Abstract
Trichuris spp. are nematode parasites infecting wild ruminants in zoological institutions worldwide. These helminths cause significant morbidity in giraffe (Giraffa camelopardalis) and other hoofstock located in zoological institutions throughout the United States. Historically, studies and institutions have used a variety of nematode detection methods with various flotation solutions. Optimization of Trichuris egg detection is necessary for monitoring collections. Fecal and soil optimized protocols were generated in this study using samples containing Trichuris eggs from multiple semi free-ranging zoological institutions. First, Sheather's sugar (specific gravity (SG) 1.27), sucrose (SG 1.40), magnesium sulfate (SG 1.26), and zinc sulfate (SG 1.18) were compared as flotation solutions by quantitative eggs per gram using a modified Stoll method. Then a soil recovery method was optimized comparing Tween 20, sodium hydroxide, Dawn™ (Procter and Gamble) detergent, and sodium chloride as liberating solutions to free eggs from the soil. We found that Sheather's sugar and sucrose solutions were the most effective for Trichuris egg detection, and either sodium hydroxide or sodium chloride liberated eggs from soil.
Keywords: Trichuris, Giraffe, Nematode, Feces, Soil, Modified stoll, Specific gravity
Graphical abstract
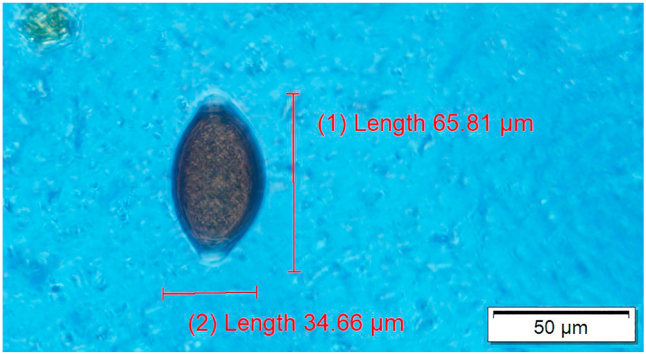
Trichuris egg seen in fecal flotation of giraffe feces.
Highlights
-
•
Optimization of giraffe Trichuris egg detection.
-
•
Soil Trichuris recovery methods analysis.
-
•
Multi-institutional giraffe Trichuris evaluation.
1. Introduction
Trichuris spp. are common nematode parasites found in a variety of host species. In zoos and conservation reserves, Trichuris eggs are commonly found on routine hoofstock fecal detection (Mir et al., 2016; Pauling et al., 2016; da Silva Barbosa et al., 2020), including in giraffe (Giraffa camelopardalis) (VanderWaal et al., 2014; Kyriánová et al., 2017). Trichuris in giraffe is a consistent clinical finding for wildlife institutions worldwide (Bertelsen, 2015). The life cycle of Trichuris makes it difficult to eradicate. Trichuris eggs are shed in feces unembryonated, and when outdoors, these feces become mixed with the soil in each enclosure. The egg then larvates in the environment to an infective stage in about three weeks. The eggs are very hardy, resistant to desiccation, and can survive for months or years in the environment. Ingestion of a larvated egg by a susceptible host allows the larvae to migrate through the gastrointestinal tract, mature into an adult, reproduce, and then female adults deposit eggs in the host's feces (Miller et al., 2012). An infected host can experience a range of clinical signs, including diarrhea, weight loss, decreased appetite, hypoproteinemia, anemia and possible mortality (Van Metre et al., 2008).
Collection giraffes are susceptible to many of the same types of parasites as confined domestic ruminants, including Trichuris (Bertelsen, 2015). Trichuris infection treatment protocol is different for each institution. Some facilities treat on a timeline schedule, performing routine deworming on a monthly basis (Pauling et al., 2016). Other facilities deworm when animals are identified as infected (Kyriánová et al., 2017). According to Fowler's Zoo and Wild Animal Medicine Volume 8, treatment for Trichuris should be instituted even if only a few eggs are found on a fecal examination, due to Trichuris' low fecundity and inconsistent shedding (Bertelsen, 2015). Thus, a single egg found on a fecal examination can still indicate that the host is infected with many adult nematodes.
Another variation among facilities is the method and the flotation solutions used to recover Trichuris eggs in detection protocols. For example, zinc sulfate (ZnSO4) with a specific gravity (SG) of 1.18 has been used for T. vulpis detection in canines (Becker et al., 2016), T. felis in felines (Geng et al., 2018), T. trichiura in gorillas (Sleeman et al., 2000) and T. ovis in soil recovery (Golek and Al-Saeed, 2019). Saturated sodium chloride (NaCl) with a SG of 1.18 has been used for T. suis detection in pigs (Tan et al., 2018). Magnesium sulfate (MgSO4) with a SG of 1.26 has been used for Trichuris spp. detection in roe deer (Body et al., 2011). Finally, sucrose solution with SG between 1.27 and 1.33 is a common flotation solution (Dryden et al., 2005) and is used for detection of Trichuris spp. at many zoo institutions.
