Abstract
The recombinant receptor-binding domain (RBD) of the viral spike protein from SARS-CoV-1 and 2 are reliable antigens for detecting viral-specific antibodies in humans. We and others have shown that the levels of RBD-binding antibodies and SARS-CoV-2 neutralizing antibodies in patients are correlated. Here, we report the expression and purification of properly folded RBD proteins from SARS and common-cold HCoVs in mammalian cells. RBD proteins were produced with cleavable tags for affinity purification from the cell culture medium and to support multiple immunoassay platforms and drug discovery efforts.
Graphic abstract:
High-Yield Production of Viral Spike RBDs for Diagnostics and Drug Discovery
Keywords: SARS-CoV-2, SARS-CoV, Coronavirus, COVID-19, Antigen, Immunoassay, Halo-tag, Spike protein, Antibody, Inhibitor screening, Drug discovery
Background
The receptor-binding domain (RBD) of the coronavirus spike protein is critical for viral attachment, fusion, and entry. It is also the primary target for antibody response and the development of entry inhibitors and vaccines. The RBDs of 2003 and 2019 SARS CoVs and the four common endemic human CoVs are poorly conserved, representing a promising antigen for detecting viral-specific antibodies in humans. We have recently shown that the RBD of SARS-CoV-2 is highly sensitive and specific for detecting antibodies nine days after the onset of symptoms ( Premkumar et al., 2020 ). Levels of RBD-binding antibodies in human sera are strongly correlated with the SARS-CoV-2 neutralizing titer in patients. Thus, RBD-based serologic assays are attractive to identify individual and environmental risk factors for severe illness and to monitor SARS-CoV-2 transmission in the community. Prior immunity to common human endemic coronaviruses (229E, NL63, OC43, and HKU1) has been reported to enhance the inflammatory response to SARS-CoV-2 ( Grifoni et al., 2020 ; Mateus et al., 2020 ). Here, we present a detailed step-by-step method for expressing and purifying the RBD of 2003 and 2019 SAR CoVs and the four common endemic human CoVs for serologic assays and inhibitor screening ( Premkumar et al., 2020 and Puhl et al., 2021 ). The technique allows the production of RBDs fused to a TEV protease cleavable self-labeling protein (HaloTag) at the N-terminus and a Twin-Strep-tag and a His-tag at the C-terminus. The tags were designed to aid affinity purification and oriented capture of antigens on solid supports incorporating streptactin, streptavidin, or nickel-nitrilotriacetic acid. The current protocol utilizes a mammalian expression system (Expi293) to produce milligram quantities of recombinant RBDs from a small cell culture volume within 5-7 days, using a single affinity purification step.
Materials and Reagents
Cryopreservation Tubes (Thermo Scientific, catalog number: 374081)
Poly-Prep Chromatography Columns (Bio-Rad, catalog number: 7311550)
Econo-Pac Chromatography Columns (Bio-Rad, catalog number: 7321010)
SnakeSkin Dialysis Tubing (Thermo Fisher Scientific, catalog number: 68700)
SnakeSkin Dialysis Clips (Thermo Fisher Scientific, catalog number: 68011)
Staples 2” Binder Clips, Large (Staples, catalog number: 10669)
5 ml Serological pipette (Thermo Fisher Scientific, catalog number: 13-678-11D)
10 ml Serological pipettes (Thermo Fisher Scientific, catalog number: 13-678-11E)
50 ml Falcon tube (Cell star, catalog number: 22761)
Precision Plus Protein Kaleidoscope 500 ml (Bio-Rad, catalog number: 161-0375)
Any kD Mini-PROTEAN TGX Stain-Free Protein Gels, 12-well, 20 μl (Bio-Rad, catalog number: 4568125)
2× Laemmli Sample Buffer (Bio-Rad, catalog number: 1610737)
Mini-PROTEAN Tetra Vertical Electrophoresis Cell for Mini Precast Gels, 2-gel (Bio-Rad, catalog number: 1658005)
2-Mercaptoethanol, 10 ml (Sigma-Aldrich, catalog number: M6250)
Coomassie Brilliant Blue R-250 Dye (Thermo Fisher Scientific, catalog number: 20278)
-
Expi293 Expression System Kit (Thermo Fisher Scientific, catalog number: A14635)
Note: Store cells in liquid nitrogen and other reagents at 2°C to 8°C.
