Abstract
In humans and rodents, the entorhinal cortical (EC) -hippocampal (HPC) circuit is crucial for the formation and recall of memory, preserving both spatial and temporal information about the occurrence of past events. Both modeling and experimental studies have revealed circuits within this network that play crucial roles in encoding space and context. However, our understanding about the time-related aspects of memory are just beginning to be understood. In this review, we first describe updates regarding recent anatomical discoveries for the EC-HPC network, as several important neural circuits critical for memory formation have been discovered by newly developed neural tracing technologies. Second, we examine the complementary roles of multiple medial entorhinal cortical inputs, including newly discovered circuits, into the hippocampus for the temporal and spatial aspects of memory. Finally, we will discuss how temporal and contextual memory information is integrated in hippocampal CA1 cells. We provide new insights into the neural circuit mechanisms for anatomical and functional segregation and integration of the temporal and spatial aspects of memory encoding in the EC-HPC networks.
Keywords: Time, Space, Cell type specific, Trace fear conditioning, Contextual fear conditioning
Introduction
The entorhinal cortical (EC)-hippocampal (HPC) network is necessary for formation of memory across mammalian species, playing a critical role in the spatial and temporal aspects of memory (Scoville & Milner, 1957; Mahut et al., 1982; Kim & Fanselow, 1992; Phillips & LeDoux, 1992; Frankland et al., 1998; Eichenbaum, 2000; Tulving, 2002; Sutherland et al., 2008; Kitamura et al., 2012; Pilkiw et al., 2017). Memory formation is typically comprised of “what”, or representation of objects (Mahut, 1971; Eacott & Norman, 2004; Knierim, 2004; Deshmukh & Knierim, 2013; Laurent et al., 2016; Connor & Knierim, 2017; Zimmermann & Eschen, 2017; Schurgin & Flombaum, 2018), “where” for spatial orientation and navigation, as well as context (O’Keefe & Dostrovsky, 1971; Morris et al., 1982; Nakazawa et al., 2004; Moser et al., 2008; Smith & Bulkin, 2014), and “when”, which is fulfilled by the timing of event sequences, and the linkage of those events across temporal gaps (McEchron et al., 2003; MacDonald et al., 2011; Eichenbaum, 2014; Kitamura et al., 2014; Kitamura et al., 2015a; Eichenbaum, 2017; Kitamura, 2017). Importantly, each of these aspects have their own distinct mechanisms for encoding and retrieval. The central focus in this review is how differential neural circuits in the EC-HPC network encode different types of memory and how these memories are integrated in the HPC. In order for each of these memory modalities to be processed and integrated, an array of local cellular microcircuits in the EC-HPC networks must interact with each other both within and across brain regions, ultimately ending in successful memory encoding. Therefore, we first examine recent advances in anatomical knowledge of the EC-HPC network, as several important neural circuits crucial for memory formation have been discovered using the anatomical gene expression atlas project, viral-mediated neural tracing, and cell type-specific optogenetic circuit stimulation (Wickersham et al., 2007; Thompson et al., 2008; Varga et al., 2010; Wickersham et al., 2010; Kitamura et al., 2014; Kohara et al., 2014; Ray et al., 2014; Kitamura et al., 2015b).
In experimental animal studies, the EC-HPC network is traditionally known for its critical role in the processing of spatial information and there has been significant progress toward understanding the neural circuit mechanisms underlying the spatial/contextual memory (O’Keefe & Dostrovsky, 1971; Morris et al., 1982; Wilson & McNaughton, 1993; Eichenbaum et al., 1999; Moser et al., 2008; Hasselmo, 2011; Buzsaki, 2013; Ekstrom & Ranganath, 2017). However, neural circuit mechanisms and their process in the EC-HPC network on temporal memory are just beginning to be understood (Buzsaki, 2013; Eichenbaum, 2014; 2017; Ekstrom & Ranganath, 2017). As our second topic, we examine the structure and function of neural circuits in the EC-HPC network involved in temporal memory by focusing on temporal association learning, which allows animals to associate two temporally segregated events (Wolff, 1966; Solomon et al., 1986; Moyer et al., 1990; Wallenstein et al., 1998; McEchron et al., 1999). As a complementary function to temporal association learning, we also examine the neural circuit activity essential for contextual memory in the EC-HPC network as a component of spatial memory (Marr, 1971; O’Reilly & McClelland, 1994; Treves & Rolls, 1994; Leutgeb et al., 2007; McHugh et al., 2007).
We review here the differential neural circuits in the EC-HPC system that contribute to temporal and spatial memory which can be integrated to form a more robust event memory (Fortin et al., 2002; MacDonald et al., 2011; Naya & Suzuki, 2011; MacDonald et al., 2013; Kitamura et al., 2014; Sakon et al., 2014; Allen et al., 2016; Aronov et al., 2017; Eichenbaum, 2017). We lastly discuss possible neural mechanisms describing how temporal and contextual memory are integrated in hippocampal CA1 pyramidal cells in order to elucidate the circuit functionality underlying these processes as they pertain to the EC-HPC network.
Multiple entorhinal cortical inputs to hippocampus
Previous studies with physical or chemical lesions and anterograde/retrograde tracing have revealed the extensive reciprocal synaptic connections between EC and HPC; superficial layers of EC (II/III) project to HPC and HPC projects back to the deep layer of EC (V/VI) (Amaral & Witter, 1989; Amaral & Lavenex, 2007; Kitamura, 2017). Specifically, the superficial layer of entorhinal cortex provides two major excitatory inputs into hippocampus (Fig 1), the direct (EC layer III → HPC CA1), and the indirect (tri-synaptic) pathway (EC layer II → HPC dentate gyrus (DG), DG → CA3 and CA2, CA3 and CA2 → HPC CA1) (Amaral & Witter, 1989; Amaral & Lavenex, 2007; Kitamura, 2017; Kitamura et al., 2017). Excitatory stellate cells in EC layer II (Reelin+ cells) project to DG, CA3 and CA2 (Tamamaki & Nojyo, 1993; Kohara et al., 2014), while Excitatory pyramidal cells in EC layer III (ECIII cells) directly project to CA1 (Amaral & Witter, 1989; Amaral & Lavenex, 2007; Kohara et al., 2014) (Fig. 1). Though previous findings made using broadly-acting electrical stimulation of fiber bundles suggested direct input from ECIII to CA2 (Chevaleyre & Siegelbaum, 2010), Kohara et al determined that such a direct connection does not exist from their more finely tuned experiments (Kohara et al., 2014). The experimenters expressed ChR2 specifically in medial ECIII (MECIII) neurons and subsequently performed whole-cell patch clamp on the downstream CA2 neurons. They then optogenetically stimulated MECIII axons with brief pulses of blue light Following these stimulatory pulses, a lack of mono-synaptic response activity was found in the CA2 pyramidal cells (Kohara et al., 2014), suggesting that rather than receiving input from MECIII, CA2 receives inputs primarily from the tri-synaptic pathway (Fig. 1). The excitatory population of cells in ECII is comprised of two morphological subpopulations; stellate and pyramidal cells (Alonso & Klink, 1993; Klink & Alonso, 1997) (Fig. 1). Stellate cells in ECII express Reelin (we refer to these as Reelin+ cells), while pyramidal cells in ECII express both CalbindinD-28K (CalB) and Wolfram syndrome 1 (Wfs1) (we refer to these as CalB+ cells) (Varga et al., 2010; Kitamura et al., 2014; Ray et al., 2014). CalB+ cell populations are not homogenously distributed, but rather CalB+ cells form clusters arranged in a hexagonal formation along the border of ECII and ECI, surrounded by Reelin+ cells (Kitamura et al., 2014; Ray et al., 2014). These clustered CalB+ cells have been referred to as “island cells”, and Reelin+ cells as “ocean cells” (Kitamura et al., 2014). In addition to morphological and broader cytoarchitectural differences in the distribution of these populations, they also possess differential physiological properties, molecular markers, and most importantly, project to different areas of the EC-HPC circuit (Alonso & Klink, 1993; Tamamaki & Nojyo, 1993; Klink & Alonso, 1997; Varga et al., 2010; Kitamura et al., 2014; Ray et al., 2014). While it has been well known that ECII stellate cells directly project to DG, CA3, and CA2, the projection patterns of ECII pyramidal cell remained unknown. In 2014, using a cell type-specific optogenetic approach, Kitamura et al. described a previously undiscovered direct projection originating from the CalB+ pyramidal cells of ECII to the GABAergic interneurons of stratum lacunosum (SL) in CA1 (SL-INs), while MECIII neurons project directly to distal pyramidal cell dendrites in stratum moleculare (SM), in close proximity to SL, (Fig. 1) (Kitamura et al., 2014). Optogenetic stimulation of the terminals of MECII ChR2-expressing CalB+ neurons coupled with whole cell patch-clamp recordings in vitro demonstrated monosynaptic glutamatergic inputs onto the SL-INs. Moreover, simultaneous recordings from SL-INs and CA1 pyramidal cells along with optogenetic stimulation of axons from MECIII cells revealed a novel gating circuit system in CA1 driven by the CalB+ cells of MECII (Kitamura et al., 2014). Entorhinal projections from CalB+ cells in ECII to hippocampal CA1 have since been confirmed by other groups (Shu et al., 2016; Surmeli et al., 2016; Yang et al., 2016; Zutshi et al., 2018; Ohara et al., 2019). In addition to excitatory neurons, GABAergic inhibitory interneurons in EC also project to the HPC. Melzer et al showed long-range projections of entorhinal interneurons onto HPC interneurons (Melzer et al., 2012). Similarly, Basu et al. found long-range projecting GABAergic interneurons in EC, which mediate gating mechanisms in CA1 (Basu et al., 2016). While it would be important to re-confirm these projections by using traditional tracing methods, newly developed viral tracing methods and cell type-specific optogenetic stimulation have significantly advanced our understanding of neural circuits, even in the heavily studied EC-HPC network.