Each species of Trichuris egg has a unique SG, for example 1.1299 for T. suis and 1.1453 for T. vulpis (David and Lindquist, 1982). Therefore, a flotation solution which is denser than the Trichuris eggs is necessary to float and detect the eggs in solution. Detection of eggs using the modified Stoll method permits quantitative fecal egg count (Williamson et al., 2014, Zajac and Conboy, 2012) and allows for comparison of different flotation methods to measure relative recovery rates. Additionally, modified Stoll is a sensitive fecal flotation method, allowing detection of 10 EPG (eggs per gram) of fecal material (Zajac and Conboy, 2012) and is well suited for low fecundity nematodes like Trichuris.
Trichuris is a soil-transmitted helminth and eggs require time to moult to an L3 infective stage in the environment, therefore soil egg recovery could serve as an additional means of routine Trichuris detection and subsequent treatment. If Trichuris eggs are detected in soil, facilities could implement measures to better prevent infection, such as quicker removal of feces or temporary movement of highly susceptible hosts from infected enclosures. Before eggs can be detected in soil samples by flotation, they must first be liberated from the soil and concentrated. Ionic solutions can be used to dissociate parasite eggs from soil through displacement of anions found on the parasite egg wall from cations found in soil (Collender et al., 2015); otherwise, if only water is used the eggs may remain adherent or associated with soil particles and not rise through the flotation solution. Different ionic, egg liberating solutions that have been used in previous studies are Tween (Steinbaum et al., 2017) and NaCl (Tun et al., 2015).
In this study, individual animal and environmental surveys of Trichuris detection in different giraffe collections was conducted and evaluation of methods occurred. Fecal and soil protocols for Trichuris detection were optimized to compare the multiple flotation and liberating solutions used in previous studies. The first aim of this study was to use modified Stoll's method to optimize a fecal flotation protocol and determine which flotation solution recovered the most Trichuris eggs. The second aim of this study was to optimize a soil egg recovery protocol to evaluate four liberating solutions to dissociate Trichuris eggs from soil and allow egg recovery. We hypothesized that sucrose solution with a SG of 1.40 would yield the most eggs, as this was the highest SG flotation solution tested. We also hypothesized that there would be no significant difference between the liberating solutions tested in their ability to dissociate for the detection of Trichuris eggs from soil.
2. Materials and methods
2.1. Study site locations
The facilities chosen in this study were White Oak Conservation (WO) in Yulee, Florida; Fossil Rim Wildlife Center (FR) in Glen Rose, Texas; The Wilds (TW) in Cumberland, Ohio; and Binder Park Zoo (BP) in Battle Creek, Michigan.
2.2. Helminth management survey of the facilities
Each study site was surveyed, and information was gathered regarding fecal flotation procedure and protocol for Trichuris, as well as feeding location and feces removal protocols. These locations were chosen in an attempt to gather samples from a range of temperate regions with differing environmental and soil conditions, and institutional management styles. Weather data over the past 30 years was reviewed and Koeppen-Geiger Climate Classifications were summarized from the Midwestern Regional Climate Center (Wilson, A.). WO and FR reside in humid, subtropical climates, however WO has an annual average total precipitation of 1.4 m while FR is more drought prone and has an annual average total precipitation of 1.0 m. Neither has dry summer months or a record minimum temperature below −11.7 °C. TW and BP reside in humid, continental climates; however, TW has slightly hotter summers compared to BP's warm summers. Both sites receive around 0.5–1.0 m of snow per year, with BP receiving a bit more than TW.
2.3. Study sites and sample collection
Giraffe Trichuris positive fecal and adjacent soil samples were obtained from each zoological facility. At the time of this study the giraffe populations sampled included 13 to 15 giraffes at WO, ten giraffes at FR, eight giraffes at TW, and seven giraffes at BP. These zoological institutions manage giraffes on large pastures when temperatures allow; and smaller yards with heated barns when temperatures or weather met specific criteria. For example, at TW, giraffes are locked in their barn when temperatures fall below 7 °C, and they are given barn access when temperatures are below 10 °C with situational adjustments based on other factors, such as wind and rain. While on open pasture there may be various interspecies interactions. For example, at FR, the giraffes share a pasture with red deer (Cervus elaphus), Arabian oryx (Oryx leucoryx), Hartmann's mountain zebra (Equus zebra hartmannae), Nile lechwe (Kobus megaceros), and fallow deer (Dama dama). Whereas BP manages its giraffe herd in the spring through fall in a 19 acre mixed species exhibit with addax (Addax nasomaculatus), bontebok (Damaliscus pygargus pygarus), Grant's zebra (Equus burchelli), addra gazelle (Nanger dama), and common waterbuck (Kobus ellipsiprymnus).
