Abstract
This study aimed to summarize the available data on the ethnomedicinal and phytopharmacological activities of Heliotropium indicum L. based on database reports. For this purpose, an up-to-date literature search was carried out in the Google Scholar, Scopus, Springer Link, Web of Science, ScienceDirect, ResearchGate, PubMed, Chem Spider, Elsevier, BioMed Central, and patent offices (e.g., USPTO, CIPO, NPI, Google patents, and Espacenet) for the published materials. The findings suggest that the plant contains many important phytochemicals, including pyrrolizidine alkaloids, indicine, echinitine, supinine, heleurine, heliotrine, lasiocarpine, acetyl indicine, indicinine, indicine N-oxide, cynoglossine, europine N-oxide, heleurine N-oxide, heliotridine N-oxide, heliotrine N-oxide, heliotrine, volatile oils, triterpenes, amines, and sterols. Scientific reports revealed that the herb showed antioxidant, analgesic, antimicrobial, anticancer, antituberculosis, antiplasmodial, anticataract, antifertility, wound healing, antiinflammatory, antinociceptive, antihyperglycemic, anthelmintic, diuretic, antitussive, antiglaucoma, antiallergic, and larvicidal activity. In conclusion, in vitro studies with animal models seem to show the potential beneficial effects of H. indicum against a wide variety of disorders and as a source of phytotherapeutic compounds. However, clinical studies are necessary to confirm the effects observed in animal models, determine the toxicity of the therapeutic dose and isolate the truly bioactive components.
1. Introduction
One of the barebones for the victory of principal health care is the accessibility and use of apposite drugs. Traditional medicine, since the early formation of human civilization, has been the most sensible and affordable source of treatment in the health care system, which is why people continue to rely on plants for multiple disorders [1]. The medicinal uses of each plant derive from the presence of significant amounts of various natural products, which can be used as alternative therapeutic or adjuvant tools. Medicinal plants play an energetic role in the discovery of new therapeutic agents, thus growing interest in the use of pharmaceutical consumption [2,3]. Medicinal plants contain many constituents such as alkaloids, flavonoids, tannins, phenols, saponins, and glycosides, with notable biological activities such as antimicrobial, analgesic, antipyretic, antitumor, wound healing, and cardioprotective, among others that can be useful against diverse human diseases [4,5].
Heliotropium indicum L. (family: Boraginaceae; Figure 1), locally known as “Hatisur” is derived from the Greek words “helios” meaning “sun” and “tropein” meaning “to turn,” indicating that the flowers and leaves turn toward the sun and known as the “Indian turnsole” [6]. It is also known as Eliopia riparia Raf., Eliopia serrata Raf., Heliophytum indicum (L.) DC., Heliotropium africanum Schumach. & Thonn., Heliotropium cordifolium Moench, Heliotropium foetidum Salisb., Heliotropium horminifolium Mill., and Tiaridium indicum (L.) Lehm. H. indicum is distributed throughout Bangladesh, Nepal, Sri Lanka, Thailand, India, and other areas of tropical Asia and in some parts of Africa [7]. H. indicum is a small annual or perennial herb with a height of about 15–50 cm in length, with the leaves always opposite, and the stem and root covered by a hairy layer [7]. Flowering time is around the whole year, and flowers are calyx green; the fruits are dried and consist of 2–4 free or almost free nutlets in 4–5 mm long [8].
Figure 1.

Different parts of Heliotropium indicum Linn:(a) whole plant, (b) leaves, (c) flowers, (d) seeds, and (e) roots.
Traditionally, this plant is widely used against many pathological disorders including wound healing, antidote, bone fracture, febrifuge, cures eye infection, menstrual disorder, nerve disorder, kidney problem, and antiseptic purpose [9–14]. H. indicum contains many important phytochemicals such as tannins, saponins, steroids, oils, and glycosides [12,15]. Schoental [16] and Hartmann and Ober [17] isolated pyrrolizidine alkaloids (e.g., indicine N-oxide, heliotrine, etc.) from this plant. Scientific reports suggest that H. indicum possesses many important pharmacological activities, including antiinflammatory [18], wound-healing [19], anticancer [15], and anticataract activities [20].
This review aims to show the current scenario on the ethnomedicinal, phytochemical, and pharmacological profiles of H. indicum.
2. Plant Taxonomy
The taxonomic hierarchy of H. indicum is the following:
Domain: Eukaryota
Kingdom: Plantae
Phylum: Spermatophyta
Subphylum: Angiospermae
Class: Dicotyledonae
Order: Boraginales
Family: Boraginaceae
Genus: Heliotropium
Species: Heliotropium indicum L
3. Plant Morphology
H. indicum is an erect, thick fetid, annual or perennial herb with hirsute ascending branches, reaching between 20 and 60 cm in height [13]. The leaves are opposite or sub-opposite, alternate or sub-alternate and straight forward, sheet-shaped from ovate to elliptical, hairy, and sharp and 5–10 cm long. The margins of the leaves are undulate; the nerves present on both sides are serrulate or cordate and clearly visible under the leaves [21]. The petiole is about 1–7 cm long, while the flowers progress apically within the cymose; at maturity, nutlets are present at the base of the inflorescence. Generally, flowers are white or whitish violet in color, regular, sessile, axillary, and nearly 5 mm in diameter. Sepals are diffused with hairs outside, deep green in color, linear to lanceolate, uneven or unequal, and about 5–3 mm long. The fruits are dry and 2–4 lobed, with or without united nutlets, and 3–6 mm long. This species grows in sunny places preferring heights around 800 m [22]. Botanical descriptions of H. indicum are given in Table 1.
Table 1.
Botanical morphology of Heliotropium indicum L.
| Habitat | The disturbed areas are garden or lawns, roadsides, anthropogenic habitats, and waste places. It is mostly found at a 1,000 m altitude. | |
|
| ||
| Foliage | Leaves | 4–10 cm long and 2–5 cm wide, opposite, or sub-opposite, alternate or sub-alternate, ovate to obovate, and acute, with a wavy or undulate, serrulate, or cordate leaf margin, nerves on either side or veins. The leaf surface is covered with short hairs, which may be quite stiff. |
| Petiole | 1–7 cm long with a sub-truncate base or ovate | |
|
| ||
| Flowers | 4–5 mm wide, regular, sessile, axillary, and slightly purple or white or whitish violet with a small yellow center and having a narrow tube with lobes formed a plate shape | |
| Inflorescence | String or twisted of beads with a prominent curl at the apex. Flowers develop apically within the cymose inflorescence. | |
| Sepals | 5 in number, 3 mm long, diffused with hairs outside, deep green in color, linear to lanceolate, and uneven or unequal | |
| Calyx lobes ciliate | 3 mm long | |
| Stamens | 5 in number and borne in a corolla tube, terminal, and corolla tube 4–6 mm long | |
| Petals | Rounded | |
| Ovary | 4 lobed | |
|
| ||
| Fruits | Fruits, also known as nutlets, are dry, indehiscent 2–4 lobed, 3–6 mm long, with or without united nutlets, ovate, and ribbed separated into two nutlets. Each nutlet is two-celled and beaked. | |
|
| ||
| Stem and roots | Wide distributed, branched or unbranched, and hirsute with hairs in the stem. The root system is a long taproot and highly branched. | |
|
| ||
| Genetics | 2n = 22, 24 | |
4. Methodology
The literature search was performed using the databases: Google Scholar, Scopus, SpringerLink, Web of Science, ScienceDirect, ResearchGate, PubMed, ChemSpider, Elsevier, BioMed Central, and USPTO, CIPO, INPI, Google Patents, and Espacenet. The scientific databases were chosen based on the topic covered (i.e., ethnobotany, ethnomedicinal uses, ethnopharmacology, pharmacology, phytochemistry, and therapeutic value) and geographical coverage (i.e., Asia and Africa). The common keyword “Heliotropium indicum” was used to search published materials, which was then paired with “traditional uses,” “ethnopharmacology,” “phytochemistry,” “pharmacology,” and “toxicity.” Other literature sources included papers published in international journals; reports from international, regional, and national organizations; conference papers; and related books. Chemical structures were drawn using the software ChemSketch (Version 14.01).
5. Traditional and Folk Values
Ethnopharmacology is the study of medicinal plant use in specific cultural groups or the study of differences in response to drugs in different cultures [23]. About 90% of native people depend on the natural products of plant origin to treat several diseases [24]. With the knowledge of ethnopharmacology, the whole plant of H. indicum has been traditionally used in different folklore systems to cure several diseases in different countries over the world. In Bangladesh, the juice or decoction of leaves and roots of H. indicum is traditionally used in chicken pox, allergy, blood purification, swelling of the knees, joint pain, and severe itchy legs and also be used as an antidote to poisoning [12,25–27]. In India, different parts of the herb, mainly leaves as a paste or beverage, are used on wounds, skin infections, ophthalmia, snakebite, and scorpion sting [28,29]. The decoction of both root and leaf is used to handle whooping cough in children in eastern Nicaragua [13].
The infusion of flowers at low doses is applied to regulate the menstrual cycle, while large doses for abortion by introducing into the vaginal cavity. In Jamaica, the flower infusion is used by females to treat menorrhagia, while in Senegal and the Philippines, it is used to treat kidney stone [9,10]. In the Philippines, the decoction of dried roots is drunk to encourage menses, while the seeds are used to heal wounds and treat cholera and malaria [30]. In African countries, it is reported that this plant is useful in treating malaria, dermatitis, abdominal pain, renal failure, and urinary infections [9,31,32]. In Thailand, the dried and powdered inflorescence (1 gm) mixed with milk or water is used for three days beginning with the fourth day of menses to yield permanent sterilization in females [33]. The whole plant is used to treat ringworm infection and counteract putrefaction in Malaysia, while the decoction of the whole plant is applied to treat gonorrhea in Burma [30]. The leaf juice is used to treat the stings and boils of scorpions and insect bites. On the other hand, the boiled juice with castor oil is used to treat mad dog bite infections [34]. Moreover, H. indicum is also used to treat rheumatism [35], ulcer, venereal disease, fever, sore throat, and sores in the rectum [36]. Traditional uses of H. indicum in different countries are summarized in Table 2.
Table 2.
Traditional uses of H. indicum L.
