Abstract
A need exists for local (ie, bladder-specific) interventions to treat overactive bladder (OAB) with low risk of unwanted postprocedural outcomes. Gene therapy targeted to leverage endogenous physiology in bladder cells may assist in restoring normal cell and organ function. Herein, we review the potential promise of gene therapy for treating OAB, focusing on gene transfer of URO-902, a non-viral naked plasmid DNA expressing the big potassium (BK) channel. We searched PubMed for articles concerning functional aspects of the BK channel and its potential use for gene transfer as local OAB treatment. Results from preclinical, phase 1, and phase 2 studies of URO-902 for erectile dysfunction and phase 1 studies of URO-902 for OAB are included. The BK channel has been extensively studied; however, URO-902 is the first gene therapy used in clinical trials directed toward treating OAB via the BK channel. In both URO-902 studies, there were no serious adverse events considered treatment related and no adverse events leading to early withdrawal. Both studies included secondary efficacy endpoints with promising results suggesting improvement in OAB symptoms, and quality of life, with use of URO-902 versus placebo. Gene therapy involving the BK channel, such as gene transfer with URO-902, has demonstrated promising safety and efficacy results in women with OAB. Findings warrant further investigation of the use of URO-902 for OAB treatment.
Keywords: big potassium channel, gene expression, ion channels, urinary bladder
Introduction
Overactive bladder (OAB) is a disorder characterized by urgency and frequency with and without urinary incontinence. It affects 15% to 25% of the general population older than 40 years of age.1–5 Current guidelines recommend non-subtype‒selective oral antimuscarinics or β3-adrenergic receptor agonists as primary pharmacotherapy.6–8 Although these agents modulate the neurotransmitter-mediated signals that cause OAB-related symptoms,9 some, such as anticholinergics, are associated with challenging adverse effects that limit compliance, and they may not be effective in all patients.6–11 Chemodenervation agents may improve symptoms with similar or even greater efficacy compared with oral agents; however, treatment with botulinum toxin may result in unwanted post-procedural outcomes such as urinary retention and urinary tract infections. Other treatments for OAB are limited by cost and invasiveness, require general anesthesia (eg, sacral neuromodulation), or have limited efficacy and require inconvenient weekly treatment sessions (eg, percutaneous tibial nerve stimulation).12 Thus, while bladder-targeted treatments are available, there remains an unmet need for locally administered interventions to treat OAB.
The large-conductance, voltage- and calcium-activated K+ channel, known as the big potassium (BK) or Maxi-K channel, is highly expressed on urinary bladder smooth muscle cells and regulates bladder detrusor muscle function.13 BK channel activation reduces smooth muscle cell excitability, and therefore modulation of this channel’s activity by the introduction of a locally instilled plasmid expressing the BK channel is a potential novel approach to OAB treatment. This article reviews the physiological importance and regulation of the BK channel in the bladder, as well as the potential of BK channel modulation with gene therapy in the management of OAB.
Bladder Contraction
When functioning normally, the bladder has a change in internal volume from nearly zero to >400 mL during its filling cycle.14 During voluntary or involuntary voiding, the detrusor smooth muscle cells go from a state of low tension to a rapid contraction in order to empty the bladder. Detrusor smooth muscle is organized in contractile units in a syncytium connected by gap junctions that facilitate the spread of contractile function throughout the detrusor muscle tissue.15 These contractile units show spontaneous depolarizations leading to uncoordinated contractile activity that has little effect on intravesical pressure but is essential for maintaining tone of the bladder wall.16 The spontaneous activity in turn generates afferent nerve activity and the sensation of bladder filling.17,18 Increases in the spontaneous activity leading to increased afferent nerve activity have been considered important in the generation of OAB symptoms.
In order to produce an emptying contraction, coordination of the activity of the muscle units is necessary, and this is provided by excitatory input from the parasympathetic system.17 Bladder emptying contraction is initiated by a massive release of acetylcholine from the pelvic nerve, which stimulates muscarinic (M3) receptors on the detrusor smooth muscle cell, and triggers an intracellular signaling cascade that leads to simultaneous membrane depolarization of all detrusor muscle cells. This results in the opening of voltage-dependent Ca2+ channels at the cell surface and a massive influx of extracellular calcium. High intracellular Ca2+ is sensed by calmodulin, an intracellular messenger protein, which leads to the activation of myosin light chain kinase and muscle contraction.17
Relaxation of detrusor smooth muscle cells is mediated, at least in part, when K+ channels open to allow the efflux of K+ ions, and hyperpolarization of the cell membrane is produced, leading to diminished intracellular calcium levels and reduction of the spontaneous activity and thus reduced detrusor muscle tone.13 This mechanism operates during the filling phase, when OAB symptoms are experienced, and has no effect on the emptying contraction. Thus, restoration/maintenance of the endogenous ionic mechanisms that govern smooth muscle cell tone, through targeted gene therapy, is an attractive approach to the treatment of any disease or disorder characterized by altered smooth muscle cell physiology—including OAB. The BK or Maxi-K channel is the K channel subtype most responsible for creating and maintaining the cell’s resting membrane potential.
Plausible Mechanism(s) for Gene Therapy Modulation of Bladder Contraction
Gene therapy can be used to treat diseases and conditions such as OAB. Specifically, we suggest that tissue or organ-specific overexpression of key modulators of smooth muscle function can mitigate pathophysiological features of diseases as diverse as hypertension, asthma, irritable bowel, erectile dysfunction, and OAB. The explicit scientific rationale, in the case of the bladder, is driven by the supposition that targeted, locally delivered, gene therapy can address the pathophysiological changes in molecular and biochemical pathways that result in dysfunctional control of detrusor smooth muscle tone, which in turn, manifests as OAB.
In this scenario, gene therapy can be used to restore, reduce, or enhance the expression of the normal gene products that control key cellular actions.19 Aging and disease can modify “normal” gene expression, for example, by elaboration of splice variants whose downstream products alter normal cellular physiology to result in dysfunctional bladder control. The introduction of carefully selected genes into a significant fraction of affected cells can return the cells and organs to more normal physiology and function.