Figure 1,
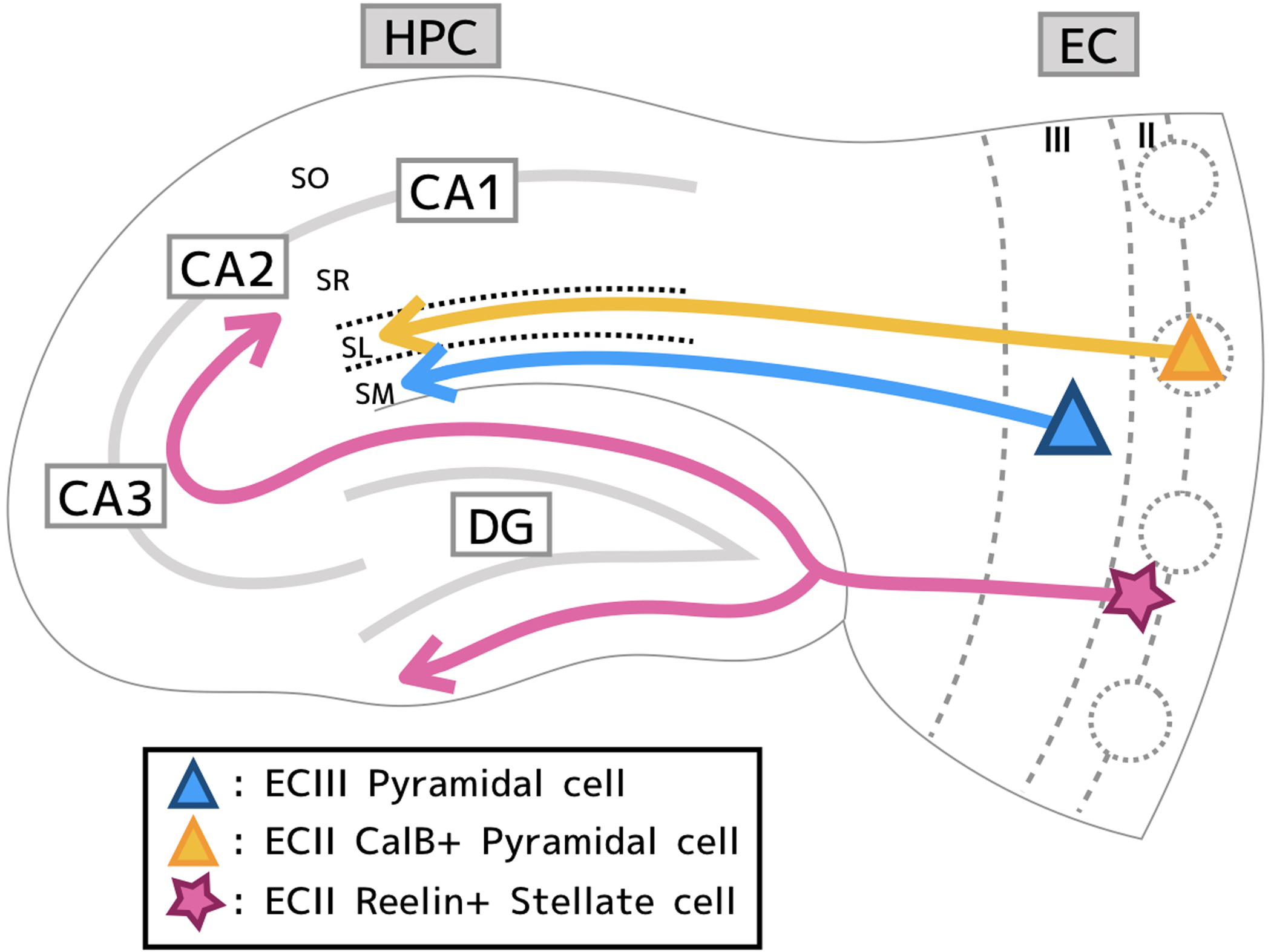
Diagram of the EC-HPC circuit depicting the layout of three major inputs from EC to HPC. I) The direct pathway from ECIII pyramidal cells (blue) to stratum moleculare (SM). II) The indirect (trisynaptic) pathway from ECII Reelin+ stellate cells (pink) projects to the granule cells of the DG. Mossy fibers project to CA3 pyramidal which then project to CA1. Mossy fibers from DG also project to CA2. III) A regulatory pathway projecting from ECII CalB+ pyramidal cells (orange) to stratum lacunosum (SL) interneurons which regulate ECIII input onto hippocampal CA1 pyramidal cells.
Pyramidal cells in hippocampal CA1 are crucial for temporal association learning
During the memory formation, temporally discontiguous events are linked by a process known as temporal association learning (Wolff, 1966; Solomon et al., 1986; Moyer et al., 1990; Wallenstein et al., 1998; McEchron et al., 1999). In experimental animal models, temporal association memory paradigms have been made possible with the application of Pavlovian conditioning (Pavlov, 1927; Solomon et al., 1986; Maren, 2001). Delay fear conditioning, in which a neutral conditioned stimulus (CS), often in the form of a tone, is paired with a shock or other aversive unconditioned stimulus (US) in such a way that the two events occur sequentially with a small overlap in timing and then terminate simultaneously, can be used to elicit a conditioned freezing response in trained animals (Weiss & Disterhoft, 2011; Tipps et al., 2014). Trace fear conditioning (TFC) introduces a temporal gap, in which the CS ends before delivery of the US begins. The time between the end of the CS and beginning of the US is called the “trace period” or “trace interval” (Solomon et al., 1986; Moyer et al., 1990; McEchron et al., 1999). These temporally based behavioral paradigms allow for assessment of differential contributions of different brain structures to temporal association learning The hippocampus has been shown to be indispensable for TFC, but delay fear conditioning does not appear to be hippocampus dependent (Solomon et al., 1986; Moyer et al., 1990; Weiss et al., 1999; Tseng et al., 2004). This suggests that the phenomenon of temporal association learning that bridges the gap between events across time is heavily dependent on the functional processes happening within the hippocampus (Meck et al., 1984; Solomon et al., 1986; Moyer et al., 1990; Lyford et al., 1993; Jackson et al., 1998; McEchron et al., 1999; Takehara et al., 2002; Takehara et al., 2003). In a CA1 specific N-methyl-D-aspartate (NMDA) receptor knockout mouse model, there is an observed deficit in TFC but not delay fear conditioning (Tsien et al., 1996; Huerta et al., 2000; Fukaya et al., 2003). Subregion specific lesions within CA1 were also found to cause similar disruption of TFC. Lesions to CA3, by contrast, did not (Kesner, 2000; Kesner et al., 2005). These findings suggest that the synaptic plasticity of CA1 pyramidal cells is necessary for TFC to bridge the temporal gap.
Input from pyramidal cells in MECIII into hippocampal CA1 drives temporal association learning
Lesions to the EC have also been shown to impair temporal association memory, specifically trace-eyeblink conditioning, but again not delay eyeblink conditioning (Ryou et al., 2001). Similar impairments were seen in TFC following EC lesions (Esclassan et al., 2009). Additionally, infusions of muscimol into the LEC specifically resulted in impaired recall during trace eyeblink testing (Morrissey et al., 2012). These findings provide evidence that in addition to CA1, the function of the EC is also necessary for temporal association memory. The use of various physical and chemical lesions, and genetic alterations to target portions of the EC-HPC network in order to determine their general contribution to a temporal association memory has been critical in uncovering the role of the hippocampus in this function, however the techniques are limited in their ability to determine the contributions of specific cell-types and local circuit activities. Two main excitatory inputs to the hippocampus arise from the EC; the tri-synaptic pathway and the direct pathway as described above (Fig. 1) (Kitamura et al., 2010; Kitamura & Inokuchi, 2014; Kohara et al., 2014; Kitamura et al., 2015a). In order to determine the importance of the inputs from ECIII to the CA1 pyramidal cells, a critical experiment was performed by Suh et al. utilizing a specifically designed transgenic mouse line expressing the Tetanus-toxin light chain (TeTX) in the dorsal MECIII. The expression of TeTX caused a blockade of signal from MECIII to CA1 pyramidal cells (Suh et al., 2011). The mutant mice showed a deficit in TFC but not delay fear conditioning. Utilizing a similar TeTX line to assess CA3 input to pyramidal cells, no deficit in TFC was shown (Nakashiba et al., 2008; Suh et al., 2011). The CA3 may still have a limited role in temporal association learning, as multiple lesion studies have shown disruptions to spatial working memory (Handelmann & Olton, 1981; Jarrard, 1983; Kesner, 2000), however, optogenetic silencing of MECII inputs from Reelin+ cells to the tri-synaptic pathway did not result in reduced TFC (Kitamura et al., 2015b), indicating that the monosynaptic inputs from MECIII to CA1 have a direct role in temporal association learning.
To better understand the circuit dynamics underlying temporal association memory, the use of optogenetics became necessary. With optogenetic approaches, either inhibition or activation of specific cell populations or terminals with precise timing on the order of milliseconds is possible (Boyden et al., 2005; Tye et al., 2011; Deisseroth, 2015), allowing for a more focused approach to understanding the individual contributions of components within the EC-HPC circuit. Importantly, this approach allows for inhibition of cellular populations at different points in a behavioral testing paradigm. Kitamura et al. in 2014 showed that optogenetic inhibition of MECIII input into HPC during TFC results in deficient TFC test performance (Kitamura et al., 2014). Oppositely, optogenetic activation of MECIII inputs to HPC by 4Hz light stimulation enhances TFC (Kitamura et al., 2014). This reveals that MECIII signal to CA1 during the gap between events is required for temporal association, and opens up a new line of questioning into what the nature of the signal itself is that allows the linkage of events across time. A possible, but still unproven, hypothesis is that persistent spiking activity of these neurons generates a consistent, pacemaker like signal that may support a framework for temporal processing downstream in the hippocampus (Frank & Brown, 2003; Major & Tank, 2004), by creating a consistent, repetitive input from MECIII to the hippocampus acting as a backdrop against which events can be ordered. This sort of activity is observed in other cortical regions, and even within the MEC itself for other functions (Kubota & Niki, 1971; Egorov et al., 2002). Persistent firing activity has been identified in MECIII cells in vitro (Yoshida et al., 2008), but not yet in vivo. Although the phenomenon has yet to be seen, the observation in vitro, which is dependent on glutamatergic activation of mGluR1 and Muscarinic 5-HT receptors (Egorov et al., 2002; Yoshida et al., 2008), is suggestive of possible persistent firing activity in vivo. Blocking cholinergic inputs to EC chemically or by deafferentation causes reduced TFC performance (Esclassan et al., 2009). Furthermore, strong inhibition of TFC is observed when antagonists for both mGluR1 and muscarinic 5-HT receptors are injected together into the MEC (Suh et al., 2011). Additionally, increased phasic multiunit activity bursts during the delay period in a delayed-non-matching-to-place task have been observed in wild type mice, and these bursts were weaker in MECIII-TeTX mice (Yamamoto et al., 2014). When all these lines of evidence are considered, it presents the possibility that the burst activity in the MECIII may be related to the observed bursting in vitro, and that this activity could be a mechanism for the association of event-related information across gaps in time. This theoretical backdrop of activity might then serve as a modifiable element to enable temporal encoding.
Input from CalB+ pyramidal cells in MECII cells into hippocampal CA1 regulates temporal association learning
Temporal association learning provides a critical means for cognitive adaptation to both dangerous and beneficial events (McEchron & Disterhoft, 1997; Kitamura, 2017). It stands to reason that such a critical process must be tightly regulated. If the association is too weak, a memory may not form. Conversely, overly strong association making may result in inappropriate linkages or the inability to distinguish useful from nonsense associations (Kitamura, 2017). A neural mechanism to regulate the formation of temporal association memories was discovered by Kitamura et al in the form of a novel circuit in the EC-HPC network, as described above (Fig. 2). CalB+ cells of ECII project to a population of interneurons within SL of CA1 (Kitamura et al., 2014). These inputs from the CalB+ cell clusters run parallel to the ECIII projections to the distal dendrite segments of CA1 pyramidal cells, forming a laminar structure (Kitamura et al., 2014). The ECII CalB+ activation of SL-interneurons near the inputs from ECIII provides the substrate for a feed-forward inhibitory circuit that regulates MECIII inputs into hippocampal CA1 (Kitamura et al., 2014) (Fig 2).
Figure 2.