| Country | Local names | Traditional use as or to treat | Part(s) used | Mode of administration | Reference(s) |
|---|---|---|---|---|---|
| Bangladesh | Hatisur | Antidote to poisoning | Leaves and stem | Decoction of leaves and stems is administered orally. | [12] |
| Swelling of knees, joint pain, and severe itching in leg | Root | Decoction or maceration of the root is used through vocal order (VO). | [25] | ||
| Chicken pox | Leaves | Juice of roots is taken orally. | [26] | ||
| Allergy | Leaves | Juice of the leaf is taken orally. | |||
| Blood purification and infections | Root | Juice of roots is used both orally and topically. | [27] | ||
| Brazil | Aguará-ciunhá-ac¸ú and jacuá-acanga | Skin ulcers and burns | Leaves | Unknown | [19,37] |
| Benin | Koklosoudèn | Dystocia | Leaves | Trituration with water and drops in eyes | [38] |
| Femal | Leaves | Leaf extract is filtered then applied through VO. | |||
| Leucorrhoea | Whole plant | The diluted juice is administered through VO. | |||
| Splenomegalia | Leaves | Unknown | |||
| Psychosis | Leaves and root | Unknown | |||
| Koclossoudinkpatcha (Fon) | Internal infection and hypertension | Stem and leaves | Decoction of stems with leaves is applied through VO. | [39] | |
| Congo | Not registered | Stomach, fever, and eye lotion | Leaves | Decoction of fresh leaves with water that is taken 1 glass/day for 1 week. | [40] |
| Colombia | Rabo de alacrán and verbena | Internal parasites | Leaves | Decoction of fresh leaves | [41] |
| Guinea | Nasinko and hogghonhwan | Diarrhea and febrifuge | Whole plant | Decoction of the whole plant | [42] |
| Antiseptic | Leaves | The decoction of leaves is allowed to administer through vocal order. | [43] | ||
| Ghana | Kɔmfemtikorɔ | Paludism and eye infections | Leaves | Decoction of leaves is used for 7 days. | [44] |
| Conakry | Not registered | Fever | Whole plant | Decoction of the whole plant | [9] |
| Gabon | (mo-)nyaka (w-)a mbumba (Eviya language) | Gingivitis | Leaves | Ground leaves of H. indicum for local application | [45] |
| India | Nakkipoo | Snakebite and scorpion sting | Leaves | The leaf juice is used by mixing with hot water. | [29] |
| Indian heliotrope and hatisundha | Wounds and skin infections | Whole plant | Paste of the whole plant is applied topically. | [28] | |
| Ophthalmia | Root | Juice of the root is taken orally. | [46] | ||
| Ivory Coast | Klaouri (Gouro), kotokorokombo (Baoule), nansifo, nosiko (Malinke), tapentiti, and taperodia (Shien) | Colds and sinusitis | Leaves | Powder of dry leaves | [47] |
| Indonesia | Bandotanlombok, djingirajam, gadjahan, tlale, and tusokkonde | Herpes and rheumatism | Leaves | Decoction of leaf is used in thrush and poultices. | [9] |
| Jamaica | Turnsoles | Menorrhagia | Flower | Infusion of the flower is taken orally. | [48] |
| Fever, ulcers, venereal diseases, and sore throat | Whole plant | Decoction of the whole plant is taken orally. | |||
| Induced abortion | Whole plant | Decoction of the whole plant is applied to the vaginal cavity. | |||
| Rectal sores | Whole plant | Decoction of the whole plant is administered rectally. | |||
| Cleansing and dressing of wounds and ulcers | Whole plant | Paste of fresh plant | |||
| Mauritius | Herbepapillon (Creole) and taylkoudougou (Tamoul) | Renal colic | Leaves | Infusion of 4 or 5 green leaves | [32,49] |
| Ophthalmia, diuretic, anthrax (poultice), and ulcers | Leaves | Diluted leaf of 1 or 2 cups | |||
| Mali | Nonsikou (Bambara) | Nausea and vomiting | Whole plant | Boiled decoction of plant bundle is taken orally. | [50] |
| Baby thinness | Leaves | Leaves decoction through VO and bath 4x/day for 10 days | [9] | ||
| Ocular infection | Leaves | Leaves decoction is used to wash eyes. | |||
| Amenorrhea | Root | Decoction of roots is applied through VO and bath for 3 days. | |||
| High blood pressure | Leaves | Leaves decoction (VO) | |||
| Mexico | Not registered | Asthma | Root | Decoction of roots or any plant part | [9] |
| Nigeria | Agogo-igun, ogbe, and akuko | Paludism, and sap is applied to gumboils. | Leaves | The decoction with water and allowed to administer through vocal order | [38] |
| Hepatitis and fever | Leaves | The decoction with water and allowed to administer through vocal order | |||
| Gonorrhea | Leaves | The leaf juice mixed with castor oil is locally applied. | [51] | ||
| Otukeyin, ekaesinono, and edisimon (Ibibio) | Boils and sore throat | Leaves | Decoction of crushed leaves is applied through VO. | [52] | |
| Nicaragua | Not registered | Skin infections | Leaves | Leaf paste is applied topically for skin infections. | [53,54] |
| Whooping cough | Leaves and root | Decoction of a combination of leaf and root is taken orally. | |||
| Philippines | Buntot-leon, pengnga-pengnga, and puntaelepante | Diuretic and kidney stone | Whole plant | Decoction of the whole plant is taken orally. | [55] |
| Rodrigues Island | Herbepapillon | Calculus | Whole plant | Decoction of the plant is applied through VO. | [56] |
| Herbepapillon (Rodrigues Creole) and Indian heliotrope (English) | Bloating and loss of appetite | Leaves | Decoction of the leaves (VO). 1 cup when needed. | [57] | |
| Siby | Nonsikou | Vomiting | Leaves | Unknown | [9] |
| Seychelles | Not registered | Chirurgical pain | Leaves | The decoction with water and allowed to administer through vocal order | [58] |
| Senegal | Manding-bambarańâgiku | Child, eczema, impetigo, and dermatitis | Leaves | The leaf powder is prepared by drying in the shadow and in the open-air that is applied in local. | [59] |
| Diuretic and kidney stone | Whole plant | Decoction of the whole plant is taken orally. | [55,60] | ||
| Sao Tome | Folhagalo | Ulcers | Leaves | The crushed leaves with palm oil are applied on the affected area. | [61] |
| Sierra Leone | Not registered | Washing the newborn babies | Leaves | Decoction of leaves | [9] |
| South America | Not registered | Insect bites and scorpion stings | Leaves and root | Paste of leaf and root together is applied externally. | [62] |
| Togo | Koklotadoe and agamassiké (Ewé) | Dermatosis | Leaves | Local application of leaves juice | [38] |
| Liver diseases | Whole plant | Decoction of the whole plant | [63] | ||
| Tanzania | Humbangara (Ngoni) | Yaws | Root | Decoction or maceration of the root through VO | [64] |
| Taiwan | Gou-wei-chung-tsan | Hepatitis | Leaves and root | Paste of leaf and root together is applied externally. | [65] |
| Thailand | Yah nguang-chang | Produce permanent sterilization in females | Inflorescence | One gram of the dried and powdered inflorescence mixed with milk or water is used for 3 days beginning with the fourth day of menses to achieve the desired result. | [33] |
| West Indies | Head lice | Whole plant | Paste of fresh whole plant | [66] |
6. Phytochemical Constituents
Based on the history of traditional and folk medicinal uses of H. indicum, many researchers have been investigating its phytochemical and pharmacological properties to identify the compounds responsible for its wide use as herbal medicines. The plant contains many important phytocomponents, including alkaloids (e.g., acetyl indicine, cynoglossine, echinitine, heleurine, heliotrine, helindicine, europine N-oxide, heleurine N-oxide, heliotridine N-oxide, heliotrine N-oxide, indicine, indicinine, indicine N-oxide, lasiocarpine, lycopsamine, trachelanthamidine, retronecine, and supinine), triterpenes (e.g., β-amyrin, lupeol, rapone, and rapanone), sterols (e.g., β-sitosterol, estradiol, chalinasterol, campesterol, hexacosane-1-ol, and stigmasterol), amines (e.g., putrescine, spermidine, and spermine), and volatile oils (e.g., 1-dodecanol, β-linalool, and phytol) [30,53,62,65,67–72]. Two new alkaloids, namely, heliotrine and indicine N-oxide, along with other alkaloids, including heleurine, supinine, echinitine, heliotrine, lasiocarpine N-oxide, acetyl indicine, indicinine, and retronecine, have been isolated from the aerial parts of H. indicum [68, 71, 73, 74]. Europine N-oxide, cynoglossine, heliotrine N-oxide, heleurine N-oxide, and heliotridine N-oxide were isolated from the seeds of this plant. Another new pyrrolizidine alkaloid, helindicine, has also been isolated from the roots of H. indicum [75]. The reported compounds are presented in Table 3, and the main representative compounds are shown in Figure 2.
Table 3.
Chemical compounds isolated from H. indicum L.
| Phytochemicals | Part(s) | Reference(s) |
|---|---|---|
| Alkaloids | ||
| Cynoglossine | Seed | [67] |
| Echinatine | Aerial | [53] |
| Heleurine | Aerial | [62] |
| Heliotrine | Aerial | [62] |
| Heliotridine | Aerial | [62] |
| Helindicine | Root | [75] |
| Europine N-oxide | Seed | [67] |
| Heleurine N-oxide | Seed | [67] |
| Heliotridine N-oxide | Seed | [67] |
| Heliotrine N-oxide | Seed | [67] |
| Indicine | Aerial | [53] |
| Indicine N-oxide | Aerial | [71] |
| Lasiocarpine | Aerial | [65] |
| Lycopsamine | Root | [75] |
| Trachelanthamidine | Leaves | [76] |
| Retronecine | Leaves and aerial | [73,76] |
| Supinine | Aerial | [53] |
| Triterpenes | ||
| β-Amyrin | Whole plant | [77] |
| Lupeol | Aerial and whole plant | [71,77] |
| Rapanone | Whole plant | [77] |
|
| ||
| Sterols | ||
| β-Sitosterol | Whole plant | [77] |
| Estradiol | Root | [78] |
| Chalinasterol | Whole plant | [77] |
| Campesterol | Whole plant | [77] |
| Hexacosane-1-ol | Whole plant | [77] |
| Stigmasterol | Whole plant | [77] |
|
| ||
| Amines | ||
| Putrescine | Leaves | [76] |
| Spermidine | Leaves | [76] |
| Spermine | Leaves | [76] |
|
| ||
| Volatile oils | ||
| 1-Dodecanol | Whole plant | [79] |
| β-Linalool | Whole plant | [79] |
| Phytol | Whole plant | [79] |
Figure 2.
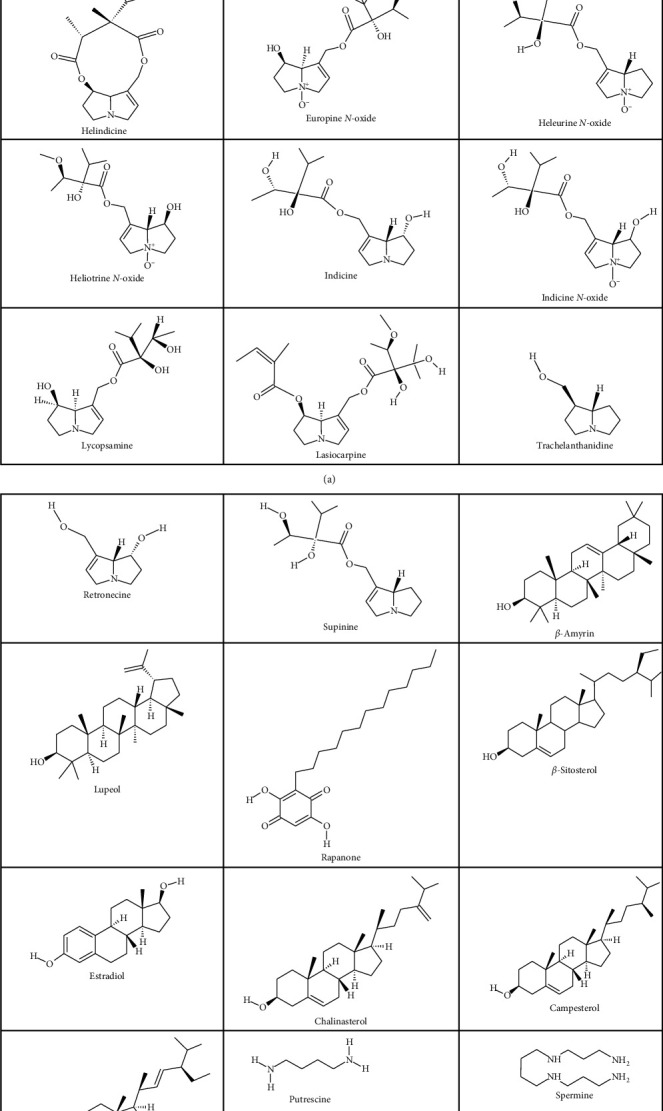
Some important isolated compounds from H. indicum L.
7. Pharmacological Activities
Various solvent extracts (e.g., aqueous, chloroform, ethanolic, methanolic, and petroleum ether) of the whole plant of H. indicum as well as its various parts (e.g., root, stem, leaf, etc.) have been investigated to validate the folk value, and the results showed diverse biological effects on experimental animals, which are described in the present section. Pharmacological activities of different parts of H. indicum have been shown in Table 4.
Table 4.
Pharmacological activities of different parts of H. indicum L.