The bladder is notably amenable to such an approach for 3 main reasons. First, the bladder is readily accessible via the urethra for delivery of therapeutic genes to the urothelium, neural network, and/or underlying detrusor myocytes.20,21 Second, therapeutic administration is local, and thus the main part of the corresponding activity should be confined to the bladder. In fact, these 2 points have recently been demonstrated in pilot clinical studies of naked DNA gene therapies for OAB, which have produced no evidence of systemic effects.22 Third, relatively modest transfection efficiency can be therapeutically tolerated, as the bladder’s myocytes communicate with each other through intercellular channels, referred to as gap junctions. This latter mechanism leverages the important role that gap junctions play in the coordinated contractile responses mediated by the extant syncytial smooth muscle cell network to ensure complete bladder emptying upon appropriate parasympathetic activation.15,20 More specifically, although only a small proportion of myocytes might incorporate and express the therapeutic naked DNA gene, the presence of an intercellular network of gap junctions allows corrective changes in gene expression to facilitate passage of current-carrying ions and secondary messenger molecules from cell to cell throughout the bladder wall.23
BK Channel Function and Channelopathy
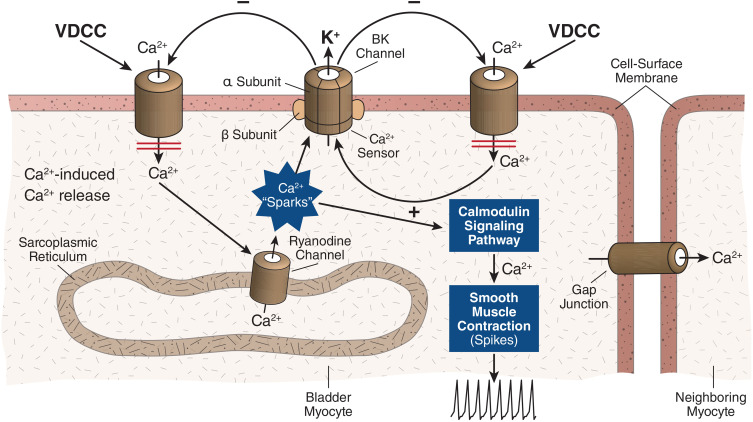
Again, it appears that the large-conductance, voltage- and calcium-activated BK (Maxi-K) channel is the K channel subtype most responsible for creating and maintaining the cell’s resting membrane potential.13 The BK channel consists of a tetramer of pore-forming α-subunits that are typically surrounded by modulatory β-subunits.24,25 The α-subunit is encoded by a single gene called Slo or KCNMA1 that is located on the long arm of chromosome 10.26 Activation by either membrane depolarization or increased intracellular Ca2+ increases the permeability of the BK channel permitting outward K+ efflux across the cell membrane down the electrochemical gradient, resulting in hyperpolarization and reduced cellular excitability (Figure 1). Since increased myogenic activity may contribute to bladder overactivity, increasing the number of BK channels (via gene transfer) has a rational scientific basis.
Figure 1.
Schematic depiction of the central role of the BK channel in regulation of myogenic tone in bladder smooth muscle. As shown, detrusor smooth muscle exhibits spontaneous myogenic contractile activity (spikes) associated with depolarizations. The depolarizations are associated with Ca2+ influx via voltage-dependent calcium channels (VDCCs). Ca2+-induced Ca2+ release from the sarcoplasmic reticulum activates BK channels via “sparks,” but BK channels are also activated via more global increases in intracellular calcium, and eventually the cell is repolarized. The repolarization closes (temporarily) the VDCC, and spike activity is reduced.
Overall, the BK channels in bladder smooth muscle act to promote detrusor relaxation and limit the amplitude and duration of spontaneous or nerve-induced detrusor contraction.13 Several lines of evidence suggest the importance of BK channels for modulating and sustaining the bladder’s resting state. In normal detrusor smooth muscle, the BK channel is expressed at high levels.27,28 In fact, the conductance of the BK channel—that is, the rate of K+ ions passing through the channel—is an order of magnitude greater than the conductance of other K+ channels.29 The BK channel is therefore unique among K+ channels in its responsiveness either to membrane depolarization or a rise in intracellular calcium, enabling it to integrate these contraction-governing signals.13 Consistent with these electrophysiological properties, in mice, for example, knockout of BK channel α-subunits is associated with increased detrusor contractility and urination frequency.30 In mouse bladder myocytes, deletion of the BK α-subunit or pharmacologic blockade of transient BK currents is sufficient to depolarize the cell membrane.31,32
Ion channel dysfunction, often referred to as channelopathy, is often associated with disorders of smooth muscle.33 In the case of the BK channel, mutations, splice variants, or low levels of channel expression in bladder myocytes would be expected to lead to increased intracellular Ca2+ levels and abnormal responsiveness to cholinergic signaling.13,34 Using detrusor smooth muscle tissue samples obtained from 33 patients during open bladder surgeries, neurogenic detrusor overactivity was associated with decreased BK channel expression and function, leading to increased detrusor smooth muscle excitability and contractility.35 BK channel dysfunction may also heighten the responsiveness of the central nervous system to sensory signals from the bladder, a phenomenon called central sensitization, which appears to occur in illnesses such as irritable bowel syndrome and fibromyalgia.36 High levels of gap junction expression would likewise be expected to make the bladder smooth muscle cells hypersensitive to cholinergic stimulation, presumably due to excessive diffusion of Ca2+ ions among detrusor coupled myocytes.15,37–39 Indeed, upregulation of connexin 43, a predominant connexin protein expressed in human bladder gap junctions, has been identified in patients with urgency incontinence.32
The use of gene therapy to treat bladder dysfunction may have intrinsic advantages over conventional pharmacotherapies when the therapeutic target is subject to epigenetic modification. It was recently demonstrated that diabetes results in epigenetic changes in the methylation pattern of the detrusor genome which are mostly, but not entirely, normalized with glycemic control.40 This phenomenon is known as hyperglycemic memory and would likely contribute to the persistence of bladder dysfunction even in diabetic patients that achieve glycemic control. The KCNMA1 gene (encoding the BK channel, α-subunit) was identified in the subset of genes encoded by genomic loci that had modulated methylation patterns with diabetes that were not reversed with glycemic control. The changes in methylation pattern correlated with expression of the BK channel α-subunit protein; its expression was downregulated with diabetes that was not reversed with insulin treatment. Although decreased expression and activity of KCNMA1 in the diabetic bladder has been reported (and used to support targeting BK channel activity to treat diabetic bladder dysfunction), expression was previously not known to be subject to hyperglycemic memory. Gene therapy overcomes hyperglycemic memory by introducing exogenous DNA into the bladder that has not been subject to epigenetic modification, restoring BK channel activity. In contrast, pharmacologic approaches to increase BK channel activity in the bladder of diabetic patients, even patients who have achieved glycemic control, would have no target to act on, or have such low levels of expression that activation is insufficient for physiological effect. Therefore, the strategy of increasing BK channel activity to treat patients with bladder dysfunction involving epigenetic modification will be more effective using gene therapy (which overexpress the KCNMA1 gene) than the use of pharmacologic agents (which may have no or little target to activate).