Mr. Frosty (Thermo Scientific, catalog number: 5100-0001)
Ni-NTA Agarose (QIAGEN, catalog number: 30230). Store at 2°C to 8°C
DMSO (Millipore Sigma, catalog number: 41640)
Opti-MEM Medium (Thermo Fisher Scientific, catalog number: 11-058-021)
Tris (MP, catalog number: 103133)
NaCl (Fisher, catalog number: S271-10)
Glycerol (VWR, catalog number: BHD 1172-1LP)
Sucrose (Fisher, catalog number: BP-220-1)
Imidazole (Thermo Fisher Scientific, catalog number: 03196-500)
Liquid nitrogen (Arc gases)
Purify Buffer (see Recipes)
Elution Buffer (see Recipes)
Dialysis Buffer (see Recipes)
Stain Buffer for SDS PAGE (see Recipes)
Destain Buffer for SDS PAGE (see Recipes)
Equipment
Fisherbrand Shaker Flasks, Plain Bottom, Vented (Thermo Fisher Scientific, catalog number: PBV12-5). Store at room temperature
4 L Beaker (Thermo Fisher Scientific, catalog number: 02-555-25K)
Forma Steri-Cycle i160 C02 Incubator (Thermo Fisher Scientific, Forma, catalog number: 51030301)
CO2 Resistant Shaker (Thermo Fisher Scientific, catalog number: 88881101)
Biological safety cabinet (Labguard, Class II, Type A2)
Precision Water Bath GP 15D–5 L and 10 L (Thermo Scientific, catalog number: TSGP15D)
Magnetic stir bar (Thermo Fisher Scientific, catalog number: 14-512-136)
Centrifuge (Sorvall, model: RC-5B)
Centrifuge (Eppendorf, model: 5810 R)
Centrifuge (Thermo, model: Sorvall Legend Micro 21R)
Freezer (Thermo Scientific Revco RLE60086A -86 °C)
Cryogenic dewar (Cole Parmer)
Mini-PROTEAN® Tetra Vertical Electrophoresis Cell for Mini Precast Gels (Bio-Rad, Catalogue number: 1658004)
Procedure
-
Establishment of the Expi293 Cell Line (Thermo Fisher Scientific)
Remove a cell aliquot from liquid nitrogen.
Immediately hand thaw the cells and place them in a 37°C water bath. Once thawed, swirl the tube gently without submerging completely until only a small amount of ice remains.
Spray hands with 70% ethanol and gently rub the cell vial to decontaminate before transferring into the laminar flow hood.
Use a serological pipette to transfer all tube contents into a plain bottom, vented Fisherbrand Shaker Flask, prewarmed with 30 ml of Expi293 Expression Medium.
Incubate cells at 37°C with ≥ 80% relative humidity and 8% CO2. Set shaking speed to 125 RPM for a 125 ml shaker flask.
-
Passage cells when the cell density reaches 1 × 106-3 × 106 cell/ml.
Note: This usually occurs 4-6 days post-thaw.
-
Proceed to transfection once the cell density reaches approximately 3 × 106-5 × 106 viable cells/ml and cell viability is ≥ 95%.
Note: To cryopreserve cells for future use, grow the cell culture to 3 × 106-5 × 106 viable cells/ml and centrifuge them at 300 × g for 5 min.
Discard the supernatant, add Expi293 Expression medium with 10% DMSO, and gently resuspend the cells by pipetting.
Dilute the cells to 1 × 107 viable cells/ml and pipet 1 ml aliquots into cryopreservation tubes and freeze with a controlled-rate freezing apparatus at -80°C freezer. After 24 h, transfer to cryogenic dewar for long-term storage and future use. Allow cells to recover in culture for two more passages post-thaw before transfecting.
Note: For general cell maintenance, passage cells at 0.5 × 106 cell/ml when they reach a density of 3 × 106-5 × 106 cell/ml. Growing past 5 × 106 cell/ml is not recommended.
-
Transfection (30 ml)
Dilute a total of 75 × 106 cells to a final density of 3 × 106 cell/ml with 25 ml of prewarmed Expi293 Expression Medium in a 125 ml shaker flask.
Dilute 25 μg of RBD expression plasmid DNA in 1.5 ml of Opti-MEM Medium.
Dilute 80 μl of ExpiFectamine 293 Reagent in 1.4 ml of Opti-MEM Medium. Incubate the solution at room temperature for 5 min.
Add diluted plasmid DNA to the solution containing ExpiFectamine 293 Reagent and incubate at room temperature 10-20 min. The volume should be approximately 3 ml.
Transfer 3 ml of the solution into a shaker flask and incubate cells at 37°C with ≥ 80% relative humidity and 8% CO2.