Samples, fecal and soil, were collected in Ziploc® bags and stored at either −15.6 °C or room temperature until shipped to The Ohio State University (OSU), College of Veterinary Medicine, Diagnostic Parasitology Laboratory no later than 2 weeks after collection. Fecal samples were collected after witnessing the individual animal defecate and when personel could safely collect the feces. Soil samples were also sent from sites where the giraffe are often housed and defecate and followed the same collection and shipping protocols as feces. An average of 100–400 g of soil was collected from each giraffe site. Coordinates from each soil sample site were run through a soil classification program (Wilson et al., 2007) at https://websoilsurvey.nrcs.usda.gov/app/WebSoilSurvey.aspx and soil types were recorded. The fecal and soil samples were all individually labelled, stored at room temperature, periodically moistened with water, and antibiotic and antimycotoxin solution (100 units/ml of penicillin, 100 μg/ml of streptomycin, and 0.25 μg/ml of Amphotericin B) in water were added to fecal samples as needed at OSU until processed. Once received at OSU, fecal and soil processing occurred anywhere from 7 days to about 3 months after receipt. Prior to processing for egg recovery, samples were well mixed to obtain a homogeneous mixture to ensure even distribution of the eggs through the representative sample.
2.4. Determination of the most effective flotation solution for fecal samples
All of the fecal samples were subjected to modified Stoll method (Zajac and Conboy, 2012) with different flotation media for an egg recovery quantitative analysis. Each sample was tested using four flotation solutions: Sheather's sugar (SG 1.27), sucrose (SG 1.40), ZnSO4 (SG 1.18), and MgSO4 (SG 1.15). These solutions were chosen based on previous study utility and the density of Trichuris eggs. Briefly, 2 g of feces were well mixed with 98 ml of water in a flask. From this flask, a quadruplicate set of 10 ml aliquots were placed into 15 ml centrifuge tubes. The tubes were spun at ~1800 g for 5 min. The water was removed, and the resulting pellet was resuspended in the designated flotation solution to be evaluated. The nearly full tubes underwent another centrifugation at ~1800 g for 5 min. After centrifugation, more flotation solution was added to make a bulging meniscus on each tube. Coverslips were placed on the meniscus and allowed to sit for 10 min before eggs were counted by microscopy. After the first coverslip was removed, more flotation solution was added to each tube to make a second bulging meniscus and another coverslip was allowed to sit for 10 min and read by microscopy to capture any additional eggs not recovered on the first coverslip. The EPG for each tube was recorded by multiplying the total number of Trichuris eggs found under both coverslips by five.
Based on preliminary results demonstrating disparate recovery of Trichuris eggs when comparing the sugar-based flotation solutions (greater quantity of eggs recovered) to the ZnSO4 and MgSO4 flotation solutions, an additional experiment was conducted. This involved using the fecal sediment material remaining at the bottom of the tube after the completion of the ZnSO4 and MgSO4 processed sample coverslips were read. This residual sediment is generally considered waste and discarded. However, fecal residual sediment samples from WO and FR sites post -ZnSO4 and post -MgSO4 were selected to undergo additional modified Stoll method using Sheather's sugar flotation to determine if the eggs remained in the residual sediment.
2.5. Determination of most efficient soil egg liberating solution
A soil recovery protocol was initially optimized testing TW-derived soil with Trichuris egg recovery and evaluated using four different detergents: 0.05% v/v Tween 20 in water, 0.1 N NaOH in water, 0.05% v/v Dawn™ (Procter and Gamble) in water, and 0.9% w/v NaCl in water on each individual soil sample. These ionic detergents were chosen based on their accessibility in a clinic setting and their non-toxic nature relative to waste generation and disposal. For BP, FR and WO, only NaCl and NaOH were used as the dispersion and first wash solution based on preliminary results of unreadable microscope slides using v/v Tween 20 and Dawn™ in water. This was followed by the use of Sheather's sugar (SG 1.27) as a flotation solution. Sheather's sugar was chosen as the flotation medium based on the results from the egg recovery from the fecal samples.
The details of the soil egg recovery involved using 50 g of soil in a glass beaker with 450 ml of the detergent or ionic solution. This was mixed by hand with a large, flat hand-held blade for 1 min, allowing the ionic solution to liberate the eggs from the soil. This slurry was then poured through a two to three mm wire weave strainer into a pilsner glass, allowed to sit for 30 s and then the supernatant containing the eggs was decanted into a 500 ml beaker. The eggs were allowed to settle in the beaker for 2 h. The supernatant was then carefully decanted, and the remaining sediment was rinsed into 15 ml centrifuge tubes. We aimed to have less than 5 ml of sediment in each centrifuge tube, so three to four centrifuge tubes were used per beaker of sample. The sediment was centrifuged (~1800 g) for 5 min and the supernatant was decanted. Approximately 3–5 ml of water was added to the centrifuge tubes and centrifuged (~1800 g) for 5 min again to wash the pellet and the supernatant was decanted. Sheather's sugar solution was added to the pellet and mixed well using mixing applicant sticks. The sediment-flotation media was centrifuged (~1800 g) for 5 min. After centrifugation, more flotation solution was added to make a bulging meniscus on each tube. Coverslips were placed on the meniscus and allowed to sit for 10 min. To obtain a number of eggs per 50 g of soil from each site, the coverslips were read by microscopy for Trichuris eggs and counts from the same site and liberating solution were summed.
2.6. Statistical analysis
To test the hypothesis that the solutions were different from one another in their ability to recover Trichuris eggs, pairwise comparisons of each solution were made using a two-sided Wilcoxon signed rank test using the EPG data on fecal samples and the egg count data on soil samples. This test was chosen due to the nonparametric data and the multiple observations for each sample. Statistical significance was set at p < 0.05.