BK α-Subunit Gene Therapy
Gene therapies are often designed for the purpose of gene augmentation or the introduction of a functional gene into a cell with deficient expression of the desired gene product.19 To ensure sufficient expression, the gene delivery system must permit uptake by the targeted cells, protect the exogenous nucleic acid from degradation by host-cell nuclease enzymes, and ensure intracellular transport of the gene to the cell nucleus. To these ends, viral vectors have often been used. However, viral-based gene therapy has been associated with severe adverse events and risk of mortality, leading to increased investigations of non-viral vectors.19,41,42
Naked plasmid DNA (pDNA) is one such non-viral vector option. Naked pDNA is significantly less immunogenic relative to viral and retroviral vectors.43 Further, naked pDNA has less potential for integration into the host cell’s genome, thereby reducing the risk of adverse events associated with insertional mutagenesis, position-effect variegation, or inflammatory immune responses.41,44 However, gene transfer via naked pDNA is also limited by low transfection efficiency and transient expression of the transgene, necessitating the administration of large quantities of the vector.41 Organs and systems successfully targeted by naked pDNA in preclinical and clinical studies have included the heart, central nervous system, pancreas, penis, and skeletal muscle.41,45–47
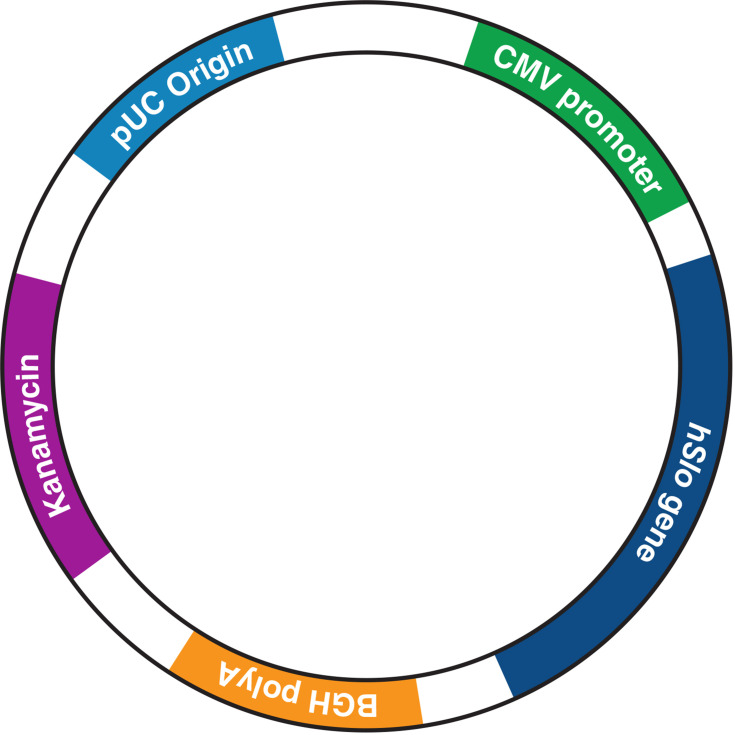
URO-902 is a 6880-base-pair naked pDNA incorporating a DNA sequence synthesized from the messenger RNA (mRNA) that encodes the human BK channel α-subunit (Figure 2).21,48 To ensure robust expression of the encoded protein in eukaryotic cells, the α-subunit code is flanked at its upstream end by a CMV promoter (from cytomegalovirus) to initiate DNA transcription and at its downstream end by a polyadenylation signal (from the bovine growth hormone gene) to terminate transcription and protect the transcript from enzymatic degradation. In addition, the plasmid incorporates sequences that are frequently used in plasmid construction to facilitate its selection and replication in Escherichia coli cultures. None of the modules enable the plasmid to replicate in eukaryotic cells, thereby confining the treatment to targeted cells transfected with the plasmid.
Figure 2.
The URO-902 plasmid construct. For further construct details, see Melman et al.48 BGH, bovine growth hormone; CMV, cytomegalovirus.
Within targeted cells, URO-902 enters the cell presumably via endocytosis and then transits the cell nucleus through a nuclear pore. The type of plasmid promoter driving BK expression determines which cells will express the protein. URO-902 uses a CMV promoter, which is a nonspecific promoter that is active in all cells. Smooth muscle cell–specific promoters, such as the smooth muscle α-actin (SMAA) promoter, also have been incorporated into the plasmid backbone and were shown to be physiologically active.49,50 In the nucleus, pDNA for the BK α-subunit is transcribed by the host cell into an mRNA, which is translated into protein in the cytoplasm. The expressed BK α-subunit channels are then inserted into the cell membrane via the cell’s endogenous machinery. Reverse transcription polymerase chain reaction (RT-PCR) measures show that the gene is expressed in erectile smooth muscle for up to 6 months.51 The hypothesis underlying the development of URO-902 as a gene therapy for OAB is that increased expression of BK channels in bladder myocytes may enhance the cellular capacity to expel K+ ions, promoting membrane hyperpolarization and reducing excitability (Figure 1). The resulting smooth muscle relaxation would be expected to reduce the symptoms associated with OAB.