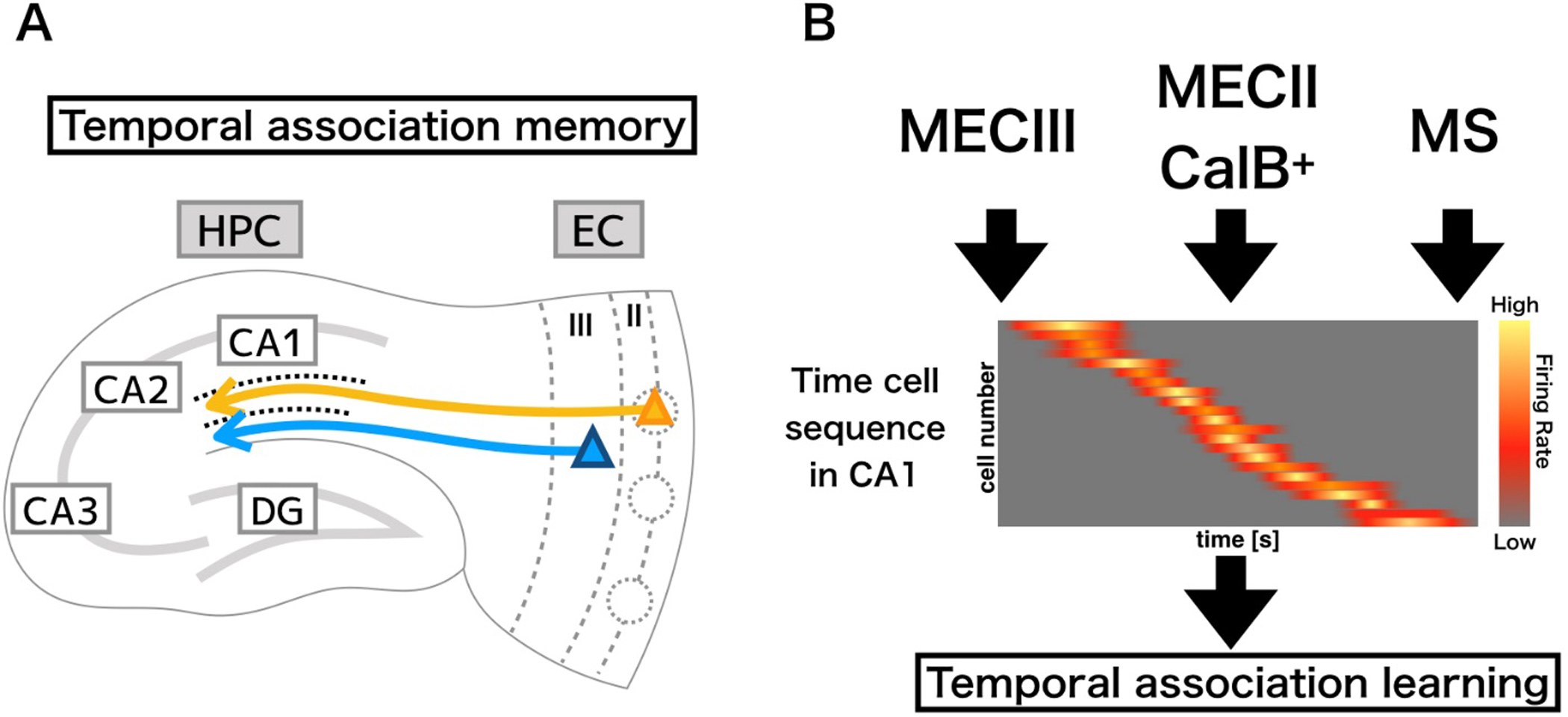
Circuitry involved in temporal association memory. A) The pyramidal cells of MECIII project directly to distal dendrites of CA1 pyramidal cells, while pyramidal cell clusters in ECIII project to interneurons in SL. B) this interplay between excitatory ECIII and inhibitory action driven ECII CalB+ inputs in CA1 tightly regulates temporal encoding, along with input from the medial septum (MS) resulting in a temporal sequence, and a refined temporal association memory. The temporal sequence can be seen in the sequential activation of individual neurons at a specific point in time within a larger time interval, with the total population covering the entirety of the interval period.
Differential contributions of both these circuits to temporal association learning were assessed using optogenetic tools for activation by Channelrhodopsin2 (ChR2) or inactivation by Archaerhodopsin (ArchT, eArch3.0) of specific cell types during TFC. (Han et al., 2011; Mattis et al., 2011; Kitamura et al., 2014; Okuyama et al., 2016). Inhibition of the MECIII input into HPC CA1 during TFC (tone + trace + shock), as was expected, caused reduced the TFC performance (Suh et al., 2011). Importantly, optogenetic activation of the CalB+ cells input into HPC CA1 during TFC inhibited TFC learning. Neither condition, however, had an effect on contextual fear conditioning (Mingote et al., 2015), indicating that both circuit components deal primarily with the transmission of temporal information and regulation of temporal association learning. Additionally, Inactivation of the CalB+ cells caused enhanced performance in TFC, showing a bidirectionality in the regulation of MECIII inputs by the CalB+ cells control of SL-interneurons, supporting the view of a feedforward inhibitory mechanism for the regulation of temporal association learning (Kitamura et al., 2014). The relative strengths of these two inputs is believed to be critical in determining the strength and longevity of these temporal associations.
Sequential time cell activity in CA1 pyramidal cells may link events across the trace period
If the MEC is the upstream driver of temporal association memory formation, the CA1 pyramidal cells, as the recipients, are likely to in some way act to encode this memory according to Hebbian principles (Hebb, 1949). This is supported by observations of temporal memory deficits with genetic knockout of NMDARs in the hippocampal CA1 pyramidal cells (Huerta et al., 2000). The hippocampus is well known for its role in memory of spatial and temporal information (Scoville & Milner, 1957; Waxler & Rosvold, 1970; Ekstrom & Ranganath, 2017). Hippocampal pyramidal cells have been observed to be responsive to specific places, acting as “place cells”, demonstrating that the pattern of hippocampal pyramidal cell activity can directly encode spatial features (O’Keefe & Dostrovsky, 1971). Interestingly, hippocampal CA1 pyramidal cells exhibit temporal organization of their neural firing (McEchron & Disterhoft, 1997; McEchron et al., 2003; Pastalkova et al., 2008; MacDonald et al., 2011). The temporal organization of this activity spans the entirety of the trace period, with individual neurons firing at specific moments within that period. For example, one cell may consistently fire at 1–2 sec from the start of the trace period, while another fires consistently at 5–6 sec. This activity, known as “internally generated cell assembly sequences” or “time cell sequences”, allows the hippocampus to form a representation of the temporal gap between events (Pastalkova et al., 2008; MacDonald et al., 2013).
It has been demonstrated that rodents run on a treadmill also showed distinct temporal tuning during repetitive runs (Kraus et al., 2013; Salz et al., 2016; Mau et al., 2018). Mau et al. utilized in vivo calcium imaging to identify time cell sequences across differential timescales (Mau et al., 2018). The activity of time cells has typically been measured in the scale of seconds (Kraus et al., 2013; Modi et al., 2014), however, Mau et al discovered differential mechanisms that allow the representation of time in the range of seconds, minutes, and days simultaneously utilizing repetitive runs on a treadmill based task. Second-scale activity is encoded by the repetitive activity of time cells at particular point in a gap phase period, as previously examined. Importantly, it was possible to decode the temporal sequences of the cellular activity to predict the temporal location with a 10s running period. Successful reconstructions of minute-scale representations are achieved by observing the activity of any individual time cell relative to the order of the trials presented. Shifts in the populations of time cells which were active across multiple days could also be decoded to indicate which day a particular trial occurred on (Mau et al., 2018).
Even though time cell activity can be observed within the delay period of timing-related behavioral tasks despite the presence of body movement in the subject animal, a more desirable condition for analysis of time cell activity would be for animals to be immobile during the delay or timing period (Kraus et al., 2013; Marks et al., 2019). Utilizing tetrode-implanted, head fixed rats MacDonald et al. performed a delayed matching to sample task for odor cues. Time-sensitive cells in CA1 that fired during the delay period between matching sets were observed, and individual cells fired consistently within the delay period at a specific temporal bin across the theta cycle (MacDonald et al., 2013). Each odor pair memory presented with a unique set of neurons firing in the delay period, with some neurons being active across multiple odor pairs. Those cells that fired across multiple memories either fired at the same point in the delay across memories or showed differential temporal tuning by odor pair memory. Hippocampal time cell activity has been also observed in head fixed animals trained in the trace eyeblink paradigm with two-photon calcium imaging. Within the delay period of the trace eyeblink task, individual neurons were observed firing sequentially at specific points within the delay period (Modi et al., 2014). More recently, Marks et al developed new behavioral paradigms, in which mice must maintain a nose poke during auditory cue presentation for up to ten seconds, and further need to discriminate the duration of cue presentation, in order to get a reward (Marks et al., 2019). These tasks have been designed for freely moving animals, and thus are compatible with activity measurement/manipulation methods free of body or head restraint. Furthermore, by combining these new tasks with head-mounted microscope imaging (Ziv et al., 2013; Sun et al., 2015; Okuyama et al., 2016; Kitamura et al., 2017; Roy et al., 2017), Marks et al successfully recorded time cell activity in the hippocampus while animals were voluntarily holding still during the nose poking period (Marks et al., 2019).
The origin of time cell activity in the hippocampal CA1 is not fully understood. Questions remain as to whether sequences are generated locally in the hippocampus and biased/modulated by EC and other brain regions, or if time cell sequences are transmitted from MEC directly. Time cell activity is abolished by inactivation of the medial septum, which generates the hippocampal theta (Lee et al., 1994; Wang et al., 2015). At the same time, optogenetic inactivation of MEC also causes loss of time sequence coherence (Robinson et al., 2017). In light of the previous findings on the role of theta and MEC inputs in memory formation, we speculate that the CA1 time cell sequences are generated by concerted action of both EC and medial septum in order to associate temporally discontinuous events and remember specific timing in memory formation (Fig. 2).