| Activity | Extract | Method | Results | References |
|---|---|---|---|---|
| Antioxidant activity | Methanolic extract of leaf, stem, and root | DPPH free radical scavenging assay | Leaf extract yields greater free radical scavenging activity than the stem and roots. | [80] |
| Aqueous leaf extract | Show high free radical scavenging activity compared with Centella asiatica, Coccinia grandis, and Euphorbia hirta. | [81] | ||
| Ethanol and water extracts of the whole plant | Ethanolic extract showed high antioxidant activity. | [14] | ||
| Analgesic activity | Aqueous and ethanol extracts of the whole plant | In vivo: Formalin-induced nociception in mice | Both extracts have analgesic activity. | [82] |
| Antinociceptive activity | Methanolic extract of the roots | Acetic-acid-induced writhing in mice | Extract produced writhing inhibition in the test animals. | [7] |
| Chloroform extract of leaves | Hot-plate model in male Swiss albino mice | Extract showed writhing inhibition in mice. | [83] | |
| Antiinflammatory activity | Methanol extracts of leaf, stem, and root | Egg-albumin- and carrageenin-induced acute paw edema models and cotton pellet granuloma sub-acute inflammation model | Extract of roots produced a significant antiinflammatory effect in acetic-acid-induced writhing in mice. | [18,84] |
| Chloroform extract of leaves | Carrageenan-induced raw paw edema | The extract showed maximum inhibition on carrageenan-induced rat paw edema. | [83] | |
| Aqueous whole plant extract | Lipopolysaccharide (LPS) induced uveitis rabbits | The extract reduced both the clinical scores of inflammation and inflammatory cells infiltration. | [85] | |
| Antimicrobial activity | Alcoholic extract of the whole plant | Agar cup plate diffusion method | The alcoholic extract was found to possess dose-dependent antimicrobial activity against bacteria, fungi, and yeasts, | [8,86] |
| Petroleum ether, chloroform, aqueous, and methanolic extracts of leaves | All extracts show effective antimicrobial activity against both Gram-positive and Gram-negative bacteria | [6,37] | ||
| Aqueous, ethanol, and chloroform extracts of the whole plant | Had significant zones of inhibition against bacteria and fungi. | [87] | ||
| Methanol whole plant extracts | Exhibited both antibacterial and antifungal activity. | [88] | ||
| Methanol leaves extract | Had an antibacterial activity. | [8] | ||
| Antituberculosis activity | Volatile oil of H. indicum from aerial parts | Alamar blue assay system with an MIC | Had profound antituberculosis activity against Mycobacterium tuberculosis H37Ra. | [79] |
| Antihyperglycemic activity | Whole plant methanol extracts | Tested on the fasting blood glucose levels of streptozotocin-induced (STZ-induced) diabetic rats | Showed a conspicuous reduction in blood glucose levels and normalization of blood glucose levels. | [89] |
| Anticataract activity | Ethanolic leaf extract | Galactose-induced cataract in rats | Significantly increased the lens glutathione. | [20] |
| Aqueous extract of the whole plant | Selenite-induced cataracts in Sprague–Dawley rats | Expressively inhibited the development of selenite-induced cataracts. | [90] | |
| Antiplasmodial properties | Dichloromethane, methanol, and total aqueous extracts of the whole plant | Tested on chloroquine-sensitive (3D7) and resistant (W2) strains of Plasmodium falciparum | Revealed no direct antiplasmodial activity. | [91] |
| Antifertility activity | Petroleum ether extract of the whole plant | In vivo test on rats | Exhibited profound activity. | [77] |
| Extract of the n-hexane and benzene fractions of whole plant | Antiimplantation and abortifacient models in rats | Had substantial antifertility activity. | [92] | |
| Anthelmintic activity | Methanolic extract of leaves | In vitro anthelmintic bioassay | The extract showed significant anthelmintic efficacy. | [93] |
| Antitumor activity | Methanolic extract of both stem and leaf | MTT assay on HeLa cell lines | Both extracts exhibited antiproliferative activity where the stem extract showed interesting results. | [94] |
| Ethanolic extract of the whole plant | MTT assay on SKBR3 human breast adenocarcinoma cell line | Showed momentous antiproliferative activity. | [91] | |
| Antitussive property | Ethanolic extract of leaves | The citric acid saturated chamber in animals | Extract syrup recorded the lowest number of coughs. | [95] |
| Antiglaucoma activity | Aqueous whole plant extract | Glaucoma of rabbits in vivo | Significantly reduced intraocular pressure in acute and chronic glaucoma. | [90] |
| Wound-healing activity | Dried parts of ethanolic extracts | Excision and restored incision wound model | Showed wound-healing capacity. | [19] |
| n-Butanol fractions aerial part (stem and leaves) | The scratch assay | The isolated compound contains profound wound-healing activity. | [96] | |
| The petroleum ether, chloroform, methanol, and aqueous extracts of leaves. | Excision (normal and infected), incision, and dead space wound models in rats | Methanol and aqueous extracts attributed intense wound-healing activity. | [37] | |
| Histo-gastroprotective activity | Aqueous extract of the dried leaves | Indomethacin-induced gastric ulcerated mucosa in rats | Had effective histo-gastroprotective activity. | [10] |
| Diuretic activity | Methanolic extract of the dried roots | Biuret, a urea derivative assayed by the electrolyte loss ratio (Na+/K+ excretion ratio) in mice | The extract revealed a marked diuretic effect. | [7] |
| Relaxant/receptor property | Ethanol extract of the roots | Guinea pig ileum and rabbit duodenum in vitro | Possess weak smooth muscle relaxant activity. | [97] |
| Dark-brown solid extract of aerial parts | Guinea pig ileum, rabbit jejunum, rat uterus, and rat anococcygeus preparations in vivo | Showed profound receptor property | [98] | |
| Clot lysis and membrane-stabilizing activities | Ethanolic, petroleum ether, carbon tetrachloride, and chloroform extracts of leaves | Membrane-stabilizing and thrombolytic activities in vitro | Had potential clot lysis and membrane-stabilizing activities. | [99] |
| Methanol extract of the whole plant | In vitro thrombolytic model and membrane-stabilizing activity assay on human RBC subjected to heat and hypotonic stress | Protected the hemolysis of RBCs induced by hypotonic solution and heat stress. | [88] | |
| Antiallergic activity | Aqueous whole plant extract | Ovalbumin-induced allergic conjunctivitis on Dunkin–Hartley guinea pigs | Exhibited antiallergic effect possibly by immunomodulation or immunosuppression. | [90] |
| Larvicidal activity | Ethanolic leaf extract | Larvicidal bioassay on mosquito larvae of Aedes aegypti | The extract showed effective mosquito larvicidal activity. | [100] |
| Pesticidal activity | Ethanol extract of aerial parts | Brine shrimp lethality bioassay | Possess potent activity against the brine shrimp nauplii. | [7] |
7.1. Antioxidant Activity
The methanolic extract of various parts of the plant, such as leaf, stem, and roots, was used to measure the total phenolic compounds and flavonoids contents as well as to determine DPPH free radical scavenging activities. The inflorescence extracts presented a higher concentration of total phenolics and flavonoids with a 21.70 mg gallic acid equivalent per gram (GAE/g) and 4.90 mg quercetin equivalent per gram (QE/g), followed by leaves, stems, and roots. The percentage of free radical scavenging activity of the methanolic extracts of inflorescence, leaves, stems, and roots followed the same response pattern, with the maximum values for inflorescence (77.78%) followed by leaves (55.25%), stems (47.49%), and roots (<20%) with respect to the standard gallic acid and ascorbic acid [80]. In another study by the same authors, the potential antioxidant activity of methanolic extracts of callus of H. indicum cultured for 30days at different temperatures (20, 25, 30, and 32°C) reported the highest DPPH scavenging activity (IC50 = 53.17 ± 1.43 μg/mL) at 30°C respect to the other temperatures [81]. In addition, another study reported that the ethanolic extract of H. indicum exerted more antioxidant capacity (EC50: 28.91 ± 4.26 μg/mL) than the water extract (EC50: >100 μg/mL) [14].
7.2. Analgesic Activity
The analgesic effect of the ethanolic and aqueous extracts of the aerial parts of H. indicum (30–300 mg/kg) in a mouse model of formalin-induced pain was compared with the standard drugs, diclofenac sodium (1–10 mg/kg), and morphine (1–10 mg/kg). The neurogenic and inflammatory phases of the formalin-induced nociception were inhibited dose-dependently by both the aqueous and ethanolic extracts, suggesting a potential analgesic application [82,101]. However, toxicity studies reported that 14-day oral administration of 1–2 g/kg of H. indicum aqueous extracts induced pathologic effects on the heart, kidney, liver, and lungs; therefore, prolonged and continuous use is not recommended.
7.3. Antinociceptive Activity
The methanol root extract of H. indicum exhibited 34.76 and 64.67% writhing inhibition in Swiss albino mice at 250 and 500 mg/kg of body weight (po), respectively, whereas the standard drug diclofenac sodium showed 66.67% writhing inhibition at the clinically established dose of 25 mg/kg for mice [7]. Another study suggested that the chloroform extract of leaves of H. indicum showed maximum antinociception effect (82.79%) at 150 mg/kg of body weight in the hot-plate test in male Swiss albino mice that was compared with the standard drug, pentazocine [83].
7.4. AntiInflammatory Activity
The antiinflammatory activity of methanolic root extracts of H. indicum (100 mg/kg) was assayed against carrageenin-induced acute paw edema and cotton pellet granuloma sub-acute inflammation models, and the standard drugs acetylsalicylic acid for the acute assay and phenylbutazone for the sub-acute assay were used as positive controls [18]. The extract evidenced a significant antiinflammatory activity with a 49.05% reduction in paw edema and 55.09% reduction in granuloma formation. These results were similar to those obtained by positive controls using the same concentration of 100 mg/kg. In another study, the ethanolic and petroleum ether extracts of H. indicum (25 mg/kg) were investigated in an egg-white-induced acute paw edema rat model [84]. Both extracts evidenced notable antiinflammatory effects, reporting similar values to the standard reference ketorolac trimethamine (10 mg/kg). The chloroform leaf extract of H. indicum extract (150 mg/kg of body weight) also showed a significant antiinflammatory effect (80.0%) on carrageenan-induced paw edema in albino Wistar rats [83]. An aqueous whole plant extract of H. indicum (30–300 mg/kg, p.o.) showed an antiinflammatory effect on the lipopolysaccharide-induced uveitic rabbits. The extract and prednisolone (positive control) expressively reduced both the clinical scores of inflammation and inflammatory cell infiltration compared with the negative control group [85]. A pharmaceutical oral product obtained from H. indicum is used against acute and chronic inflammation, particularly against inflammatory diseases of the intestines [102].
7.5. Antimicrobial Activity
The alcoholic extract with a percentage yield of 7.2% w/w of the whole plant showed a concentration-dependent (1–100 mg/mL) antibacterial activity against Bacillus subtilis, Bacillus pumilus, Staphylococcus aureus, Micrococcus glutamicus, Pseudomonas aeruginosa, Proteus vulgaris, Serratia marcescens, and Escherichia coli. The alcoholic extract also showed antifungal activity against Aspergillus niger, Aspergillus wentii, Rhizopus oryzae, Saccharomyces cerevisiae, and Candida albicans [8,86]. However, as high extract concentrations are required to observe inhibitory effects, activity-directed assays are necessary to isolate and characterize the active metabolite responsible for the observed activity. The petroleum ether, chloroform, aqueous, and methanolic extracts of H. indicum leaves showed antimicrobial activity against both Gram-positive and Gram-negative bacteria, such as B. subtilis, S. aureus, P. aeruginosa, and E. coli [6,37]. In a wound infection model with S. aureus and P. aeruginosa, the methanolic and aqueous extracts of leaves mixed with a simple ointment (10% w/w) presented the most promising activity favoring the healing similarly to the reference standard nitrofurazone [37]. In another study, the antimicrobial screening of petroleum and methanolic extracts of the aerial parts of the plant evidenced significant zones of inhibition against the three previously mentioned microorganisms [6].
The aqueous ethanol and chloroform extract of the whole plant of H. indicum showed antibacterial and antifungal activities, where it produced significant zones of inhibition against 70% of the tested organisms, using amikacin (5 g/disc) as a positive control [87]. Among the different extracts, chloroform is the one that showed the best results, although the zone of inhibition was always lower than for amikacin (e.g., for S. aureus, the inhibition diameter was 19 mm for the control and 12 mm for chloroform extract). The methanol extract of the whole plant also showed activity against five Gram-positive and eight Gram-negative bacteria and three fungi, using the standard antibiotic, ciprofloxacin, as a positive control [88]. In addition, the carbon tetrachloride soluble materials obtained by the fractionation of the methanolic extract using a rotary evaporator revealed notable activity against a number of microbes with zones of inhibition ranging from 7 to 20 mm, showing the highest inhibitory capacity for Bacillus cereus (20.0 mm) [88]. The methanol extract of H. indicum leaves (6.25, 12.5, 25, 50, 100, and 200 mg/mL) showed activity against S. aureus, P. aeruginosa, Proteus mirabilis, and E. coli, where the diameters of the zones of inhibition were 6 mm [8]. However, the high concentration required to obtain inhibition, compared with the positive control (gentamycin, 10 mg/ml), suggests a low antimicrobial capacity of the extract. The volatile oil isolated from the aerial parts of H. indicum with phytol (49.1%), 1-dodecanol (6.4%), and β-linalool (3.0%) as main compounds showed antituberculosis activity against Mycobacterium tuberculosis H37Ra with an MIC value of 20.8 μg/mL, using the drugs, isoniazid, and kanamycin, as positive controls [79].
7.6. Antihyperglycemic Effect
Administration of the whole plant methanol extract among the different solvent extracts of H. indicum (250, 500, 750, or 1,000 mg/kg) on the fasting blood glucose levels of streptozotocin-induced (STZ-induced) diabetic rats showed a significant reduction (31.5%) but less antihyperglycemic activity in comparison with the aqueous extract (47%) and methanol active fraction (750 mg/kg of body weight) of the plant (60%) [89].
7.7. Anticataract Effect
The ethanolic leaf extract of H. indicum (200 mg/kg of body weight) showed a significant anticataract activity in rats. The results showed that there was a significant increase in the lens glutathione, soluble protein, and water content in the groups of H. indicum and vitamin-E-treated animals than the galactose-containing control group [20]. Another study showed that the aqueous extracts of the whole plant (including aerial and root parts) significantly inhibited the development of selenite-induced cataracts in Sprague–Dawley rats [90].
7.8. Antiplasmodial Properties
In order to find out its scientific relevance to the traditional use in malaria, the extracts of H. indicum were undergone for the evaluation of antiplasmodial activity. However, H. indicum methanolic extracts had not shown clear antiplasmodial effects assayed in vitro against chloroquine-resistant (K1) and sensitive (FCR3) strains, and antiTrypanosoma effects were assayed in Trypanosoma brucei brucei GUT at 3.1 strain [91]. Its use in traditional medicine can be explained by its activity in reducing hyperthermia and colic, which are two symptoms of malaria [103].