URO-902 Gene Transfer Studies for Urologic Applications: Erectile Dysfunction and OAB
First Test in Erectile Dysfunction
Transfer of DNA encoding the BK channel α-subunit was first tested as a potential treatment for erectile dysfunction.21 In 2 rat models, intracavernosal injection of a plasmid incorporating the α-subunit gene yielded improvement in the ratio of intracavernous pressure to systemic arterial blood pressure.51–53 A dose-dependent effect on erectile response to cavernous nerve stimulation was seen for up to 6 months. Improvement in erectile function also was identified in atherosclerotic male cynomolgus monkeys, where intracorporal BK channel gene transfer also enhanced sexual behavior—implying that erectile function per se may lead to increased sexual function.49
In a phase 1 safety study, 11 men with severe erectile dysfunction who were unresponsive to pharmacotherapy but otherwise in good health were treated with open-label URO-902 administered as a single dose by intracavernosal injection.47,48 The tested dose levels were 0.5, 1, 5, and 7.5 mg. Patients were monitored for 6 months with annual follow-up for 2 years. The therapy was well tolerated, with no adverse events considered to be gene-transfer-related and no clinically significant changes in physical, laboratory, or electrocardiographic parameters. Semen samples obtained up to 4 weeks after URO-902 dosing showed no evidence of the plasmid. At the 2 highest doses, a clinical response persisted throughout the 6-month study period. A phase 2 study in 26 men confirmed the tolerability of URO-902, with all events being mild and considered not related to study drug.54
Further Application to OAB
To evaluate potential use in OAB, preclinical research established the ability of bladder instillation of pDNA encoding the BK α-subunit to ameliorate the bladder overactivity observed in rats following partial urethral obstruction.55 These studies were followed by phase 1 safety studies of female patients with OAB.22 In each of 2 such trials, the participants were otherwise healthy, non-fertile women with non-neurogenic (idiopathic) OAB and associated detrusor overactivity of at least 6-month duration (Table 1). Moreover, study participants were required to document at least 8 micturitions per day and at least 5 urgency urinary incontinence episodes per week at baseline, without clinically significant stress incontinence. Each woman received a single double-blind dose of URO-902 or placebo administered by intravesical instillation in one trial (ION-02 [NCT00495053]) and by direct detrusor injection in another trial (ION-03 [NCT01870037]). For instilled URO-902, the tested dose levels were 5 and 10 mg. For injected URO-902, dose levels were 16 and 24 mg divided among 20 to 30 injection sites. Patients were assessed and monitored for 6 months with periodic follow-up for an additional 18 months.
Table 1.
Baseline Characteristics by Study Drug Treatment in Women Participating in Phase 1 URO-902 Trials
| Characteristic | ION-02 Study | ION-03 Study | ||||
|---|---|---|---|---|---|---|
| URO-902 by Intravesical Instillation 5 mg (n=10) | URO-902 by Intravesical Instillation 10 mg (n=6) | Placebo (n=5) | URO-902 by Detrusor Injection 16 mg (n=6) | URO-902 by Detrusor Injection 24 mg (n=3) | Placebo (n=4) | |
| Age (years), mean (SD) | 62.6 (15.2) | 65.8 (14.4) | 69.8 (9.8) | 55.8 (4.6) | 65.1 (9.2) | 57.0 (6.8) |
| Race, n (%) | ||||||
| White | 9 (90.0) | 6 (100.0) | 4 (80.0) | 2 (33.3) | 2 (66.7) | 4 (100.0) |
| Black/African American | 1 (10.0) | 0 | 0 | 4 (66.7) | 1 (33.3) | 0 |
| Ethnicity, n (%) | ||||||
| Latino/Hispanic | 0 | 0 | 1 (20.0) | 0 | 1 (33.3) | 0 |
| Micturitions/day, mean (SD) | 11.5 (3.4) | 11.2 (4.7) | 10.1 (3.2) | 11.3 (2.7) | 17.2 (7.1) | 10.2 (4.8) |
| Urgency episodes/day, mean (SD) | 11.5 (3.2) | 11.2 (4.7) | 10.1 (3.2) | 10.2 (3.6) | 17.2 (7.1) | 9.8 (5.2) |
| Urgency incontinence episodes/day, mean (SD) | 2.7 (2.3) | 2.2 (2.2) | 5.3 (3.6) | 1.9 (0.8) | 3.8 (3.3) | 1.8 (1.5) |
Note: Adapted from Rovner E, Chai TC, Jacobs S, et al. Evaluating the safety and potential activity of URO-902 (hMaxi-K) gene transfer by intravesical instillation or direct injection into the bladder wall in female participants with idiopathic (non-neurogenic) overactive bladder syndrome and detrusor overactivity from two double-blind, imbalanced, placebo-controlled randomized phase 1 trials. Neurourol Urodyn. 2020;39(2):744–753. doi:10.1002/nau.24272. © 2020 The Authors. Neurourology and Urodynamics published by Wiley Periodicals, Inc.22
Abbreviation: SD, standard deviation.
In ION-02 (intravesical installation), the 90-mL dose of URO-902 was instilled through a small-diameter catheter into the lumen of the bladder, and patients were requested to retain the solution in the bladder for at least 2 hours.22 In ION-03 (direct injection), URO-902 was injected approximately 2 mm into the detrusor muscle with a BoNee needle through a rigid cystoscope, without general or regional anesthesia, and 20 injections of either 0.2 mL (16-mg dose) or 30 injections of 0.2 mL (24-mg dose) each were spaced approximately 1 cm apart.22 Prior to direct injection, 40 mL of 2% lidocaine was instilled into the bladder, and 10 mL of 2% xylocaine gel was instilled into the urethra.
In both studies, few treatment-related adverse events were noted. Of 34 participants in both studies, only 3 had treatment-related adverse events, and 1 of those patients received placebo. Furthermore, there were no adverse events leading to an early withdrawal from the study (Tables 2 and 3).22 One serious adverse event was reported in ION-03: exacerbation of pre-existing asthma in a patient treated with 16 mg URO-902. This event was not considered related to the investigational agent. Across both trials, no patients experienced urinary retention as a reported adverse event.22
Table 2.