Inputs from Reelin+ stellate cells in MECII into hippocampus are crucial for contextual memory
The ECII-DG-CA3 pathway has historically been considered to be crucial for the generation of discriminatory representations of spaces and contexts bearing strong similarity to each other, with processing occurring at each step along the tri-synaptic pathway. The paradigms used in this context separation were understood to occur along this pathway based in part on observations of the large amount of DG granule cells, the sparse activity of those same cells, and the low redundancy observed in DG-CA3 synaptic connections (Marr, 1971; O’Reilly & McClelland, 1994; Treves & Rolls, 1994; Leutgeb et al., 2007; Bakker et al., 2008). These theories are supported with experimental data showing the physiological responses of CA3 pyramidal cells during alternating exposure to pairs of closely related contexts (Leutgeb, 2004; Leutgeb et al., 2007; McHugh et al., 2007; Wintzer et al., 2014), and data showing the behavioral phenotype of mice without functional NMDAR expression in the dentate granule cells (McHugh et al., 2007). More recent studies utilizing mice with impaired adult neurogenesis (Altman & Das, 1965; Schlessinger et al., 1975; Seki & Arai, 1993; Eriksson et al., 1998) and/or blockade of DG-CA3 inputs, however, showed that the input from the mossy fibers from DG to CA3 were not necessary for the discrimination of similar contexts (Nakashiba et al., 2012). Instead, a relatively small contingent of the larger granule cell population, comprised of newly generated granule cells (Kitamura et al., 2009; Arruda-Carvalho et al., 2011; Gage & Temple, 2013; Kitamura & Inokuchi, 2014; Alam et al., 2018; Terranova et al., 2019), was found to be responsible for the contextual discrimination (Clelland et al., 2009; Scobie et al., 2009; Creer et al., 2010; Sahay et al., 2011; Nakashiba et al., 2012). Mutant mice lacking neural transmission from CA3 outputs due to expression of TeTX in CA3 pyramidal cells also showed deficits in contextual fear conditioning. These studies have all been directed towards understanding the roles of the various HPC subfields and their activity patterns as the driving force underlying contextual memory. However, these studies have not touched on upstream circuits, like those in ECII, and their potential contributions to contextual memory and context discrimination. The possibility exists, however, that EC cells may demonstrate a heterogenous pattern of activity such that differential contexts drive differential patterns of neuronal activity in the region. This type of activity may be capable of driving the discrimination of contextual cues downstream in the HPC, and in that way contributing to contextual memory encoding. Kitamura et al in 2014 applied a novel approach to differentiate two populations of MECII excitatory neurons, and examine their unique functionalities (Kitamura et al., 2014). Their study focused on the respective roles of Reelin+ and CalB+ neurons of MECII in the process of contextual discrimination by applying in vivo Ca2+ imaging to observe the activity patterns of Reelin+ and CalB+ cells while freely moving mice were exposed to two different contexts with an alternating presentation. Cell type-specific in vivo Ca2+ imaging revealed that Reelin+ cells rapidly form a distinct representation for novel contexts, while CalB+ cells in MEC did not have any context-specific activity (Kitamura et al., 2015b). Consistent with these findings, other groups using single unit tetrode neural recording found similar context-specific neural firing in the MEC (Pérez-Escobar et al., 2016; Diehl et al., 2017). What neural inputs generate such context-specific neural activity of Reelin+ cells in MECII? One possibility is that context-specific activity is generated by a diverse set of external inputs from outside of the EC-HPC networks including postrhinal cortex, visual cortex, medial septum, para-subiculum, and pre-subiculum (Canto et al., 2008). Another possibility is that the back projection from hippocampal CA1 into ECV/VI could contribute to the context-specific activity of Reelin+ cells, however,, Kitamura et al showed pharmacological inhibition of CA1 activity did not have a strong effect on the context-specific activity of Reelin+ cells in MECII (Kitamura et al., 2015b), indicating that MECII rapidly processes contextual information and sends it to the hippocampal DG, CA3, and CA2 neurons (Fig 3).
Figure 3.
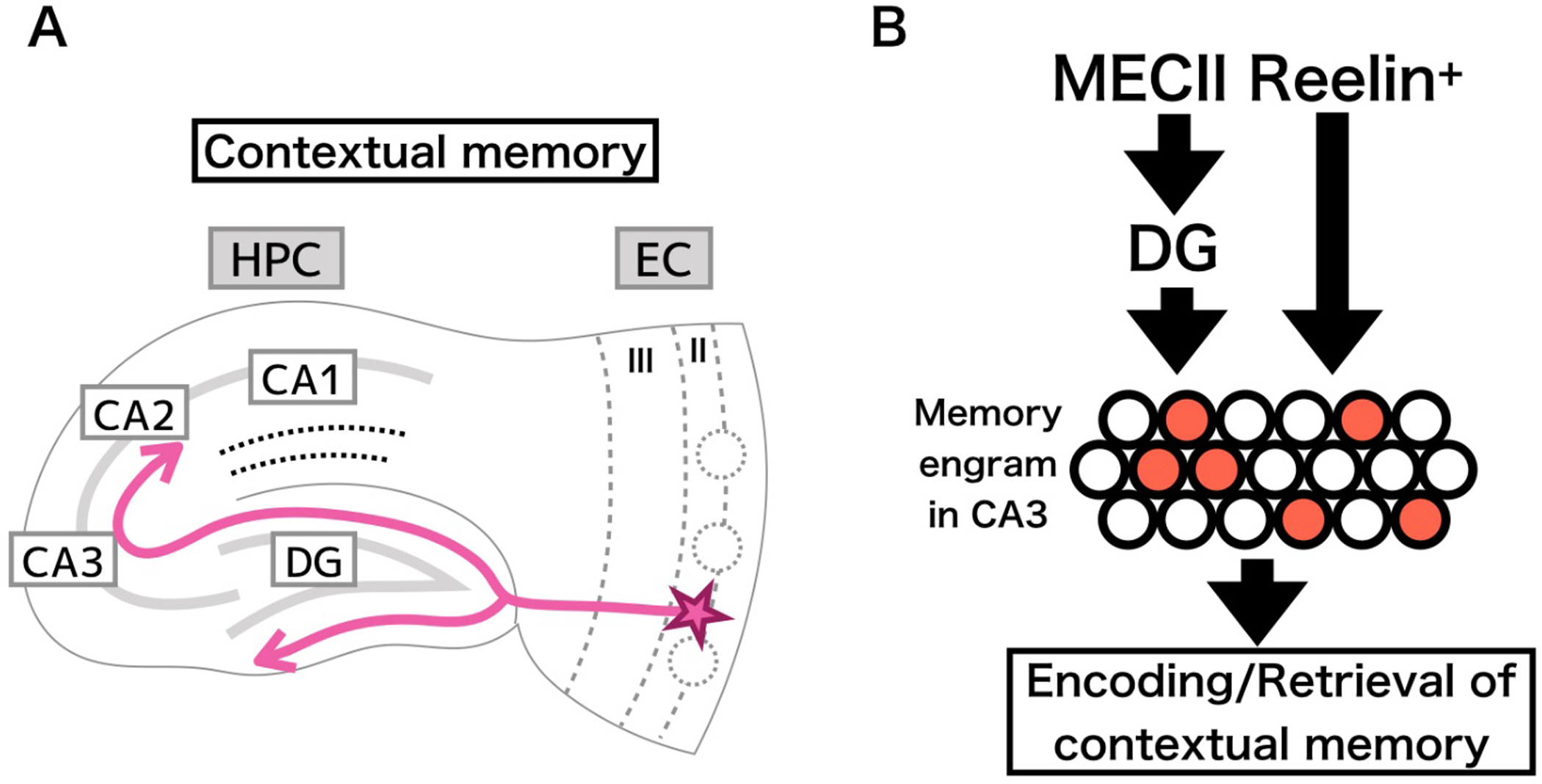
Circuitry involved in contextual memory. A) Reelin+ stellate cells in ECII act on CA1 by means of the traditional tri-synaptic pathway. B) parallel inputs along the tri-synaptic pathway and collateral direct inputs to CA1 enables the encoding of a contextual representation in the form of an engram, a specific subset of cells activated by a particular stimulus (indicated by red filled circles) that can be reactivated to initiate memory recall.
To investigate the role of MEC inputs into the HPC on the formation and recall of contextual memory, Kitamura et al, further examined optogenetic manipulation of each type of HPC-projecting MEC excitatory neuron during contextual fear conditioning (CFC), which is an association between context and aversive foot shock (Pavlov, 1927; Kim & Fanselow, 1992; Phillips & LeDoux, 1992; Frankland et al., 1998; Amaral & Lavenex, 2007; Sutherland et al., 2008; Maren et al., 2013; Kitamura et al., 2015b; Kitamura et al., 2017). They showed that optogenetic cell body inhibition of Reelin+ cells during CFC disrupted the formation of CFC memory, while optogenetic inhibition of CalB+ cells or MECIII cells during CFC did not affect CFC memory (Kitamura et al., 2014; Kitamura et al., 2015b). Optogenetic inhibition of Reelin+ cells during testing of CFC also disrupted the recall of CFC memory, indicating that neural activity of Reelin+ cells is essential for both formation and recall of CFC memory. While Reelin+ cells in MECII have been also found as a critical functional component for spatial navigation memory (Kanter et al., 2017; Qin et al., 2018; Tennant et al., 2018), importantly, Reelin+ cells in MECII did not have a critical role in trace fear conditioning (Kitamura et al., 2014), which requires two MEC direct inputs (MECIII cells and CalB+ cells) into CA1, indicating that the multiple MEC inputs into the HPC have complementary roles for temporal and contextual memory formation respectively (Fig. 2–3).
Inputs from Reelin+ stellate cells in MECII into hippocampus are crucial for the generation and reactivation of hippocampal memory engram cells for a specific context
Approaches for targeting specific cell populations, in this case active cells as a functional subset rather than an anatomical or biochemical classification, have yielded crucial information about the encoding and recall of contextual memories in the form of engram cells (Guzowski et al., 1999; Han et al., 2007; Reijmers et al., 2007; Han et al., 2009) Richard Semon first developed the physical theory of human memory in the early 19th century (Semon, 1921).To define a hypothetical physical substrate of memory, he developed the term “engram”. The currently accepted idea of what defines a true memory engram can be described by a set of three criteria (Josselyn, 2010; Tonegawa et al., 2015; Tonegawa et al., 2018). An engram i) is a substantive and long-term alteration to a neural network either in its physical structure or chemical activity/composition, ii) results from induction of activity in a subpopulation of neurons by event stimulus, and iii) can be reactivated by a stimulus that was a part of the original set of stimuli to initiate recall of the memory. Activity-dependent cell labeling, made possible by using immediate early gene promoters can be paired with optogenetics to drive expression of an opsin protein in specific neurons activated during an event, has resulted in the first observations of an “engram cell” (Reijmers et al., 2007; Liu et al., 2012). Labeling of DG cells active during CFC with ChR2 was performed by Liu et al in 2012, utilizing a doxycycline dependent ChR2 expression paradigm. They observed that a 20Hz pulse stimulation with blue light along implanted optic fibers terminating in DG was able to induce memory recall in the absence of the original cue in a context specific manner (Liu et al., 2012). All three of the criteria for a true engram are met by this activity in the dentate gyrus granule cells (Josselyn, 2010; Tonegawa et al., 2015; Tonegawa et al., 2018). These studies highlight the idea that a specific subset of neurons activated during a particular event can be artificially reactivated with optogenetic stimulation is sufficient for recall of a memory, and furthermore provide evidence for engrams in the dentate gyrus. Subsequent studies also showed that memory engram cells can be also formed in CA3 and CA1 for contextual memory (Ramirez et al., 2013a; Ramirez et al., 2013b; Denny et al., 2014; Ohkawa et al., 2015; Ryan et al., 2015; Guo, 2018; Tanaka et al., 2018; Ghandour et al., 2019; Oishi et al., 2019). Furthermore, , Choi et al recently found that engram cells preferentially form synapses with other engram cells in the HPC (Choi et al., 2018). Therefore, engram cells are described as cell networks which physically store the information pertinent to a memory, and activation of a local engram network is necessary and sufficient to recall memory (Tonegawa et al., 2015; Denny et al., 2017).