7.9. Antifertility Activity
Antifertility and abortifacient activity of petroleum ether extract of H. indicum were significant in rats, which validated the ethnomedicinal use of this plant as an antifertility agent [77]. The n-hexane and benzene fractions of the ethanol extract of the whole plant also showed antifertility activity using antiimplantation and abortifacient models in rats [92].
7.10. Anthelmintic Effect
The anthelmintic effects of methanolic and aqueous leaf extracts of H. indicum (25, 50, and 100 mg/mL) were tested against the Indian adult earthworm, Pheretima posthuma. Mebendazole was used as a reference standard using the same concentrations as the extract. The time to paralysis and death progressively decreased in parallel with the increase in the concentrations of the methanolic extract, showing results similar to those of the standard drug mebendazole [93]. On the contrary, the effects of the aqueous extract were much smaller and not very effective against P. posthuma.
7.11. Anticancer Effect
The methanolic extract of H. indicum roots (10, 20, 40, 80, and 160 μg/mL) showed a potent cytotoxic effect on the brine shrimp nauplii [7]. The LC50 values were ranged from 2.57 to 31.44 μg/mL. The crude methanol extract also showed cytotoxic effects on brine shrimp nauplii with the LC50 value of 2.57 ± 0.22 μg/mL as compared with 0.45 μg/mL for positive control vincristine sulphate [88]. In another study, the anticancer effects of the methanolic extracts of stem and leaves were investigated against HeLa cell line [94]. Both methanolic extracts exhibited antiproliferative activity after 48 h of treatment, evidencing a relative death percentage of 64.5% for the methanolic extract of stem at 200 μg/mL and 49.7% for the leaf extract at the same concentration with respect to control cell supplemented only with the vehicle [94]. The ethanolic extract of the whole plant was also found to exert a significant antiproliferative effect on SKBR3 human breast adenocarcinoma cell line [91]. Indicine N-oxide, which is the principal pyrrolizidine alkaloid isolated from this plant has reached phase 1 clinical trial in advanced cancer patients with the risk of hepatotoxicity [104].
7.12. Antitussive Effect
The ethanolic leaf extract of H. indicum showed an antitussive effect on experimental animals. While statistically comparable with dextromethorphan, the results of the investigation showed that 50% and 100% ethanolic extract syrup reduced the coughing score by 4.67 and 2.0, respectively [95].
7.13. Antiglaucoma Activity
The aqueous whole plant extract of H. indicum (30–300 mg/kg of body weight) significantly reduced the intraocular pressure in acute and chronic glaucoma, preserved glutathione levels, and glutamate concentration in rabbits [90].
7.14. Wound Healing Capacity
The alcoholic extract of H. indicum showed wound-healing activity in animal models. In a rat model, topical application of 10% w/v H. indicum showed a complete wound-healing capacity on the 14th day [19]. Two alkaloids, pestalamide B and glycinamide, N-(1-oxooctadecyl) glycyl-lalanylglycyl-L-histidyl, isolated from the n-butanol crude extract of H. indicum showed excellent wound-healing activity on H292 human lung cells [96]. The n-butanol extract of H. indicum also showed a significant wound-healing activity on H292 human lung cells in vitro [96]. Another experiment proved that the methanol and aqueous extracts of H. indicum revealed significant wound-healing activities than the other extracts (e.g., petroleum ether and chloroform) in rats [37].
7.15. Gastroprotective Effect
The aqueous extract of the dried leaves of H. indicum showed a dose-dependent gastroprotective effect in indomethacin-induced (80 mg/kg of body weight) gastric ulcer mucosa in rats [10]. Histological observations of the different components of the mucosa layer of the stomach evidenced normal morphological appearance in the H. indicum groups, whereas in the control group, significant erosions in the mucosa were observed. It was also supposed that this effect may be due to the presence of tannins, alkaloids, and saponins in the leaves of the plant that may induce the release of prostaglandins in gastric mucosa maintaining gastric microcirculation through mucus and bicarbonate production.
7.16. Diuretic Effect
The methanolic root extract of H. indicum at 200 and 400 mg/kg revealed a marked diuretic effect of the electrolyte loss ratio (Na+/K+ excretion ratio was 1.38 and 1.45, respectively) as compared with the standard diuretic furosemide (1.37) in mice [7,105].
7.17. Relaxant/Receptor Property
The ethanol (95%) extract of the roots showed weak smooth muscle-relaxant activity on guinea pig ileum and rabbit duodenum [97]. Another study performed on isolated guinea pig ileum, rabbit jejunum, rat uterus, and rat anococcygeus preparations with several agonists, antagonists, and the aqueous plant extract showed a dose-dependent activity of the acetylcholine, methylcholine, carbamylcholine, nicotine, histamine, oxytocin, and plasma cholinesterase [98].
7.18. Antithrombotic Effects
Different extracts of H. indicum exhibited a potential lysis of clots and stabilizing activities of the membrane, which is why traditionally the leaves of H. indicum have been used as a remedy for thrombosis. The ethanol, petroleum ether, carbon tetrachloride, and chloroform extracts of H. indicum leaves showed 23.78, 35.40, 32.48, and 18.95% clot lysis activity, respectively, in the blood of healthy male subjects [99]. In this study, streptokinase, used as a positive control, showed a 65.15% clot lysis activity. In another study, the methanolic extract of the whole plant showed mild-to-moderate thrombolytic activity at a concentration of 1.0 mg/mL protecting red blood cells against hypotonic and heat-induced hemolysis [88]. In addition, the carbon tetrachloride soluble fraction obtained from this extract showed a 41.47 ± 1.12 and 37.97 ± 0.14% of red blood cell lysis induced by hypotonic solution and heat, respectively, while acetylsalicylic acid used as positive control showed 71.92 and 42.12% of lysis [88].
7.19. Larvicidal Activity
H. indicum is a potential plant for the control of Aedes aegypti, which is a potential vector of the dengue virus. Veerakumar et al. [106] suggested that H. indicum can be an ideal eco-friendly plant for the control of Anopheles stephensi and A. aegypti. The alcoholic extracts of H. indicum at different concentrations (0.30, 0.25, 0.20, 0.15, 0.10, 0.075, 0.050, and 0.025 mg/mL) were found to act against the mosquito larvae of A. aegypti [100]. In this study, an inability to come to the surface, restlessness, loss of equilibrium, and finally the death of the larvae were observed with the treatment of H. indicum extracts. The results showed a mortality of 10% already in the lowest concentration of 0.025 mg/mL, reaching 100% in the concentration of 0.25 mg/mL. However, no positive control was used in the study, making it difficult to compare the real efficacy of the extract.
7.20. Miscellaneous Effects
The aqueous and ethanol extracts of the H. indicum roots exhibited a strong uterine stimulant effect in rats [107]. Bero et al. [103] reported that the aqueous extract of H. indicum possesses antileukemic and ganglion-blocking activities. The leaf extract of H. indicum is also evident to be used in ophthalmic disorders, erysipelas, and pharyngodynia [108]. An aqueous whole plant extract of H. indicum (30–300 mg/kg of body weight) exhibited an antiallergic effect on Dunkin–Hartley guinea pigs possibly by immunomodulation pathway [90].
7.21. Toxicological Profile
The aqueous and ethanolic extracts of the whole plant exhibited cumulative toxic effects on the kidney, liver, and lungs on prolonged use [82,101]. Heliotrine is evident to cause liver damage in experimental animals [109], while lasiocarpine developed malignant tumors in rats [110]. Retrorsine exerted a toxic effect on human embryo liver cells [111].
In a five-month toxicity study, an oral administration of the ethanol extract of H. indicum caused dose-dependent mortality (LD50: 9.78 g/kg of body weight) in Swiss albino mice [112].
Pyrrolizidine alkaloids are evident to produce highly reactive adducts, such as 2,3-dihydro-1H-pyrrolizine protein, through the hepatic cytochrome P450 system. These adducts bind to proteins and genetic materials (e.g., DNA and RNA) and induce veno-occlusive disease in the liver [113]. The acute intoxication of pyrrolizidine alkaloids is characterized by hemorrhagic necrosis, hepatomegaly, and ascites, while chronic exposure is characterized by necrosis, fibrosis, cirrhosis, liver failure, and even death [114]. Due to photosensitization in animals upon their consumption and metabolism, pyrrolizidine alkaloids may initiate skin cancer [115]. Moreover, these substances can cause neurotoxicity and encephalitis, which is characterized by vertigo, headaches, delirium, and loss of consciousness [116].
8. Discussion
H. indicum has long been used in traditional medicine systems to treat various ailments; therefore, this review summarized the botany, traditional uses, phytochemistry, and pharmacology of this plant and its components. A number of phytochemical classes have been isolated from this medicinal plant. Available pharmacological studies on the ingredients and crude extracts indicated broad biological effects of H. indicum, providing basic evidence for traditional claims. However, as viewed from the current findings, some areas still require scientific evaluation and exploration. First, the leaves of H. indicum are the main medicinal part used in Bangladesh, while in other countries (e.g., India and Thailand), different parts are used for different purposes. Therefore, it is convenient to investigate the differences between plant parts regarding phytochemistry and pharmacology. Second, alkaloids are considered as the main bioactive constituents, particularly heliotrine and heleurine N-oxide. Numerous bioactivities of other bioactive constituents have been reported to be of prominent pharmacological activities and are worth to be given more attention. In addition, more research on the identification and isolation can be done on extracts, with reported bioactivities to discover new active phytochemicals and elucidate their structure-activity relationships and possible synergistic effects. Third, the reliability of the herb to treat coronary heart disease, kidney diseases, hemorrhagic diseases, and vitiligo has been justified by the long history, but current findings are not enough to ascertain these traditional claims from the perspective of modern pharmacology. Moreover, the evaluation of representative and appropriate cell or animal models is equally important to assess these traditional uses precisely. Fourth, the anticancer activity of H. indicum indicated that the plant could be a natural source to find promising and cost-effective lead compounds with little side effects for cancer treatment. The cytotoxic effects are mainly due to the action of the pyrrolizidine alkaloid, indicine N-oxide, which alters the assembly of tubulin into microtubules, inducing DNA damage [117]. However, the appearance of liver toxicity and even bone marrow aplasia has led to the withdrawal of this compound from the development of clinical trials [118,119]. Thus, it will be necessary to find new compounds in H. indicum with anticancer potential. Finally, acute and chronic toxicity should be comprehensively studied in order to establish safety and toxicological limits and provide guidance for clinical applications.
Phytochemical research has led to the isolation and identification of 32 compounds in H. indicum [13, 22]. Different classes of compounds have been detected, including alkaloids, triterpenes, sterols, amines, and volatile oils (Table 3 and Figure 2). H. indicum contains a large class of alkaloids with antiinflammatory, analgesic, antibacterial, antitumor, and other activities. Among them, acetyl indicine, echinitine, heleurine, heliotrine, indicine, indicinine, indicine N-oxide, lasiocarpine, retronecine, supinine, and trachelanthamidine were isolated from the aerial parts of the plant, while cynoglossine, europine N-oxide, heleurine N-oxide, and heliotridine N-oxide were separated from the seed, and heliotrine and lycopsamine were separated from the root [30, 53, 62, 67, 69, 71, 73, 75, 76]. The chemical structures of alkaloids are shown in Figure 2. Indicine N-oxide, which is the principal pyrrolizidine alkaloid isolated from H. indicum, has the potential risk of hepatotoxicity [104], and because of the presence of a high amount of pyrrolizidine alkaloids, this plant exerts potent anticancer activity [94]. The plasma cholinesterase receptor activity of H. indicum validates some of its traditional folk values such as relieving abdominal pain, hypertension, and impotence and sexual weakness [98].
Triterpenes are the second class of molecules that have been well-studied in H. indicum evidencing a wide variety of biological functions. Among them, β-amyrin, lupeol, and rapanone have been evidenced to possess biological functions, including defense against herbivores, microbial attack, or other sources of injury [71, 77]. β-Amyrin also showed potential antihyperglycemic and hypolipidemic effects, suggesting that it could be a lead compound for drug development for diabetes and atherosclerosis [120]. Lupeol is a novel antiinflammatory and anticancer dietary triterpene, which has strong antioxidant, antimutagenic, antiinflammatory, and antiarthritic characteristics with potential pharmaceutical applications [121]. Rapanone has been reported to exert significant antioxidant, antiinflammatory, and cytotoxic activities against a panel of human tumor cells [122]. Toxicity studies have observed some alterations in rats such as tremor, ataxia, increased respiratory rate, and decreased activity at concentrations of β-amyrin above 30 mg/kg for 4 weeks, while no toxicity has been observed for lupeol at doses up to 200 mg/kg [121, 123]. Although no significant effects of rapanone have been shown in non-cancer cells, at doses of 60 and 120 mg/kg, it induced anovulatory effects in female mice [124, 125].