3. Results
3.1. Survery results
Survey results for each study site are summarized (Table 1). Each facility differed on their detection protocols for Trichuris in giraffe. At WO, giraffe have access to elevated grain and hay rack feeding areas and hanging browse, and it is estimated that the heaviest defecation is between 100 and 300 feet from these feeding areas. At FR, giraffe are provided elevated feed troughs. At TW, in the case of new arrivals, weekly fecal exams are conducted while the giraffe is in quarantine and held in the same facility as the current collection. Clinical signs warranting fecal testing include abnormal stool production or decreasing weight or body condition. Giraffe are provided hanging browse and elevated feeders. At BP, clinical signs warranting fecal testing include soft stool or decreasing weight or body condition. Giraffe are also provided hanging browse and elevated feeders. Information collected from the study site surveys was used as background descriptive information, with no further comparative analysis relative to the soil and fecal data collected and analyzed.
Table 1.
Site location survey results.
| Location | Flotation procedure | Scheduled protocols | Feces removal and feeding methods |
|---|---|---|---|
| WO | Modified McMaster | Monthly fecal exam of all individuals and fecal examination based on clinical signs | Feces removed from giraffe barn daily, corral monthly, and never from pasture. Elevated feeding areas provided. |
| FR | Modified McMaster | Twice per year fecal examination of all individuals and fecal examination based on clinical signs | Feces removed from inhabited giraffe barns and yards weekly and never from pasture. Elevated feeding areas provided. |
| TW | Modified Stoll | Monthly fecal examination of all individuals and fecal examination based on clinical signs. Weekly fecal examination while in quarantine | Feces removed from inhabited giraffe barns daily and never from pastures. Elevated feeding areas provided. |
| BP | Passive flotation | Quarterly fecal examination on all individuals and fecal examination based on clinical signs. Monthly fecal examination on giraffe <1 yr old and new arrivals during their first year after exiting quarantine period. Weekly fecal examinations while in quarantine | Feces removed from giraffe indoor stalls and outdoor holding yards daily and never from pasture. Elevated feeding areas provided. |
3.2. Sample collection
Two individual fecal samples were collected from WO, three individual fecal samples were collected from FR, five individual fecal samples were collected from TW, and three individual fecal samples were collected from BP. Additionally, soil samples were collected from two sites at WO, two sites from FR, four sites from TW, and three sites from BP. The collection sites, coordinates, and soil types are presented (Table 2).
Table 2.
Description, coordinates, and soil types from study sites.
| Location | Enclosure | Coordinates | Soil Type |
|---|---|---|---|
| WO | Swale (in pasture) and behind corral | 30.742643, −81.730845 | Chaires fine sand and Goldhead fine sand |
| FR | Main pasture | 32.169325, −97.805368 | Sunev clay loam and Maloterre gravelly clay loam |
| FR | Giraffe pasture | 32.160912, −97.798373 | Brackett soils, Venus loam, and Frio silty clay |
| TW | Feeder in pasture, water trough in pasture, and giraffe barn fence line | 39.8367, −81.7189 | Morristown silty clay loam, reclaimed |
| BP | G1, G2, and G4a | 42.241512, −85.167761 | Oshtemo sandy loam |
Facility outdoor holding yard designation.
3.3. Egg recoveries from feces and soil
The Trichuris EPG of the modified Stoll's fecals are presented (Table 3a). EPGs of fecal flotation where ZnSO4 and MgSO4 flotation were initially used followed by immediate re-processing of sediment using Sheather's sugar flotation is presented (Table 3b). The Trichuris egg counts recorded from soil recovery are presented (Table 4).
Table 3a.
Individual modified Stoll procedures results.