-
Eighteen to twenty-two hours post-transfection, add 150 μl of ExpiFectamine 293 Transfection Enhancer 1 and 1.5 ml of ExpiFectamine 293 Enhancer 2 to the shaker flask. Incubate cells for up to 5 days post-transfection.
Note: The procedure can be scaled up proportionally for larger transfections. Cell viability should be above 50% on day 5.
-
Harvest and Dialysis
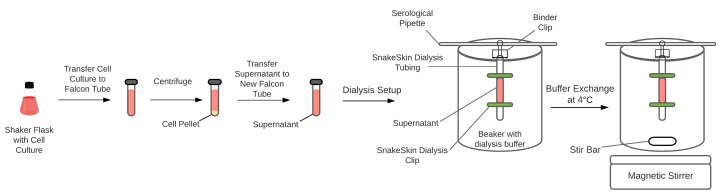
Transfer the cell culture to a 50 ml Falcon tube and centrifuge at 3,000-5,000 × g for 5 min at 25°C (Figure 1).
Transfer the supernatant into a fresh 50 ml Falcon tube and keep it on ice.
Prepare 3 L of dialysis buffer.
-
Use about 7-8 inches of SnakeSkin Dialysis Tubing and SnakeSkin Dialysis Clips to transfer 30 ml of harvested supernatant into the dialysis buffer.
Note: Hydrate the membrane with the dialysis buffer before transferring the supernatant.
Place the sealed snakeskin tubing and a magnetic stir bar in a 4-L beaker.
Place the beaker on a magnetic stirrer and allow buffer exchange at 4°C overnight.
Transfer the buffer-exchanged supernatant into a 50 ml Falcon tube.
Note: Upon harvesting, it is possible to evaluate the success of transfection and protein expression before the affinity chromatography step by SDS-PAGE with the cell culture supernatant (optional). To perform this:
Mix 50 µl of 2× Laemmli Sample Buffer with 2-mercaptoethanol and 50 µl of cell culture supernatant and boil the sample at 95°C for 5 min.
Load 8-15 μl of the reduced sample onto the SDS-PAGE.
Run sample at 170 v in the Mini-PROTEAN Tetra Vertical Electrophoresis Cell for 35 min and visualize the bands after Coomassie staining using the manufacturer’s protocol.
-
Immobilized metal affinity chromatography
-
Take 0.5 ml of Ni-NTA resin in a poly-prep chromatography column and equilibrate with 5 ml of purifying buffer in 1 ml increments. Close the column and resuspend the resin in 1 ml of purifying buffer.
Note: Proportionally adjust the amount of resin needed for larger transfections.
Transfer the equilibrated resin into a 50 ml Falcon tube containing the buffer exchanged supernatant.
Incubate the resin with the supernatant on a rocking shaker for 1 h at 4°C.
Remove the 50 ml Falcon tube from the rocker and place it on a stand. Allow the resin to settle for 20 min.
Transfer the supernatant into a new 50 ml Falcon tube without disturbing the resin.
Transfer the resin directly onto the bed of the poly-prep chromatography column.
-
Wash the resin with 6 ml of purifying buffer in 1 ml increments by pipetting on the chromatography column wall.
Note: Allow the buffer to flow through the column completely before adding another ml of wash buffer.
Elute the protein by adding 200 μl of elution buffer onto the column wall for a total of 7 fractions.
Quantify the protein by measuring the absorbance of the fraction at 280 nm.
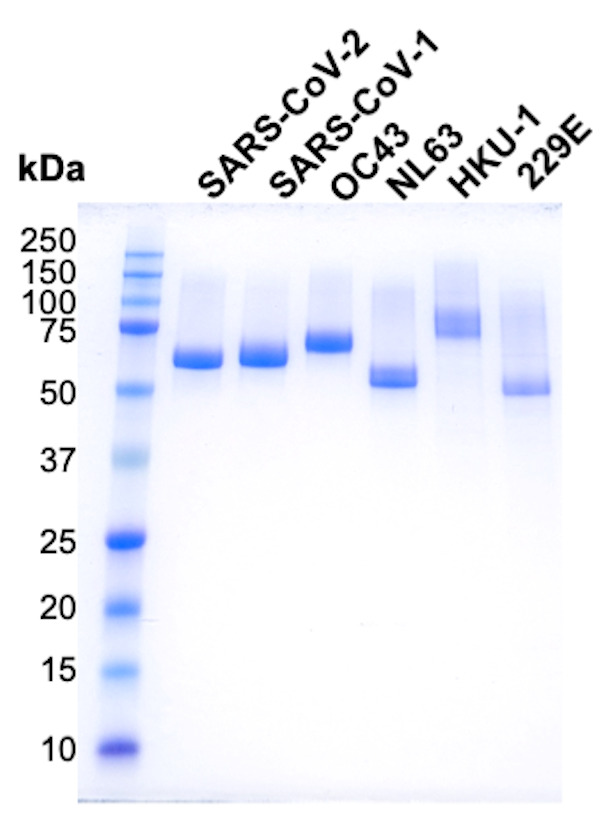
Assess the protein purity by SDS PAGE run under reducing conditions (Figure 2).