Reported Adverse Events by Study Drug Treatment Among Women Participating in the Phase 1 ION-02 URO-902 Trial
| AE, % | URO-902 by Intravesical Instillation 5 mg (n=10) | URO-902 by Intravesical Instillation 10 mg (n=6) | Placebo (n=5) |
|---|---|---|---|
| Any AE | 36 | 11 | 7 |
| Any treatment-related AE | 2 | 0 | 1 |
| Any serious AE | 0 | 0 | 0 |
| Any AE leading to premature discontinuation | 0 | 0 | 0 |
| Total participants with at least 1 AE | 6 (60.0) | 5 (83.3) | 3 (60.0) |
| Most common AEs, n (%) | |||
| Urinary tract infection | 5 (50.0) | 1 (16.7) | 2 (40.0) |
| Constipation | 1 (10.0) | 1 (16.7) | 0 |
| Gastroesophageal reflux disease | 2 (20.0) | 0 | 0 |
| Back pain | 0 | 1 (16.7) | 0 |
| Blood creatinine increased | 1 (10.0) | 0 | 0 |
| Blood creatinine phosphokinase increased | 1 (10.0) | 0 | 0 |
| Blood pressure increased | 0 | 1 (16.7) | 0 |
| Diarrhea | 1 (10.0) | 0 | 0 |
| Dysuria | 0 | 1 (16.7) | 0 |
| Hepatitis C | 0 | 1 (16.7) | 0 |
| Hypertensive nephropathy | 1 (10.0) | 0 | 0 |
| Nasopharyngitis | 0 | 1 (16.7) | 0 |
| Mobitz type II second block | 1 (10.0) | 0 | 0 |
| Tendonitis | 1 (10.0) | 0 | 0 |
| Urinary retention | 0 | 0 | 0 |
Note: Adapted from Rovner E, Chai TC, Jacobs S, et al. Evaluating the safety and potential activity of URO-902 (hMaxi-K) gene transfer by intravesical instillation or direct injection into the bladder wall in female participants with idiopathic (non-neurogenic) overactive bladder syndrome and detrusor overactivity from two double-blind, imbalanced, placebo-controlled randomized phase 1 trials. Neurourol Urodyn. 2020;39(2):744–753. doi:10.1002/nau.24272. © 2020 The Authors. Neurourology and Urodynamics published by Wiley Periodicals, Inc.22
Abbreviation: AE, adverse event.
Table 3.
Reported Adverse Events by Study Drug Treatment Among Women Participating in the Phase 1 ION-03 URO-902 Trial
| AE, % | URO-902 by Detrusor Injection 16 mg (n=6) | URO-902 by Detrusor Injection 24 mg (n=3) | Placebo (n=4) |
|---|---|---|---|
| Any AE | 13 | 8 | 17 |
| Any treatment-related AE | 0 | 0 | 0 |
| Any serious AE | 1 | 0 | 0 |
| Any AE leading to premature discontinuation | 0 | 0 | 0 |
| Total participants with at least 1 AE | 4 (66.7) | 3 (100.0) | 4 (100.0) |
| Most common AEs, n (%) | |||
| Hyperglycemia | 3 (50.0) | 2 (66.7) | 4 (100.0) |
| Hematuria | 2 (33.3) | 1 (33.3) | 2 (50.0) |
| Abdominal pain | 1 (16.7) | 1 (33.3) | 1 (25.0) |
| Blood creatine phosphokinase increased | 1 (16.7) | 0 | 1 (25.0) |
| Dysuria | 0 | 1 (33.3) | 1 (25.0) |
| Urinary tract infection | 1 (16.7) | 1 (33.3) | 0 |
| Upper respiratory tract infection | 1 (16.7) | 0 | 0 |
| Bladder pain | 0 | 1 (33.3) | 0 |
| Arthralgia | 0 | 1 (33.3) | 0 |
| Antinuclear antibody positive | 1 (16.7) | 0 | 0 |
| Electrocardiogram QT prolonged | 0 | 0 | 1 (25.0) |
| Hematocrit decreased | 0 | 0 | 1 (25.0) |
| Hemoglobin decreased | 0 | 0 | 1 (25.0) |
| Hypoglycemia | 1 (16.7) | 0 | 0 |
| Asthma | 1 (16.7) | 0 | 0 |
| Urinary retention | 0 | 0 | 0 |
Note: Adapted from Rovner E, Chai TC, Jacobs S, et al. Evaluating the safety and potential activity of URO-902 (hMaxi-K) gene transfer by intravesical instillation or direct injection into the bladder wall in female participants with idiopathic (non-neurogenic) overactive bladder syndrome and detrusor overactivity from two double-blind, imbalanced, placebo-controlled randomized phase 1 trials. Neurourol Urodyn. 2020;39(2):744–753. doi:10.1002/nau.24272. © 2020 The Authors. Neurourology and Urodynamics published by Wiley Periodicals, Inc.22
Abbreviation: AE, adverse event.
Among the 16 women receiving URO-902 by instillation, post-dose urine samples showed no evidence of the plasmid.22 Among the 9 women receiving URO-902 by injection, post-dose samples obtained after dosing detected the plasmid in the urine of 4 patients and in the blood of 1 patient 15 minutes after dosing. There was no evidence of the plasmid in later samples.22
In both ION-02 and ION-03, the efficacy of URO-902 for the treatment of OAB was assessed by several endpoints.22 In ION-02, patients receiving URO-902 by intravesical instillation exhibited no significant changes in the mean number of voids or urgency incontinence episodes at either dose. Nevertheless, several efficacy signals were detected. Across URO-902 recipients, the mean reduction in detrusor contractions from baseline to week 24 was trending toward significance (P<0.0508). At week 8, the 5-mg subgroup showed a >40% mean reduction in urgency incontinence episodes.22
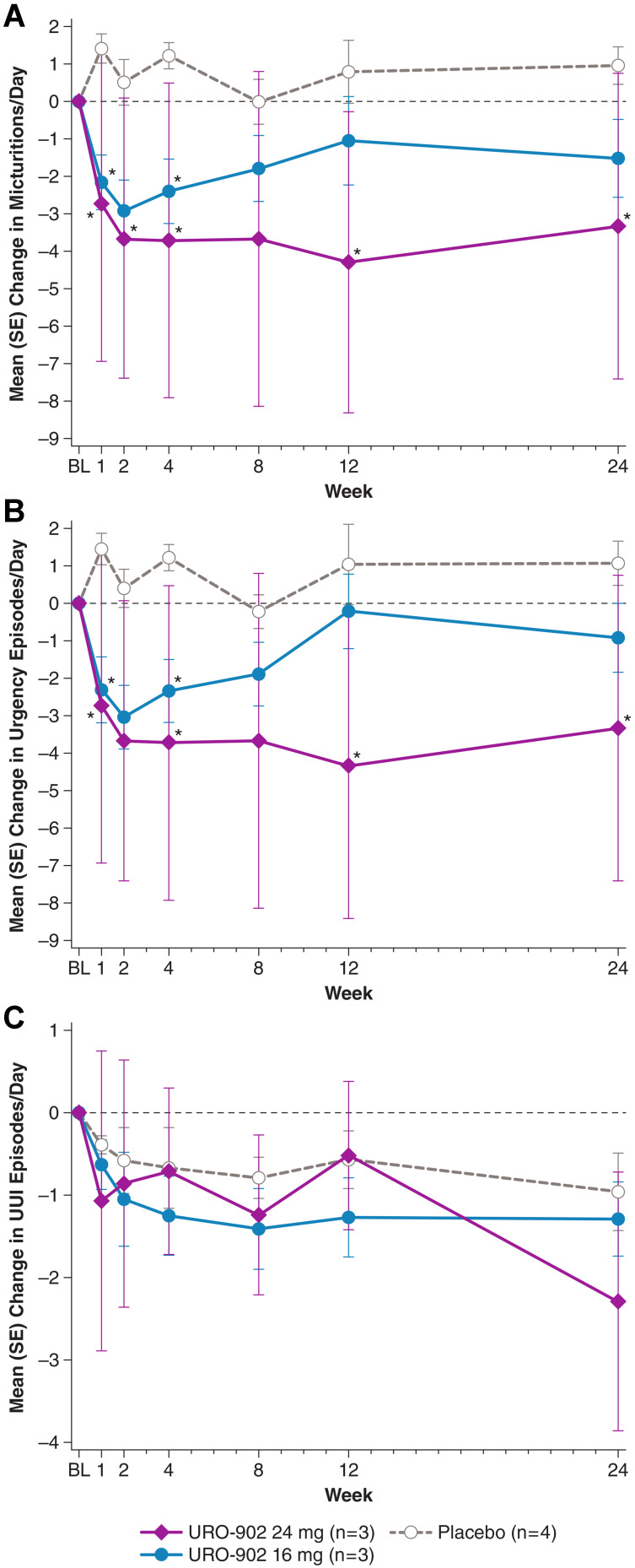
