immunity in a manner that increases disease resistance, birds encountering sick individuals might be investing in immune activation rather than behavioural avoidance to capitalize on foraging. Indeed, a separate study found that house finches balance investment in immune defenses and behavioural avoidance strategies, where individuals investing less in behavioural defenses had stronger immune defenses [35]. While the present study included both male and female focal birds, we did not have sufficient sample sizes to investigate sex differences in physiological responses to cues of infection. Future work should investigate how visual cues of disease shape investment in behavioural and immune defense strategies, whether there are sex-specific differences in these strategies, and explore how variation in these strategies relates to disease transmission dynamics.
Our results demonstrate that social cues of infection can alter immunity in birds, and that the observed physiological changes in healthy individuals seeing sick conspecifics coincide with the severity of visual signs of infection in infected conspecifics. While shifts in behaviour following the perception of sick conspecifics can influence how organisms interact with their environment and other individuals [7–9], shifts in physiology may mediate the likelihood of infection or disease severity. Future research should investigate whether immune activation following a social cue of infection confers any protection against infection, such as decreased recovery time or reduced disease severity, and whether this alters disease transmission potential. Finally, this research has important implications for experimental design and animal housing practices, as sick animals could influence the physiology of their neighbours.
Acknowledgements
We would like to thank Anna Smith, Aimee Nash, Natali Edwards, Jill Murray and Ryan Shannon for assistance with data collection and animal care. We would also like to thank three anonymous reviewers for their suggestions and improvements made to the original manuscript.
Ethics
All research protocols were approved by the Oklahoma State University Institutional Animal Care and Use Committee (AS-14-18).
Data accessibility
The data are provided in electronic supplementary material [36].
Authors' contributions
A.C.L. designed the experiment, collected and analysed data and wrote the manuscript. S.E.D. provided resources and laboratory space and assisted with experimental design, data analysis and editing the manuscript. C.G.G., K.G. and J.B.K. assisted with data collection and revising the manuscript. C.G.G. also assisted with primer design and statistical analyses. A.C.L., K.G. and J.B.K. performed immune assays. All authors approved the manuscript and agree to be accountable for the work performed herein.
Competing interests
We declare we have no competing interests
Funding
This work was supported by the NSF Graduate Research Fellowship Program (A.C.L.), a Sigma Xi GIAR (A.C.L.) and an Oklahoma State University start up fund to S.E.D.
References
- 1.Sarabian C, Curtis V, McMullan R. 2018. Evolution of pathogen and parasite avoidance behaviours. Phil. Trans. R. Soc. B 373, 20170256. ( 10.1098/rstb.2017.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiesecker JM, Skelly DK, Beard KH, Preisser E. 1999. Behavioral reduction of infection risk. Proc. Natl Acad. Sci. USA 96, 9165-9168. ( 10.1073/pnas.96.16.9165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Roode JC, Lefèvre T. 2012. Behavioral immunity in insects. Insects 3, 789-820. ( 10.3390/insects3030789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis VA. 2014. Infection-avoidance behaviour in humans and other animals. Trends Immunol. 35, 457-464. ( 10.1016/j.it.2014.08.006) [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. 2005. Superspreading and the effects of individual variation on disease emergence. Nature 438, 355-359. ( 10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore J. 2002. Parasites and the behavior of animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Patterson JE, Ruckstuhl KE. 2013. Parasite infection and host group size: a meta-analytical review. Parasitology 140, 803-813. ( 10.1017/S0031182012002259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber I, Dingemanse NJ. 2010. Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077-4088. ( 10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck JC, Weinstein SB, Young HS. 2018. Ecological and evolutionary consequences of parasite avoidance. Trends Ecol. Evol. 33, 619-632. ( 10.1016/j.tree.2018.05.001) [DOI] [PubMed] [Google Scholar]
- 10.Schaller M, Miller GE, Gervais WM, Yager S, Chen E. 2010. Mere visual perception of others' disease symptoms facilitates a more aggressive immune response. Psychol. Sci. 21, 649-652. ( 10.1177/0956797610368064) [DOI] [PubMed] [Google Scholar]
- 11.Stevenson RJ, Hodgson D, Oaten MJ, Barouei J, Case TI. 2011. The effect of disgust on oral immune function. Psychophysiology 48, 900-907. ( 10.1111/j.1469-8986.2010.01165.x) [DOI] [PubMed] [Google Scholar]
- 12.Stevenson RJ, Hodgson D, Oaten MJ, Moussavi M, Langberg R, Case TI, Barouei J. 2012. Disgust elevates core body temperature and up-regulates certain oral immune markers. Brain Behav. Immun. 26, 1160-1168. ( 10.1016/j.bbi.2012.07.010) [DOI] [PubMed] [Google Scholar]
- 13.Wilson K, Reeson AF. 1998. Density-dependent prophylaxis: evidence from Lepidoptera–baculovirus interactions? Ecol. Entomol. 23, 100-101. ( 10.1046/j.1365-2311.1998.00107.x) [DOI] [Google Scholar]