| Location | Giraffe Identifier | Flotation Solution | First Coverslip | Second Coverslip | EPG in Feces |
|---|---|---|---|---|---|
| WO | 1 | Sheather's | 29 | 1 | 150 |
| Sucrose | 23 | 2 | 125 | ||
| ZnSO4 | 0 | 6 | 30 | ||
| MgSO4 | 2 | 1 | 15 | ||
| WO | 2 | Sheather's | 0 | 0 | 0 |
| Sucrose | 0 | 0 | 0 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 1 | 5 | ||
| FR | 3 | Sheather's | 28 | 3 | 155 |
| Sucrose | 58 | 5 | 315 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 0 | 0 | ||
| FR | 4 | Sheather's | 9 | 2 | 55 |
| Sucrose | 14 | 7 | 105 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 0 | 0 | ||
| FR | 5 | Sheather's | 3 | 0 | 15 |
| Sucrose | 1 | 3 | 20 | ||
| ZnSO4 | 1 | 3 | 20 | ||
| MgSO4 | 0 | 1 | 5 | ||
| TW | 6 | Sheather's | 0 | 0 | 0 |
| Sucrose | 1 | 1 | 10 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 0 | 0 | ||
| TW | 7 | Sheather's | 0 | 0 | 0 |
| Sucrose | 1 | 1 | 10 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 0 | 0 | ||
| TW | 8 | Sheather's | 0 | 1 | 5 |
| Sucrose | 0 | 0 | 0 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 0 | 0 | ||
| TW | 9 | Sheather's | 2 | 0 | 10 |
| Sucrose | 8 | 0 | 40 | ||
| ZnSO4 | 2 | 0 | 10 | ||
| MgSO4 | 0 | 0 | 0 | ||
| TW | 10 | Sheather's | 1 | 0 | 5 |
| Sucrose | 0 | 0 | 0 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 0 | 0 | ||
| BP | 11Aa | Sheather's | 1 | 0 | 5 |
| Sucrose | 0 | 0 | 0 | ||
| ZnSO4 | 1 | 0 | 5 | ||
| MgSO4 | 0 | 0 | 0 | ||
| BP | 11Ba | Sheather's | 3 | 1 | 20 |
| Sucrose | 5 | 1 | 30 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 1 | 0 | 5 | ||
| BP | 12 | Sheather's | 0 | 0 | 0 |
| Sucrose | 0 | 0 | 0 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 0 | 0 | ||
| BP | 13Ab | Sheather's | 1 | 0 | 5 |
| Sucrose | 2 | 0 | 10 | ||
| ZnSO4 | 0 | 0 | 0 | ||
| MgSO4 | 0 | 0 | 0 | ||
| BP | 13Bb | Sheather's | 7 | 0 | 35 |
| Sucrose | 5 | 0 | 25 | ||
| ZnSO4 | 0 | 2 | 10 | ||
| MgSO4 | 0 | 0 | 0 |
11A and 11B were taken 1 week apart from the same individual.
13A and 13B were taken 1 week apart from the same individual.
Table 3b.
Results of initial and reprocessing of selected samples.
| Location | Giraffe | Initiala | EPGb | EPGc |
|---|---|---|---|---|
| WO | 1 | ZnSO4 | 0 | 205 |
| WO | 1 | MgSO4 | 10 | 215 |
| FR | 3 | ZnSO4 | 0 | 350 |
| FR | 3 | MgSO4 | 0 | 450 |
Flotation solution used for initial processing.
Eggs per gram recovered after initial processing.
Eggs per gram results from residual sediment reprocessed using Sheather's sugar (SG 1.27).
Table 4.
Results of soil recovery protocol.
| Location | Enclosure | Suspensory fluid | Egg count | Observations |
|---|---|---|---|---|
| TW | Feeder | Tween | 0 | |
| Dawn | 0 | Too many bubbles | ||
| NaOH | 1 | 57.5 μm x 32.5 μma | ||
| NaCl | 1 | |||
| TW | Water trough | Tween | 0 | |
| Dawn | 0 | |||
| NaOH | 0 | |||
| NaCl | 0 | |||
| TW | Barn fence line | Tween | 0 | |
| Dawn | 0 | Too many bubbles | ||
| NaOH | 0 | Too many bubbles | ||
| NaCl | 0 | |||
| FR | Main pasture | NaOH | 0 | Too many bubbles |
| NaCl | 0 | |||
| FR | Pasture | NaOH | 0 | Many mites and strongyles |
| NaCl | 0 | Many mites and strongyles | ||
| BP | G1b | NaOH | 7 | |
| NaCl | 87 | 67.5 μm × 35.0 μm | ||
| BP | G2b | NaOH | 5 | |
| NaCl | 34 | 72.5 μm × 35.0 μm | ||
| BP | G4b | NaCl | 10 | |
| WO | Swale | NaOH | 1 | 55.0 μm × 37.5 μm |
| NaCl | 0 | |||
| WO | Behind corral | NaOH | 0 | |
| NaCl | 2 | 70.0 μm × 37.5 μm |
Recovered egg length by width measurements in microns.
Facility outdoor holding yard designation.
3.4. Statistical analysis and egg recovery comparison
The results of the fecal EPG statistical analysis are recorded (Table 5) and support the hypothesis that the solutions are different regarding egg recovery. Sheather's sugar and sucrose solutions consistently recovered significantly more eggs compared to MgSO4 and ZnSO4. Often, a solution recovered no eggs using MgSO4 or ZnSO4, whereas eggs were recovered using Sheather's sugar or sucrose. The Sheather's sugar and sucrose solutions were not statistically different, meaning one solution did not detect more eggs than the other. However, sucrose was more technically difficult to obtain a good mixture of the sediment due to its intense viscosity as compared to Sheather's sugar solution. Additionally, MgSO4 and ZnSO4 were not significantly different in their egg recoveries compared to one another.
Table 5.
Two sided Wilcoxon signed rank test p-values for pairwise comparisons.
Values less than 0.05 were significantly different.
For soil recovery of Trichuris eggs, NaCl and NaOH dispersion solutions were not significantly different (p = 0.25). This may be due to the low number of Trichuris positive soil samples. Initially after processing soil samples from TW, it was determined that Tween 20 and Dawn™ (Procter and Gamble) detergent generated too many bubbles under the coverslip for readable slides, thus their use was discontinued.