-
-
Storage
Purified proteins can be stored at 4°C for a few weeks. For long-term storage, protein samples can be aliquoted, flash-frozen in liquid nitrogen, and stored at -80°C. Frozen protein samples can be quickly hand thawed before use.
Figure 1. Schematics of harvesting cell culture and dialysis of supernatant.
Figure 2. SDS-PAGE analysis of purified spike RBD proteins.
Recipes
-
Purify buffer
50 mM Tris pH 8
105 mM NaCl
10% glycerol
10% sucrose
-
Dialysis buffer
50 mM Tris-HCl, pH 8
100 mM NaCl
-
Elution buffer
50 mM Tris pH 8
105 mM NaCl
10% glycerol
10% sucrose
300 mM imidazole
-
Stain Buffer for SDS PAGE (1 L)
500 ml deionized water
100 ml methanol
100 ml glacial acetic acid
3 g brilliant blue
-
Destain Buffer for SDS PAGE (1 L)
500 ml deionized water
400 ml methanol
100 ml glacial acetic acid
Notes
Codon-optimized nucleotide sequences encoding the RBDs of SARS-CoV-1 (318-514 aa, P59594), SARS-CoV-2 (331-528 aa, QIS60558.1), OC43 (329-613 aa, P36334.1), HKU-1 (310-611 aa, Q0ZME7.1), 229E (295-433 aa, P15423.1), and NL63 (480-617 aa, Q6Q1S2.1) are available in GenBank under the accession codes MT649401, MT649402, MT649403, MT649404, MT649405, and MT649406. The genes encoding the proteins above were cloned between KpnI and XhoI sites of the mammalian expression plasmid pαH. The mammalian expression plasmids will also be made available by the authors from the plasmid repository (Addgene).
Acknowledgments
This work was funded by the University of North Carolina School of Medicine and NCI U54 CA260543-01 (L.P. and A.D). This protocol was adapted with minor modification from previous study published by Premkumar et al. (2020).
Competing interests
The authors have declared no competing interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Grifoni A., Weiskopf D., Ramirez S. I., Mateus J., Dan J. M., Moderbacher C. R., Rawlings S. A., Sutherland A., Premkumar L., Jadi R. S., Marrama D., de Silva A. M., Frazier A., Carlin A. F., Greenbaum J. A., Peters B., Krammer F., Smith D. M., Crotty S. and Sette A.(2020). Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181(7): 1489-1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S. I., Dan J. M., Burger Z. C., Rawlings S. A., Smith D. M., Phillips E., Mallal S., Lammers M., Rubiro P., Quiambao L., Sutherland A., Yu E. D., da Silva Antunes R., Greenbaum J., Frazier A., Markmann A. J., Premkumar L., de Silva A., Peters B., Crotty S., Sette A. and Weiskopf D.(2020). Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 370(6512): 89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Premkumar L., Segovia-Chumbez B., Jadi R., Martinez D. R., Raut R., Markmann A., Cornaby C., Bartelt L., Weiss S., Park Y., Edwards C. E., Weimer E., Scherer E. M., Rouphael N., Edupuganti S., Weiskopf D., Tse L. V., Hou Y. J., Margolis D., Sette A., Collins M. H., Schmitz J., Baric R. S. and de Silva A. M.(2020). The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 5(48): eabc8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puhl A. C., Fritch E. J., Lane T. R., Tse L. V., Yount B. L., Sacramento C. Q., Fintelman-Rodrigues N., Tavella T. A., Maranhao Costa F. T., Weston S., Logue J., Frieman M., Premkumar L., Pearce K. H., Hurst B. L., Andrade C. H., Levi J. A., Johnson N. J., Kisthardt S. C., Scholle F., Souza T. M. L., Moorman N. J., Baric R. S., Madrid P. B. and Ekins S.(2021). Repurposing the Ebola and Marburg Virus Inhibitors Tilorone, Quinacrine, and Pyronaridine: In Vitro Activity against SARS-CoV-2 and Potential Mechanisms . ACS Omega 6(11): 7454-7468. [DOI] [PMC free article] [PubMed] [Google Scholar]