Six main sterol compounds have been isolated from H. indicum: β-sitosterol, chalinasterol, campesterol, stigmasterol, hexacosane-1-ol, and estradiol [77, 78]. Sterols have a wide variety of functions in plant physiology, including the regulation of Na+/K+-ATPase, cell differentiation, and proliferation or membrane fluidity and permeability [126–128]. In addition, plant-derived sterols have been reported to exert antiinflammatory effects useful in the treatment of non-alcoholic fatty liver, inflammatory bowel diseases, and allergic asthma [129]. However, no studies have specifically evaluated the effects of sterols isolated from H. indicum against these diseases.
Amines are an important class of molecules in H. indicum that display pesticidal, fungicidal, herbicidal, analgesic, and antioxidant activities. Putrescine, spermidine, and spermine were separated from the leaves of H. indicum [76]. Putrescine scavenges reactive oxygen species and regulates DNA and protein synthesis, cell proliferation, and differentiation of tissues, thereby supporting placental development and embryogenesis in mammals [130]. Spermidine is a polyamine compound that counteracts aging and promotes cellular longevity [131]. The compound induces autophagy in a mammalian target of rapamycin (mTOR) independent manner by inhibiting the acetyltransferase EP300, resulting in hypoacetylation of several core autophagy proteins, including ATG5, ATG7, ATG12, and LC3 [132]. Spermine is a natural polyamine known to be essential regulators of various cellular processes, including DNA stability, cellular growth, differentiation, and apoptosis, and also used to treat cancer, other pathologies, inflammation, immunity, infection, and aging [133].
Three volatile oils were separated from the whole plant of H. indicum [79]. Among them, linalool (acyclic monoterpene alcohol) exerted its antiproliferative activity against various cancer cells through the mevalonate pathway [134]. Linalool has nutraceutical anticancer, antioxidant, antimicrobial, antidiabetic, antinociceptive, antiinflammatory, and hypolipidemic effects [135]. Phytol, diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress and also provides antinociceptive activities [136, 137], and it has many biomedical applications [138, 139], including antimicrobial, cytotoxic, anticancer, non-mutagenic, antiteratogenic, antibioticchemotherapeutic, antidiabetic, lipid-lowering, antispasmodic, anticonvulsant, antinociceptive, antioxidant, antiinflammatory, anxiolytic, antidepressant, immune-adjuvant, hair growth facilitator, hair fall defense, and antidandruff activities [140]. Moreover, it has antipyretic [141] and clot lysis activities [142].
Diabetes mellitus is a chronic metabolic disease caused by an absolute or relative lack of insulin and/or reduced physiological insulin activity, resulted in hyperglycemia and abnormalities in carbohydrate, protein, and fat metabolism [143]. The methanol extract of H. indicum showed a dose-dependent antidiabetic effect on STZ-induced diabetic rats [89]. Different compounds present in this herb, for example, lupeol [144], phytol [140], and stigmasterol [145], have been found to show antidiabetic effects in experimental animals.
Rapanone has cytotoxic effects on MCF-7 cells, where it induced apoptosis through mitochondrial membrane potential loss [146]. Although effective therapeutic interventions are yet to be found out, it has been seen that estradiol positively impacts some aspects of cognitive function in humans and other animals [147]. Stigmasterol also possesses many biological activities, including immune-modulatory [148], anticancer [149], neuroprotective [150], hypolipidemic [151], and other effects. Putrescine has antiaging property [152] and can reduce antibiotic-induced oxidative stress in Burkholderia cenocepacia [153]. Spermidine alleviated autoimmune encephalomyelitis by inducing inhibitory macrophages [154]. It has several important biological activities, including antioxidant [155], cardioprotective, [156], neuroprotective [157], and other effects.
Spermine is a polyamine, initially discovered as crystals in human semen by Antonie van Leeuwenhoek in 1678 [158], and evokes olfactory responses in teleost fish [159] and possibly humans [160]. In a study, it has been found to act as a specific semen-derived sex pheromone in sea lamprey and promotes mating behaviors [161]. It is also evident to show antiinflammatory [162], mitochondrial protein synthesis [163], cardioprotective [164], and other effects. Lycopsamine exerted protective effects in spinal cord injury in rats by improving functional recovery and suppressing apoptosis [165]. Lupeol, a triterpene, found in this plant has several bioactivities, including antidiabetic, antiinflammatory, antioxidant [166, 167], skin protective [168], anticancer [149], and so on.
9. Conclusion and Future Directions
Medicinal plants and traditional medicine comprise about 90% of newly discovered pharmaceuticals, thus ensuring the safety, quality, and effectiveness of medicinal plants and herbal drugs that have gained much attention nowadays [169, 170]. Numerous results of experiments developed by researchers around the world support the biological activities associated with the traditional uses of H. indicum. In this sense, it can be concluded that H. indicum is a potential source of chemical compounds with promising biological activities. However, nowadays, clinical trials are scarce, which makes it difficult to translate them into routine clinical practice, making it necessary to carry out additional studies. In addition, several pyrrolizidine alkaloids isolated from the plant have been evident to show hepatotoxic effects on experimental animals; hence, further studies are required to ensure the safety of internal use of this plant. We hope that the information provided here could be helpful for the safe traditional uses and beneficial for further research.
The use of plant extracts in experimentation involves many drawbacks, including changes in their constituents depending on the climate or form of cultivation, presence of compounds with adverse or antagonistic effects, or changes in bioactivity during their handling, storage, or preparation of materials. Thus, working with pure compounds with known bioactivity makes it possible to obtain a targeted therapeutic effect and determine effective doses, toxic doses, and selectivity indexes to control the quality of the therapeutic formulation [171]. In addition, working with isolated compounds will reduce the risk of infections in the plant that could end up affecting patients and the presence of contaminants such as heavy metals [172].
Loss of medicinal plant species over time is another challenge for us. Among 80,000 flowering plant species that are used for pharmaceutical purposes, about 15,000 species are exposed to a risk of extinction due to high harvesting and destruction of habitats [173], and 20% of their wildlife resources are decreasing due to growing human populations and excessive consumption of plants [174]. Thus, the environmental code of ethics should be strictly followed to preserve the biodiversity of medicinal plants [175]. The good agricultural practice may be helpful for the production and quality assurance of medicinal plants [176]. For example, China has promoted the growth of conventional medicinal plants [177].
Nowadays, many people believe that using herbal medicines is good for health, but there are still many concerns about its safety and efficacy. The ethnobotanical record of H. indicum indicates that this plant is used in many countries around the world for various diseases. Upon going through the scientific reports on this plant, it should be claimed that H. indicum contains many important phytochemicals and possesses diverse biological activities, suggesting it as an important medicinal plant. More studies are necessary on its phytochemical analysis. Furthermore, the biological activities evaluated on its phytoconstituents are not sufficient. Although H. indicum can potentially contribute to the advancement of health care, to date, only a few studies have been conducted on its isolated constituents, limiting its translation to clinical practice. Another factor that hinders its clinical use is the presence of some components, such as heliotrine, lasiocarpine, and retrorsine, with evidence of toxic effects on experimental animals or human-derived cells. In addition, to build credibility for the use of this medicinal plant in conventional medicine, the empirical arguments should be converted into evidence-based arguments. Finally, several issues about safety, effective dosing, treatment duration, side or adverse effects, acute and chronic toxicities, as well as the standardization of H. indicum herbal preparations and phytoconstituent products should be resolved properly by conducting adequate research on this hopeful medicinal plant. If these issues are properly resolved, this medicinal plant can be used as a safe, effective, and affordable form of health care.
Acknowledgments
This work was supported by CONICYT PIA/APOYO CCTE AFB170007. A. Sureda was granted by the Instituto de Salud Carlos III (CIBEROBN CB12/03/30038).
Abbreviations
- AA:
Ascorbic acid
- BHT:
Butylated hydroxytoluene
- DPPH:
1-Diphenyl-2 picrylhydrazyl
- EC50:
Half-maximal effective concentration
- GC-FID:
Gas chromatography–flame ionization detector
- GC-MS:
Gas chromatography–mass spectrometry
- IC50:
Half-maximal inhibitory concentration
- LPS:
Lipopolysaccharide
- MIC:
Minimum inhibitory concentration
- mTOR:
Mammalian target of rapamycin
- NMR:
Nuclear magnetic resonance
- RBC:
Red blood cell
- STZ:
Streptozotocin
- VO:
Vocal order
- WHO:
World Health Organization.
Contributor Information
Muhammad Torequl Islam, Email: dmt.islam@bsmrstu.edu.bd.
Miquel Martorell, Email: martorellpons@gmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Mukherjee P. K., Venkatesh P., Ponnusankar S. Ethnopharmacology and integrative medicine-let the history tell the future. Journal of Ayurveda and Integrative Medicine. 2010;1(2):100–109. doi: 10.4103/0975-9476.65077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calixto J. B., Santos A. R., Cechinel Filho V., Yunes R. A. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Medicinal Research Reviews. 1998;18(4):225–258. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi-Rad M., Lankatillake C., Dias D. A., et al. Impact of natural compounds on neurodegenerative disorders: from preclinical to pharmacotherapeutics. Journal of Clinical Medicine. 2020;9(4):p. 1061. doi: 10.3390/jcm9041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabricant D. S., Farnsworth N. R. The value of plants used in traditional medicine for drug discovery. Environmental Health Perspectives. 2001;109(1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salehi B., Calina D., Docea A. O., et al. Curcumin’s nanomedicine formulations for therapeutic application in neurological diseases. Journal of Clinical Medicine. 2020;9(2):p. 430. doi: 10.3390/jcm9020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oluwatoyin S., Ndukwe G. I., Joseph A. Phytochemical and antimicrobial studies on the aerial parts of Heliotropium indicum Linn. Annals of Biological Research. 2011;2(2):129–136. [Google Scholar]
- 7.Rahman M. A., Mia M., Shahid I. Pharmacological and phytochemical screen activities of roots of Heliotropium indicum Linn. PharmacologyOnLine. 2011;1(1):185–192. [Google Scholar]
- 8.Osungunna M. O., Adedeji K. A. Phytochemical and antimicrobial screening of methanol extract of Heliotropium indicum leaf. Journal of Microbiology and Antimicrobials. 2016;3(8):213–216. [Google Scholar]
- 9.Togola A., Diallo D., Dembélé S., Barsett H., Paulsen B. S. Ethnopharmacological survey of different uses of seven medicinal plants from Mali, (West Africa) in the regions Doila, Kolokani and Siby. Journal of Ethnobiology and Ethnomedicine. 2005;1:p. 7. doi: 10.1186/1746-4269-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adelaja A. A., Ayoola M. D., Otulana J. O., Akinola O. B., Olayiwola A., Ejiwunmi A. B. Evaluation of the histo - gastroprotective and antimicrobial activities of Heliotropium indicum Linn (boraginaceae) Malaysian Journal of Medical Sciences. 2008;15(3):22–30. [PMC free article] [PubMed] [Google Scholar]