Using targeted approaches can allow for a more in-depth analysis of how MEC inputs into the HPC contribute to the formation of engram networks for encoding contextual memory. Kitamura et al examined the effects of optogenetic inhibition of Reelin+ or CalB+ cell activity on the generation of contextual memory engrams in HPC sub-regions (Kitamura et al., 2015b). They found that optogenetic inhibition of Reelin+ cells inhibited the number of c-Fos+ cells activated by novel context exposure in both hippocampal DG and CA3, while optogenetic inhibition of CalB+ cells in MEC did not, indicating that MECII input into hippocampal DG and CA3 is crucial for the generation for contextual memory engrams. They also tested the reactivation of the memory engrams upon re-exposure to the same context. Memory engram cells in CA3 were reactivated when an animal re-explored the same context after its initial presentation (Nakashiba et al., 2009; Denny et al., 2014; Kitamura et al., 2015b; Lacagnina et al., 2019). Kitamura et al demonstrated that the MECII Reelin+ input into the HPC is crucial for the reactivation of CA3 engram cells (Kitamura et al., 2015b), which is essential for the recall of contextual memory. In summary, contextual memory for specific contexts are stored in a small population (5–10%) of neurons activated during contextual learning as memory engrams which are necessary and sufficient for the recall of contextual memory. Reelin+ cells in MECII drive context-specific activation to downstream hippocampal circuits to generate and reactivate hippocampal memory engrams cells to form and recall contextual memory, respectively (Kitamura et al., 2015b) (Fig. 3).
Integration mechanisms of temporal and contextual memory in hippocampal CA1 pyramidal cells
While previous sections examined how differential entorhinal inputs contribute to temporal or contextual memory, how these memories are integrated to in successful memory encoding remains an open question. Eichenbaum theorized that the integration process would involve a “mixed selectivity” of neurons within a population, meaning that elements are independently selected from encoded sequences rather than having a unified memory engram. In this model, A memory would be constructed by the activation of differentially coded data sets within the same population (Eichenbaum, 2017). We believe that this integration would be conducted within the population of CA1 pyramidal cells. Multiple studies demonstrate that time-responsive and place or movement responsive cell populations can overlap in CA1 (MacDonald et al., 2011; Kraus et al., 2015; Mau et al., 2018), however, some data exists that suggests that this may be an integrated response; for example, a context-dependent time cell, or a place-dependent speed cell. This is likely due not to a complete unification of data, but rather a stratification in selection of a particular ensemble during encoding and recall, with the overlap occurring between population subsets serving as a functional linkage. Evidence of such activity dependent ensemble selection has been observed in the form of object-relative spatial coding (Connor & Knierim, 2017).
MacDonald et al in 2011 demonstrated that while some neurons are responsive to time regardless of location, a subpopulation of time cells were directly influenced by an animals’ location (MacDonald et al., 2011).This is supported by a more recent study showing that the CA1 network fluctuates between spatially and temporally permissive states, usually occupying a state permissive to both (Haimerl et al., 2019). Close examination of active time cells between different odor presentations in MacDonald et als’ 2013 examination of time cell activity in rats shows that some time cells maintain a specific temporal profile across two different odors, while one slightly shifts its activation time across differing presentations (MacDonald et al., 2013). Furthermore, analysis of the time cell activity in Sabariego et als’ 2019 study suggests that there is a contextual element overlapping the temporal processing (Sabariego et al., 2019). These data stand in support of variable, overlapping activity in CA1 pyramidal cells. In addition, mathematical modeling suggests the possibility of the already differential activity of place cells in a history or path dependent manner behaving differentially across time, indicating that the processing boundary between specific places or contexts and temporal activity may occur on a longer scale of time (minutes to hours vs seconds) (Howard et al., 2014). Gradual changing of time cell sequences across hours and days has been observed, which was found to be encoded by a general shift in the population of pyramidal cells acting as time cells from one day to the next (Mau et al., 2018). CA2 place cells have been observed to have a greater level of instability across time than place cells in CA1 (Mankin et al., 2015; Hainmueller & Bartos, 2018). within hours, it may be possible for CA2 to generate a gradual shift of time cell activity concerted with MECIII and MECII CalB+ inputs. Could this process be occurring in CA1 as well? CA1 serving as the site of integration for these data streams is probable, considering the tight regulation of inputs by a vast array of local interneuron interactions (Ali et al., 1998; Klausberger & Somogyi, 2008; Cutsuridis et al., 2010; Stark et al., 2014; Amilhon et al., 2015; Del Pino et al., 2017). While the CA1 interneuron network functions as a large network, anatomically, a few specific subpopulations are more obvious contributors to the integration processes due to their known associations with dendritic sub-compartments of the CA1 pyramidal cells. Firstly, Somatostatin positive cells of the stratum oriens, the oriens-lacunosum moleculare cells (OLMs), project to stratum lacunosum moleculare and inhibit the distal terminals of CA1 pyramidal cells (Fig 1B) (Leao et al., 2012; Orban-Kis et al., 2015) which receive temporal information from the entorhinal cortex layer III (Fig 1) (Kitamura et al., 2014). The Parvalbumin (PV) positive cells of the stratum radiatum, which include bistratified cells and PV+ basket cells (Ali et al., 1998; Klausberger & Somogyi, 2008), preferentially innervate the more proximal apical dendrites of CA1 pyramidal cells in the stratum radiatum (Sik et al., 1997; Ali et al., 1998; Muller & Remy, 2014; Pelkey et al., 2017) which receive contextual information via CA3 (Fig. 1) (Kitamura et al., 2015b). While the anatomical evidence alone is compelling, the OLMs and at least one subset of PV+ interneurons have been shown to interact with each other to form a feedback control/input gating loop that differentially interacts with the ECIII and CA3 inputs in a theta dependent manner (Buhl et al., 1996; Sik et al., 1997; Klausberger et al., 2004; Leao et al., 2012; Muller & Remy, 2014). Our interest in the hippocampal representation of memory is the differential processing of different information types within the hippocampus, and the integration strategies used to link the different memory components. For example, while contextual memory components discussed in this review were examined in terms of engrammatic storage, no true time engram has yet been identified. It would be reasonable to conclude that a continuous variable like time would need to be encoded by a more dynamic process, as it is not a discrete event or object that can be recalled like a snapshot, but rather acts more as a linkage between snapshots. The time engram may be widely distributed to allow its integration into various memory engram structures. This is not far off the theory proposed by Yntema & Trask in 1963, and further refined by Wolff in 1966, that a sort of temporal stamp is somehow associated with a particular object or event to enable sequential orientation of remembered units (Yntema & Trask, 1963; Wolff, 1966). It has been demonstrated that dendritic plateau potentials in CA1 pyramidal cells originating from ECIII can act to predispose a pyramidal cell to burst firing activity resulting from concurrent CA3 input activity (Bittner et al., 2015; Grienberger et al., 2017). Bittner et al demonstrated that an individual pyramidal cell is not tuned to a given location prior to place field formation, but rather is recruited by these concurrent inputs, which they suggest is driven in part by local plasticity in CA1 and differential grid cell firing patterns from ECIII (Bittner et al., 2015), which in addition to GABAergic interneuron regulation (Cutsuridis & Hasselmo, 2012; Grienberger et al., 2017), causes a long term alteration in the local microcircuit environment to support integrative activity for these two signals in a given spatial environment. Calcium imaging in the dorsal hippocampus reveals that stable place fields can persist on the scale of days to weeks (Rubin et al., 2015; Kinsky et al., 2018), however periodic place independent representations and spatial remapping events occur which were discovered to contain functional timestamps as assessed by the rate of change in overlap between representations against the stable rate of place field remapping. These decoded time sequences were able to predict sequential order of events, and were able to link events in different locations across various timescales (Rubin et al., 2015). Moreover, time fields have been observed to shift across the scale of days, such that over a given period of time the identity of individual time cells will change, however, some overlap across timescales will remain, potentially allowing the linkage of events across time as individual cells have active representations in multiple timescales (Mau et al., 2018). In conjunction with observations of differing engram stability across hippocampal subregions (Mankin et al., 2015; Hainmueller & Bartos, 2018), these findings may support the idea that the imposition of sequential timing information onto memory structures involves an interplay between the rate of change of relatively static vs. dynamic engrams, allowing the linkage of events across time into a set of associated recallable units. This makes sense in light of the findings from Susumu Takahashi’s 2013 study, which showed that place fields can represent the same place across multiple tasks by changing the frequency of their firing, or rate remapping, rather than a complete spatial remapping. The observed rate remapping was tied to a hierarchical representation of different contexts (Takahashi, 2013). It is not a far leap to consider similar processes may be proceeding with other forms of information, and that a hierarchical approach to rate remapping across related events within existing engrams is a more broadly active component of the hippocampal integration strategy. Indeed, the mechanisms behind the integration of individual spatial components are still severely understudied, in part due to the complexities of the processing of these individual components themselves.
Here, we speculate contextual activity may influence which cells become active time cells during an event, and that those contextual memory engram cells can work as an index to retrieve context-dependent time cell sequences (Fig. 4). For example, if memory engram cells for context A in HPC CA3 are activated, Context A dependent time cell sequences are generated in hippocampal CA1 cells. If memory engram cells for context B in HPC CA3 are activated, Context B dependent time cell sequences are generated in hippocampal CA1 cells. This differential temporal encoding dependent on contextual representations could be a mechanism for the linkage of temporal information with engrammatic storage of event information.
Figure 4.