It was determined that the number of eggs recovered from individual giraffe feces did not parallel the number of eggs recovered from the corresponding soil location. Individual giraffes with the highest EPG came from FR and WO; however, the most soil eggs were recovered from BP. The Wilds had the highest number of positive individuals (n = 5), but neither the highest EPG or soil egg recovery.
4. Discussion
The survey results of this study found that many sites were currently utilizing passive flotation techniques to analyze their fecal samples. The use of a modified Stoll technique, which utilizes centrifugation, rather than a passive flotation protocol for Trichuris egg detection is further supported by other fecal detection protocol comparison studies. In a study comparing passive fecal flotation results to ZnSO4 (SG 1.2) centrifugation, passive fecal flotation identified Trichuris positive results in only 54.2% of T. vulpis positive canine feces, thereby missing 45.8% of Trichuris positive individuals (Gates and Nolan, 2009). In another study which compared camelid fecal examination using a modified McMasters technique to a centrifugation-flotation procedure, the centrifugation-flotation procedure yielded more positive Trichuris results, as well as more positive results for Eimeria macusaniensis oocysts, Nematodirus, and capillarids-type eggs (Cebra and Stang, 2008). The mean egg recovery rate for the modified McMasters procedure was determined to be 46.4% with reliable positive results as of 500 EPG, whereas centrifugation-flotation technique with ZnSO4 flotation (SG 1.3) had reliable positive results as of 80 EPG (Becker et al., 2016). These studies all support that the use of a centrifugation-flotation procedure is more effective at identifying positive Trichuris egg shedding individuals than a passive fecal flotation, and that eggs from low fecundity parasites like Trichuris are less likely to be detected using a modified McMaster technique.
Though some samples were kept at room temperature for 2 weeks without any preserving solution before processing, Trichuris eggs were still recovered from held soil and fecal samples. This egg recovery ability is consistent with other studies which found that Trichuris has the ability to remain in the environment for years (Burden and Hammet, 1979; Burden et al., 1987). This finding also supports that enclosure and soil contamination with Trichuris eggs poses a risk to susceptible host species within that enclosure for potentially years.
In a study comparing flotation-centrifugation with zinc chloride (SG 1.45) to flotation-centrifugation with sucrose (SG 1.2), zinc chloride was able to identify more Trichuris positive results and recover more Trichuris eggs (Taglioretti et al., 2014). This differs from our finding that sucrose SG 1.40 flotation recovered statistically similar numbers of eggs to Sheather's sugar SG 1.27. Additionally, a soil egg recovery method has been previously described by Horiuchi and Uga (2016) which found that using 0.05% Tween as a detergent and sucrose (SG 1.2) was effective at recovering Toxocara eggs in soil, and also detected Trichuris eggs in soil. However, we found that 0.05% v/v Tween produced unreadable slides. The 0.1N NaOH or 0.9% w/v NaCl produced readable slides as compared to the detergent based dispersion solution which did not.
Possible variables which could alter the number of Trichuris eggs found in fecal and soil samples include host treatment protocol for Trichuris being utilized at each site, other direct hosts or aberrant hosts for Trichuris that may be sharing enclosures with the giraffe affected, and population density of the giraffe relative to enclosure size and soil substrate matrix of the environment. In a study where soil samples were gathered from Kenya and Bangladesh, it was found that texture of the soil effected soil transmitted helminths (STH) egg recovery, where sandy samples had higher recovery than loamy samples (Steinbaum et al., 2016). According to Collender et al. (2015), the recovery efficiency from highest to lowest would be sand, loam, and then clay. In this study, Trichuris eggs were found in clay, loam soil at TW and sandy, loam soil at BP. Eggs were not found in fine sand soil at WO or in clay, loam at FR.
Individual susceptibility parameters for parasitism may also contribute to increased parasite loads, such as age, body mass, and pregnancy status (Pauling et al., 2016). Additionally, due to the fecal-oral spread of Trichuris, other factors which can impact exposure include type and quality of forage supplied, how long and on what substrate animals are allowed to graze, and time at which feces are removed from the grazing area and enclosures. Feed or feces which is left near egg contaminated soil for longer than three weeks is at greater risk of containing infective Trichuris eggs. At all sites, giraffe are provided elevated feeding areas and feces are removed from indoor areas daily or weekly. However, routine feces removal on pasture is not conducted. Therefore, giraffe on pasture are at greater risk of ingesting infective eggs, especially those which graze. In a wild environment, giraffe are known to be browsers which may reduce their exposure to STH, such as Trichuris (VanderWaal et al., 2014). However, at all sites sampled in this study the giraffe are allowed grazing on pasture thereby increasing their exposure to STH, though some individuals graze more than others. For example, at TW giraffe are often seen grazing on pasture, whereas at FR and WO grazing is not witnessed often.