- 11.Ayyanar M., Ignacimuthu S. Herbal medicines for wound healing among tribal people in Southern India: ethnobotanical and scientific evidences. International Journal of Applied Research in Natural Products. 2009;2(3):29–42. [Google Scholar]
- 12.Nawaz A. H., Hossain M., Karim M., Khan M., Jahan R., Rahmatullah M. An ethnobotanical survey of Rajshahi district in Rajshahi division, Bangladesh. American-Eurasian Journal of Sustainable Agriculture. 2009;3(2):143–150. [Google Scholar]
- 13.Dash G. K., Abdullah M. S. A review on Heliotropium indicum L. (Boraginaceae) International Journal of Pharmaceutical Sciences and Research. 2012;4(4):p. 1253. [Google Scholar]
- 14.Chunthorng-Orn J., Dechayont B., Phuaklee P., Prajuabjinda O., Juckmeta T., Itharat A. Cytotoxic, anti-inflammatory and antioxidant activities of Heliotropium indicum extracts. Journal of the Medical Association of Thailand. 2016;99(4):S102–S109. [PubMed] [Google Scholar]
- 15.Kugelman M., Liu W. C., Axelrod M., McBride T. J., Rao K. V. Indicine-N-oxide: the antitumor principle of Heliotropium indicum. Lloydia. 2015;39(2-3):125–128. [PubMed] [Google Scholar]
- 16.Schoental R. Toxicology and carcinogenic action of pyrrolizidine alkaloids. Cancer Research. 1968;28(11):2237–2246. [PubMed] [Google Scholar]
- 17.Hartmann T., Ober D. Biosynthesis and Metabolism of Pyrrolizidine Alkaloids in Plants and Specialized Insect Herbivores. Berlin, Heidelberg, Germany: Springer; 2000. [Google Scholar]
- 18.Srinivas K., Rao M. E. B., Rao S. Anti-inflammatory activity of Heliotropium indicum Linn and Leucas aspera Spreng. in albino rats. Indian Journal of Pharmacology. 2000;32(1):37–38. [Google Scholar]
- 19.Reddy J. S., Rao P. R., Reddy M. S. Wound healing effects of Heliotropium indicum, Plumbago zeylanicum and Acalypha indica in rats. Journal of Ethnopharmacology. 2002;79(2):249–251. doi: 10.1016/s0378-8741(01)00388-9. [DOI] [PubMed] [Google Scholar]
- 20.Veda V. T., Sasi K. S., Asokan B. R., Sengottuvelu S., Jaikumar S. Anticataract activity of ethanolic extract of Heliotropium indicum leaves on galactose induced cataract in rats. International Journal of Pharmacology & Toxicology. 2016;5:18–20. [Google Scholar]
- 21.Kandemir N., Çelik A., Shah S. N., Razzaq A. Comparative micro-anatomical investigation of genus Heliotropium (Boraginaceae) found in Turkey. Flora. 2020;262 doi: 10.1016/j.flora.2019.151495.151495 [DOI] [Google Scholar]
- 22.Ghosh P., Das P., Das C., Mahapatra S., Chatterjee S. Morphological characteristics and phytopharmacological detailing of hatishur (Heliotropium indicum Linn.): a concise review. Journal of Pharmacognosy and Phytochemistry. 2018;7(5):1900–1907. [Google Scholar]
- 23.Reyes-García V. The relevance of traditional knowledge systems for ethnopharmacological research: theoretical and methodological contributions. Journal of Ethnobiology and Ethnomedicine. 2010;6:p. 32. doi: 10.1186/1746-4269-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nisar M. F., Jaleel F., Waseem M., Ismail S., Toor Y., Mujtaba Haider S. Ethno-medicinal uses of plants from district Bahawalpur, Pakistan. Current Research Journal of Biological Sciences. 2014;6(5):183–190. doi: 10.19026/crjbs.6.5191. [DOI] [Google Scholar]
- 25.Kamal Z., Bairage J., Moniruzzaman, et al. Ethnomedicinal practices of a folk medicinal practitioner in Pabna district, Bangladesh. World Journal of Pharmacy and Pharmaceutical Sciences. 2014;3(12):73–85. [Google Scholar]
- 26.Shahnaj S., Asha U., Mim T., et al. A survey on the ethnomedicinal practices of a folk medicinal practitioner in Manikganj district, Bangladesh. Journal of Chemical and Pharmaceutical Research. 2015;7(8):690–696. [Google Scholar]
- 27.Akhter J., Khatun R., Akter S., et al. Ethnomedicinal practices in Natore district, Bangladesh. World Journal of Pharmacy and Pharmaceutical Sciences. 2021;5(8):212–222. [Google Scholar]
- 28.Muthu C., Ayyanar M., Raja N., Ignacimuthu S. Medicinal plants used by traditional healers in Kancheepuram district of Tamil Nadu, India. Journal of Ethnobiology and Ethnomedicine. 2006;2:p. 43. doi: 10.1186/1746-4269-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alagesaboopathi C. Ethnomedicinal plants and their utilization by villagers in Kumaragiri hills of Salem district of Tamilnadu, India. African Journal of Traditional, Complementary and Alternative Medicines. 2009;6(3):222–227. doi: 10.4314/ajtcam.v6i3.57157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiart C. Medicinal Plants of the Asia-Pacific. Boca Raton, FL, USA: CRC Press; 2006. [Google Scholar]
- 31.Odugbemi T. O., Akinsulire O. R., Aibinu I. E., Fabeku P. O. Medicinal plants useful for malaria therapy in Okeigbo, Ondo state, Southwest Nigeria. African Journal of Traditional, Complementary and Alternative Medicines. 2007;4(2):191–198. doi: 10.4314/ajtcam.v4i2.31207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suroowan S., Pynee K. B., Mahomoodally M. F. A comprehensive review of ethnopharmacologically important medicinal plant species from Mauritius. South African Journal of Botany. 2019;122:189–213. doi: 10.1016/j.sajb.2019.03.024. [DOI] [Google Scholar]
- 33.Berhaut J. Flore illustrée du Sénégal. Gouvernement du Sénégal, Ministère du développement rural, Direction des eaux et forêts. 9 [Google Scholar]
- 34.Dey K. L. The Indigenous Drugs of India. Thacker, Spink, & Co., Calcutta, India: 1896. [Google Scholar]
- 35.Chopra R. N., Nayar S. L., Chopra I. C., et al. Glossary of Indian Medicinal Plants. New Delhi, India: Council of Scientific & Industrial Research; 1956. [Google Scholar]
- 36.Dahanukar S. A., Kulkarni R. A., Rege N. N. Pharmacology of medicinal plants and natural products. Indian Journal of Pharmacology. 2000;32(4):S81–S118. [Google Scholar]
- 37.Dash G. K., Murthy P. N. Studies on wound healing activity of Heliotropium indicum Linn. leaves on rats. ISRN Pharmacology. 2011;2011 doi: 10.5402/2011/847980.847980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adjanohoun E. Le processus de rénovation de la pharmacopée africaine. Bulletin de la Société Botanique de France Actualités Botaniques. 2014;136(3-4):35–39. doi: 10.1080/01811789.1989.10826952. [DOI] [Google Scholar]
- 39.Apema R., Mozouloua D., Kosh-Komba E., Ngoule Y. Les plantes médicinales utilisées dans le traitement de l’hypertension artérielle par les tradipraticiens à Bangui
- 40.Kalanda K., Omasombo W. D. Contribution à la connaissance des plantes médicinales du Haut Zaïre: plantes utilisées dans le traitement des maux d’estomac dans la ville de Kisangani. Revue de Médecine et de Pharmacie. 2019;9(1):59–69. [Google Scholar]
- 41.Agudelo-Lopez S., Gomez-Rodriguez L., Coronado X., et al. [Prevalence of intestinal parasitism and associated factors in a village on the Colombian Atlantic Coast] Revista de Salud Pública. 2008;10(4):633–642. doi: 10.1590/s0124-00642008000400013. [DOI] [PubMed] [Google Scholar]
- 42.Carrière M. CIRAD-EMVT; Plantes de Guinée à l’usage des éleveurs et des vétérinaires. Annexes. [Google Scholar]
- 43.Magassouba F. B., Diallo A., Kouyate M., et al. Ethnobotanical survey and antibacterial activity of some plants used in Guinean traditional medicine. Journal of Ethnopharmacology. 2007;114(1):44–53. doi: 10.1016/j.jep.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Komlaga G., Agyare C., Dickson R. A., et al. Medicinal plants and finished marketed herbal products used in the treatment of malaria in the Ashanti region, Ghana. Journal of Ethnopharmacology. 2015;172:333–346. doi: 10.1016/j.jep.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 45.Walker R. Usages pharmaceutiques des plantes spontanées du Gabon. Institut d’Études Centrafricaines. 1986;4(6):232–262. [Google Scholar]
- 46.Das A. K., Dutta B. K., Sharma G. D. Medicinal plants used by different tribes of Cachar district, Assam. Indian Journal of Traditional Knowledge. 2008;7(3):446–454. [Google Scholar]
- 47.Bouquet A. Plantes médicinales de la Côte d’Ivoire. ORSTOM. 1974;165:38–39. [Google Scholar]
- 48.Asprey G. F., Thornton P. Medicinal plants of Jamaica. Parts III. West Indian Medical Journal. 1955;4(4):69–82. [PubMed] [Google Scholar]
- 49.Daruty C. Plantes médicinales de I’lle Maurice et des pays intertropicaux. Mauritius: Mauritius Stationery and Printing Establishment; 2018. [Google Scholar]
- 50.Nordeng H., Al-Zayadi W., Diallo D., Ballo N., Paulsen B. S. Traditional medicine practitioners’ knowledge and views on treatment of pregnant women in three regions of Mali. Journal of Ethnobiology and Ethnomedicine. 9(1):p. 67. doi: 10.1186/1746-4269-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ainslie J. R. The List of Plants Used in Native Medicine in Nigeria, Imp. Vol. 7. Forest Inst Oxford Inst Paper; [Google Scholar]
- 52.Ajibesin K. K., Ekpo B. A., Bala D. N., Essien E. E., Adesanya S. A. Ethnobotanical survey of Akwa Ibom state of Nigeria. Journal of Ethnopharmacology. 2008;115(3):387–408. doi: 10.1016/j.jep.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Coe F. G., Anderson G. J. Ethnobotany of the garífuna of Eastern Nicaragua. Economic Botany. 1996;50(1):71–107. doi: 10.1007/BF02862114. [DOI] [Google Scholar]
- 54.Barrett B. Medicinal plants of Nicaragua’s atlantic coast. Economic Botany. 1994;48(1):8–20. [Google Scholar]
- 55.Quisumbing E. Medicinal plants of the Philippines. Tech Bull. 16:p. 126. [Google Scholar]
- 56.Gurib-Fakim A., Gueho J., Sewraj-Bissoondoyal M. The medicinal plants of Mauritius–part 1. International Journal of Pharmacognosy. 2008;35(4):237–254. doi: 10.1076/phbi.35.4.237.13313. [DOI] [Google Scholar]
- 57.Samoisy A. K., Mahomoodally M. F. Ethnopharmacological analysis of medicinal plants used against non-communicable diseases in Rodrigues Island, Indian Ocean. Journal of Ethnopharmacology. 2015;173:20–38. doi: 10.1016/j.jep.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 58.Adjanohoun E. J., Abel A., Aké Assi L., et al. Paris, France: Agence de Coopération Culturelle et Technique; Médecine traditionelle et pharmacopée-contribution aux études ethnobotaniques et floristiques aux Seychelles; p. p. 170. [Google Scholar]
- 59.Kerharo J., Adam J. G. La pharmacopée sénégalaise traditionnelle: plantes médicinales et toxiques. Editions Vigot Frères. 1974;1011 [Google Scholar]
- 60.Berhault J. Floore Illustree du Senegal. Govt Senegal, Min Rural Development, Water and Forest Division, Dakar. 2:110–114. [Google Scholar]
- 61.Sequeira V. Medicinal plants and conservation in São Tomé. Biodiversity & Conservation. 3(9):910–926. [Google Scholar]
- 62.Duke J. A. Amazonia Ethnobotanical Dictionary. Boca Raton, FL, USA: CRC Press; 1994. [Google Scholar]
- 63.Kpodar M. S., Karou S. D., Katawa G., et al. An ethnobotanical study of plants used to treat liver diseases in the maritime region of Togo. Journal of Ethnopharmacology. 2015;181:263–273. doi: 10.1016/j.jep.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 64.Kokwaro J. O. Medicinal plants of east Africa. East African Literature Bureau. 1976:p. 232. [Google Scholar]
- 65.Lin C. C., Kan W. S. Medicinal plants used for the treatment of hepatitis in Taiwan. The American Journal of Chinese Medicine. 1990;18(1-2):35–43. doi: 10.1142/S0192415X9000006X. [DOI] [PubMed] [Google Scholar]
- 66.Ayensu E. S. Medicinal Plants of the West Indies. Algonac, MI, USA: Reference Publications, Inc; 1981. [Google Scholar]
- 67.Williaman J. J., Schubert B. G. Alkaloid-bearing plants and their contained alkaloids. Technical Bulletin No. 1234. Journal of Pharmaceutical Sciences. 1962;51(3):p. 296. [Google Scholar]
- 68.Mattocks A. R., Schoental R., Crowley H. C., Culvenor C. C. J. Indicine: the major alkaloid of Heliotropium indicum L. Journal of the Chemical Society. 1961;1961:5400–5403. [Google Scholar]
- 69.Hoque M. S., Ghani A., Rashid H. Alkaloids of Heliotropium indicum L. grown in Bangladesh. Bangladesh Pharmaceutical Journal. 1976;5:13–15. [Google Scholar]
- 70.Pandey V. B., Singh J. P., Rao Y. V., Acharya S. B. Isolation and pharmacological action of heliotrine, the major alkaloid of Heliotropium indicum seeds. Planta Medica. 1982;45(4):229–233. doi: 10.1055/s-2007-971378. [DOI] [PubMed] [Google Scholar]
- 71.Pandey D. P., Singh J. P., Roy R., Singh V. P., Pandey V. B. Constituents of Heliotropium indicum. Oriental Journal of Chemistry. 1996;12:321–322. [Google Scholar]
- 72.Sivagnanam S., Singh M. K., Satish M. K., Rao M. R. K. Preliminary phytochemical analysis of Amaranthus polygonoides. Research Journal of Pharmaceutical, Biological, and Chemical Sciences. 2014;5(3):p. 82. [Google Scholar]