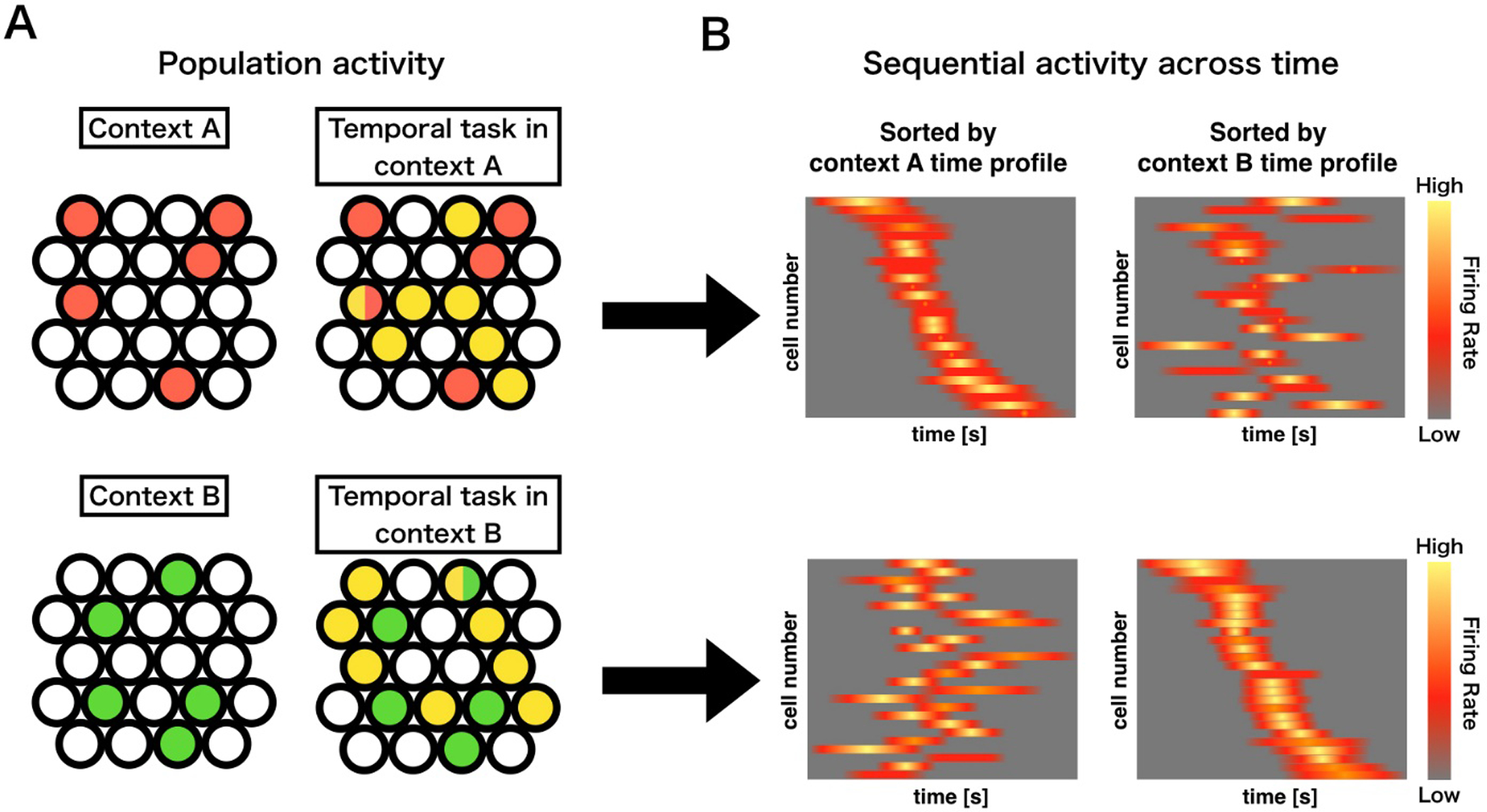
A hypothetical process for context-time integration in CA1. Panel A depicts the summation of population activity within two different hypothetical contexts; A (upper), and B (lower). Differential exposure to contexts will result in formation of a particular memory engram specific to that context (red or green). Active timing or temporal task performance within a specific context will result in a time cell assembly unique to that particular context (Yellow) and having some overlap with the contextual engram cells (mixed color). Panel B depicts the sequential activity across time of the time cell populations specific to the differential contexts in panel A. The resultant time cell sequences will be indistinguishable in a different context, allowing for a specific linkage between contextual memory units and temporal information
Conclusions
In this review, we have addressed the current understanding of the circuit mechanisms behind temporal association learning and contextual memory in the EC-HPC networks. Crucial inputs from MEC to CA1 were observed using cell-type specific TeTX or optogenetic approaches. MECIII inputs to the distal dendrites of CA1 were shown to be crucial for temporal association, while MECII CalB+ cell inputs to SL interneurons in CA1 engage in feed-forward regulation of the temporal signal from MECIII. Reelin+ cells in MECII, by contrast did not carry temporal information, but were involved in contextual memory formation via the hippocampal tri-synaptic pathway (Nakashiba et al., 2008; Suh et al., 2011; Kitamura et al., 2014; Kitamura et al., 2015b). In this way, the MEC presents the hippocampus with two distinct information streams; context via the tri-synaptic synapses, and time, primarily via the temporoammonic pathway and regulated by a novel feedforward circuit originating in MECII island clusters. These two modalities and others (e.g. smell, object) may be integrated at the CA1 subfield, with both sequential neural activity and engrammatic neural activity being combined into a singular, but multi-faceted representation during memory formation.
Acknowledgements
This work was supported by grants from Endowed Scholar Program to T.K, Human Frontier Science Program to T.K (RGY0072/2018), Brain Research Foundation to T.K (BRFSG-2018-04), Faculty Science and Technology Acquisition and Retention Program to T.K, the Brain & Behavior Research Foundation to T.K (26391), The Whitehall Foundation to T.K (2019-05-38), National Institute of Mental Health to T.K (R01MH120134) and WDM (T32MH076690-10), and Japan Society for the Promotion of Science to N.Y (201860573).
List of Abbreviations
- CA1
Cornu Ammonis 1
- CA2
Cornu Ammonis 2
- CA3
Cornu Ammonis 3
- CalB
Calbindin
- CFC
Contextual fear conditioning
- DG
Dentate gyrus
- EC
Entorhinal cortex
- HPC
Hippocampus
- Wfs1
Wolfram Syndrome 1
- LEC
Lateral entorhinal cortex
- MEC
Medial entorhinal cortex
- OLM
Oriens – Lacunosum Moleculare projecting cell
- PV
Parvalbumin
- SST
Somatostatin
- TeTX
Tetanus toxin light chain
- TFC
trace fear conditioning
References
- Alam MJ, Kitamura T, Saitoh Y, Ohkawa N, Kondo T & Inokuchi K (2018) Adult Neurogenesis Conserves Hippocampal Memory Capacity. The Journal of Neuroscience, 38, 6854–6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Deuchars J, Pawelzik H & Thomson AM (1998) CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol, 507 (Pt 1), 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Salz DM, McKenzie S & Fortin NJ (2016) Nonspatial Sequence Coding in CA1 Neurons. J Neurosci, 36, 1547–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A & Klink R (1993) Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol, 70, 128–143. [DOI] [PubMed] [Google Scholar]
- Altman J & Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol, 124, 319–335. [DOI] [PubMed] [Google Scholar]
- Amaral D & Lavenex P (2007) Hippocampal Neuroanatomy. In Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J (eds) The Hippocampus Book. Oxford University Press, New York, pp. 37–109. [Google Scholar]
- Amaral DG & Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience, 31, 571–591. [DOI] [PubMed] [Google Scholar]
- Amilhon B, Huh CY, Manseau F, Ducharme G, Nichol H, Adamantidis A & Williams S (2015) Parvalbumin Interneurons of Hippocampus Tune Population Activity at Theta Frequency. Neuron, 86, 1277–1289. [DOI] [PubMed] [Google Scholar]
- Aronov D, Nevers R & Tank DW (2017) Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature, 543, 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA & Frankland PW (2011) Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci, 31, 15113–15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M & Stark CE (2008) Pattern separation in the human hippocampal CA3 and dentate gyrus. Science, 319, 1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A & Siegelbaum SA (2016) Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science, 351, aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S & Magee JC (2015) Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci, 18, 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G & Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci, 8, 1263–1268. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Szilagyi T, Halasy K & Somogyi P (1996) Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus, 6, 294–305. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2013) Cognitive neuroscience: Time, space and memory. Nature, 497, 568–569. [DOI] [PubMed] [Google Scholar]
- Canto CB, Wouterlood FG & Witter MP (2008) What does the anatomical organization of the entorhinal cortex tell us? Neural plasticity, 2008, 381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V & Siegelbaum SA (2010) Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron, 66, 560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Sim SE, Kim JI, Choi DI, Oh J, Ye S, Lee J, Kim T, Ko HG, Lim CS & Kaang BK (2018) Interregional synaptic maps among engram cells underlie memory formation. Science, 360, 430–435. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH & Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science, 325, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE & Knierim JJ (2017) Integration of objects and space in perception and memory. Nat Neurosci, 20, 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H & Bussey TJ (2010) Running enhances spatial pattern separation in mice. Proceedings of the National Academy of Sciences, 107, 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsuridis V, Cobb S & Graham BP (2010) Encoding and retrieval in a model of the hippocampal CA1 microcircuit. Hippocampus, 20, 423–446. [DOI] [PubMed] [Google Scholar]
- Cutsuridis V & Hasselmo M (2012) GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations. Hippocampus, 22, 1597–1621. [DOI] [PubMed] [Google Scholar]
- Deisseroth K (2015) Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci, 18, 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pino I, Brotons-Mas JR, Marques-Smith A, Marighetto A, Frick A, Marin O & Rico B (2017) Abnormal wiring of CCK(+) basket cells disrupts spatial information coding. Nat Neurosci, 20, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A & Hen R (2014) Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron, 83, 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Lebois E & Ramirez S (2017) From Engrams to Pathologies of the Brain. Front Neural Circuits, 11, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS & Knierim JJ (2013) Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus, 23, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl GW, Hon OJ, Leutgeb S & Leutgeb JK (2017) Grid and Nongrid Cells in Medial Entorhinal Cortex Represent Spatial Location and Environmental Features with Complementary Coding Schemes. Neuron, 94, 83–92.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ & Norman G (2004) Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci, 24, 1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME & Alonso AA (2002) Graded persistent activity in entorhinal cortex neurons. Nature, 420, 173–178. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci, 1, 41–50. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2014) Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci, 15, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017) On the Integration of Space, Time, and Memory. Neuron, 95, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M & Tanila H (1999) The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron, 23, 209–226. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD & Ranganath C (2017) Space, time, and episodic memory: The hippocampus is all over the cognitive map. Hippocampus. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA & Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med, 4, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G & Marchand AR (2009) A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning. J Neurosci, 29, 8087–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL & Eichenbaum HB (2002) Critical role of the hippocampus in memory for sequences of events. Nat Neurosci, 5, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM & Brown EN (2003) Persistent activity and memory in the entorhinal cortex. Trends Neurosci, 26, 400–401. [DOI] [PubMed] [Google Scholar]
- Frankland P, Cestari V, Filipkowski R, McDonald R & Silva A (1998) The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav. Neurosci, 112, 863–874. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Kato A, Lovett C, Tonegawa S & Watanabe M (2003) Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci U S A, 100, 4855–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH & Temple S (2013) Neural stem cells: generating and regenerating the brain. Neuron, 80, 588–601. [DOI] [PubMed] [Google Scholar]
- Ghandour K, Ohkawa N, Fung CCA, Asai H, Saitoh Y, Takekawa T, Okubo-Suzuki R, Soya S, Nishizono H, Matsuo M, Osanai M, Sato M, Ohkura M, Nakai J, Hayashi Y, Sakurai T, Kitamura T, Fukai T & Inokuchi K (2019) Orchestrated ensemble activities constitute a hippocampal memory engram. Nat Commun, 10, 2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Milstein AD, Bittner KC, Romani S & Magee JC (2017) Inhibitory suppression of heterogeneously tuned excitation enhances spatial coding in CA1 place cells. Nat Neurosci, 20, 417–426. [DOI] [PubMed] [Google Scholar]
- Guo N (2018) Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat. Med, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA & Worley PF (1999) Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci, 2, 1120–1124. [DOI] [PubMed] [Google Scholar]
- Haimerl C, Angulo-Garcia D, Villette V, Reichinnek S, Torcini A, Cossart R & Malvache A (2019) Internal representation of hippocampal neuronal population spans a time-distance continuum. Proc Natl Acad Sci U S A, 116, 7477–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainmueller T & Bartos M (2018) Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature, 558, 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ & Josselyn SA (2007) Neuronal competition and selection during memory formation. Science, 316, 457–460. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW & Josselyn SA (2009) Selective erasure of a fear memory. Science, 323, 1492–1496. [DOI] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R & Boyden ES (2011) A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci, 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelmann GE & Olton DS (1981) Spatial memory following damage to hippocampal CA3 pyramidal cells with kainic acid: impairment and recovery with preoperative training. Brain Res, 217, 41–58. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME (2011) Neural Dynamics of Episodic Memory How We Remember. The MIT Press, pp. 31–81. [Google Scholar]
- Hebb DO (1949) The organization of behavior; a neuropsychological theory. Wiley, Oxford, England. [Google Scholar]
- Howard MW, MacDonald CJ, Tiganj Z, Shankar KH, Du Q, Hasselmo ME & Eichenbaum H (2014) A unified mathematical framework for coding time, space, and sequences in the hippocampal region. J Neurosci, 34, 4692–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA & Tonegawa S (2000) Formation of Temporal Memory Requires NMDA Receptors within CA1 Pyramidal Neurons. Neuron, 25, 473–480. [DOI] [PubMed] [Google Scholar]