The small number of animals sampled with respect to total herd sizes is a limitation of this study. However, this study determined that a modified Stoll method utilizing higher specific gravity Sheather's sugar or sucrose flotation solution was significantly more effective than ZnSO4 or MgSO4 for detection of Trichuris eggs from the sampled giraffe feces. Both Sheather's sugar SG 1.27 and sucrose SG 1.40 identified positive individuals, recovering statistically similar EPGs. As soil dispersion solutions, the NaOH and NaCl were not statistically different and trended towards being more effective at liberating the giraffe Trichuris eggs from soil compared to Tween 20 or Dawn™ (Procter and Gamble) due to their ability to produce readable slides. Another limitation of this study was the low number of positive Trichuris soil samples. Statistical significance may have been determined between the NaOH and NaCl had more positive soil samples been analyzed. Additionally, the different sized Trichuris eggs recovered from each site suggest the presence of different Trichuris spp. contaminating the enclosures or among giraffe populations.
The fecal and soil protocols optimized in this study can be utilized by institutions for routine detection of Trichuris and subsequent treatment. In the cases where Trichuris infection is a chronic herd problem, routine fecal and soil monitoring can support decisions which limit exposure of susceptible individuals. For example, Trichuris positive individuals should have their feces removed from enclosures as soon as possible. Routine monitoring of Trichuris is important for host health, as well as judicious treatment protocols.
In conclusion, this study demonstrates that the use of ZnSO4 SG 1.18 or MgSO4 SG 1.15 for centrifugation fecal flotation will underestimate or miss animals shedding Trichuris eggs. The study results support the use of Sheather's sugar or sucrose flotation solution with at least SG 1.27 along with centrifugation for fecal egg detection. In addition, NaOH and NaCl are successful ionic solutions for liberating eggs from the soil types analyzed in this study.
Funding
This work was supported by a T35 NIH Training Grant (T35OD010977) and The Ohio State University College of Veterinary Medicine. The funding source had no involvement in study design; collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declaration of competing interest
To the best of the authors’ knowledge regarding conflict of interest, declarations of interest: none.
Acknowledgments
We thank the Departments of Animal Management, Animal Health and Wildlife Ecology at The Wilds for technical assistance. We thank veterinary and giraffe management staff at White Oak Conservation, Dr. Holly Haefele at Fossil Rim Wildlife Center, and Dr. Kimberly Thompson at Binder Park Zoo for sample collection and submission, and for insight into the project. We thank Cathy Bremer (OSU) for assistance in receiving samples and Faith Hagelberger (OSU) for aid in sample processing. We thank Dr. Aaron Wilson at the State Climate Office of Ohio for data interpretation.
References
- Becker A.C., Kraemer A., Epe C., Strube C. Sensitivity and efficiency of selected coproscopical methods—sedimentation, combined zinc sulfate sedimentation-flotation, and McMaster method. Parasitol. Res. 2016;115:2581–2587. doi: 10.1007/s00436-016-5003-8. [DOI] [PubMed] [Google Scholar]
- Bertelsen M.F. vol. 8. W. B. Saunders; Philadelphia: 2015. pp. 602–610. (Giraffidae. Fowler's Zoo and Wild Animal Medicine). [Google Scholar]
- Body G., Ferté H., Gaillard J., Delorme D., Klein F., Gilot-Fromont E. Population density and phenotypic attributes influence the level of nematode parasitism in roe deer. Oecologia. 2011;167(3):635–646. doi: 10.1007/s00442-011-2018-9. [DOI] [PubMed] [Google Scholar]
- Burden D.J., Hammet N.C. The development and survival of Trichuris suis ova on pasture plots in the south of England. Res. Vet. Sci. 1979;26:66–70. [PubMed] [Google Scholar]
- Burden D.J., Hammet N.C., Brooks P.A. Field Observations on the longevity of Trichuris suis ova. Vet. Rec. 1987;121:43. doi: 10.1136/vr.121.2.43. [DOI] [PubMed] [Google Scholar]
- Cebra C.K., Stang B.V. Comparison of methods to detect gastrointestinal parasites in llamas and alpacas. J. Am. Vet. Med. Assoc. 2008;232(5):733–741. doi: 10.2460/javma.232.5.733. [DOI] [PubMed] [Google Scholar]
- Collender P.A., Kirby A.E., Addiss D.G., Freeman M.C., Remais J.V. Methods for quantification of soil-transmitted helminths in environmental media: current techniques and recent advances. Trends Parasitol. 2015;31(12):625–639. doi: 10.1016/j.pt.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Barbosa A., Pinheiro J.L., Dos Santos C.R., de Lima C.S.C.C., Dib L.V., Echarte G.V., Augusto A.M., Bastos A.C.M.P., Uchôa C.M.A., Bastos O.M.P., Santos F.N., Fonseca A.B.M., Amendoeira M.R.R. Gastrointestinal parasites in captive animals at the rio de Janeiro zoo. Acta Parasitol. 2020;65:237–249. doi: 10.2478/s11686-019-00145-6. [DOI] [PubMed] [Google Scholar]