In ION-03, URO-902 administered by direct detrusor injection showed greater efficacy compared with results of ION-02.22 Improvement of OAB manifestations included a dose-dependent reduction in the mean number of micturitions and urgency episodes per day, with statistical significance versus placebo for the 24-mg dose at post-dose time points including weeks 12 and 24 (Figure 3A and B). These improvements were accompanied by a decrease in urgency incontinence episodes per day, with dose dependence observed at week 24 (Figure 3C). For micturitions per day and urgency episodes per day, the mean improvement at both URO-902 doses was statistically significant versus placebo at week 1, whereas placebo recipients showed no improvement throughout the trial. In future trials, these higher doses of URO-902 may lead to further improved response. URO-902 recipients also reported improvements versus baseline in quality of life (QoL) as measured by the King’s Health Questionnaire, a health‐related QoL instrument specific for urinary incontinence. At multiple post-dose visits, statistically significant improvements were reported in domain scores measuring impact on life, physical limitations, role limitations, social limitations, and sleep/energy.22
Figure 3.
Mean changes from baseline in (A) micturitions, (B) urgency episodes, and (C) UUI episodes by treatment group during the ION-03 study of URO-902.22 Data on file from Dr. Melman. *P<0.05 versus placebo. P values are derived from a linear mixed model with the number of urgency episodes or number of voids as dependent variables, treatments, time point, and interaction of time and treatment.
Abbreviations: BL, baseline; SE, standard error; UUI, urge urinary incontinence.
It has been demonstrated that large molecular weight proteins, such as nerve growth factor, botulinum toxin,56 and wheat germ agglutinin conjugated to horseradish peroxidase,57 are capable of retrograde transport to dorsal root ganglia from intradetrusor injection sites. A similar transport of the injected plasmid in ION-03 is possible, and, provided that this is the case, a contribution to the lack of dose dependence in toxicity and in efficacy cannot be excluded. However, in a recently published study,58 it was the absence of the BK channel and not increased expression that resulted in decreased neuromuscular transmission. Additionally, because URO-902 uses a nonspecific promoter, it is possible that there may be an effect on neurons in the bladder.
As different injection techniques and doses were used for delivery of URO-902 in ION-02 and ION-03, results from these studies cannot be compared. At present, it is not known whether direct detrusor injection or intravesical instillation will yield the best gene expression in patients with OAB, nor is it established which technique may ultimately be more effective at reducing the symptoms of OAB. As long-term animal studies may pose challenges with multiple survival surgeries, further in-human studies are required to optimize and refine the use of URO-902.
Conclusions
Among transmembrane ion channels in bladder smooth muscle cells, the BK channel has a particularly crucial role in modulating smooth muscle excitability and thus detrusor tone and contraction. The rationale for URO-902 as a gene therapy for OAB is that enhanced expression of BK channel α-subunits in bladder myocytes may decrease detrusor smooth muscle cell excitability leading to decreased afferent activity. In turn, this leads to decreased urgency, urinary frequency, and urge urinary incontinence. In preclinical research, gene transfer via naked pDNA encoding the BK channel α-subunit led to improvement in animal models of detrusor overactivity. In adult female patients with OAB, secondary analyses of efficacy endpoints suggested sustained benefits through 24 weeks of post-dose monitoring, especially with direct detrusor injection of the gene therapy, which were accompanied by improvements in QoL. These findings warrant continued investigation of URO-902 in larger-scale clinical studies.
Acknowledgments
Medical writing and editorial support was provided to the authors by The Curry Rockefeller Group, LLC, Tarrytown, NY, and was funded by Urovant Sciences.
Funding Statement
ION-03 was supported in part by National Institute on Aging grant R44DK093279. Urovant Sciences provided funding for medical writing and editorial support, which was provided by The Curry Rockefeller Group, LLC.
Disclosure
K-EA has nothing to disclose. GJC is co-founder, director, and shareholder of Ion Channel Innovations, LLC, and has a US Patent Application Appl. No. 16/612286; 371(c) Date: November 8, 2019 (US National Phase of Int’l Appl. No. PCT/US2018/032574; Int’l Filing Date: May 14, 2018) For: Compositions And Methods For Treating Idiopathic Overactive Bladder Syndrome And Detrusor Overactivity, pending to Urovant. KD is a shareholder of Ion Channel Innovations, LLC, and received research funds from Urovant. ER reports relationships with Ion Channel Innovations, LLC, during the conduct of the study and with Urovant in the form of consulting honoraria. AM reports an NIH grant for Ion Channel Innovations, LLC, and consultant relationship with Urovant; he was co-founder of Ion Channel Innovations, LLC.