- Jackson PA, Kesner RP & Amann K (1998) Memory for duration: role of hippocampus and medial prefrontal cortex. Neurobiol Learn Mem, 70, 328–348. [DOI] [PubMed] [Google Scholar]
- Jarrard LE (1983) Selective hippocampal lesions and behavior: effects of kainic acid lesions on performance of place and cue tasks. Behav Neurosci, 97, 873–889. [DOI] [PubMed] [Google Scholar]
- Josselyn SA (2010) Continuing the search for the engram: examining the mechanism of fear memories. Journal of psychiatry & neuroscience : JPN, 35, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter BR, Lykken CM, Avesar D, Weible A, Dickinson J, Dunn B, Borgesius NZ, Roudi Y & Kentros CG (2017) A Novel Mechanism for the Grid-to-Place Cell Transformation Revealed by Transgenic Depolarization of Medial Entorhinal Cortex Layer II. Neuron, 93, 1480–1492 e1486. [DOI] [PubMed] [Google Scholar]
- Kesner R (2000) Testing neural network models of memory with behavioral experiments. Current Opinion in Neurobiology, 10, 260–265. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR & Gilbert PE (2005) The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behav Neurosci, 119, 781–786. [DOI] [PubMed] [Google Scholar]
- Kim J & Fanselow M (1992) Modality-specific retrograde amnesia of fear. Science, 256, 675–677. [DOI] [PubMed] [Google Scholar]
- Kinsky NR, Sullivan DW, Mau W, Hasselmo ME & Eichenbaum HB (2018) Hippocampal Place Fields Maintain a Coherent and Flexible Map across Long Timescales. Current Biology, 28, 3578–3588.e3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T (2017) Driving and regulating temporal association learning coordinated by entorhinal-hippocampal network. Neurosci Res, 121, 1–6. [DOI] [PubMed] [Google Scholar]
- Kitamura T & Inokuchi K (2014) Role of adult neurogenesis in hippocampal-cortical memory consolidation. Mol Brain, 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Macdonald CJ & Tonegawa S (2015a) Entorhinal-hippocampal neuronal circuits bridge temporally discontiguous events. Learn Mem, 22, 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL & Tonegawa S (2017) Engrams and circuits crucial for systems consolidation of a memory. Science, 356, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Okubo-Suzuki R, Takashima N, Murayama A, Hino T, Nishizono H, Kida S & Inokuchi K (2012) Hippocampal function is not required for the precision of remote place memory. Mol Brain, 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K & Tonegawa S (2014) Island cells control temporal association memory. Science, 343, 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Murayama A, Sugiyama H & Inokuchi K (2010) LTP induction within a narrow critical period of immature stages enhances the survival of newly generated neurons in the adult rat dentate gyrus. Mol Brain, 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H & Inokuchi K (2009) Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell, 139, 814–827. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Sun C, Martin J, Kitch LJ, Schnitzer MJ & Tonegawa S (2015b) Entorhinal Cortical Ocean Cells Encode Specific Contexts and Drive Context-Specific Fear Memory. Neuron, 87, 1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ & Somogyi P (2004) Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci, 7, 41–47. [DOI] [PubMed] [Google Scholar]
- Klausberger T & Somogyi P (2008) Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science, 321, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R & Alonso A (1997) Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus, 7, 571–583. [DOI] [PubMed] [Google Scholar]
- Knierim JJ (2004) How to avoid going bump in the night: object and place representations in the hippocampus. J Gen Physiol, 124, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, Wickersham IR & Tonegawa S (2014) Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci, 17, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Brandon MP, Robinson RJ 2nd, Connerney MA, Hasselmo ME & Eichenbaum H (2015) During Running in Place, Grid Cells Integrate Elapsed Time and Distance Run. Neuron, 88, 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ 2nd, White JA, Eichenbaum H & Hasselmo ME (2013) Hippocampal “time cells”: time versus path integration. Neuron, 78, 1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K & Niki H (1971) Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol, 34, 337–347. [DOI] [PubMed] [Google Scholar]
- Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, Lim SC, Santos SL, Denny CA & Drew MR (2019) Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci, 22, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent X, Ensslin A & Mari-Beffa P (2016) An action to an object does not improve its episodic encoding but removes distraction. Journal of experimental psychology. Human perception and performance, 42, 494–507. [DOI] [PubMed] [Google Scholar]
- Leao RN, Mikulovic S, Leao KE, Munguba H, Gezelius H, Enjin A, Patra K, Eriksson A, Loew LM, Tort AB & Kullander K (2012) OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci, 15, 1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG & Buzsaki G (1994) Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience, 62, 1033–1047. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB & Moser EI (2007) Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315, 961–966. [DOI] [PubMed] [Google Scholar]
- Leutgeb S (2004) Distinct Ensemble Codes in Hippocampal Areas CA3 and CA1. Science, 305, 1295–1298. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K & Tonegawa S (2012) Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature, 484, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Gutnikov SA, Clark AM & Rawlins JN (1993) Determinants of non-spatial working memory deficits in rats given intraventricular infusions of the NMDA antagonist AP5. Neuropsychologia, 31, 1079–1098. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R & Eichenbaum H (2013) Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J Neurosci, 33, 14607–14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT & Eichenbaum H (2011) Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron, 71, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahut H (1971) Spatial and object reversal learning in monkeys with partial temporal lobe ablations. Neuropsychologia, 9, 409–424. [DOI] [PubMed] [Google Scholar]
- Mahut H, Zola-Morgan S & Moss M (1982) Hippocampal resections impair associative learning and recognition memory in the monkey. J Neurosci, 2, 1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G & Tank D (2004) Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol, 14, 675–684. [DOI] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Sparks FT, Leutgeb S & Leutgeb JK (2015) Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron, 85, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci, 24, 897–931. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL & Liberzon I (2013) The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks WD, Osanai H, Yamamoto J, Ogawa SK & Kitamura T (2019) Novel nose poke-based temporal discrimination tasks with concurrent in vivo calcium imaging in freely moving mice. Molecular Brain, 12, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1971) Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B, 262. [DOI] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, Yizhar O & Deisseroth K (2011) Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods, 9, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau W, Sullivan DW, Kinsky NR, Hasselmo ME, Howard MW & Eichenbaum H (2018) The Same Hippocampal CA1 Population Simultaneously Codes Temporal Information over Multiple Timescales. Curr Biol, 28, 1499–1508 e1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C & Disterhoft JF (1999) Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus, 8, 638–646. [DOI] [PubMed] [Google Scholar]
- McEchron MD & Disterhoft JF (1997) Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J Neurophysiol, 78, 1030–1044. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W & Disterhoft JF (2003) Single Neurons in CA1 Hippocampus Encode Trace Interval Duration during Trace Heart Rate (Fear) Conditioning in Rabbit. The Journal of Neuroscience, 23, 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA & Tonegawa S (2007) Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science, 317, 94–99. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM & Olton DS (1984) Hippocampus, time, and memory. Behav Neurosci, 98, 3–22. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA & Monyer H (2012) Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science, 335, 1506–1510. [DOI] [PubMed] [Google Scholar]
- Mingote S, Chuhma N, Kusnoor SV, Field B, Deutch AY & Rayport S (2015) Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions. J Neurosci, 35, 16259–16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi MN, Dhawale AK & Bhalla US (2014) CA1 cell activity sequences emerge after reorganization of network correlation structure during associative learning. Elife, 3, e01982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN & O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature, 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Morrissey MD, Maal-Bared G, Brady S & Takehara-Nishiuchi K (2012) Functional dissociation within the entorhinal cortex for memory retrieval of an association between temporally discontiguous stimuli. J Neurosci, 32, 5356–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E & Moser M-B (2008) Place Cells, Grid Cells, and the Brain’s Spatial Representation System. Annual Review of Neuroscience, 31, 69–89. [DOI] [PubMed] [Google Scholar]
- Moyer JR Jr., Deyo RA & Disterhoft JF (1990) Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci, 104, 243–252. [DOI] [PubMed] [Google Scholar]
- Muller C & Remy S (2014) Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Front Synaptic Neurosci, 6, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Buhl DL, McHugh TJ & Tonegawa S (2009) Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron, 62, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman Jesse D., Pelkey Kenneth A., Renaudineau S, Buhl Derek L., McHugh Thomas J., Barrera Vanessa R., Chittajallu R, Iwamoto Keisuke S., McBain Chris J., Fanselow Michael S. & Tonegawa S (2012) Young Dentate Granule Cells Mediate Pattern Separation, whereas Old Granule Cells Facilitate Pattern Completion. Cell, 149, 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL & Tonegawa S (2008) Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science, 319, 1260–1264. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA & Tonegawa S (2004) NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci, 5, 361–372. [DOI] [PubMed] [Google Scholar]
- Naya Y & Suzuki WA (2011) Integrating what and when across the primate medial temporal lobe. Science, 333, 773–776. [DOI] [PubMed] [Google Scholar]
- O’Keefe J & Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34, 171–175. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC & McClelland JL (1994) Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus, 4, 661–682. [DOI] [PubMed] [Google Scholar]
- Ohara S, Gianatti M, Itou K, Berndtsson CH, Doan TP, Kitanishi T, Mizuseki K, Iijima T, Tsutsui KI & Witter MP (2019) Entorhinal Layer II Calbindin-Expressing Neurons Originate Widespread Telencephalic and Intrinsic Projections. Front Syst Neurosci, 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa N, Saitoh Y, Suzuki A, Tsujimura S, Murayama E, Kosugi S, Nishizono H, Matsuo M, Takahashi Y, Nagase M, Sugimura YK, Watabe AM, Kato F & Inokuchi K (2015) Artificial association of pre-stored information to generate a qualitatively new memory. Cell Rep, 11, 261–269. [DOI] [PubMed] [Google Scholar]