- 14.Ruiz-González MX, Moret Y, Brown MJ. 2009. Rapid induction of immune density-dependent prophylaxis in adult social insects. Biol. Lett. 5, 781-783. ( 10.1098/rsbl.2009.0505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotter SC, Hails RS, Cory JS, Wilson K. 2004. Density-dependent prophylaxis and condition-dependent immune function in Lepidopteran larvae: a multivariate approach. J. Anim. Ecol. 73, 283-293. ( 10.1111/j.0021-8790.2004.00806.x) [DOI] [Google Scholar]
- 16.Hawley DM, Grodio J, Frasca S, Kirkpatrick L, Ley DH. 2011. Experimental infection of domestic canaries (Serinus canaria domestica) with Mycoplasma gallisepticum: a new model system for a wildlife disease. Avian Pathol. 40, 321-327. ( 10.1080/03079457.2011.571660) [DOI] [PubMed] [Google Scholar]
- 17.Love AC, Foltz SL, Adelman JS, Moore IT, Hawley DM. 2016. Changes in corticosterone concentrations and behavior during Mycoplasma gallisepticum infection in house finches. Gen. Comp. Endocrinol. 235, 70-77. ( 10.1016/j.ygcen.2016.06.008) [DOI] [PubMed] [Google Scholar]
- 18.Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1-18. ( 10.18637/jss.v025.i01) [DOI] [Google Scholar]
- 19.Adelman JS, Kirkpatrick L, Grodio JL, Hawley DM. 2013. House finch populations differ in early inflammatory signaling and pathogen tolerance at the peak of Mycoplasma gallisepticum infection. Am. Nat. 181, 674-689. ( 10.1086/670024) [DOI] [PubMed] [Google Scholar]
- 20.Janeway C, Travers P, Walport M, Shlomchik M. 2005. Immunobiology: the immune system in health and disease, 6th edn. New York, NY: Garland Science. [Google Scholar]
- 21.Ram S, Lewis LA, Rice PA. 2010. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin. Microbiol. Rev. 23, 740-780. ( 10.1128/CMR.00048-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genovese KJ, He H, Swaggerty CL, Kogut MH. 2013. The avian heterophil. Dev. Comp. Immunol. 41, 334-340. ( 10.1016/j.dci.2013.03.021) [DOI] [PubMed] [Google Scholar]
- 23.Ferro PJ, Swaggerty CL, Kaiser P, Pevzner IY, Kogut MH. 2004. Heterophils isolated from chickens resistant to extraintestinal Salmonella enteritidis infection express higher levels of pro-inflammatory cytokine mRNA following infection than heterophils from susceptible chickens. Epidemiol. Infect. 132, 1029-1037. ( 10.1017/S0950268804002687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shebl FM, Pinto LA, García-Piñeres A, Lempicki R, Williams M, Harro C, Hildesheim A. 2010. Comparison of mRNA and protein measures of cytokines following vaccination with HPV-16 L1 virus-like particles. Cancer Epidemiol. Biomarkers Prev. 19, 978-981. ( 10.1158/1055-9965.EPI-10-0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Beyer A, Aebersold R. 2016. On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535-550. ( 10.1016/j.cell.2016.03.014) [DOI] [PubMed] [Google Scholar]
- 26.Demas GE, Chefer V, Talan MI, Nelson RJ. 1997. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6 J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 273, R1631-R1637. ( 10.1152/ajpregu.1997.273.5.R1631) [DOI] [PubMed] [Google Scholar]
- 27.Martin LB, Scheuerlein A, Wikelski M. 2003. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs. Proc. R. Soc. Lond. B 270, 153-158. ( 10.1098/rspb.2002.2185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lochmiller RL, Deerenberg C. 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87-98. ( 10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- 29.Swift KN, Marzluff JM. 2015. Wild American crows gather around their dead to learn about danger. Anim. Behav. 109, 187-197. ( 10.1016/j.anbehav.2015.08.021) [DOI] [Google Scholar]
- 30.Balthazart J, Taziaux M. 2009. The underestimated role of olfaction in avian reproduction? Behav. Brain Res. 200, 248-259. ( 10.1016/j.bbr.2008.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchanan KL, Catchpole CK, Lewis JW, Lodge A. 1999. Song as an indicator of parasitism in the sedge warbler. Anim. Behav. 57, 307-314. ( 10.1006/anbe.1998.0969) [DOI] [PubMed] [Google Scholar]
- 32.Spencer KA, Buchanan KL, Leitner S, Goldsmith AR, Catchpole CK. 2005. Parasites affect song complexity and neural development in a songbird. Proc. R. Soc. B 272, 2037-2043. ( 10.1098/rspb.2005.3188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adelman JS, Carter AW, Hopkins WA, Hawley DM. 2013. Deposition of pathogenic Mycoplasma gallisepticum onto bird feeders: host pathology is more important than temperature-driven increases in food intake. Biol. Lett. 9, 20130594. ( 10.1098/rsbl.2013.0594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouwman KM, Hawley DM. 2010. Sickness behaviour acting as an evolutionary trap? Male house finches preferentially feed near diseased conspecifics. Biol. Lett. 6, 462-465. ( 10.1098/rsbl.2010.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zylberberg M, Klasing KC, Hahn TP. 2012. House finches (Carpodacus mexicanus) balance investment in behavioural and immunological defences against pathogens. Biol. Lett. 9, 20120856. ( 10.1098/rsbl.2012.0856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Love AC, Grisham K, Krall JB, Goodchild CG, DuRant SE. 2021. Perception of infection: disease-related social cues influence immunity in songbirds. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Love AC, Grisham K, Krall JB, Goodchild CG, DuRant SE. 2021. Perception of infection: disease-related social cues influence immunity in songbirds. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in electronic supplementary material [36].