References
- 1.Abrams P, Artibani W, Cardozo L, et al. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn. 2009;28(4):287. doi: 10.1002/nau.20737 [DOI] [PubMed] [Google Scholar]
- 2.Van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardization of terminology in nocturia: report from the standardization subcommittee of the International Continence Society. BJU Int. 2002;90(suppl 3):11–15. doi: 10.1046/j.1464-410X.90.s3.3.x [DOI] [PubMed] [Google Scholar]
- 3.Reynolds WS, Fowke J, Dmochowski R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8–13. doi: 10.1007/s11884-016-0344-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. doi: 10.1007/s00345-002-0301-4 [DOI] [PubMed] [Google Scholar]
- 5.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138. doi: 10.1111/j.1464-410X.2010.09993.x [DOI] [PubMed] [Google Scholar]
- 6.Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 Suppl):2455–2463. doi:10.1016/j.juro.2012.09.079 [DOI] [PubMed] [Google Scholar]
- 7.Lightner DJ, Gomelsky A, Souter L, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment 2019. J Urol. 2019;202(3):558–563. doi: 10.1097/ju.0000000000000309 [DOI] [PubMed] [Google Scholar]
- 8.Maman K, Aballea S, Nazir J, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65(4):755–765. doi:10.1016/j.eururo.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 9.Yamada S, Ito Y, Nishijima S, Kadekawa K, Sugaya K. Basic and clinical aspects of antimuscarinic agents used to treat overactive bladder. Pharmacol Ther. 2018;189:130–148. doi:10.1016/j.pharmthera.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 10.Yeowell G, Smith P, Nazir J, Hakimi Z, Siddiqui E, Fatoye F. Real-world persistence and adherence to oral antimuscarinics and mirabegron in patients with overactive bladder (OAB): a systematic literature review. BMJ Open. 2018;8(11):e021889. doi: 10.1136/bmjopen-2018-021889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084–1093. doi: 10.1001/jamainternmed.2019.0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo C-W, Wu M-Y, Yang SS-D, Jaw F-S, Chang S-J. Comparing the efficacy of onabotulinumtoxinA, sacral neuromodulation, and peripheral tibial nerve stimulation as third line treatment for the management of overactive bladder symptoms in adults: systematic review and network meta-analysis. Toxins. 2020;12(2):128. doi: 10.3390/toxins12020128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petkov GV. Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R571–584. doi: 10.1152/ajpregu.00142.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukacz ES, Sampselle C, Gray M, et al. A healthy bladder: a consensus statement. Int J Clin Pract. 2011;65(10):1026–1036. doi: 10.1111/j.1742-1241.2011.02763.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H-Z, Brink PR, Christ GJ. Gap junction channel activity in short-term cultured human detrusor myocyte cell pairs: gating and unitary conductances. Am J Physiol Cell Physiol. 2006;291(6):C1366–1376. doi: 10.1152/ajpcell.00027.2006 [DOI] [PubMed] [Google Scholar]
- 16.Andersson K-E. Detrusor myocyte activity and afferent signaling. Neurourol Urodyn. 2010;29(1):97–106. doi: 10.1002/nau.20784 [DOI] [PubMed] [Google Scholar]
- 17.Andersson K-E, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84(3):935–986. doi: 10.1152/physrev.00038.2003 [DOI] [PubMed] [Google Scholar]
- 18.Chakrabarty B, Bijos DA, Vahabi B, et al. Modulation of bladder wall micromotions alters intravesical pressure activity in the isolated bladder. Front Physiol. 2019;9:1937. doi: 10.3389/fphys.2018.01937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anguela XM, High KA. Entering the modern era of gene therapy. Annu Rev Med. 2019;70(1):273–288. doi: 10.1146/annurev-med-012017-043332 [DOI] [PubMed] [Google Scholar]
- 20.Christ GJ, Hodges S. Molecular mechanisms of detrusor and corporal myocyte contraction: identifying targets for pharmacotherapy of bladder and erectile dysfunction. Br J Pharmacol. 2006;147(Suppl S2):S41–55. doi: 10.1038/sj.bjp.0706627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melman A, Davies K. Gene therapy for erectile dysfunction: what is the future? Curr Urol Rep. 2010;11(6):421–426. doi: 10.1007/s11934-010-0145-1 [DOI] [PubMed] [Google Scholar]
- 22.Rovner E, Chai TC, Jacobs S, et al. Evaluating the safety and potential activity of URO-902 (hMaxi-K) gene transfer by intravesical instillation or direct injection into the bladder wall in female participants with idiopathic (non-neurogenic) overactive bladder syndrome and detrusor overactivity from two double-blind, imbalanced, placebo-controlled randomized phase 1 trials. Neurourol Urodyn. 2020;39(2):744–753. doi: 10.1002/nau.24272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campos de Carvalho AC, Roy C, Moreno AP, et al. Gap junctions formed of connexin43 are found between smooth muscle cells of human corpus cavernosum. J Urol. 1993;149(6):1568–1575. doi: 10.1016/S0022-5347(17)36455-8 [DOI] [PubMed] [Google Scholar]
- 24.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem. 1994;269(6):3921–3924. doi: 10.1016/S0021-9258(17)41720-0 [DOI] [PubMed] [Google Scholar]
- 25.Latorre R, Castillo K, Carrasquel-Ursulaez W, et al. Molecular determinants of BK channel functional diversity and functioning. Physiol Rev. 2017;97(1):39–87. doi: 10.1152/physrev.00001.2016 [DOI] [PubMed] [Google Scholar]
- 26.Tseng-Crank J, Foster CD, Krause JD, et al. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13(6):1315–1330. doi: 10.1016/0896-6273(94)90418-9 [DOI] [PubMed] [Google Scholar]
- 27.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. β1-Subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohi Y, Yamamura H, Nagano N, et al. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol. 2001;534:313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latorre R, Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1–2):11–30. doi: 10.1007/BF01870671 [DOI] [PubMed] [Google Scholar]
- 30.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279(35):36746–36752. doi: 10.1074/jbc.M405621200 [DOI] [PubMed] [Google Scholar]
- 31.Mimata H, Nomura Y, Emoto A, Latifpour J, Wheeler M, Weiss RM. Muscarinic receptor subtypes and receptor-coupled phosphatidylinositol hydrolysis in rat bladder smooth muscle. Int J Urol. 1997;4(6):591–596. doi: 10.1111/j.1442-2042.1997.tb00315.x [DOI] [PubMed] [Google Scholar]