- 73.Mattocks A. R. Minor alkaloids of Heliotropium indicum L. Journal of the Chemical Society C: Organic. 1967:329–331. doi: 10.1039/j39670000329. [DOI] [PubMed] [Google Scholar]
- 74.Birecka H., Frohlich M. W., Glickman L. M. Free and esterified necines in Heliotropium species from Mexico and Texas. Phytochemistry. 1983;22(5):1167–1171. doi: 10.1016/0031-9422(83)80214-3. [DOI] [Google Scholar]
- 75.Souza J. S. N., Machado L. L., Pessoa O. D. L., et al. Pyrrolizidine alkaloids from Heliotropium indicum. Journal of the Brazilian Chemical Society. 2005;16(6B):1410–1414. doi: 10.1590/S0103-50532005000800019. [DOI] [Google Scholar]
- 76.Birecka H., DiNolfo T. E., Martin W. B., Frohlich M. W. Polyamines and leaf senescence in pyrrolizidine alkaloid-bearing Heliotropium plants. Phytochemistry. 1984;23(5):991–997. doi: 10.1016/S0031-9422(00)82598-4. [DOI] [Google Scholar]
- 77.Andhiwal C. K., Has C., Varshney R. P. Chemical and pharmacological studies of Heliotropium indicum. Indian Drugs. 2013;22(11):567–569. [Google Scholar]
- 78.Mannan A., Ahmad K. Preliminary study of sex hormones of medical importance in Bangladeshi plants. Bangladesh Medical Research Council Bulletin. 1978;4(2):78–85. [PubMed] [Google Scholar]
- 79.Machan T., Korth J., Liawruangrath B., Liaewruangrath S., Pyne S. Composition and antituberculosis activity of the volatile oil of Heliotropium indicum Linn. growing in Phitsanulok, Thailand. Flavour and Fragrance Journal. 2005;21(2):265–267. doi: 10.1002/ffj.1577. [DOI] [Google Scholar]
- 80.Kumar M. S., Chaudhury S., Balachandran S. In vitro callus culture of Heliotropium indicum Linn. for assessment of total phenolic and flavonoid content and antioxidant activity. Applied Biochemistry and Biotechnology. 2014;174(8):2897–2909. doi: 10.1007/s12010-014-1235-1. [DOI] [PubMed] [Google Scholar]
- 81.Kumar M., Kumar S., Balachandran S., Chaudhury S. Influence of incubation temperatures on total phenolic, flavonoids content and free radical scavenging activity of callus from Heliotropium indicum L. Asian Journal of Pharmaceutical Research. 2012;2(4):2231–5683. [Google Scholar]
- 82.Boye A., Koffuor G. A., Amoateng P., Ameyaw E. O., Abaitey A. K. Analgesic activity and safety assessment of Heliotropium indicum Linn. (Boraginaceae) in rodents. International Journal of Pharmacology. 2012;8(2):91–100. doi: 10.3923/ijp.2012.91.100. [DOI] [Google Scholar]
- 83.Betanabhatla K. S., Jasmin S. R., Raamamurthy J., Christina A. J., Sasikumar S. Anti-inflammatory and anyinociceptive activities of Heliotropium indicum Linn. experimental animal models. PharmacologyOnLine. 2007;3:438–445. [Google Scholar]
- 84.Shalini S., Kaza R., Shaik F. Study on the anti-inflammatory activity of Heliotropium indicum. Journal of Innovative Trends in Pharmaceutical Sciences. 2010;1(1):p. 43. [Google Scholar]
- 85.Kyei S., Koffuor G. A., Ramkissoon P., Ameyaw E. O., Asiamah E. A. Anti-inflammatory effect of Heliotropium indicum Linn on lipopolysaccharide-induced uveitis in New Zealand white rabbits. International Journal of Ophthalmology. 2016;9(4):528–535. doi: 10.18240/ijo.2016.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rao P. R., Nammi S., Raju A. D. V. Studies on the antimicrobial activity of Heliotropium indicum Linn. Journal of Natural Remedies. 2002;2(2):195–198. [Google Scholar]
- 87.Premnath D., Gomez P. Antifungal and anti bacterial activities of chemical constituents from Heliotropium indicum Linn. Plant. Drug Invention Today. 2012;4(11):564–568. [Google Scholar]
- 88.Mourin N. A., Sharmin T., Chowdhury S. R., Islam F., Rahman M. S., Rashid M. A. Evaluation of bioactivities of Heliotropium indicum, a medicinal plant of Bangladesh. Pharma Innovation. 2013;2:217–221. [Google Scholar]
- 89.Mohammad S. A., Abdul Nabi S., Marella S., et al. Phytochemical screening and antihyperglycemic activity of Heliotropium indicum whole plant in streptozotocin induced diabetic rats. Journal of Applied Pharmaceutical Science. 2015;4(12):65–71. doi: 10.7324/JAPS.2014.41212. [DOI] [Google Scholar]
- 90.Kyei S., Koffuor G. A., Ramkissoon P., Afari C., Asiamah E. A. The claim of anti-cataract potential of Heliotropium indicum: a myth or reality? Ophthalmology and Therapy. 2015;4(2):115–128. doi: 10.1007/s40123-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goyal N., Sharma S. Bioactive phytoconstituents and plant extracts from genus Heliotropium. International Journal of Green Pharmacy. 2014;8(4):217–225. doi: 10.4103/0973-8258.142674. [DOI] [Google Scholar]
- 92.Savadi R. V., Alagawadi K. R., Darade S. S. Antifertility activity of ethanolic extract and its n-hexane and benzene fractions of Heliotropium indicum leaves on albino rats. Journal of Pharmacy Research. 2009;2(5):927–930. [Google Scholar]
- 93.Mahato K., Kakoti B. B., Borah S., Kumar M. Evaluation of in-vitro anthelmintic activity of Heliotropium indicum Linn. leaves in Indian adult earthworm. Asian Pacific Journal of Tropical Disease. 2014;4:S259–S262. doi: 10.1016/S2222-1808(14)60451-5. [DOI] [Google Scholar]
- 94.Sivajothi V., Shruthi D. S., Sajini J. R. Cytotoxic effect of Heliotropium indicum extracts on Hela cell line. International Journal of Pharmacy and Pharmaceutical Sciences. 2015;7(6):412–414. [Google Scholar]
- 95.Villa M. H., Peria J. N. T., Mangansat N. J. M., Dulay R. M. R. Antitussive and antibacterial activity of Trompang elepante (Heliotropium indicum Linn.) Asian Journal of Plant Science and Research. 2016;6(1):30–34. [Google Scholar]
- 96.Dodehe Y., Barthelemy A., Calixte B., Jean D. N., Allico J. D., Nelly F. In vitro wound healing effect of n-butanol fractions from Heliotropium indicum. Just-in-Time Production Systems. 2013;2:1–7. [Google Scholar]
- 97.Vieira J. E. V., Barros G. S. G., Medeiros M. C., Matos F. J. A., Souza M. P., Medeiros M. J. Pharmacologic screening of plants from Northeast Brazil. II. Revista brasileira de farmácia. 1972;49:67–75. [Google Scholar]
- 98.Koffuor G. A., Boye A., Amoateng P., Ameyaw E. O., Abaitey A. K. Investigating the site of action of an aqueous extract of Heliotropium indicum Linn (Boraginaceae) on smooth muscles. Research Journal of Pharmacology. 2012;6(1):12–19. doi: 10.3923/rjpharm.2012.12.19. [DOI] [Google Scholar]
- 99.Samira K., Laboni F. R., Julie A. S., Jalal U., Labu Z. K. Biological investigations of medicinal plants of Heliotropium indicum indigenous to Bangladesh. Journal of Coastal Life Medicine. 2016;4(11):874–878. doi: 10.12980/jclm.4.2016J6-175. [DOI] [Google Scholar]
- 100.Ramamurthy V., Krishnaveni S. Larvicidal efficacy of leaf extracts of Heliotropium Indicum and Mukia maderaspatana against the dengue fever mosquito vector Aedes aegypti. Journal of Entomology and Zoology Studies. 2014;2:40–45. [Google Scholar]
- 101.Roy A. Pharmacological activities of Indian Heliotrope (Heliotropium indicum L.): a review. Journal of Pharmacognosy and Phytochemistry. 2015;4(3):101–104. [Google Scholar]
- 102.Pianowski L. F., Calixto J. B., Chaves C. P. Pharmaceutical Oral Product Obtained from Parts of Heliotropium Plants. 2011. [Google Scholar]
- 103.Bero J., Ganfon H., Jonville M. C., et al. In vitro antiplasmodial activity of plants used in Benin in traditional medicine to treat malaria. Journal of Ethnopharmacology. 2009;122(3):439–444. doi: 10.1016/j.jep.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 104.Ohnuma T., Sridhar K. S., Ratner L. H., Holland J. F. Phase I study of indicine N-oxide in patients with advanced cancer. Cancer Treatment Reviews. 1982;66(7):1509–1515. [PubMed] [Google Scholar]
- 105.Bose A., Mondal S., Gupta J., Dash G., Ghosh T., Si S. Studies on diuretic and laxative activity of ethanolic extract and its fractions of Cleome rutidosperma aerial parts. Pharmacognosy Magazine. 2006;2(7):178–182. [Google Scholar]
- 106.Veerakumar K., Govindarajan M., Rajeswary M., Muthukumaran U. Mosquito larvicidal properties of silver nanoparticles synthesized using Heliotropium indicum (Boraginaceae) against Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus (Diptera: Culicidae) Parasitology Research. 2014;113(6):2363–2373. doi: 10.1007/s00436-014-3895-8. [DOI] [PubMed] [Google Scholar]
- 107.Barros G. S., Matos F. J., Vieira J. E., Sousa M. P., Medeiros M. C. Pharmacological screening of some Brazilian plants. Journal of Pharmacy and Pharmacology. 1970;22(2):116–122. doi: 10.1111/j.2042-7158.1970.tb08403.x. [DOI] [PubMed] [Google Scholar]
- 108.Kumarasamyraja D., Jeganathan N. S., Manavalan R. A review on medicinal plants with potential wound healing activity. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;2:105–111. [Google Scholar]
- 109.Christie G. S., Le Page R. N. Liver damage in acute heliotrine poisoning. 1. The intracellular distribution of pyridine nucleotides. Biochemical Journal. 1962;84(1):25–38. doi: 10.1042/bj0840025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rao M. S., Reddy J. K. Malignant neoplasms in rats fed lasiocarpine. British Journal of Cancer. 1978;37(2):289–293. doi: 10.1038/bjc.1978.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Armstrong S. J., Zuckerman A. J. The effects of lasiocarpine, retrorsine and retronecine pyrrole on human embryo lung and liver cells in culture. British Journal of Experimental Pathology. 1972;53(2):138–144. [PMC free article] [PubMed] [Google Scholar]
- 112.Owolabi M. A., Oribayo O. O., Ukpo G. E., Mbaka G. O., Akindehin O. E. A 5-month toxicity study of the ethanol extract of the leaves of Heliotropium indicum in Sprague Dawley rats after oral administration. Nigerian Quarterly Journal of Hospital Medicine. 2015;25(3):184–192. [PubMed] [Google Scholar]
- 113.Mattocks A. R. Toxicity of pyrrolizidine alkaloids. Nature. 1968;217(5130):723–728. doi: 10.1038/217723a0. [DOI] [PubMed] [Google Scholar]
- 114.Moreira R., Pereira D. M., Valentao P., Andrade P. B. Pyrrolizidine alkaloids: chemistry, pharmacology, toxicology and food safety. International Journal of Molecular Sciences. 2018;19(6):p. 1668. doi: 10.3390/ijms19061668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao Y., Xia Q., Yin J. J., Lin G., Fu P. P. Photoirradiation of dehydropyrrolizidine alkaloids--formation of reactive oxygen species and induction of lipid peroxidation. Toxicology Letters. 2011;205(3):302–309. doi: 10.1016/j.toxlet.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 116.Huxtable R. J. Human health implications of pyrrolizidine alkaloids and herbs containing them. Toxicants of Plant Origin. 1989;1:41–86. [Google Scholar]
- 117.Appadurai P., Rathinasamy K. Indicine N-oxide binds to tubulin at a distinct site and inhibits the assembly of microtubules: a mechanism for its cytotoxic activity. Toxicology Letters. 2013;225(1):66–77. doi: 10.1016/j.toxlet.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 118.Cook B. A., Sinnhuber J. R., Thomas P. J., et al. Hepatic failure secondary to indicine N-oxide toxicity. A pediatric oncology group study. Cancer. 1983;52(1):61–63. doi: 10.1002/1097-0142(19830701)52:1<61::aid-cncr2820520112>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 119.Letendre L., Ludwig J., Perrault J., Smithson W. A., Kovach J. S. Hepatocellular toxicity during the treatment of refractory acute leukemia with indicine N-oxide. Cancer. 1984;54(7):1256–1259. doi: 10.1002/1097-0142(19841001)54:7<1256::aid-cncr2820540704>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 120.Santos F. A., Frota J. T., Arruda B. R., et al. Antihyperglycemic and hypolipidemic effects of alpha, beta-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids in Health and Disease. 2012;11:p. 98. doi: 10.1186/1476-511X-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Letters. 2009;285(2):109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pardo Andreu G. L., Reis F. Z. D., González-Durruthy M., et al. Rapanone, a naturally occurring benzoquinone, inhibits mitochondrial respiration and induces HepG2 cell death. Toxicology in Vitro. 2020;63 doi: 10.1016/j.tiv.2019.104737.104737 [DOI] [PubMed] [Google Scholar]
- 123.Thirupathi A., Silveira P. C., Nesi R. T., Pinho R. A. β-Amyrin, a pentacyclic triterpene, exhibits anti-fibrotic, anti-inflammatory, and anti-apoptotic effects on dimethyl nitrosamine-induced hepatic fibrosis in male rats. Human & Experimental Toxicology. 2016;36(2):113–122. doi: 10.1177/0960327116638727. [DOI] [PubMed] [Google Scholar]