- Oishi N, Nomoto M, Ohkawa N, Saitoh Y, Sano Y, Tsujimura S, Nishizono H, Matsuo M, Muramatsu SI & Inokuchi K (2019) Artificial association of memory events by optogenetic stimulation of hippocampal CA3 cell ensembles. Mol Brain, 12, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S & Tonegawa S (2016) Ventral CA1 neurons store social memory. Science, 353, 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban-Kis K, Szabadi T & Szilagyi T (2015) The loss of Ivy cells and the hippocampal input modulatory O-LM cells contribute to the emergence of hyperexcitability in the hippocampus. Rom J Morphol Embryol, 56, 155–161. [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A & Buzsaki G (2008) Internally generated cell assembly sequences in the rat hippocampus. Science, 321, 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP (1927) Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford Univ. Press, Oxford, England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC & McBain CJ (2017) Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev, 97, 1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Escobar JA, Kornienko O, Latuske P, Kohler L & Allen K (2016) Visual landmarks sharpen grid cell metric and confer context specificity to neurons of the medial entorhinal cortex. eLife, 5, e16937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R & LeDoux J (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci, 106, 274–285. [DOI] [PubMed] [Google Scholar]
- Pilkiw M, Insel N, Cui Y, Finney C, Morrissey MD & Takehara-Nishiuchi K (2017) Phasic and tonic neuron ensemble codes for stimulus-environment conjunctions in the lateral entorhinal cortex. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Fu L, Hu B, Liao X, Lu J, He W, Liang S, Zhang K, Li R, Yao J, Yan J, Chen H, Jia H, Zott B, Konnerth A & Chen X (2018) A Visual-Cue-Dependent Memory Circuit for Place Navigation. Neuron, 99, 47–55 e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ & Tonegawa S (2013a) Creating a false memory in the hippocampus. Science, 341, 387–391. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Tonegawa S & Liu X (2013b) Identification and optogenetic manipulation of memory engrams in the hippocampus. Front Behav Neurosci, 7, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Naumann R, Burgalossi A, Tang Q, Schmidt H & Brecht M (2014) Grid-layout and theta-modulation of layer 2 pyramidal neurons in medial entorhinal cortex. Science, 343, 891–896. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N & Mayford M (2007) Localization of a stable neural correlate of associative memory. Science, 317, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Robinson NTM, Priestley JB, Rueckemann JW, Garcia AD, Smeglin VA, Marino FA & Eichenbaum H (2017) Medial Entorhinal Cortex Selectively Supports Temporal Coding by Hippocampal Neurons. Neuron, 94, 677–688.e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Kitamura T, Okuyama T, Ogawa SK, Sun C, Obata Y, Yoshiki A & Tonegawa S (2017) Distinct Neural Circuits for the Formation and Retrieval of Episodic Memories. Cell, 170, 1000–1012 e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A, Geva N, Sheintuch L & Ziv Y (2015) Hippocampal ensemble dynamics timestamp events in long-term memory. Elife, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A & Tonegawa S (2015) Memory. Engram cells retain memory under retrograde amnesia. Science, 348, 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryou JW, Cho SY & Kim HT (2001) Lesions of the entorhinal cortex impair acquisition of hippocampal-dependent trace conditioning. Neurobiol Learn Mem, 75, 121–127. [DOI] [PubMed] [Google Scholar]
- Sabariego M, Schonwald A, Boublil BL, Zimmerman DT, Ahmadi S, Gonzalez N, Leibold C, Clark RE, Leutgeb JK & Leutgeb S (2019) Time Cells in the Hippocampus Are Neither Dependent on Medial Entorhinal Cortex Inputs nor Necessary for Spatial Working Memory. Neuron, 102, 1235–1248 e1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A & Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature, 472, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakon JJ, Naya Y, Wirth S & Suzuki WA (2014) Context-dependent incremental timing cells in the primate hippocampus. Proc Natl Acad Sci U S A, 111, 18351–18356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz DM, Tiganj Z, Khasnabish S, Kohley A, Sheehan D, Howard MW & Eichenbaum H (2016) Time Cells in Hippocampal Area CA3. J Neurosci, 36, 7476–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM & Gottlieb DI (1975) An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol, 159, 149–175. [DOI] [PubMed] [Google Scholar]
- Schurgin MW & Flombaum JI (2018) Properties of visual episodic memory following repeated encounters with objects. Learn Mem, 25, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R & Sahay A (2009) Krüppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29, 9875–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB & Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry, 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T & Arai Y (1993) Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci, 13, 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon R (1921) The Mneme. George Allen & Unwin, London. [Google Scholar]
- Shu S, Zhu H, Tang N, Chen W, Li X, Li H, Pei L, Liu D, Mu Y, Tian Q, Zhu LQ & Lu Y (2016) Selective Degeneration of Entorhinal-CA1 Synapses in Alzheimer’s Disease via Activation of DAPK1. J Neurosci, 36, 10843–10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M & Buzsaki G (1997) Interneurons in the hippocampal dentate gyrus: an in vivo intracellular study. Eur J Neurosci, 9, 573–588. [DOI] [PubMed] [Google Scholar]
- Smith DM & Bulkin DA (2014) The form and function of hippocampal context representations. Neurosci Biobehav Rev, 40, 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF & Weisz DJ (1986) Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci, 100, 729–744. [DOI] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S & Buzsaki G (2014) Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron, 83, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T & Tonegawa S (2011) Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science, 334, 1415–1420. [DOI] [PubMed] [Google Scholar]
- Sun C, Kitamura T, Yamamoto J, Martin J, Pignatelli M, Kitch LJ, Schnitzer MJ & Tonegawa S (2015) Distinct speed dependence of entorhinal island and ocean cells, including respective grid cells. Proc Natl Acad Sci U S A, 112, 9466–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeli G, Marcu DC, McClure C, Garden DLF, Pastoll H & Nolan MF (2016) Molecularly Defined Circuitry Reveals Input-Output Segregation in Deep Layers of the Medial Entorhinal Cortex. Neuron, 92, 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, O’Brien J & Lehmann H (2008) Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus, 18. [DOI] [PubMed] [Google Scholar]
- Takahashi S (2013) Hierarchical organization of context in the hippocampal episodic code. Elife, 2, e00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S & Kirino Y (2003) Time-dependent reorganization of the brain circuitry underlying memory retention in trace eyeblink conditioning. Seibutsu Butsuri, 43, S238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Takatsuki K & Kirino Y (2002) Time-limited role of the hippocampus in the memory for trace eyeblink conditioning in mice. Brain Res, 951, 183–190. [DOI] [PubMed] [Google Scholar]
- Tamamaki N & Nojyo Y (1993) Projection of the entorhinal layer II neurons in the rat as revealed by intracellular pressure-injection of neurobiotin. Hippocampus, 3, 471–480. [DOI] [PubMed] [Google Scholar]
- Tanaka KZ, He H, Tomar A, Niisato K, Huang AJY & McHugh TJ (2018) The hippocampal engram maps experience but not place. Science, 361, 392–397. [DOI] [PubMed] [Google Scholar]
- Tennant SA, Fischer L, Garden DLF, Gerlei KZ, Martinez-Gonzalez C, McClure C, Wood ER & Nolan MF (2018) Stellate Cells in the Medial Entorhinal Cortex Are Required for Spatial Learning. Cell Rep, 22, 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JI, Ogawa SK & Kitamura T (2019) Adult hippocampal neurogenesis for systems consolidation of memory. Behav Brain Res, 372, 112035. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT, Cusick A, Riley ZL, Sunkin SM, Bernard A, Puchalski RB, Gage FH, Jones AR, Bajic VB, Hawrylycz MJ & Lein ES (2008) Genomic Anatomy of the Hippocampus. Neuron, 60, 1010–1021. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ & Lattal KM (2014) Delay and trace fear conditioning in C57BL/6 and DBA/2 mice: issues of measurement and performance. Learn Mem, 21, 380–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S, Morrissey MD & Kitamura T (2018) The role of engram cells in the systems consolidation of memory. Nat Rev Neurosci, 19, 485–498. [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Pignatelli M, Roy DS & Ryan TJ (2015) Memory engram storage and retrieval. Curr Opin Neurobiol, 35, 101–109. [DOI] [PubMed] [Google Scholar]
- Treves A & Rolls ET (1994) Computational analysis of the role of the hippocampus in memory. Hippocampus, 4, 374–391. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF & Weiss C (2004) Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus, 14, 58–65. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT & Tonegawa S (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell, 87, 1327–1338. [DOI] [PubMed] [Google Scholar]
- Tulving E (2002) Episodic memory: from mind to brain. Annu Rev Psychol, 53, 1–25. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C & Deisseroth K (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature, 471, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Lee SY & Soltesz I (2010) Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci, 13, 822–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Hasselmo ME & Eichenbaum H (1998) The hippocampus as an associator of discontiguous events. Trends in Neurosciences, 21, 317–323. [DOI] [PubMed] [Google Scholar]
- Wang Y, Romani S, Lustig B, Leonardo A & Pastalkova E (2015) Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci, 18, 282–288. [DOI] [PubMed] [Google Scholar]
- Waxler M & Rosvold HE (1970) Delayed alternation in monkeys after removal of the hippocampus. Neuropsychologia, 8, 137–146. [DOI] [PubMed] [Google Scholar]
- Weiss C & Disterhoft JF (2011) Exploring prefrontal cortical memory mechanisms with eyeblink conditioning. Behav Neurosci, 125, 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Knuttinen MG, Power JM, Patel RI, O’Connor MS & Disterhoft JF (1999) Trace eyeblink conditioning in the freely moving rat: Optimizing the conditioning parameters. Behavioral Neuroscience, 113, 1100–1105. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK & Callaway EM (2007) Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods, 4, 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Sullivan HA & Seung HS (2010) Production of glycoprotein-deleted rabies viruses for monosynaptic tracing and high-level gene expression in neurons. Nat Protoc, 5, 595–606. [DOI] [PubMed] [Google Scholar]
- Wilson M & McNaughton B (1993) Dynamics of the hippocampal ensemble code for space. Science, 261, 1055–1058. [DOI] [PubMed] [Google Scholar]
- Wintzer ME, Boehringer R, Polygalov D & McHugh TJ (2014) The Hippocampal CA2 Ensemble Is Sensitive to Contextual Change. The Journal of Neuroscience, 34, 3056–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff P (1966) Trace quality in the temporal ordering of events. Perceptual and motor skills, 22, 283–286. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Suh J, Takeuchi D & Tonegawa S (2014) Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell, 157, 845–857. [DOI] [PubMed] [Google Scholar]
- Yang X, Yao C, Tian T, Li X, Yan H, Wu J, Li H, Pei L, Liu D, Tian Q, Zhu LQ & Lu Y (2016) A novel mechanism of memory loss in Alzheimer’s disease mice via the degeneration of entorhinal-CA1 synapses. Mol Psychiatry, 23, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema DB & Trask FP (1963) Recall as a search process. Journal of Verbal Learning and Verbal Behavior, 2, 65–74. [Google Scholar]
- Yoshida M, Fransen E & Hasselmo ME (2008) mGluR-dependent persistent firing in entorhinal cortex layer III neurons. Eur J Neurosci, 28, 1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K & Eschen A (2017) Brain regions involved in subprocesses of small-space episodic object-location memory: a systematic review of lesion and functional neuroimaging studies. Memory, 25, 487–519. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A & Schnitzer MJ (2013) Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci, 16, 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutshi I, Fu ML, Lilascharoen V, Leutgeb JK, Lim BK & Leutgeb S (2018) Recurrent circuits within medial entorhinal cortex superficial layers support grid cell firing. Nat Commun, 9, 3701. [DOI] [PMC free article] [PubMed] [Google Scholar]