- 32.Neuhaus J, Pfeiffer F, Wolburg H, Horn L-C, Dorschner W. Alterations in connexin expression in the bladder of patients with urge symptoms. BJU International. 2005;96(4):670–676. doi: 10.1111/j.1464-410X.2005.05703.x [DOI] [PubMed] [Google Scholar]
- 33.Bailey CS, Moldenhauer HJ, Park SM, Keros S, Meredith AL. KCNMA1-linked channelopathy. J Gen Physiol. 2019;151(10):1173–1189. doi: 10.1085/jgp.201912457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies KP, Zhao W, Tar M, et al. Diabetes-induced changes in the alternative splicing of the slo gene in corporal tissue. Eur Urol. 2007;52(4):1229–1237. doi: 10.1016/j.eururo.2006.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hristov KL, Afeli SAY, Parajuli SP, Cheng Q, Rovner ES, Petkov GV. Neurogenic detrusor overactivity is associated with decreased expression and function of the large conductance voltage- and Ca2+-activated K+ channels. PLoS One. 2013;8(7):e68052. doi: 10.1371/journal.pone.0068052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds WS, Dmochowski R, Wein A, Bruehl S. Does central sensitization help explain idiopathic overactive bladder? Nat Rev Urol. 2016;13(8):481–491. doi:10.1038/nrurol.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou F, Li H, Zhou C, et al. Structural and functional changes in gap junctional intercellular communication in a rat model of overactive bladder syndrome induced by partial bladder outlet obstruction. Exp Ther Med. 2016;11(6):2139–2146. doi:10.3892/etm.2016.3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okinami T, Imamura M, Nishikawa N, et al. Altered detrusor gap junction communications induce storage symptoms in bladder inflammation: a mouse cyclophosphamide-induced model of cystitis. PLoS One. 2014;9(8):e104216. doi:10.1371/journal.pone.0104216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imamura M, Negoro H, Kanematsu A, et al. Basic fibroblast growth factor causes urinary bladder overactivity through gap junction generation in the smooth muscle. Am J Physiol Renal Physiol. 2009;297(1):F46–54. doi:10.1152/ajprenal.90207.2008 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Tar MT, Davies KP. Hyperglycemic memory in the rat bladder detrusor is associated with a persistent hypomethylated state. Physiol Rep. 2020;8:e14614.doi:10.14814/phy2.14614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayant RD, Sosa D, Kaushik A, et al. Current status of non-viral gene therapy for CNS disorders. Expert Opin Drug Deliv. 2016;13(10):1433–1445. doi:10.1080/17425247.2016.1188802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chira S, Jackson CS, Oprea I, et al. Progresses towards safe and efficient gene therapy vectors. Oncotarget. 2015;6(31):30675–30703.doi:10.18632/oncotarget.5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardee CL, Arévalo-Soliz LM, Hornstein BD, Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes. 2017;8(2):65. doi: 10.3390/genes8020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Athanasopoulos T, Munye MM, Yanez-Munoz RJ. Nonintegrating gene therapy vectors. Hematol Oncol Clin North Am. 2017;31(5):753–770. doi:10.1016/j.hoc.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 45.Hagstrom JE, Hegge J, Zhang G, et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther. 2004;10(2):386–398. doi: 10.1016/j.ymthe.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 46.Yamada Y, Tabata M, Abe J, Nomura M, Harashima H. In vivo transgene expression in the pancreas by the intraductal injection of naked plasmid DNA. J Pharm Sci. 2018;107(2):647–653. doi:10.1016/j.xphs.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 47.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum Gene Ther. 2006;17(12):1165–1176. doi:10.1089/hum.2006.17.1165 [DOI] [PubMed] [Google Scholar]
- 48.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. The first human trial for gene transfer therapy for the treatment of erectile dysfunction: preliminary results. Eur Urol. 2005;48(2):314–318. doi:10.1016/j.eururo.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 49.Christ GJ, Andersson KE, Williams K, et al. Smooth-muscle-specific gene transfer with the human maxi-K channel improves erectile function and enhances sexual behavior in atherosclerotic cynomolgus monkeys. Eur Urol. 2009;56(6):1055–1066. doi:10.1016/j.eururo.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 50.Melman A, Biggs G, Davies K, Zhao W, Tar MT, Christ GJ. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther. 2008;15(5):364–370. doi:10.1038/sj.gt.3303093 [DOI] [PubMed] [Google Scholar]
- 51.Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol. 2003;170(1):285–290. doi:10.1097/01.ju.0000063375.12512.6e [DOI] [PubMed] [Google Scholar]
- 52.Christ GJ, Day N, Santizo C, et al. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004;287(4):H1544–1553. doi:10.1152/ajpheart.00792.2003 [DOI] [PubMed] [Google Scholar]
- 53.Christ GJ, Rehman J, Day N, et al. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am J Physiol. 1998;275(2):H600–608. doi:10.1152/ajpheart.1998.275.2.H600 [DOI] [PubMed] [Google Scholar]
- 54.Arun N. Treatment of erectile dysfunction with hMaxi-K gene transfer: safety report from phase IIA study. Eur Urol. 2018;12(2):e1706. [Google Scholar]
- 55.Christ GJ, Day NS, Day M, et al. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1699–1709. doi:10.1152/ajpregu.2001.281.5.R1699 [DOI] [PubMed] [Google Scholar]
- 56.Papagiannopoulou D, Vardouli L, Dimitriadis F, Apostolidis A. Retrograde transport of radiolabelled botulinum neurotoxin type A to the CNS after intradetrusor injection in rats. BJU Int. 2016;117(4):697–704. doi:10.1111/bju.13163 [DOI] [PubMed] [Google Scholar]
- 57.Kruse MN, Erdman SL, Puri G, de Groat WC. Differences in Fluorogold and wheat germ agglutinin-horseradish peroxidase labelling of bladder afferent neurons. Brain Res. 1993;613(2):352–356. doi:10.1016/0006-8993(93)90926-e [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Burke SRA, Talmadge RJ, Voss AA, Rich MM. Depressed neuromuscular transmission causes weakness in mice lacking BK potassium channels. J Gen Physiol. 2020;152(5):e201912526. doi: 10.1085/jgp.201912526 [DOI] [PMC free article] [PubMed] [Google Scholar]