- David E., Lindquist W. Determination of the specific gravity of certain helminth eggs using sucrose density gradient centrifugation. J. Parasitol. 1982;68(5):916–919. [PubMed] [Google Scholar]
- Dryden M.W., Payne P.A., Ridley R., Smith V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet. Therapeut. 2005;6:15–28. [PubMed] [Google Scholar]
- Gates M.C., Nolan T.J. Comparison of passive fecal flotation run by veterinary students to zinc-sulfate centrifugation flotation run in a diagnostic Parasitology laboratory. J. Parasitol. 2009;95(5):1213–1214. doi: 10.1645/GE-2058.1. [DOI] [PubMed] [Google Scholar]
- Geng J., Elsemore D.A., Oudin N., Ketzis J.K. Diagnosis of feline whipworm infection using a coproantigen ELISA and the prevalence in feral cats in southern Florida. Vet Parasitol Reg Stud Rep. 2018;14:181–186. doi: 10.1016/j.vprsr.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Golek H.I., Al-Saeed A.T. Contamination of soil with Toxocara and other helminths in soils of amadyia district, duhok governorate, Kurdistan region - Iraq. Appl. Ecol. Environ. Res. 2019;17(6):14883–14891. [Google Scholar]
- Horiuchi S., Uga S. Modified flotation method, an effective technique for recovering helminth eggs in soil. Parasitol. Int. 2016;65(5):576–579. doi: 10.1016/j.parint.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Kyriánová I., Drnek J., Langrová I., Peřinková P., Nechybová S. Gastrointestinal parasites in giraffes kept in zoological gardens of the Czech republic. Sci. Agric. Bohem. 2017;48(3):122–126. [Google Scholar]
- Miller J.E., Kaplan R.M., Pugh D.G. second ed. W. B. Saunders; Philadelphia: 2012. Internal Parasites. Sheep and Goat Medicine; pp. 106–125. [Google Scholar]
- Mir A.Q., Dua K., Singla L.D., Sharma S., Singh M.P. Prevalence of parasitic infection in captive wild animals in Bir Moti Bagh mini zoo (Deer Park), Patiala, Punjab. Vet. World. 2016;9(6):540–543. doi: 10.14202/vetworld.2016.540-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C.D., Oller A.R., Jackson V. Fecal parasite identification by microscopy and PCR in scimitar-horned oryx, Oryx dammah, managed at two sites. Int J Parasitol Parasites Wildl. 2016;5(3):312–320. doi: 10.1016/j.ijppaw.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman J., Meader L., Mudakikwa A., Foster J., Patton S. Gastrointestinal Parasites of Mountain Gorillas (Gorilla gorilla beringei) in the Parc National des Volcans, Rwanda. J. Zoo Wildl. Med. 2000;31(3):322–328. doi: 10.1638/1042-7260(2000)031[0322:GPOMGG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Steinbaum L., Kwong L.H., Ercumen A., Negash M.S., Lovely A.J., Njenga S.M., Nelson K.L. Detecting and enumerating soil-transmitted helminth eggs in soil: new method development and results from field testing in Kenya and Bangladesh. PLoS Neglected Trop. Dis. 2017;11(4) doi: 10.1371/journal.pntd.0005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglioretti V., Sardella N., Fugassa M. Effectiveness of coproscopic concentration techniques. Helminthologia. 2014;51(3):210–214. [Google Scholar]
- Tan L., Wang A., Yi J., Liu Y., Li J., Liu W. Prevalence and phylogenetic analyses of Trichuris suis in pigs in hunan province, subtropical China. Kor. J. Parasitol. 2018;56(5):495–500. doi: 10.3347/kjp.2018.56.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun S., Ithoi I., Mahmud R., Samsudin N.I., Kek Heng C., Yee Ling L. Detection of helminth eggs and identification of hookworm species in stray cats, dogs and soil from Klang valley, Malaysia. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0142231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Metre D.C., Tennant B.C., Whitlock R.H., Divers Thomas J., Peek Simon F. Rebhun's Diseases of Dairy Cattle. second ed. W.B. Saunders; 2008. Chapter 6 - infectious diseases of the gastrointestinal tract; pp. 200–294. ISBN 9781416031376. [Google Scholar]
- VanderWaal K., Omondi G.P., Obanda V. Mixed-host aggregations and helminth parasite sharing in an East African wildlife–livestock system. Vet. Parasitol. 2014;205(1–2):224–232. doi: 10.1016/j.vetpar.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Williamson Lisa H., Van Saun Robert J., Johnson LaRue W. Chapter 6 - anthelmintic resistance in camelid parasites. In: Cebra Christopher, Anderson David E., Tibary Ahmed, Llama, Care Alpaca, Saunders W.B., editors. 2014. pp. 16–22. 9781437723526. [Google Scholar]
- Wilson, A. State Climate Office of Ohio. The Ohio State University. NOAA Cooperative Network Data Obtained from the Midwestern Regional Climate Center, Cli-MATE (MRCC Application Tools Environment, on/CLIMATE/”). Accessed online on April 12, 2021.
- Wilson M.A., Burt R., Indorante S.J., Jenkins A.B., Chiaretti J.V., Ulmer M.G., Scheyer J.M. Geochemistry in the modern soil survey program. Environ. Monit. Assess. 2007;139(1–3):151–171. doi: 10.1007/s10661-007-9822-z. [DOI] [PubMed] [Google Scholar]
- Zajac A.M., Conboy G.A. John Wiley & Sons, Incorporated; Ames, IA: 2012. Veterinary Clinical Parasitology. ISBN 9781118292037. [Google Scholar]