- 124.Calle J., Olarte J., Pinzon R., Ospina L. F., Mendoza M. C., Orozco M. J. Alterations in the reproduction of mice induced by rapanone. Journal of Ethnopharmacology. 2000;71(3):521–525. doi: 10.1016/s0378-8741(99)00214-7. [DOI] [PubMed] [Google Scholar]
- 125.Wróbel-Biedrawa D., Grabowska K., Galanty A., Sobolewska D., Żmudzki P., Podolak I. Anti-melanoma potential of two benzoquinone homologues embelin and rapanone-a comparative in vitro study. Toxicology in Vitro. 2020;65 doi: 10.1016/j.tiv.2020.104826.104826 [DOI] [PubMed] [Google Scholar]
- 126.Hartmann M.-A. 5 Sterol metabolism and functions in higher plants. In: Daum G., editor. Lipid Metabolism and Membrane Biogenesis. Berlin, Heidelberg, Germany: Springer; 2004. [Google Scholar]
- 127.Ferrer A., Altabella T., Arro M., Boronat A. Emerging roles for conjugated sterols in plants. Progress in Lipid Research. 2017;67:27–37. doi: 10.1016/j.plipres.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 128.Stillwell W. An Introduction to Biological Membranes. 2nd. Berlin, Heidelberg, Germany: Elsevier; 2016. [Google Scholar]
- 129.Plat J., Baumgartner S., Vanmierlo T., et al. Plant-based sterols and stanols in health & disease: consequences of human development in a plant-based environment? Progress in Lipid Research. 2019;74:87–102. doi: 10.1016/j.plipres.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 130.Wang X., Wu G., Bazer F. W. Molecules to Medicine with mTOR. Cambridge, MA USA: Academic Press; 2016. mTOR: the master regulator of conceptus development in response to uterine histotroph during pregnancy in ungulates. [Google Scholar]
- 131.Minois N., Rockenfeller P., Smith T. K., Carmona-Gutierrez D. Spermidine feeding decreases age-related locomotor activity loss and induces changes in lipid composition. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102435.e102435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grootaert M. O. J., Kurdi A., De Munck D. G., Martinet W., De Meyer G. R. Y. Autophagy in atherosclerosis. In: Hayat M. A., editor. Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Cambridge, MA, USA: Academic Press; 2016. [Google Scholar]
- 133.Aliwaini S., Bleloch J., Kimani S., Prince S. Induction of autophagy and apoptosis in melanoma treated with palladacycle complexes. In: Hayat M. A., editor. Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Cambridge, MA, USA: Academic Press; 2016. [Google Scholar]
- 134.Abdelaziz H. M., Freag M. S., Elzoghby A. O. Solid lipid nanoparticle-based drug delivery for lung cancer. In: Kesharwani P., editor. Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer. Cambridge, MA, USA: Academic Press; 2019. [Google Scholar]
- 135.Mughal M. H. Linalool: a mechanistic treatise. Journal of Nutrition, Food Research and Technology. 2019;2(1):1–5. doi: 10.30881/jnfrt.00014. [DOI] [Google Scholar]
- 136.Silva R. O., Sousa F. B., Damasceno S. R., et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundamental & Clinical Pharmacology. 2014;28(4):455–464. doi: 10.1111/fcp.12049. [DOI] [PubMed] [Google Scholar]
- 137.Santos C. C., Salvadori M. S., Mota V. G., et al. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Journal of Neuroscience. 2013;2013 doi: 10.1155/2013/949452.949452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alencar M., Islam M. T., Ali E. S., et al. Association of phytol with toxic and cytotoxic activities in an antitumoral perspective: a meta-analysis and systemic review. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(13):1828–1837. doi: 10.2174/1871520618666180821113830. [DOI] [PubMed] [Google Scholar]
- 139.Islam M. T., Ali E. S., Uddin S. J., et al. Phytol: a review of biomedical activities. Food and Chemical Toxicology. 2018;121:82–94. doi: 10.1016/j.fct.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 140.Islam M. T., de Alencar M. V., da Conceicao Machado K., et al. Phytol in a pharma-medico-stance. Chemico-Biological Interactions. 2015;240:60–73. doi: 10.1016/j.cbi.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 141.Islam M. T. Antipyretic effect of phytol, possibly via 5KIR-dependent COX-2 inhibition pathway. Inflammopharmacology. 2019;27(4):857–862. doi: 10.1007/s10787-019-00574-9. [DOI] [PubMed] [Google Scholar]
- 142.Islam M. T., Molla S., Das A. K., Zaman F., Khan R. In vitro anti-atherothrombosis activity of Nigella sativa oil, phytol and their combinations. Indian Journal of Pharmaceutical Education and Research. 2019;53(4):670–674. doi: 10.5530/ijper.53.4.129. [DOI] [Google Scholar]
- 143.Blair M. Diabetes mellitus review. Urologic Nursing. 2016;36(1):27–36. [PubMed] [Google Scholar]
- 144.Hashmi W. J., Ismail H., Mehmood F., Mirza B. Neuroprotective, antidiabetic and antioxidant effect of Hedera nepalensis and lupeol against STZ + AlCl3 induced rats model. Daru. 2018;26(2):179–190. doi: 10.1007/s40199-018-0223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang J., Huang M., Yang J., et al. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food & Nutrition Research. 2017;61(1) doi: 10.1080/16546628.2017.1364117.1364117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kuete V., Omosa L. K., Tala V. R., et al. Cytotoxicity of plumbagin, rapanone and 12 other naturally occurring quinones from Kenyan flora towards human carcinoma cells. BMC Pharmacology and Toxicology. 2016;17(1):p. 60. doi: 10.1186/s40360-016-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Le G., Novotny S. A., Mader T. L., et al. A moderate oestradiol level enhances neutrophil number and activity in muscle after traumatic injury but strength recovery is accelerated. The Journal of Physiology. 2018;596(19):4665–4680. doi: 10.1113/JP276432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Antwi A. O., Obiri D. D., Osafo N., Essel L. B., Forkuo A. D., Atobiga C. Stigmasterol alleviates cutaneous allergic responses in rodents. BioMed Research International. 2018;2018 doi: 10.1155/2018/3984068.3984068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kangsamaksin T., Chaithongyot S., Wootthichairangsan C., Hanchaina R., Tangshewinsirikul C., Svasti J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-alpha. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189628.e0189628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Adebiyi O. E., Olopade J. O., Olayemi F. O. Sodium metavanadate induced cognitive decline, behavioral impairments, oxidative stress and down regulation of myelin basic protein in mice hippocampus: ameliorative roles of beta-spinasterol, and stigmasterol. Brain and Behavior. 2018;8(7) doi: 10.1002/brb3.1014.e01014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao C. H., Zhao C., Ye H. Q., et al. Hypolipidemic activity of low-cholesterol ovum oil of Rana chensinensis and phytosterol (stigmasterol) in rats. Journal of Zhejiang University Science B. 2019;20(7):613–616. doi: 10.1631/jzus.B1900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu W., Li L., Sun J., et al. Putrescine delays postovulatory aging of mouse oocytes by upregulating PDK4 expression and improving mitochondrial activity. Aging. 2018;10(12):4093–4106. doi: 10.18632/aging.101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.El-Halfawy O. M., Valvano M. A. Putrescine reduces antibiotic-induced oxidative stress as a mechanism of modulation of antibiotic resistance in Burkholderia cenocepacia. Antimicrobial Agents and Chemotherapy. 2014;58(7):4162–4171. doi: 10.1128/AAC.02649-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Q., Zheng C., Cao J., et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death & Differentiation. 2016;23(11):1850–1861. doi: 10.1038/cdd.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ohashi K., Kageyama M., Shinomiya K., et al. Spermidine oxidation-mediated degeneration of retinal pigment epithelium in rats. Oxidative Medicine and Cellular Longevity. 2017;2017 doi: 10.1155/2017/4128061.4128061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eisenberg T., Abdellatif M., Schroeder S., et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nature Medicine. 2016;22(12):1428–1438. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang Y., Chen S., Zhang Y., et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated beclin 1 cleavage. Cell Death & Disease. 2017;8(4):p. e2738. doi: 10.1038/cddis.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tabor C. W., Tabor H. Polyamines. Annual Review of Biochemistry. 1984;53(1):749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 159.Rolen S. H., Sorensen P. W., Mattson D., Caprio J. Polyamines as olfactory stimuli in the goldfish Carassius auratus. The Journal of Experimental Biology. 2003;206(10):1683–1696. doi: 10.1242/jeb.00338. [DOI] [PubMed] [Google Scholar]
- 160.Lefevre P. L., Palin M. F., Murphy B. D. Polyamines on the reproductive landscape. Endocrine Reviews. 32(5):694–712. doi: 10.1210/er.2011-0012. [DOI] [PubMed] [Google Scholar]
- 161.Scott A. M., Zhang Z., Jia L., et al. Spermine in semen of male sea lamprey acts as a sex pheromone. PLoS Biology. 2019;17(7) doi: 10.1371/journal.pbio.3000332.e3000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang M., Caragine T., Wang H., et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. Journal of Experimental Medicine. 1997;185(10):1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Christian B. E., Haque M. E., Spremulli L. L. The effect of spermine on the initiation of mitochondrial protein synthesis. Biochemical and Biophysical Research Communications. 2010;391(1):942–946. doi: 10.1016/j.bbrc.2009.11.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wei C., Li H., Wang Y., et al. Exogenous spermine inhibits hypoxia/ischemia-induced myocardial apoptosis via regulation of mitochondrial permeability transition pore and associated pathways. Experimental Biology and Medicine. 2016;241(14):1505–1515. doi: 10.1177/1535370216643417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jin J., Li H., Zhao G., Jiang S. Lycopsamine exerts protective effects and improves functional outcome after spinal cord injury in rats by suppressing cell death. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2018;24:7444–7450. doi: 10.12659/MSM.912978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Geetha T., Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. Journal of Ethnopharmacology. 2001;76(1):77–80. doi: 10.1016/S0378-8741(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 167.Prasad S., Kalra N., Shukla Y. Hepatoprotective effects of lupeol and mango pulp extract of carcinogen induced alteration in Swiss albino mice. Molecular Nutrition & Food Research. 2007;51(3):352–359. doi: 10.1002/mnfr.200600113. [DOI] [PubMed] [Google Scholar]
- 168.Malinowska M., Miroslaw B., Sikora E., et al. New lupeol esters as active substances in the treatment of skin damage. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0214216.e0214216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jamshidi-Kia F., Lorigooini Z., Amini-Khoei H. Medicinal plants: past history and future perspective. Journal of Herbmed Pharmacology. 2018;7(1):1–7. doi: 10.15171/jhp.2018.01. [DOI] [Google Scholar]
- 170.Sarker S. D., Nahar L. Chemistry for Pharmacy Students: General, Organic, and Natural Product Chemistry. Hoboken, NJ, USA: John Wiley Sons Inc; 2007. [Google Scholar]
- 171.Zhang H. Bioactive natural products: detection, isolation, and structural determination. Phytomedicine. 2008;18(10):902–903. [Google Scholar]
- 172.Clark A. M. Natural products as a resource for new drugs. Pharmaceutical Research. 1996;13(8):1133–1141. doi: 10.1023/a:1016091631721. [DOI] [PubMed] [Google Scholar]
- 173.Bentley R. E. Medicinal Plants. London, UK: Domville-Fife Press; 2018. [Google Scholar]
- 174.Ross I. A. Constituents, Medicinal Plants of the World (Volume 3): Chemical Traditional and Modern Medicinal Uses. Totowa, NJ, USA: Humana Press; 1999. [Google Scholar]
- 175.Pan S.-Y., Zhou S.-F., Gao S.-H., et al. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/627375.627375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chan K., Shaw D., Simmonds M. S., et al. Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. Journal of Ethnopharmacology. 2012;140(3):469–475. doi: 10.1016/j.jep.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 177.Ma J., Rong K., Cheng K. Research and practice on biodiversity in situ conservation in China: pro-gress and prospect. Biodiversity Science. 2012;20(5):551–558. doi: 10.3724/SP.J.1003.2012.08118. [DOI] [Google Scholar]


